Mussel Shell-Supported Yttrium-Doped Bi2MoO6 Composite with Superior Visible-Light Photocatalytic Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Y-Doped Bi2MoO6/CMS Photocatalyst
2.3. Characterization
2.4. Photocatalytic Activity Test
2.5. Cycle Experiment
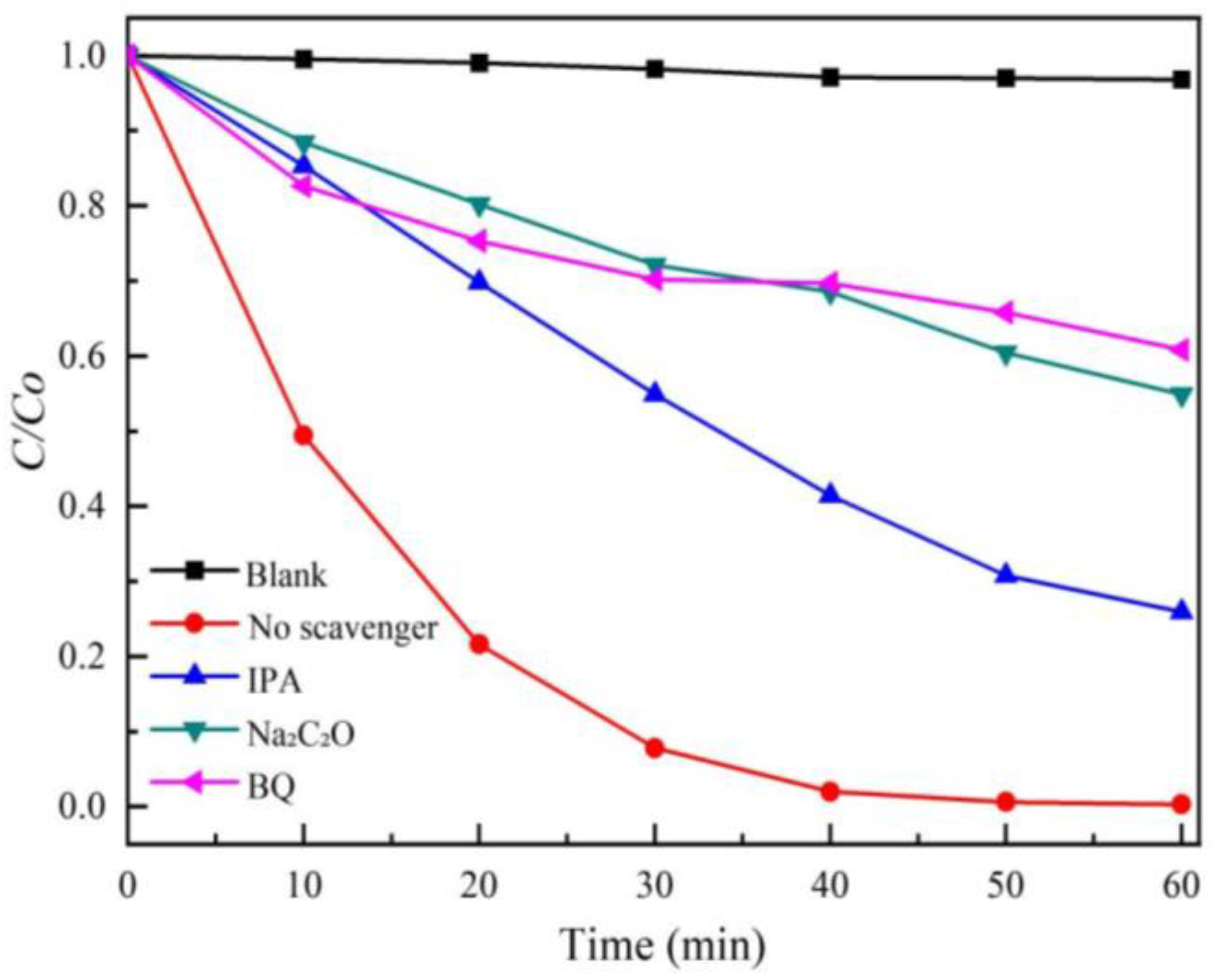
2.6. Active Species Trapping Experiments
3. Results
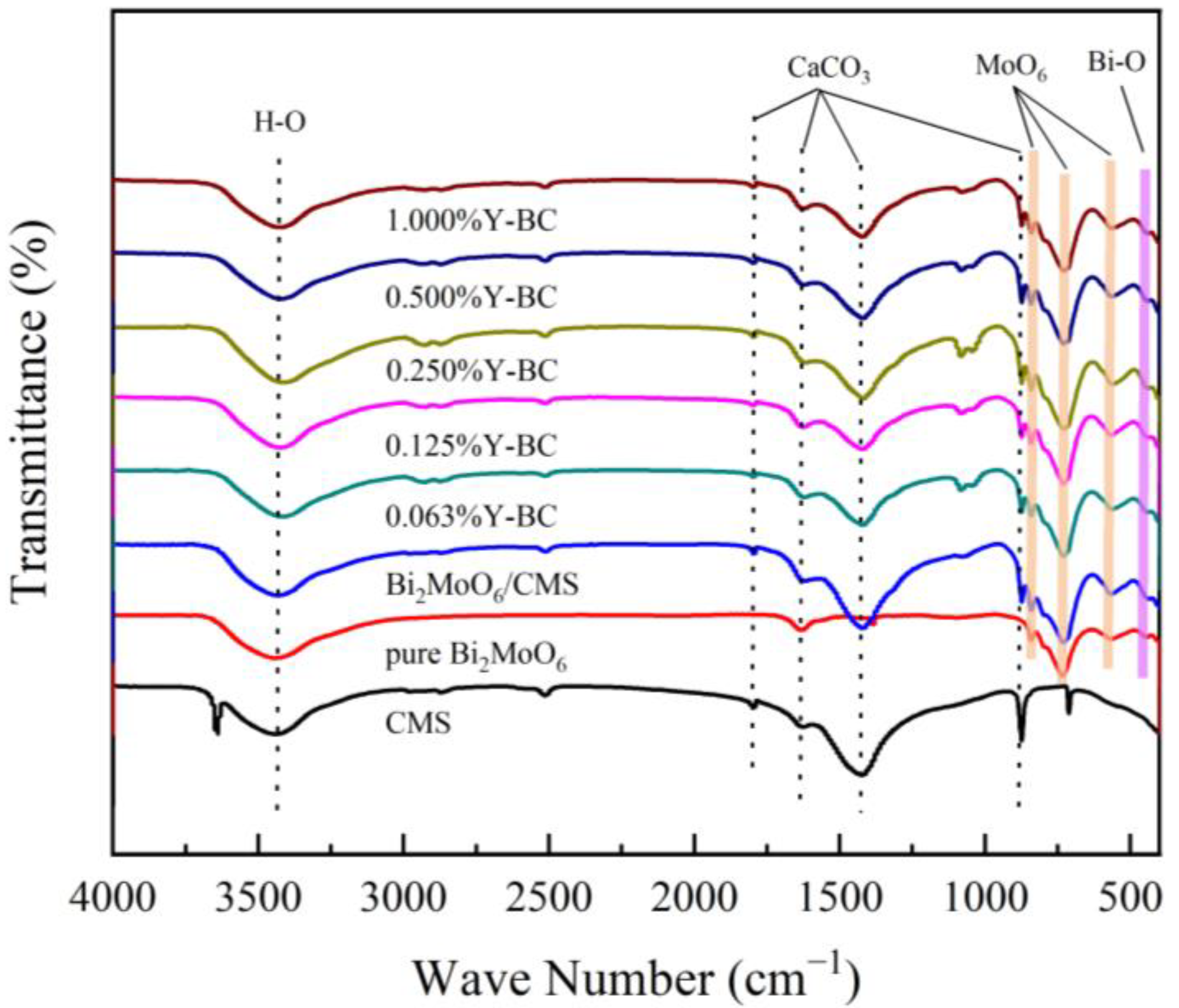
3.1. Chemical Composition Analysis
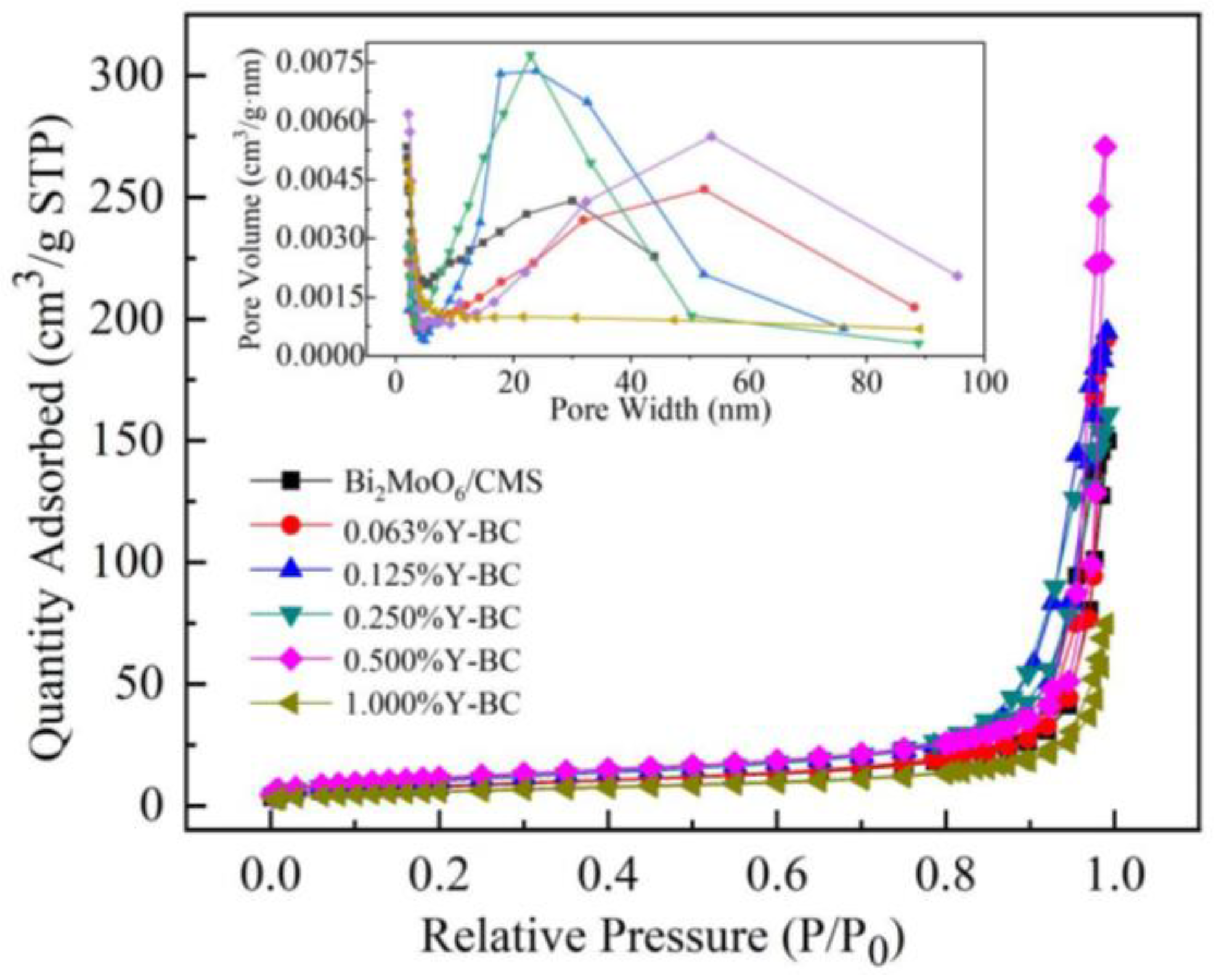
3.2. Morphology and Texture Analysis
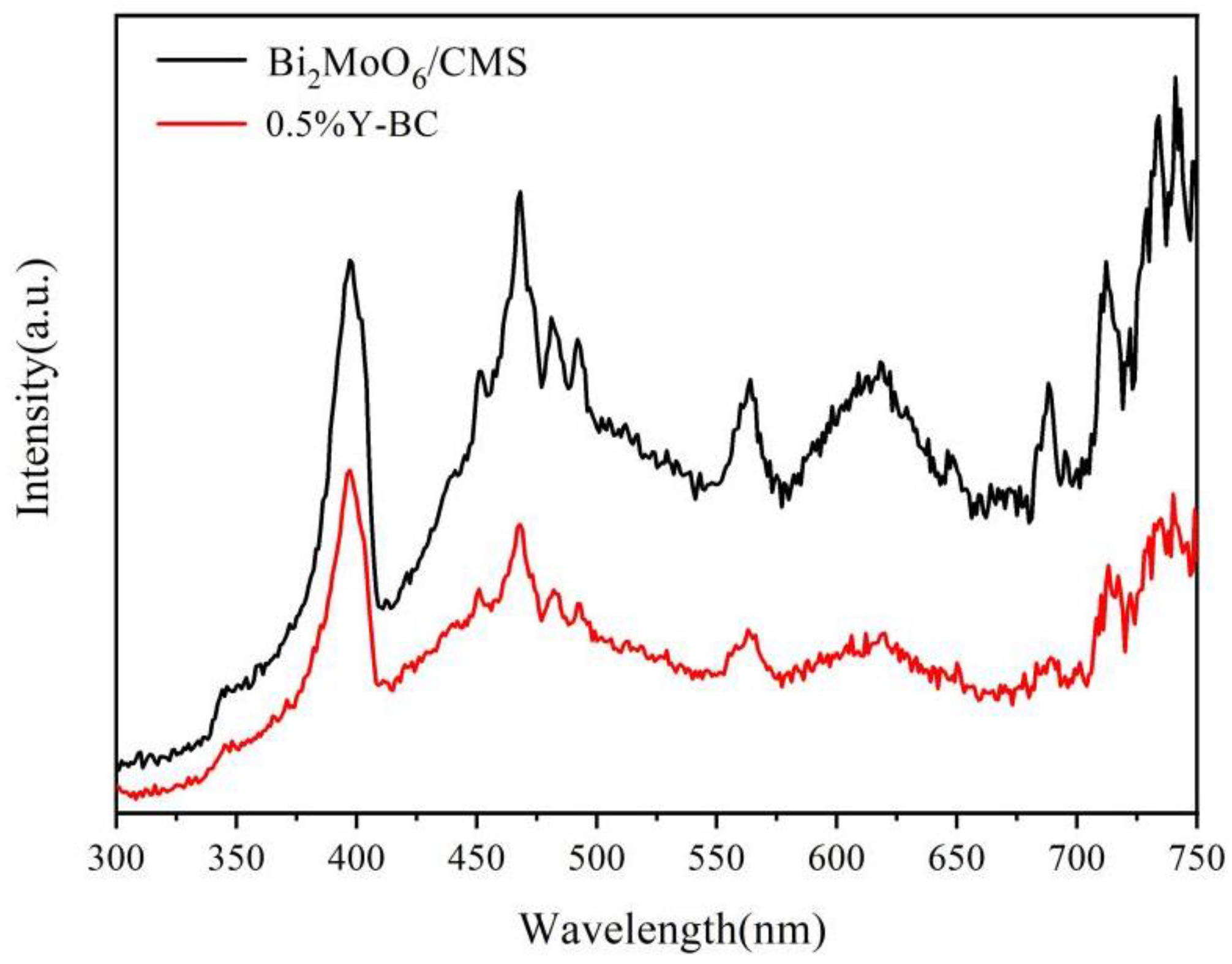
3.3. Optical Properties
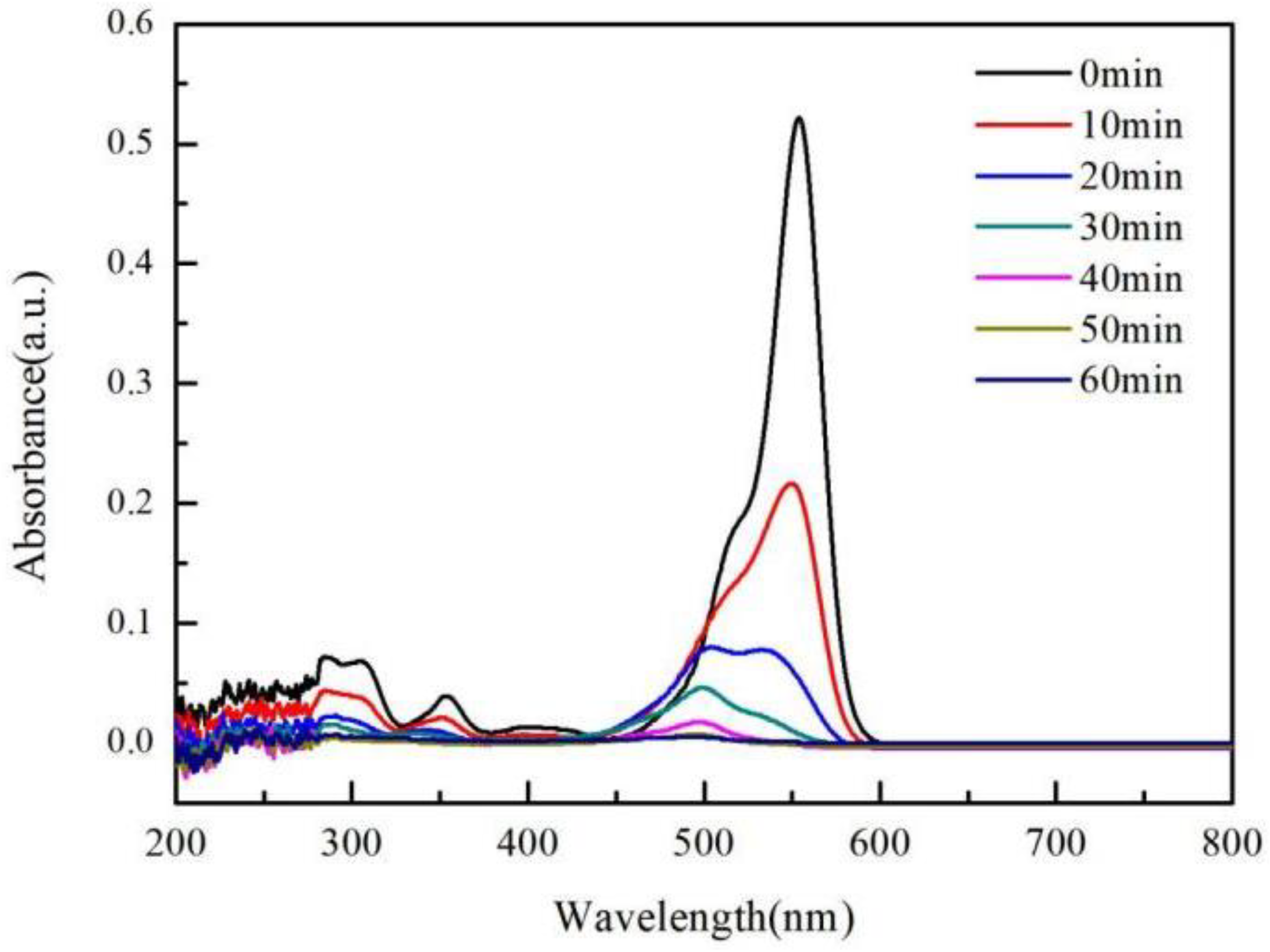
3.4. Photocatalytic Degradation of RhB
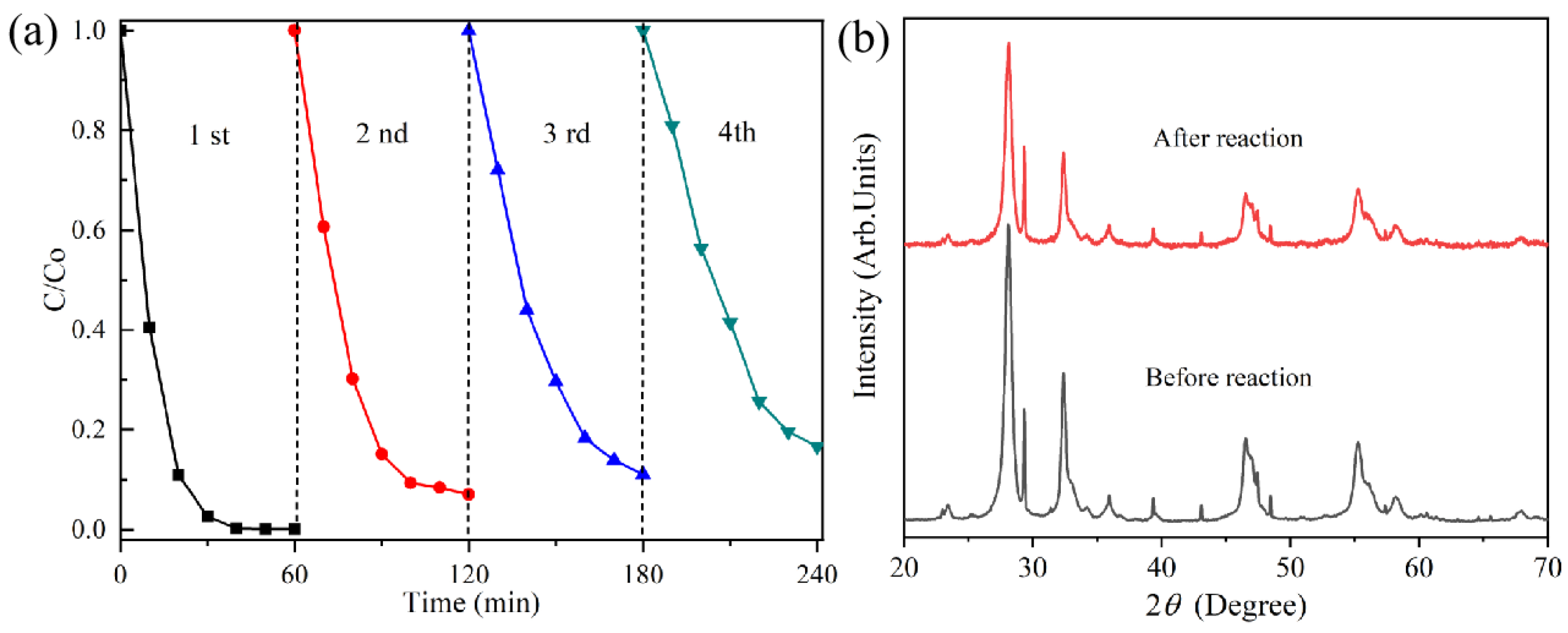
3.5. Cycle Experiment
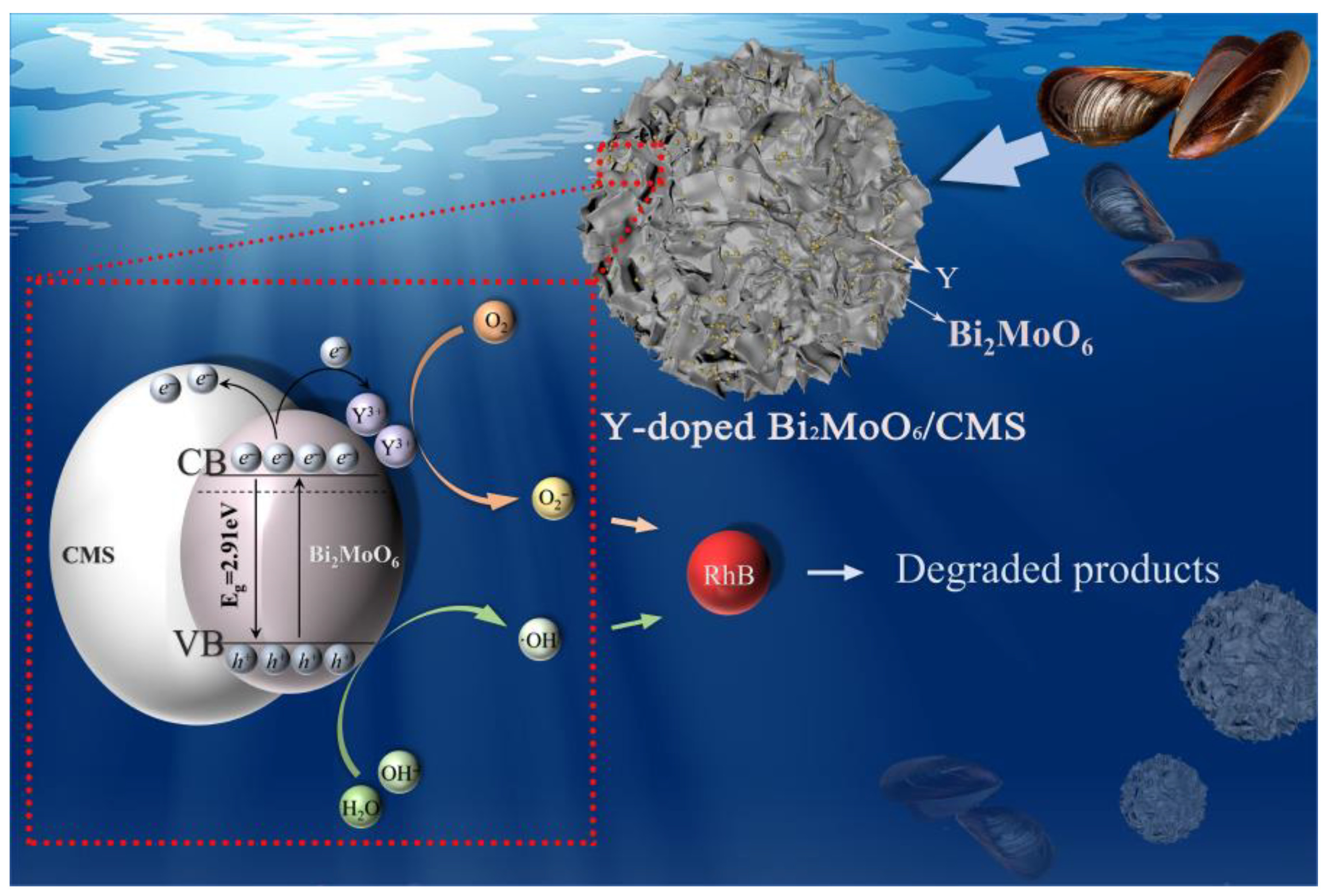
3.6. Possible Photocatalytic Mechanism of Photocatalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Fu, X.; Li, B.; Zhao, H.; Yuan, D.; Na, B. Highly efficient organic dyes capture using thiol-functionalized porous organic polymer. ACS Omega 2022, 7, 17941–17947. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, W.; Zhao, S.; Li, Y.; Chen, K. Enhanced photocatalytic performance of NiS2/g-C3N4/SnS2 by improving the charge diffusion on both valence band and conduction band of carbon nitride. Chem. Sel. 2021, 6, 4440–4447. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Xing, H.; Li, W.; He, H.; Li, W.; Chu, P.K. Interfacial self-assembled dual-functional nanocomposite films for SERS monitoring of visible-light photocatalytic degradation of organic dye pollutants. Surf. Interfaces 2023, 38, 102808. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Alasri, T.M.; Ali, S.L.; Salama, R.S.; Alshorifi, F.T. Band-structure engineering of TiO2 photocatalyst by AuSe quantum dots for efficient degradation of malachite green and phenol. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1729–1740. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zou, Z. Recent advances in designing ZnIn2S4-based heterostructured photocatalysts for hydrogen evolution. J. Mater. Sci. Technol. 2023, 139, 167–188. [Google Scholar] [CrossRef]
- Hu, J.; Xia, K.; Yang, A.; Zhang, Z.; Xiao, W.; Liu, C.; Zhang, Q. Interfacial engineering of ultrathin 2D/2D NiPS3/C3N5 heterojunctions for boosting photocatalytic H2 evolution. Acta Phys.-Chim. Sin. 2023, 40, 2305043. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Mannaa, M.A.; Alotaibi, M.T.; El-Bahy, S.M.; Salama, R.S. Facile and green synthesis of silver quantum dots immobilized onto a polymeric CTS-PEO blend for the photocatalytic degradation of p-Nitrophenol. ACS Omega 2021, 6, 30432–30441. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Ali, S.L.; Salama, R.S. Promotional synergistic effect of Cs–Au NPs on the performance of Cs–Au/MgFe2O4 catalysts in catalysis 3,4-dihydropyrimidin-2(1H)-ones and degradation of RhB dye. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3765–3776. [Google Scholar] [CrossRef]
