Carotenogenic Activity of Two Hypersaline Greek Dunaliella salina Strains under Nitrogen Deprivation and Salinity Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Strain Isolation
2.2. Experimental Design
2.3. Analytical Procedures
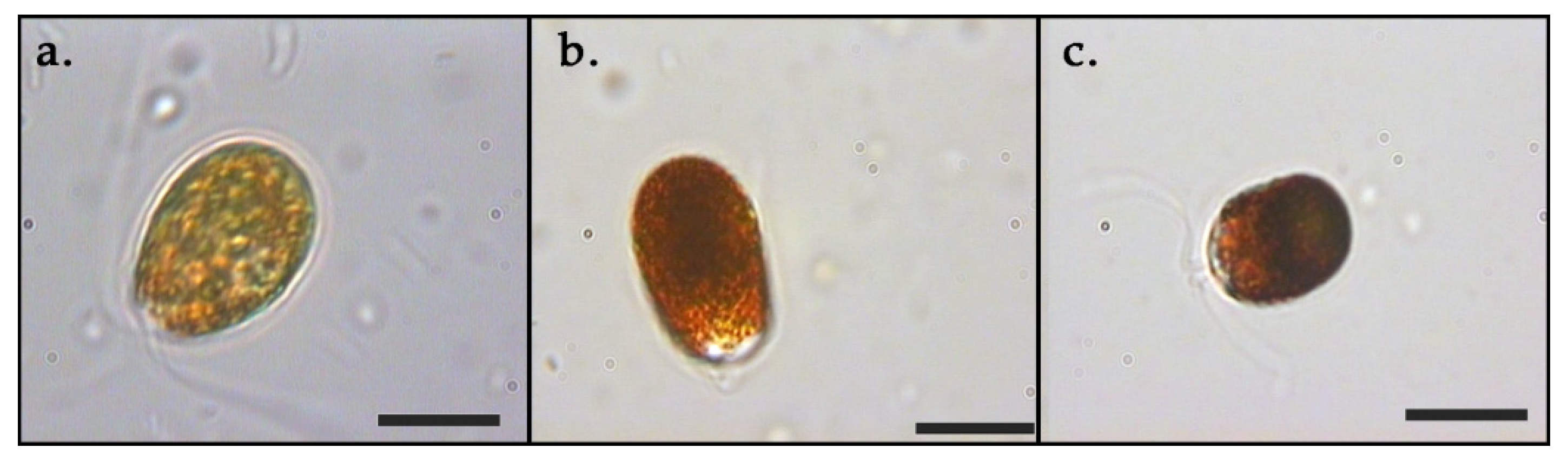
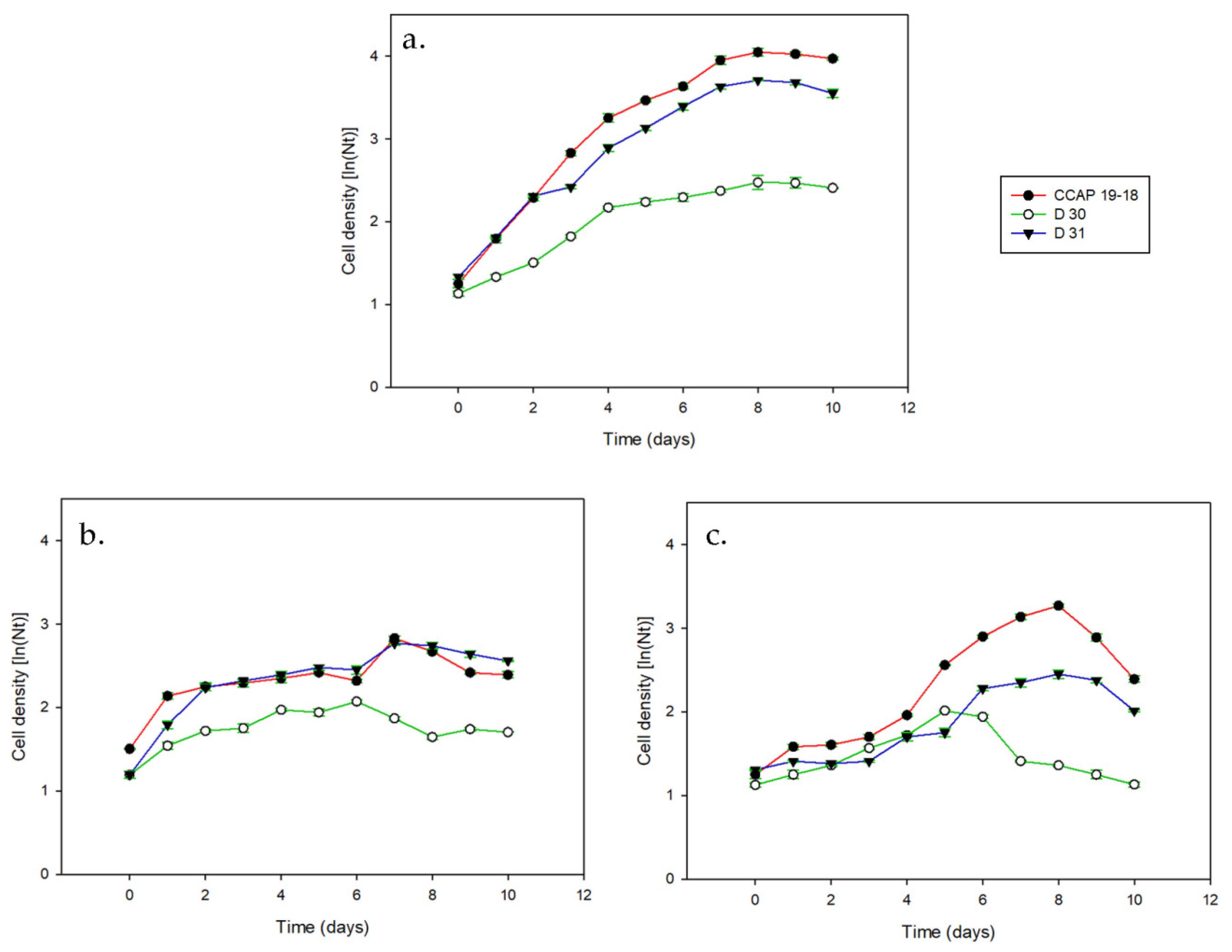
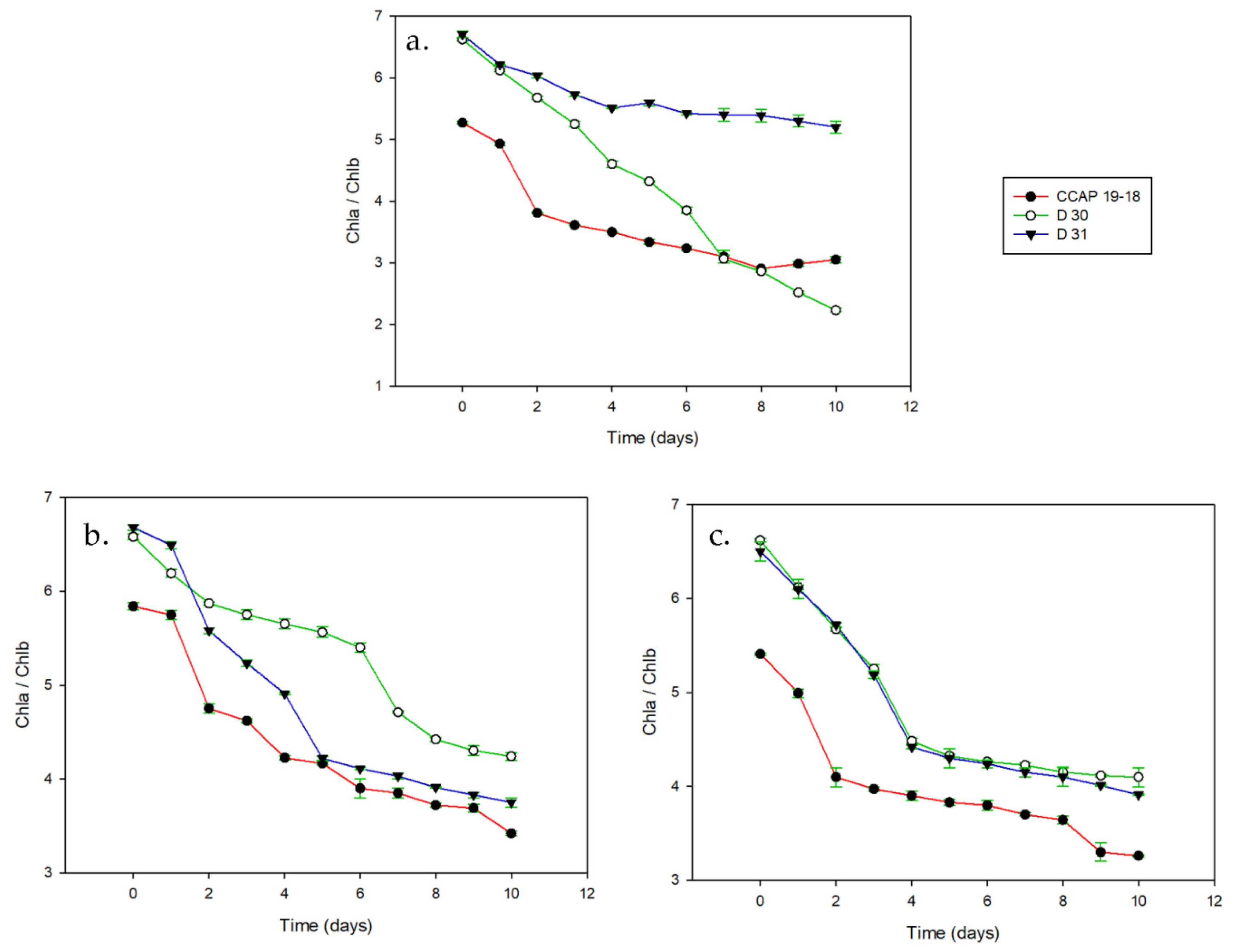
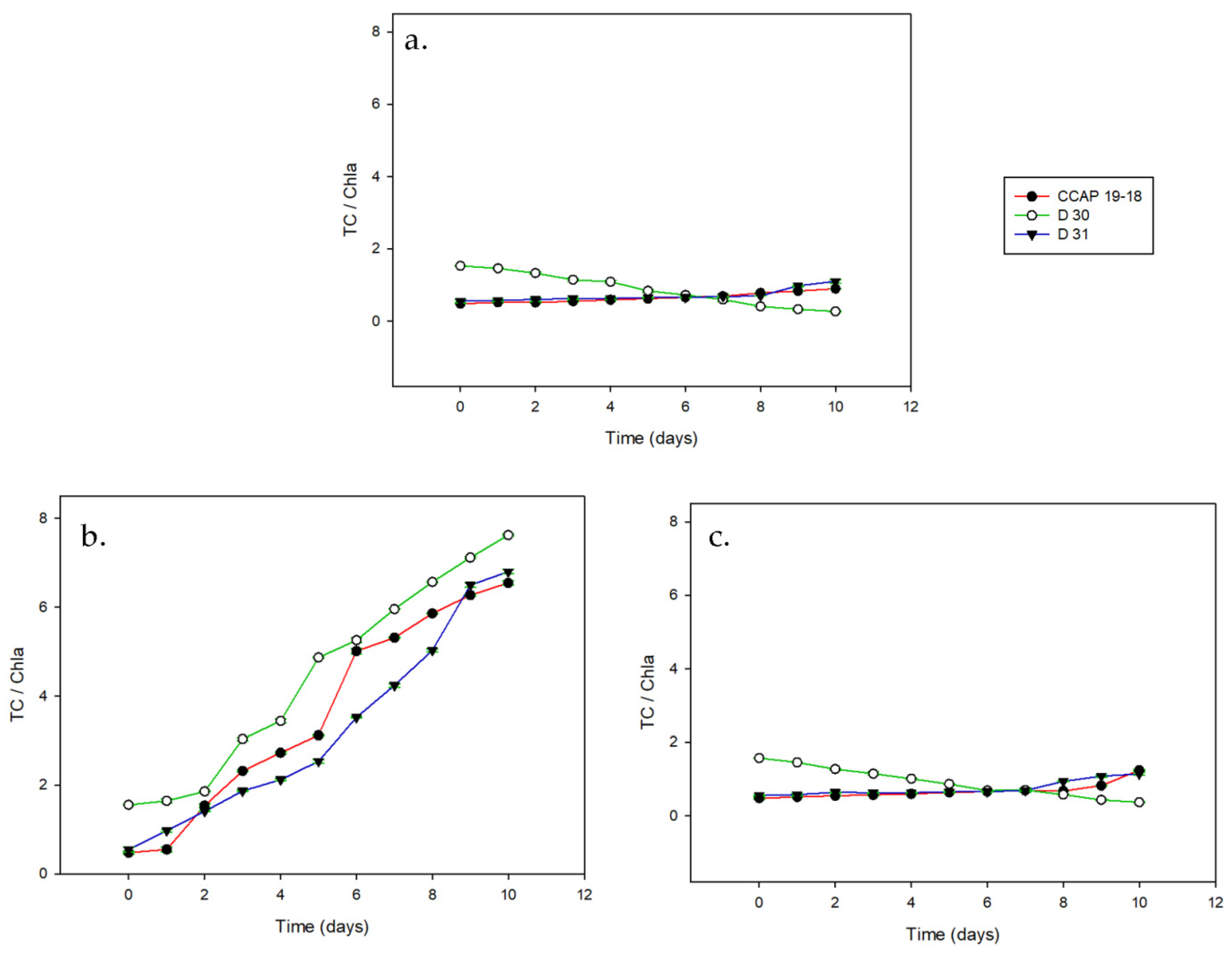
3. Results
3.1. Growth and Pigments in Daily Basis
3.2. Carotenoid Content on a Dry Basis
3.3. Carotenoids Composition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borowitzka, L.J.; Moulton, T.P.; Borowitzka, M.A. The mass culture of Dunaliella salina for fine chemicals: From laboratory to pilot plant. In Proceedings of the Eleventh International Seaweed Symposium, Developments in Hydrobiology, Qingdao, China, 19–25 June 1983. [Google Scholar] [CrossRef]
- Saha, S.K.; Murray, P. Exploitation of microalgae species for nutraceutical purposes: Cultivation aspects. Fermentation 2018, 4, 46. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Commercial-scale production of microalgae for bioproducts. In Blue Biotechnology: Production and Use of Marine Molecules; La Barre, S., Bates, S.S., Eds.; Wiley-VCH: Weinheim, Germany, 2018; Volume 1, pp. 33–65. [Google Scholar] [CrossRef]
- Winwood, R.J. Recent developments in the commercial production of DHA and EPA rich oils from micro-algae. Ocl 2013, 20, D604. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Raja, R.; Hemaiswarya, S.; Rengasamy, R. Exploitation of Dunaliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007, 74, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Teodoresco, E.C. Organisation et développement du Dunaliella, nouveau genre de Volvocacée-Polyblepharidée. Beih. Z Bot. Centralbl. 1905, 18, 215–232. [Google Scholar]
- Ben-Amotz, A.; Avron, M. On the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant. Physiol. 1983, 72, 593–597. [Google Scholar] [CrossRef]
- Hashemi, A.; Moslemi, M.; Pajoum Shariati, F.; Delavari Amrei, H. Beta-carotene production within Dunaliella salina cells under salt stress condition in an indoor hybrid helical-tubular photobioreactor. Can. J. Chem. Eng. 2020, 98, 69–74. [Google Scholar] [CrossRef]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliella salina, a unicellular green microalga. J. Biotechnol. 2012, 162, 21–27. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Siva, C.J. The taxonomy of the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis on the marine and halophilic species. J. Appl. Phycol. 2007, 19, 567–590. [Google Scholar] [CrossRef]
- Oren, A. The ecology of Dunaliella in high-salt environments. J. Biol. Res. (Thessalon) 2014, 21, 23. [Google Scholar] [CrossRef]
- Massjuk, N.P. Morphology, Systematics, Ecology, Geographic Distribution of Genus Dunaliella Teod, and Perspectives of Its Practical Use; Nauk, Dumka Press: Kiev, Ukraine, 1973. [Google Scholar]
- Ben-Amotz, A.; Avron, M. The role of glycerol in the osmotic regulation of the halophilic alga Dunaliella parva. Plant. Physiol. 1973, 51, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Montazeri-Najafabady, N.; Negahdaripour, M.; Salehi, M.H.; Morowvat, M.H.; Shaker, S.; Ghasemi, Y. Effects of osmotic shock on production of β-carotene and glycerol in a naturally isolated strain of Dunaliella salina. J. Appl. Pharm. Sci. 2016, 8, 160–163. [Google Scholar] [CrossRef]
- Larcher, W. Streß bei Pflanzen. Naturwissenschaften 1987, 74, 158–167. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Katz, A.; Avron, M. Accumulation of β-carotene in halotolerent algae: Purification and characterization of β-carotene-rich globules from Dunaliella bardawil (Chlorophyceae). J. Phycol. 1982, 18, 529–537. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Shaish, A.; Avron, M. Mode of action of the massively accumulated β-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol. 1989, 91, 1040–1043. [Google Scholar] [CrossRef]
- Loeblich, L.A. Action spectra and effect of light-intensity on growth, pigments and photosynthesis in Dunaliella salina. J. Protozool. 1974, 21, 420. [Google Scholar] [CrossRef]
- Loeblich, L.A. Growth limitation of Dunaliella salina by CO2 at high salinity. J. Phycol. 1970, 6, 9. [Google Scholar]
- Shaish, A.; Avron, M.; Pick, U.; Ben-Amotz, A. Are active oxygen species involved in induction of β-carotene in Dunaliella bardawil? Planta. 1993, 190, 363–368. [Google Scholar] [CrossRef]
- Xi, Y.; Kong, F.; Chi, Z. ROS induce β-carotene biosynthesis caused by changes of photosynthesis efficiency and energy metabolism in Dunaliella salina under stress conditions. Front. Bioeng. Biotechnol. 2021, 8, 1447. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, X.; Wang, M.; Zhang, W.; Zhou, B.; Wang, Y. ROS and calcium signaling mediated pathways involved in stress responses of the marine microalgae Dunaliella salina to enhanced UV-B radiation. J. Photochem. Photobiol. B Biol. 2017, 173, 360–367. [Google Scholar] [CrossRef]