- Guo, C.; Xu, J.; Wang, S.; Li, L.; Zhang, Y.; Li, X. Facile synthesis and photocatalytic application of hierarchical mesoporous Bi2MoO6 nanosheet-based microspheres. CrystEngComm 2012, 14, 3602–3608. [Google Scholar] [CrossRef]
- Jing, T.; Dai, Y.; Wei, W.; Ma, X.; Huang, B. Near-infrared photocatalytic activity induced by intrinsic defects in Bi2MO6(M = W, Mo). Phys. Chem. Chem. Phys. 2014, 16, 18596–18604. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, C.; Yuan, S.; Li, X.; Zhang, J.; Hu, X.; Lin, H.; Wu, Y.; He, Y. One-step fabrication of Cu-doped Bi2MoO6 microflower for enhancing performance in photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2023, 638, 427–438. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Wang, J.; Chen, T.; Wang, S.; Ding, X.; Chen, H. Insights into the surface/interface modifications of Bi2MoO6: Feasible strategies and photocatalytic applications. Sol. RRL 2020, 5, 2000442. [Google Scholar] [CrossRef]
- Hao, Y.; Dong, X.; Wang, X.; Ma, H.; Zhang, X. Ultrathin-nanosheet-assembled Bi2MoO6 mesoporous hollow framework for realizing optimized sunlight-driven photocatalytic water oxidation. RSC Adv. 2016, 6, 102155–102158. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, L.; Wang, H.; Huang, B.; Yuan, X.; Huang, J.; Zhang, J.; Zeng, G. Modulation of Bi2MoO6-based materials for photocatalytic water splitting and environmental application: A critical review. Small 2019, 15, 1901008. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W. Advanced chemical compositions and nanoarchitectures of bismuth based complex oxides for solar photocatalytic application. RSC Adv. 2014, 4, 47136–47152. [Google Scholar] [CrossRef]
- Dutta, D.P.; Ballal, A.; Chopade, S.; Kumar, A. A study on the effect of transition metal (Ti4+, Mn2+, Cu2+ and Zn2+)-doping on visible light photocatalytic activity of Bi2MoO6 nanorods. J. Photochem. Photobiol. A Chem. 2017, 346, 105–112. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, R.; Xu, L.; Liu, C.; Cheng, Y.; Jiang, Z.; Liu, Y.; Zhang, T.; Li, J.; Liu, X. Highly efficient flower-like Dy3+-doped Bi2MoO6 photocatalyst under simulated sunlight: Design, fabrication and characterization. Opt. Mater. 2021, 116, 111094. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Liu, X.; Ren, C.; Miao, X.; Li, X. Engineering of Gd/Er/Lu-triple-doped Bi2MoO6 to synergistically boost the photocatalytic performance in three different aspects: Oxidizability, light absorption and charge separation. Appl. Surf. Sci. 2018, 463, 556–565. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Z.; Yu, X.; Tang, W.Z. Rare earth elements (lanthanum, cerium and erbium) doped black oxygen deficient Bi2O3-Bi2O3–x as novel photocatalysts enhanced photocatalytic performance. J. Rare Earth 2022, 40, 1053–1062. [Google Scholar] [CrossRef]