- Dring, M.J. Stress resistance and disease resistance in seaweeds: The role of reactive oxygen metabolism. Adv. Bot. Res. 2006, 43, 175–207. [Google Scholar] [CrossRef]
- Tammam, A.A.; Fakhry, E.M.; El-Sheekh, M. Effect of salt stress on antioxidant system and the metabolism of the reactive oxygen species in Dunaliella salina and Dunaliella tertiolecta. Afr. J. Biotechnol. 2011, 10, 3795–3808. [Google Scholar]
- Mueller, L.; Boehm, V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Harvey, P.J.; Ben-Amotz, A. Towards a sustainable Dunaliella salina microalgal biorefinery for 9-cis β-carotene production. Algal Res. 2020, 50, 102002. [Google Scholar] [CrossRef]
- Wolf, L.; Cummings, T.; Müller, K.; Reppke, M.; Volkmar, M.; Weuster-Botz, D. Production of β-carotene with Dunaliella salina CCAP19/18 at physically simulated outdoor conditions. Eng. Life Sci. 2021, 21, 115–125. [Google Scholar] [CrossRef]
- Neves, M.I.; Silva, E.K.; Meireles, M.A. Trends and challenges in the industrialization of natural colorants. Food Public Health 2019, 9, 33–44. [Google Scholar] [CrossRef]
- Kleinegris, D.M.; Janssen, M.; Brandenburg, W.A.; Wijffels, R.H. The selectivity of milking of Dunaliella salina. Mar. Biotechnol. 2010, 12, 14–23. [Google Scholar] [CrossRef][Green Version]
- Morowvat, M.H.; Ghasemi, Y. Culture medium optimization for enhanced β-carotene and biomass production by Dunaliella salina in mixotrophic culture. Biocatal. Agric. Biotechnol. 2017, 7, 217–223. [Google Scholar] [CrossRef]
- Gallego-Cartagena, E.; Castillo-Ramírez, M.; Martínez-Burgos, W. Effect of stressful conditions on the carotenogenic activity of a Colombian strain of Dunaliella salina. Saudi J. Biol. Sci. 2019, 26, 1325–1330. [Google Scholar] [CrossRef]
- Gómez, P.I.; González, M.A. Genetic polymorphism in eight Chilean strains of the carotenogenic microalga Dunaliella salina Teodoresco (Chlorophyta). Biol. Res. 2001, 34, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.I.; González, M.A. Genetic variation among seven strains of Dunaliella salina (Chlorophyta) with industrial potential, based on RAPD banding patterns and on nuclear ITS rDNA sequences. Aquaculture 2004, 233, 149–162. [Google Scholar] [CrossRef]
- Guevara, M.; Lodeiros, S.; Gómez, O.; Lemus, N.; Núñez, P.; Romero, L.; Vásquez, A.; Rosales, N. Carotenogénesis de cinco cepas del alga Dunaliella sp. (Chlorophyceae) aisladas de lagunas hipersalinas de Venezuela. Rev. Biol. Trop. 2005, 53, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Walne, P.R. Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria and Mytilus. Fish. Invest. Ser. 2 1970, 26, 5. [Google Scholar]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Andersen, R.A.; Kawachi, M. Traditional Microalgae Isolation Techniques. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elservier Academic Press: Oxford, UK, 2005; pp. 90–92. [Google Scholar]
- Koletti, K.; Chantzistrountsiou, X.; Ntzouvaras, A.; Tzovenis, I.; Flemetakis, E.; Economou-Amilli, A. Identification and phylogeny of microalgae strains from Greek coastal lagoons (tentative title). A. EconomoU-Amilli, Sector of Ecology & Systematics, Department of Biology, School of Science, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15784 Athens, Greece. 2022; manuscript in preparation. [Google Scholar]
- Kleinegris, D.M.; Janssen, M.; Brandenburg, W.A.; Wijffels, R.H. Continuous production of carotenoids from Dunaliella salina. Enzyme Microb. Technol. 2011, 48, 253–259. [Google Scholar] [CrossRef]
- Hashemi, A.; Pajoum Shariati, F.; Delavari Amrei, H.; Heydari Nasab, A. The Effect of Instantaneous and Slow-Release Salt Stress Methods on Beta-Carotene Production within Dunaliella Salina Cells. IJCCE. 2021, 40, 1642–1652. [Google Scholar] [CrossRef]
- He, Q.; Lin, Y.; Tan, H.; Zhou, Y.; Wen, Y.; Gan, J.; Zhang, Q. Transcriptomic profiles of Dunaliella salina in response to hypersaline stress. BMC Genomics 2020, 21, 115. [Google Scholar] [CrossRef]
- Borovkov, A.B.; Gudvilovich, I.N.; Avsiyan, A.L.; Memetshaeva, N.O.A.; Lelekov, A.S.; Novikova, T.M. Production characteristics of Dunaliella salina at two-phase pilot cultivation (Crimea). Turkish J. Fish. Aquat. Sci. 2019, 20, 401–408. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Britton, G.; Liaanen-Jensen, S.; Pfander, H. Carotenoids. Handbook; Springer Science & Business Media: Berlin, Germany, 2004. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 99, 114. [Google Scholar] [CrossRef]
- Ben-Amotz, A. Effect of irradiance and nutrient deficiency on the chemical composition of Dunaliella bardawil Ben-Amotz and Avron (Volvocales, Chlorophyta). J. Plant Physiol. 1987, 131, 479–487. [Google Scholar] [CrossRef]
- Lamers, P.P.; van de Laak, C.C.W.; Kaasenbrood, P.S.; Lorier, J.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol. Bioeng. 2010, 106, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Semenenko, V.E.; Abdullayev, A.A. Parametric control of β-carotene biosynthesis in Dunaliella salina cells under conditions of intensive cultivation. Fiz. Rast. 1980, 27, 31–41. [Google Scholar]