- Wang, F.; He, T.; Gao, Y.; Li, Y.; Cui, S.; Huang, H.; Yang, J. Z-scheme heterojunction Bi2MoO6/NH2-UiO-66 (Zr/Ce) for efficient photocatalytic degradation of oxytetracycline: Pathways and mechanism. Sep. Purif. Technol. 2023, 21, 124596. [Google Scholar] [CrossRef]
- Ge, W.; Liu, K.; Yang, P.; Deng, S.; Shen, L. Synthesis and upconversion luminescent properties of Bi2MoO6: 20%Yb3+, 2%Er3+ hollow microsphere with different W6+ ions doping. J. Solid State Chem. 2021, 297, 122064. [Google Scholar] [CrossRef]
- Alemi, A.A.; Kashfi, R.; Shabani, B. Preparation and characterization of novel Ln (Gd3+, Ho3+ and Yb3+)-doped Bi2MoO6 with Aurivillius layered structures and photocatalytic activities under visible light irradiation. J. Mol. Catal. A Chem. 2014, 392, 290–298. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Li, F.; Yu, C. Eu3+ doped Bi2MoO6 nanosheets fabricated via hydrothermal-calcination route and their superior performance for aqueous volatile phenols removal. J. Taiwan Inst. Chem. Eng. 2021, 125, 276–284. [Google Scholar] [CrossRef]
- Moscow, S.; Kavinkumar, V.; Sriramkumar, M.; Jothivenkatachalam, K.; Saravanan, P.; Rajamohan, N.; Vasseghian, Y.; Rajasimman, M. Impact of Erbium (Er) and Yttrium (Y) doping on BiVO4 crystal structure towards the enhancement of photoelectrochemical water splitting and photocatalytic performance. Chemosphere 2022, 299, 134343. [Google Scholar] [CrossRef]
- Usai, S.; Obregón, S.; Becerro, A.I.; Colón, G. Monoclinic-tetragonal heterostructured BiVO4 by yttrium-doping with improved photocatalytic activity. J. Phys. Chem. C 2013, 117, 24479–24484. [Google Scholar] [CrossRef]
- Vaddi, D.R.; Vinukonda, K.; Patnala, R.K.; Kanithi, Y.; Gurugubelli, T.R.; Bae, J.; Koutavarapu, R.; Lee, D.Y.; Shim, J. Effect of yttrium doping on the crystal structure, optical, and photocatalytic properties of hydrothermally synthesized ZnO nanorods. Mater. Sci. Eng. B 2023, 296, 116664. [Google Scholar] [CrossRef]
- Vadivel, S.; Paul, B.; Maruthamani, D.; Kumaravel, M.; Vijayaraghavan, T.; Hariganesh, S.; Pothu, R. Synthesis of yttrium doped BiOF/RGO composite for visible light Photocatalytic applications. Mater. Sci. Energy Technol. 2019, 2, 112–116. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Fang, B.; Cui, Y.; Xing, Z.; Li, Z.; Zhou, W. Self-floating biomass charcoal supported flower-like plasmon silver/carbon, nitrogen co-doped defective TiO2 as robust visible light photocatalysts. J. Clean. Prod. 2021, 329, 129723. [Google Scholar] [CrossRef]
- Deng, C.; Ling, X.; Peng, L.; Wang, T.; Xu, R.; Zhu, Y.; Zhang, W.; Sun, P.; Wu, Y.; Hu, H.; et al. Constructing nano CdS-decorated porous biomass-derived carbon for multi-channel synergetic photocatalytic hydrogen evolution under solar lighting. Appl. Surf. Sci. 2023, 632, 157065. [Google Scholar] [CrossRef]
- Alsaiari, M. Biomass-derived active carbon (AC) modified TiO2 photocatalyst for efficient photocatalytic reduction of chromium (VI) under visible light. Arab. J. Chem. 2021, 14, 103258. [Google Scholar] [CrossRef]