- Bonneford, H.; Moelants, N.; Talec, A.; Mayzaud, P.; Bernard, O.; Sciandra, A. Coupling and uncoupling of triglyceride and beta-carotene production by Dunaliella salina under nitrogen limitation and starvation. Biotechnol. Biofuels 2017, 10, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, X.; Kapoore, R.V.; Xu, C.; Vaidyanathan, S. Influence of nutrient status on the accumulation of biomass and lipid in Nannochloropsis salina and Dunaliella salina. Energy Convers. Manag. 2015, 106, 61–72. [Google Scholar] [CrossRef]
- Marín, N.; Morales, F.; Lodeiros, C.; Tamigneaux, E. Effect of nitrate concentration on growth and pigment synthesis of Dunaliella salina cultivated under low illumination and preadapted to different salinities. J. Appl. Phycol. 1998, 10, 405–411. [Google Scholar] [CrossRef]
- Jimenez, C.; Pick, U. Differential reactivity of [beta]-carotene isomers from Dunaliella bardawil toward oxygen radicals. Plant Physiol. 1993, 101, 385–390. [Google Scholar] [CrossRef]
- Zhekisheva, M.; Boussiba, S.; Khozin-Goldberg, I.; Zarka, A.; Cohen, Z. Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters1. J. Phycol. 2002, 38, 325–331. [Google Scholar] [CrossRef]
- Gómez, P.I.; Barriga, A.; Cifuentes, A.S.; Gonzalez, M.A. Effect of salinity on the quantity and quality of carotenoids accumulated by Dunaliella salina (strain CONC-007) and Dunaliella bardawil (strain ATCC 30861) Chlorophyta. Biol. Res. 2003, 36, 185–192. [Google Scholar] [CrossRef]
- Pisal, D.S.; Lele, S.S. Carotenoid production from microalga, Dunaliella salina. Indian J. Biotechnol. 2005, 4, 476–483. [Google Scholar]
- Geider, R.J.; Macintyre, H.L.; Graziano, L.M.; McKay, R.M.L. Responses of the photosynthetic apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorus limitation. Eur. J. Phycol. 1998, 33, 315–332. [Google Scholar] [CrossRef]
- Phadwal, K.; Singh, P.K. Effect of nutrient depletion on β-carotene and glycerol accumulation in two strains of Dunaliella sp. Bioresour. Technol. 2003, 90, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A.; Borowitzka, L.J.; Kessly, D. Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J. Appl. Phycol. 1990, 2, 111–119. [Google Scholar] [CrossRef]
- Cifuentes, A.S.; Gonzalez, M.A.; Parra, O.O. The effect of salinity on the growth and carotenogenesis in two Chilean strains of Dunaliella salina Teodoresco. Biol. Res. 1996, 29, 227–236. [Google Scholar]
- Lv, H.; Cui, X.; Wahid, F.; Xia, F.; Zhong, C.; Jia, S. Analysis of the physiological and molecular responses of Dunaliella salina to macronutrient deprivation. PLoS ONE 2016, 11, e0152226. [Google Scholar] [CrossRef]
- Srinivasan, R.; Mageswari, A.; Subramanian, P.; Suganthi, C.; Chaitanyakumar, A.; Aswini, V.; Gothandam, K.M. Bicarbonate supplementation enhances growth and biochemical composition of Dunaliella salina V-101 by reducing oxidative stress induced during macronutrient deficit conditions. Sci. Rep. 2018, 8, 6972. [Google Scholar] [CrossRef]
- Dale, M.P.; Causton, D.R. Use of the Chlorophyll a/b Ratio as a Bioassay for the Light Environment of a Plant. Funct. Ecol. 1992, 6, 190. [Google Scholar] [CrossRef]
- Mirshekari, M.; Einali, A.; Valizadeh, J. Metabolic changes and activity pattern of antioxidant enzymes induced by salicylic acid treatment in green microalga Dunaliella salina under nitrogen deficiency. J. Appl. Phycol. 2019, 31, 1709–1719. [Google Scholar] [CrossRef]
- Young, E.B.; Beardall, J. Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle. J. Phycol. 2003, 5, 897–905. [Google Scholar] [CrossRef]
- Borovkov, A.B.; Gudvilovich, I.N.; Avsiyan, A.L. Scale-up of Dunaliella salina cultivation: From strain selection to open ponds. J. Appl. Phycol. 2020, 32, 1545–1558. [Google Scholar] [CrossRef]
- Fazeli, M.R.; Tofighi, H.; Samadi, N.; Jamalifar, H.; Fazeli, A. Carotenoids accumulation by Dunaliella tertiolecta (Lake Urmia isolate) and Dunaliella salina (ccap 19/18 & wt) under stress conditions. DARU J. Pharm. Sci. 2006, 14, 146–150. [Google Scholar]
- Hadi, M.R.; Shariati, M.; Afsharzadeh, S. Microalgal biotechnology: Carotenoid and glycerol production by Dunaliella sp. algae isolated from the Gave khooni salt marsh, Iran. Biotech. Bioproc. Eng. 2008, 13, 540–544. [Google Scholar] [CrossRef]
- Cifuentes, A.S.; González, M.; Conejeros, M.; Dellarossa, V.; Parra, O. Growth and carotenogenesis in eight strains of Dunaliella salina Teodoresco from Chile. J. Appl. Phycol. 1992, 4, 111–118. [Google Scholar] [CrossRef]
- Fu, W.; Paglia, G.; Magnúsdóttir, M.; Steinarsdóttir, E.A.; Gudmundsson, S.; Palsson, B.Ø.; Andrésson, O.S.; Brynjólfsson, S. Effects of abiotic stressors on lutein production in the green microalga Dunaliella salina. Microb. Cell Factories 2014, 13, 1–9. [Google Scholar] [CrossRef]