- Saleh, T.S.; Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Design and Development of Novel Composites Containing Nickel Ferrites Supported on Activated Carbon Derived from Agricultural Wastes and Its Application in Water Remediation. Materials 2023, 16, 2170. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Zhu, Z.; Lu, G.Q. Nanoporous carbon electrode from waste coffee beans for high performance supercapacitors. Electrochem. Commun. 2008, 10, 1594–1597. [Google Scholar] [CrossRef]
- Ramya, S.; Vidhya, E.; Bukhari, N.A.; Hatamleh, A.A.; Nilavukkarasi, M.; Vijayakumar, S. TiO2 nanoparticles derived from egg shell waste: Eco synthesis, characterization, biological and photocatalytic applications. Environ. Res. 2022, 214, 113829. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, X.; Shen, X.; Yu, X. Synergetic effect of photocatalytic oxidation plus catalytic oxidation on the performance of coconut shell fiber biochar decorated α-MnO2 under visible light towards BPA degradation. J. Environ. Manag. 2023, 345, 118911. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lin, F.; Yan, B.; Cheng, Z.; Chen, G.; Kuang, M.; Yang, C.; Hou, L. The role of seashell wastes in TiO2/Seashell composites: Photocatalytic degradation of methylene blue dye under sunlight. Environ. Res. 2020, 188, 109831. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Xue, B.; Chen, J.; Wang, H.; Liu, Y. Facile Preparation of a Novel Bi2WO6/Calcined Mussel Shell Composite Photocatalyst with Enhanced Photocatalytic Performance. Catalysts 2020, 10, 1166. [Google Scholar] [CrossRef]
- Echabbi, F.; Hamlich, M.; Harkati, S.; Jouali, A.; Tahiri, S.; Lazar, S.; Lakhmiri, R.; Safi, M. Photocatalytic degradation of methylene blue by the use of titanium-doped Calcined Mussel Shells CMS/TiO2. J. Environ. Chem. Eng. 2019, 7, 103293. [Google Scholar] [CrossRef]
- Wanahari, W.A.; Waiho, K.; Azwar, E.; Fazhan, H.; Peng, W.; Ishak, S.D.; Tabatabaei, M.; Yek, P.N.Y.; Almomani, F.; Aghbashlo, M.; et al. A state-of-the-art review on producing engineered biochar from shellfish waste and its application in aquaculture wastewater treatment. Chemosphere 2022, 288, 132559. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.C.; Magán, A.; Valiño, S.; Bello, P.M.; Casares, J.J.; Blanco, J.M. Identification of best available techniques in the seafood industry: A case study. J. Clean. Prod. 2009, 17, 391–399. [Google Scholar] [CrossRef]
- Cao, J.; Ju, P.; Chen, Z.; Dou, K.; Li, J.; Zhang, P.; Zhu, Z.; Sun, C. Trash to treasure: Green synthesis of novel Ag2O/Ag2CO3 Z-scheme heterojunctions with highly efficient photocatalytic activities derived from waste mussel shells. Chem. Eng. J. 2023, 454, 140259. [Google Scholar] [CrossRef]
- Cai, L.; Zhou, Y.; Wang, Z.; Chen, J.; Ji, L.; Guo, J.; Wang, Y.; Liu, J. Preparation and evaluation of a hierarchical Bi2MoO6/MSB composite for visible-light-driven photocatalytic performance. RSC Adv. 2019, 9, 38280–38288. [Google Scholar] [CrossRef]
- Mannaa, M.; Qasim, K.; Shorifi, F.A.; Salama, R. Role of NiO nanoparticles in enhancing structure properties of TiO2 and its applications in photodegradation and hydrogen evolution. ASC Omega 2021, 6, 30386–30400. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, N.; Zhang, L.; Jiang, K.; Chen, Y.; Chen, Z. Synthesis of Yb3+/Er3+, co-doped Bi2WO6, nanosheets with enhanced photocatalytic activity. Mater. Lett. 2016, 163, 16–19. [Google Scholar] [CrossRef]
- He, M.; Li, W.; Xia, J.; Xu, L.; Di, J.; Xu, H.; Yin, S.; Li, H.; Li, M. The enhanced visible light photocatalytic activity of yttrium-doped BiOBr synthesized via a reactable ionic liquid. Appl. Surf. Sci. 2015, 331, 170–178. [Google Scholar] [CrossRef]
- Cao, R.; Huang, H.; Tian, N.; Zhang, Y.; Guo, Y.; Zhang, T. Novel Y doped Bi2WO6 photocatalyst: Hydrothermal fabrication, characterization and enhanced visible-light-driven photocatalytic activity for Rhodamine B degradation and photocurrent generation. Mater. Charact. 2015, 101, 166–172. [Google Scholar] [CrossRef]
- El-Hakam, S.A.; ALShorifi, F.T.; Salama, R.S.; Gamal, S.; El-Yazeed, W.S.A.; Ibrahim, A.A.; Ahmed, A.I. Application of nanostructured mesoporous silica/ bismuth vanadate composite catalysts for the degradation of methylene blue and brilliant green. J. Mater. Res. Technol. 2022, 18, 1963–1976. [Google Scholar] [CrossRef]
- Shkir, M.; Alshahrani, T. Enhancement in photo response of spray deposited Yttrium doped Bi2S3 thin films. Sens. Actuators A Phys. 2023, 351, 114169. [Google Scholar] [CrossRef]
- Liu, X.; Deng, H.; Yao, W.; Jiang, Q.; Shen, J. Preparation and photocatalytic activity of Y-doped Bi2O3. J. Alloys Compd. 2015, 651, 135–142. [Google Scholar] [CrossRef]
- Zhang, Q.; Rao, G.; Xiao, Y.; Dong, H.; Liu, G.; Zhang, Y.; Liang, J. Crystal structure, magnetic and electrical-transport properties of rare-earth-doped Sr2FeMoO6. Phys. B Condens. Matter 2006, 381, 233–238. [Google Scholar] [CrossRef]
- Maarisetty, D.; Mary, R.; Hang, D.R.; Mohapatra, P.; Baral, S.S. The role of material defects in the photocatalytic CO2 reduction: Interfacial properties, thermodynamics, kinetics and mechanism. J. CO2 Util. 2022, 64, 102175. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, X.; He, Q.; Zhou, W.; Yang, K.; Tao, L.; Li, F.; Yu, C. Preparation and characterization of Sm3+/Tm3+ co-doped BiVO4 micro-squares and their photocatalytic performance for CO2 reduction. J. Taiwan Inst. Chem. Eng. 2023, 144, 104737. [Google Scholar] [CrossRef]
- Qin, Z.; Tian, H.; Su, T.; Ji, H.; Guo, Z. Soft template inducted hydrothermal BiYO3 catalysts for enhanced formic acid formation from the photocatalytic reduction of carbon dioxide. RSC Adv. 2016, 6, 52665–52673. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, J.; Yin, S.; Li, H.; Xu, H.; He, M.; Huang, L.; Zhang, Q. Improvement of visible light photocatalytic activity over flower-like BiOCl/BiOBr microspheres synthesized by reactable ionic liquids. Colloids Surf. A Physicochem. Eng. Asp. 2013, 420, 89–95. [Google Scholar] [CrossRef]