- Liang, M.H.; Liang, Z.C.; Chen, H.H.; Jiang, J.G. The bifunctional identification of both lycopene β-and ε-cyclases from the lutein-rich Dunaliella bardawil. Enzyme Microb. Technol. 2019, 131, 109426. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Ho, S.H.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, W.L.; Lee, D.J.; Chang, J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Xie, Y.; Ho, S.H.; Chen, C.N.N.; Chen, C.Y.; Ng, I.S.; Jing, K.J.; Chang, J.S.; Lu, Y. Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: Effects of nitrate concentration, light intensity and fed-batch operation. Bioresour. Technol. 2013, 144, 435–444. [Google Scholar] [CrossRef]
- Kim, S.H.; Liu, K.H.; Lee, S.Y.; Hong, S.J.; Cho, B.K.; Lee, H.; Lee, C.G.; Choi, H.K. Effects of light intensity and nitrogen starvation on glycerolipid, glycerophospholipid, and carotenoid composition in Dunaliella tertiolecta culture. PLoS ONE 2013, 8, e72415. [Google Scholar] [CrossRef]
- Yeh, T.J.; Tseng, Y.F.; Chen, Y.C.; Hsiao, Y.; Lee, P.C.; Chen, T.J.; Chen, C.Y.; Cao, C.Y.; Chang, J.S.; Chen, J.C.; et al. Transcriptome and physiological analysis of a lutein-producing alga Desmodesmus sp. reveals the molecular mechanisms for high lutein productivity. Algal Res. 2017, 21, 103–119. [Google Scholar] [CrossRef]
- Highfield, A.; Ward, A.; Pipe, R.; Schroeder, D.C. Molecular and phylogenetic analysis reveals new diversity of Dunaliella salina from hypersaline environments. J. Mar. Biol. Assoc. UK 2021, 101, 27–37. [Google Scholar] [CrossRef]
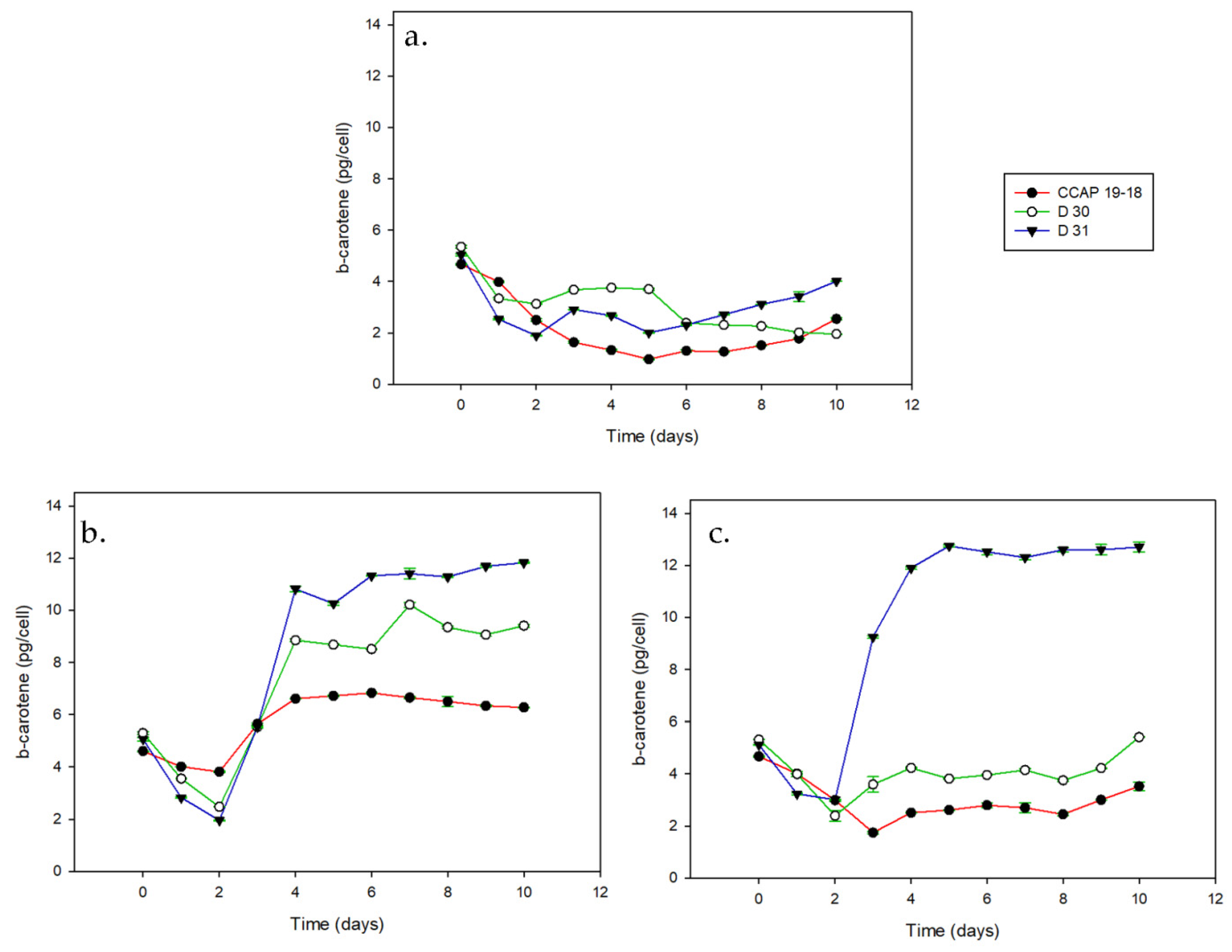
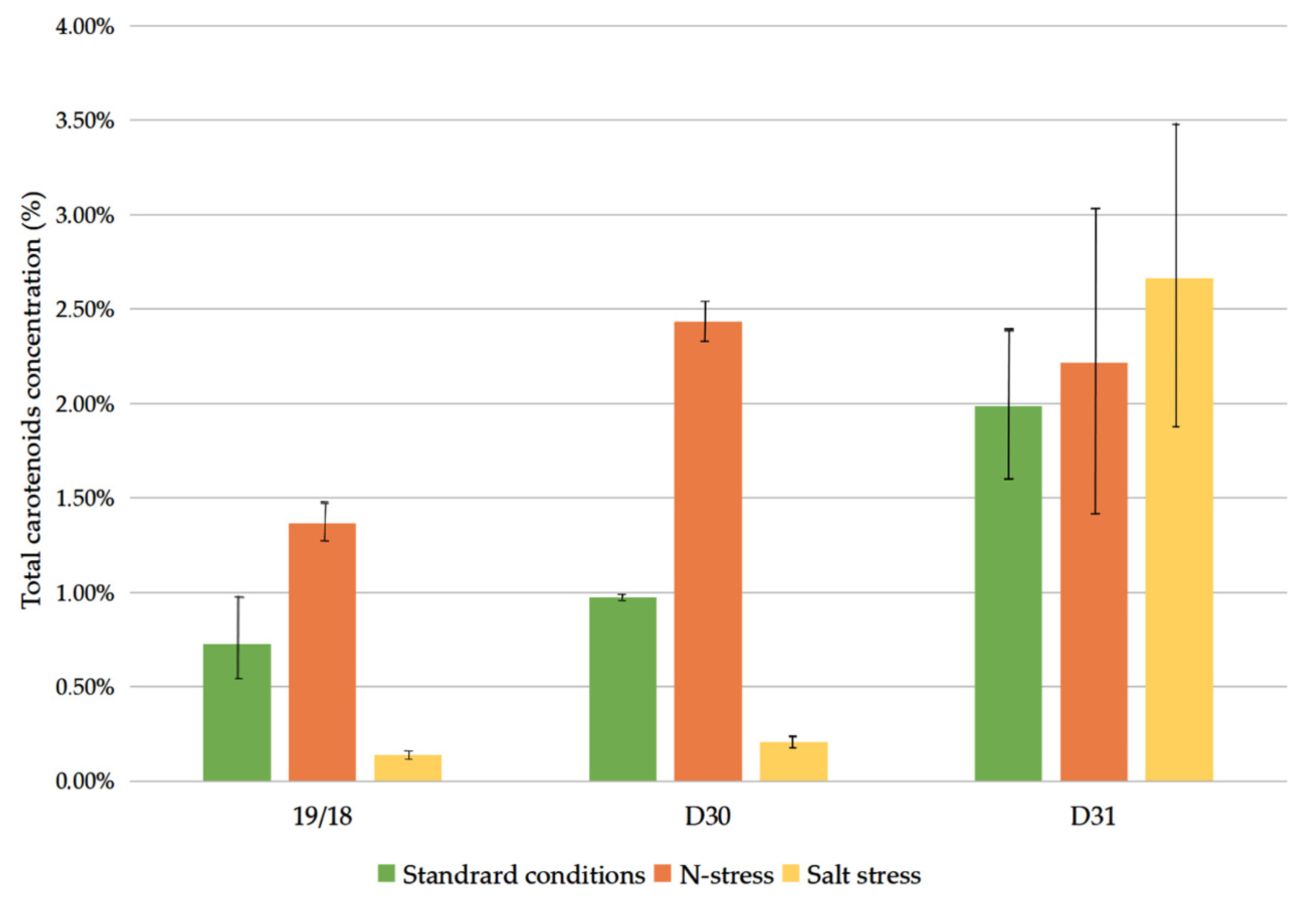
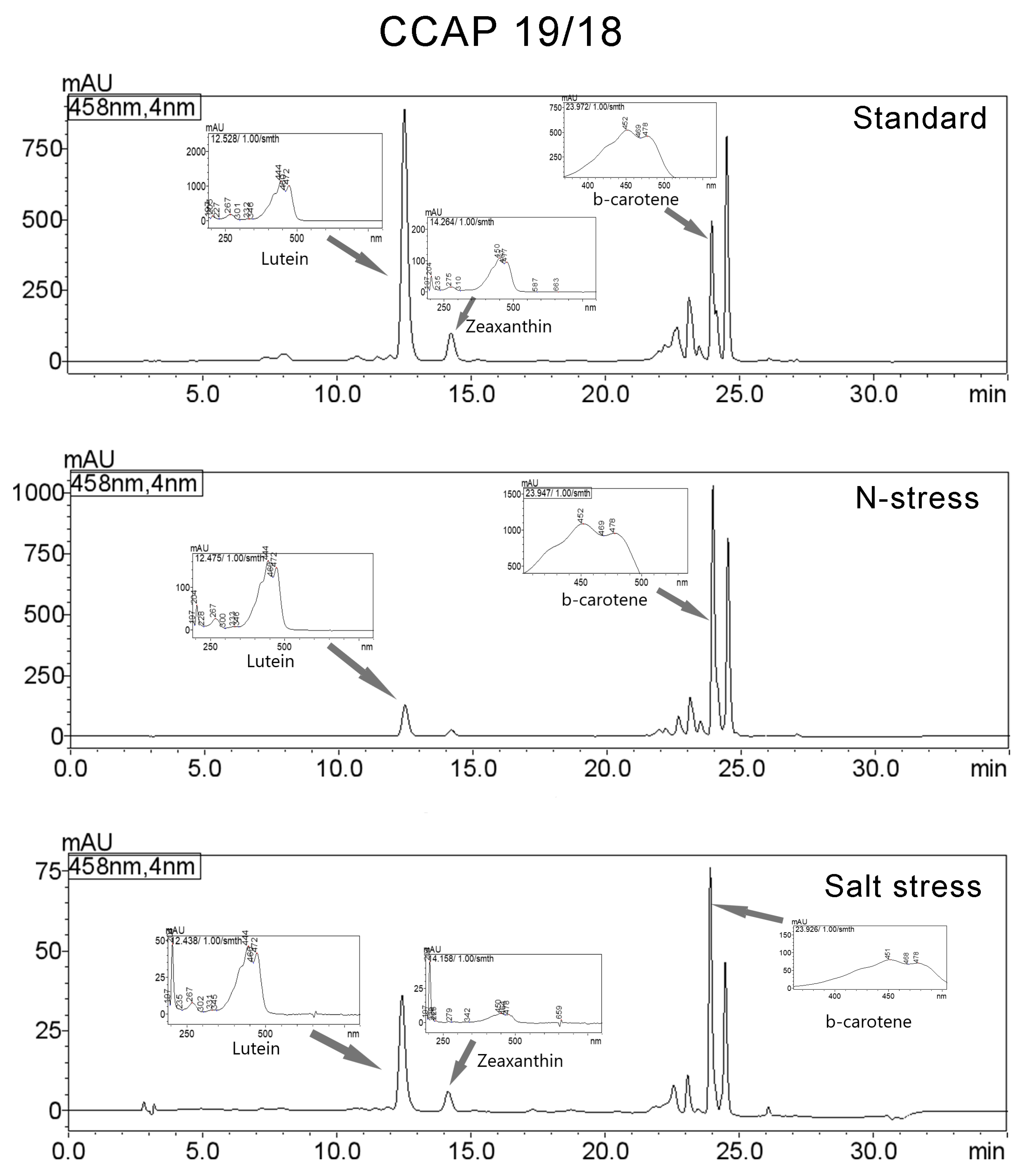

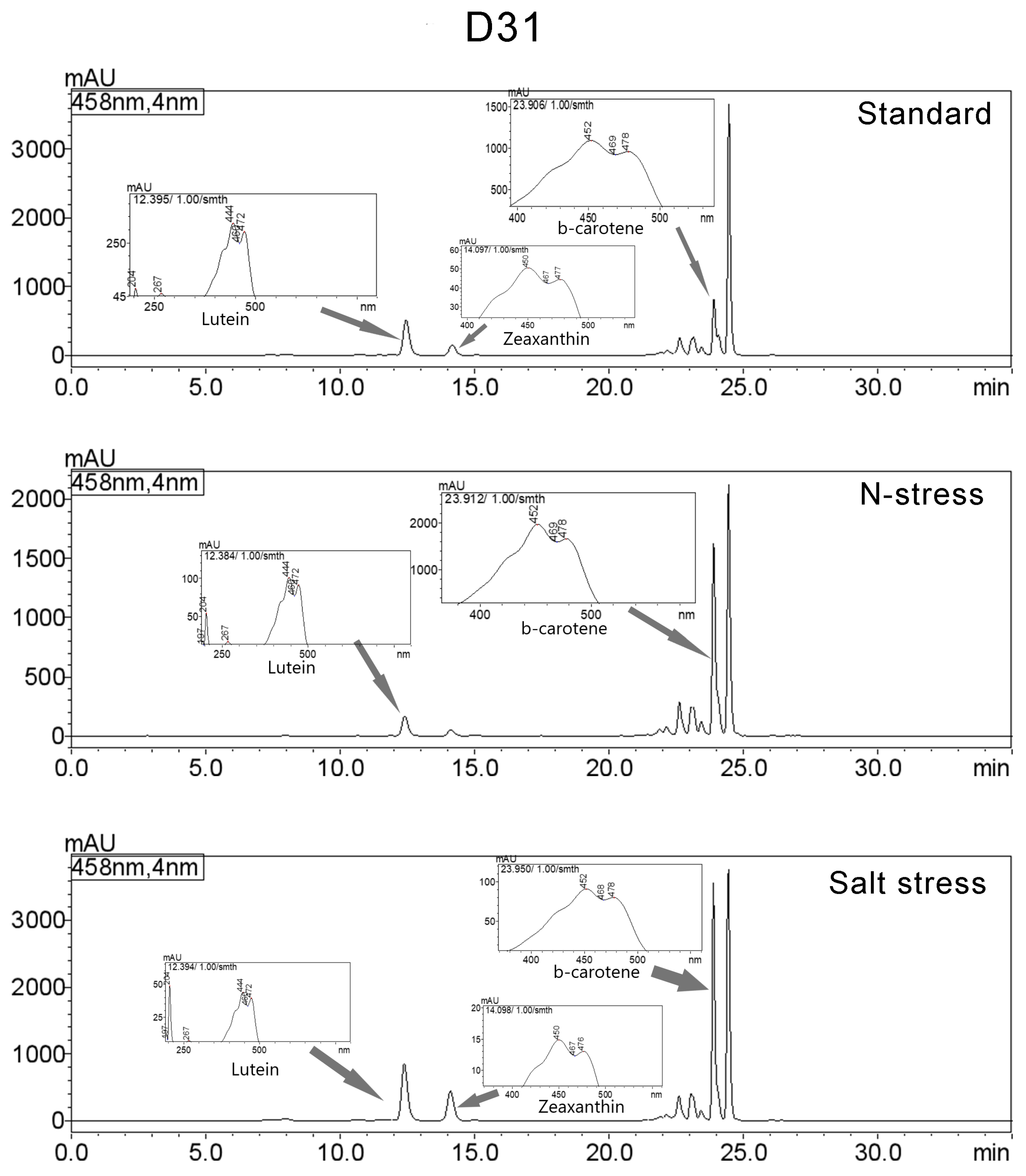
| Strain | μmax (d−1) | ||
|---|---|---|---|
| Standard Conditions | Nitrogen Deprivation | Salinity Shock | |
| CCAP 19\18 | 0.513 a,b (0.010) | 0.197 a,c (0.013) | 0.165 a,b,c (0.007) |
| AthU-Al D30 | 0.255 a (0.006) | 0.188 a,c (0.013) | 0.162 a,b,c (0.017) |
| AthU-Al D31 | 0.390 b (0.004) | 0.288 b (0.011) | 0.112 a,b,c (0.025) |
| Sample | Carotenoids | Ret. Time (min) | Area | Height | Area% | Lamda Max |
|---|---|---|---|---|---|---|
| CCAP 19/18 | ||||||
| Standard | Lutein | 12.528 | 14821899 | 890778 | 36.844 | 444/472/267/205/332 |
| Zeaxanthin | 14.264 | 1826232 | 99707 | 4.54 | 450/477/204/275/663 | |
| β-carotene | 23.972 | 4194955 | 497210 | 10.428 | 452/478/204/273/659 | |
| N-Stress | Lutein | 12.475 | 2086890 | 127377 | 8.285 | 444/472/204/267/333 |
| β-carotene | 23.947 | 9928467 | 1031593 | 39.416 | 452/478/204/273/601 | |
| Salt Stress | Lutein | 12.438 | 610784 | 36318 | 24.293 | 204/444/472/267/331 |
| Zeaxanthin | 14.158 | 108323 | 6245 | 4.308 | 204/450/478/659/225 | |
| β-carotene | 23.926 | 700634 | 77453 | 27.867 | 204/451/478/270/659 | |
| AthuAl D30 | ||||||
| Standard | Lutein | 12.395 | 4238251 | 256546 | 15.098 | 444/472/204/267/333 |