- Krishna, R.J.; Srinivas, B.; Kumari, V.D.; Subrahmanyam, M. Sm3+-doped Bi2O3 photocatalyst prepared by hydrothermal synthesis. ChemCatChem 2009, 1, 492–496. [Google Scholar]
- Hu, Z.; Chen, D.; Wang, S.; Zhang, N.; Qin, L.; Huang, Y. Facile synthesis of Sm-doped BiFeO3 nanoparticles for enhanced visible light photocatalytic performance. Mater. Sci. Eng. B 2017, 220, 1–12. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, X.; Ma, Z.; Li, Y.; Yue, S.; Liu, X.; Zhang, J. W5+-W5+ pair induced LSPR of W18O49 to sensitize ZnIn2S4 for full-spectrum solar-light-driven photocatalytic hydrogen evolution. Adv. Funct. Mater. 2022, 32, 2203638. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhao, X.; Zhu, Y. Controllable synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocatalytic activities. Appl. Catal. B Environ. 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Hou, W.; Du, N.; Zhang, R.; Tao, X. Synthesis and characterization of g-C3N4/Bi2MoO6 heterojunctions with enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2014, 160–161, 89–97. [Google Scholar] [CrossRef]
- Simpson, L.J. Electrochemically generated CaCO3 deposits on iron studied with FTIR and Raman spectroscopy. Electrochim. Acta 1998, 43, 2543–2547. [Google Scholar] [CrossRef]
- Huang, N.; Wang, J. A TGA-FTIR study on the effect of CaCO3 on the thermal degradation of EBA copolymer. J. Anal. Appl. Pyrolysis 2009, 84, 124–130. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, P.; Kuang, S. Fabrication and enhanced visible-light photocatalytic activities of BiVO4/Bi2WO6 composites. RSC Adv. 2014, 4, 46054–46059. [Google Scholar] [CrossRef]
- Yassin, A.Y.; Abdelghany, A.M.; Salama, R.S.; Tarabiah, A.E. Structural, Optical and Antibacterial Activity Studies on CMC/PVA Blend Filled with Three Different Types of Green Synthesized ZnO Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1855–1867. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Salama, R.S.; El-Hakam, S.A.; Khder, A.S.; Ahmed, A.I. Synthesis of sulfated zirconium supported MCM-41 composite with high-rate adsorption of methylene blue and excellent heterogeneous catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126361. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Cheng, B.; Zhou, M.; Zhao, X. Enhanced photocatalytic activity of TiO2 powder (P25) by hydrothermal treatment. J. Mol. Catal. A Chem. 2006, 253, 112–118. [Google Scholar] [CrossRef]
- Wang, M.; You, M.; Guo, P.; Tang, H.; Lv, C.; Zhang, Y.; Zhu, T.; Han, J. Hydrothermal synthesis of Sm-doped Bi2MoO6 and its high photocatalytic performance for the degradation of Rhodamine B. J. Alloys Compd. 2017, 728, 739–746. [Google Scholar] [CrossRef]
- Wang, C.; Gu, C.; Zeng, T.; Zhang, Q.; Luo, X. Bi2WO6 doped with rare earth ions: Preparation, characterization and photocatalytic activity under simulated solar irradiation. J. Rare Earth 2021, 39, 58–66. [Google Scholar] [CrossRef]
- Huang, H.; Wang, S.; Tian, N.; Zhang, Y. A one-step hydrothermal preparation strategy for layered BiIO4/Bi2WO6 heterojunctions with enhanced visible light photocatalytic activities. RSC Adv. 2014, 11, 5561–5567. [Google Scholar] [CrossRef]
- Tian, G.; Chen, Y.; Zhai, R.; Zhou, J.; Zhou, W.; Wang, R.; Pan, K.; Tian, C.; Fu, H. Hierarchical flake-like Bi2MoO6/TiO2 bilayer films for visible-light-induced self-cleaning applications. J. Mater. Chem. A 2013, 1, 6961–6968. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Yu, G.; Jiang, H.; Mu, X.; Liu, H. A Bi2WO6-based hybrid photocatalyst with broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Adv. Mater. 2013, 25, 5075–5080. [Google Scholar] [CrossRef]
- Yu, C.; Wu, Z.; Liu, R.; He, H.; Fan, W.; Xue, S. The effects of Gd3+ doping on the physical structure and photocatalytic performance of Bi2MoO6 nanoplate crystals. J. Phys. Chem. Solids 2016, 93, 7–13. [Google Scholar] [CrossRef]
- Xue, M.; Meng, F.; Ma, Y.; Zhou, S. Growing of ultra-thin Bi2MoO6 nanoflowers on Co/N-doped graphitic carbon nanoshells as attractive custom supports: Excellent photocatalytic degradation activity for pollutants. Appl. Surf. Sci. 2023, 613, 156100. [Google Scholar] [CrossRef]
- Varma, R.; Chaurasia, S.; Patel, N.; Bhanage, B.M. Interplay of adsorption, photo-absorption, electronic structure and charge carrier dynamics on visible light driven photocatalytic activity of Bi2MoO6/rGO (0D/2D) heterojunction. J. Environ. Chem. Eng. 2020, 8, 104551. [Google Scholar] [CrossRef]
- Xu, C.; Wu, H.; Gu, F.L. Efficient adsorption and photocatalytic degradation of Rhodamine B under visible light irradiation over BiOBr/montmorillonite composites. J. Hazard. Mater. 2014, 275, 185–192. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, W.; Liu, X.; Wang, Q.; Hu, J.; Zhang, S.; Xu, J.; Zhang, Q.; Zou, Z. Rationally designed Ti3C2 MXene/CaIn2S4 Schottky heterojunction for enhanced photocatalytic Cr (VI) reduction: Performance, influence factors and mechanism. J. Mater. Sci. Technol. 2023, 161, 123–135. [Google Scholar] [CrossRef]
- Bouattour, S.; Rego, A.M.B.D.; Ferreira, L.F.V. Photocatalytic activity of Li+–Rb+–Y3+ doped or codoped TiO2 under sunlight irradiation. Mater. Res. Bull. 2010, 45, 818–825. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, Y.; Wu, Y.; Zhang, Y.N.; Zuo, T. Low-cost Y-doped TiO2 nanosheets film with highly reactive {001} facets from CRT waste and enhanced photocatalytic removal of Cr (VI) and methyl orange. Acs Sustain. Chem. Eng. 2016, 4, 1794–1803. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Y.; Liu, J.; Zhang, Z.; Xu, Y.; Ren, H. B and Y co-doped TiO2 photocatalyst with enhanced photodegradation efficiency. J. Alloys Compd. 2016, 695, 1462–1469. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, W.; Wang, B.; Yan, H. Synthesis, characterization and photocatalytic properties of the Y-doped polar borate photocatalysts: Bi2ZnOB2O6: xY3+. Chem. Phys. Lett. 2019, 734, 136707. [Google Scholar] [CrossRef]