| Zeaxanthin | 14.097 | 806390 | 46474 | 2.873 | 204/450/477/274/659 | |
| β-carotene | 23.906 | 9118255 | 1036987 | 32.483 | 452/478/204/273/659 | |
| N-Stress | Lutein | 12.384 | 1301617 | 79794 | 3.86 | 444/472/204/267/332 |
| Zeaxanthin | 14.08 | 439293 | 25564 | 1.303 | 204/450/476/274/630 | |
| β-carotene | 23.912 | 15360003 | 1846875 | 45.55 | 452/478/204/273/659 | |
| Salt Stress | Lutein | 12.394 | 579670 | 34787 | 20.048 | 204/444/472/267/335 |
| Zeaxanthin | 14.098 | 230176 | 13772 | 7.961 | 204/450/476/273/629 | |
| β-carotene | 23.95 | 782976 | 87623 | 27.079 | 204/452/478/272/659 | |
| AthuAl D31 | ||||||
| Standard | Lutein | 12.457 | 8527333 | 519453 | 14.461 | 444/472/267/204/333 |
| Zeaxanthin | 14.173 | 2669667 | 152510 | 4.527 | 450/477/204/274/659 | |
| β-carotene | 23.908 | 6770635 | 812780 | 11.482 | 452/478/204/273/659 | |
| N-Stress | Lutein | 12.411 | 2735242 | 166483 | 5.774 | 444/472/204/267/333 |
| β-carotene | 23.896 | 15389583 | 1629503 | 32.485 | 451/478/204/273/630 | |
| Salt Stress | Lutein | 12.393 | 13798884 | 850817 | 14.126 | 444/472/267/205/332 |
| Zeaxanthin | 14.105 | 7717916 | 441817 | 7.901 | 450/477/204/274/659 | |
| β-carotene | 23.891 | 27417197 | 3572706 | 28.067 | 449/478/273/204/659 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chantzistrountsiou, X.; Ntzouvaras, A.; Papadaki, S.; Tsirigoti, A.; Tzovenis, I.; Economou-Amilli, A. Carotenogenic Activity of Two Hypersaline Greek Dunaliella salina Strains under Nitrogen Deprivation and Salinity Stress. Water 2023, 15, 241. https://doi.org/10.3390/w15020241
Chantzistrountsiou X, Ntzouvaras A, Papadaki S, Tsirigoti A, Tzovenis I, Economou-Amilli A. Carotenogenic Activity of Two Hypersaline Greek Dunaliella salina Strains under Nitrogen Deprivation and Salinity Stress. Water. 2023; 15(2):241. https://doi.org/10.3390/w15020241
Chicago/Turabian StyleChantzistrountsiou, Xanthi, Alexandros Ntzouvaras, Sofia Papadaki, Amersa Tsirigoti, Ioannis Tzovenis, and Athena Economou-Amilli. 2023. "Carotenogenic Activity of Two Hypersaline Greek Dunaliella salina Strains under Nitrogen Deprivation and Salinity Stress" Water 15, no. 2: 241. https://doi.org/10.3390/w15020241
APA StyleChantzistrountsiou, X., Ntzouvaras, A., Papadaki, S., Tsirigoti, A., Tzovenis, I., & Economou-Amilli, A. (2023). Carotenogenic Activity of Two Hypersaline Greek Dunaliella salina Strains under Nitrogen Deprivation and Salinity Stress. Water, 15(2), 241. https://doi.org/10.3390/w15020241