| Photocatalysts | Average Crystallite Size (nm) | BET Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) | Band Gap Energy (eV) |
|---|---|---|---|---|---|
| Bi2MoO6/CMS | 10.81 | 28.64 | 9.63 | 0.07 | 3.00 |
| 0.063%Y-BC | 10.65 | 29.91 | 9.76 | 0.07 | 2.98 |
| 0.125%Y-BC | 8.49 | 37.94 | 14.71 | 0.14 | 2.94 |
| 0.25%Y-BC | 8.38 | 38.23 | 14.07 | 0.13 | 2.92 |
| 0.5%Y-BC | 8.91 | 42.50 | 7.92 | 0.08 | 2.91 |
| 1%Y-BC | 9.15 | 20.60 | 8.15 | 0.04 | 2.83 |
| Photocatalyst | Catalyst Dosage | Light Source | Irradiation Time | Pollutants | Efficiency | K (min−1) | Ref. |
|---|---|---|---|---|---|---|---|
| 0.5%Y-BC | 20 mg | 300 W Xe lamp | 60 min | RhB, 6 mg/L, 40 mL | 99.7% | 0.1022 | This work |
| Bi2WO6/Calcined mussel Shell | 20 mg | 300 W Xe lamp | 150 min | RhB, 10.0 mg/L, 100 mL | 98.4% | 0.0248 | [36] |
| TiO2/Seashell | 100 mg | Xe lamp | 140 min | MB, 10 mg/L, 100 mL | 96% | / | [35] |
| TiO2/Calcined Mussel Shell | 40 mg | UV light | 300 min | MB, 10 mg/L, 20 mL | 97% | / | [39] |
| Co/N-graphitic carbon@Bi2MoO6 | 10 mg | 300 W Xe lamp | 75 min | RhB/MO, 10 mg/L, 200 mL | 99.54% | 0.0335 | [74] |
| Bi2MoO6/rGO | 20 mg | 150 W Xe lamp | 180 min | RhB, 10 mg/L,/50 mL | 100% | / | [75] |
| Gd3+-doped Bi2MoO6 | 100 mg | 300 W Xe lamp | 180 min | RhB, 10 mg/L,/50 mL | 90.2% | 0.0122 | [24] |
| Ho3+-doped Bi2MoO6 | 100 mg | 300 W Xe lamp | 180 min | RhB, 10 mg/L,/50 mL | 81.9% | 0.0078 | [24] |
| Yb3+-doped Bi2MoO6 | 100 mg | 300 W Xe lamp | 180 min | RhB, 10 mg/L,/50 mL | 79.8% | 0.0091 | [24] |
| Dy3+-doped Bi2MoO6 | 100 mg | 300 W Xe lamp | 40 min | RhB, 10 mg/L, 100 mL | 100% | / | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Zhou, Y.; Guo, J.; Sun, J.; Ji, L. Mussel Shell-Supported Yttrium-Doped Bi2MoO6 Composite with Superior Visible-Light Photocatalytic Performance. Water 2023, 15, 3478. https://doi.org/10.3390/w15193478
Cai L, Zhou Y, Guo J, Sun J, Ji L. Mussel Shell-Supported Yttrium-Doped Bi2MoO6 Composite with Superior Visible-Light Photocatalytic Performance. Water. 2023; 15(19):3478. https://doi.org/10.3390/w15193478
Chicago/Turabian StyleCai, Lu, Yarui Zhou, Jian Guo, Jiaxing Sun, and Lili Ji. 2023. "Mussel Shell-Supported Yttrium-Doped Bi2MoO6 Composite with Superior Visible-Light Photocatalytic Performance" Water 15, no. 19: 3478. https://doi.org/10.3390/w15193478
APA StyleCai, L., Zhou, Y., Guo, J., Sun, J., & Ji, L. (2023). Mussel Shell-Supported Yttrium-Doped Bi2MoO6 Composite with Superior Visible-Light Photocatalytic Performance. Water, 15(19), 3478. https://doi.org/10.3390/w15193478





