Magnetic Metal–Organic Framework Enhanced Inorganic Coagulation for Water Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PFS-MMOFs
2.3. Coagulation Test
2.4. Analytical Methods
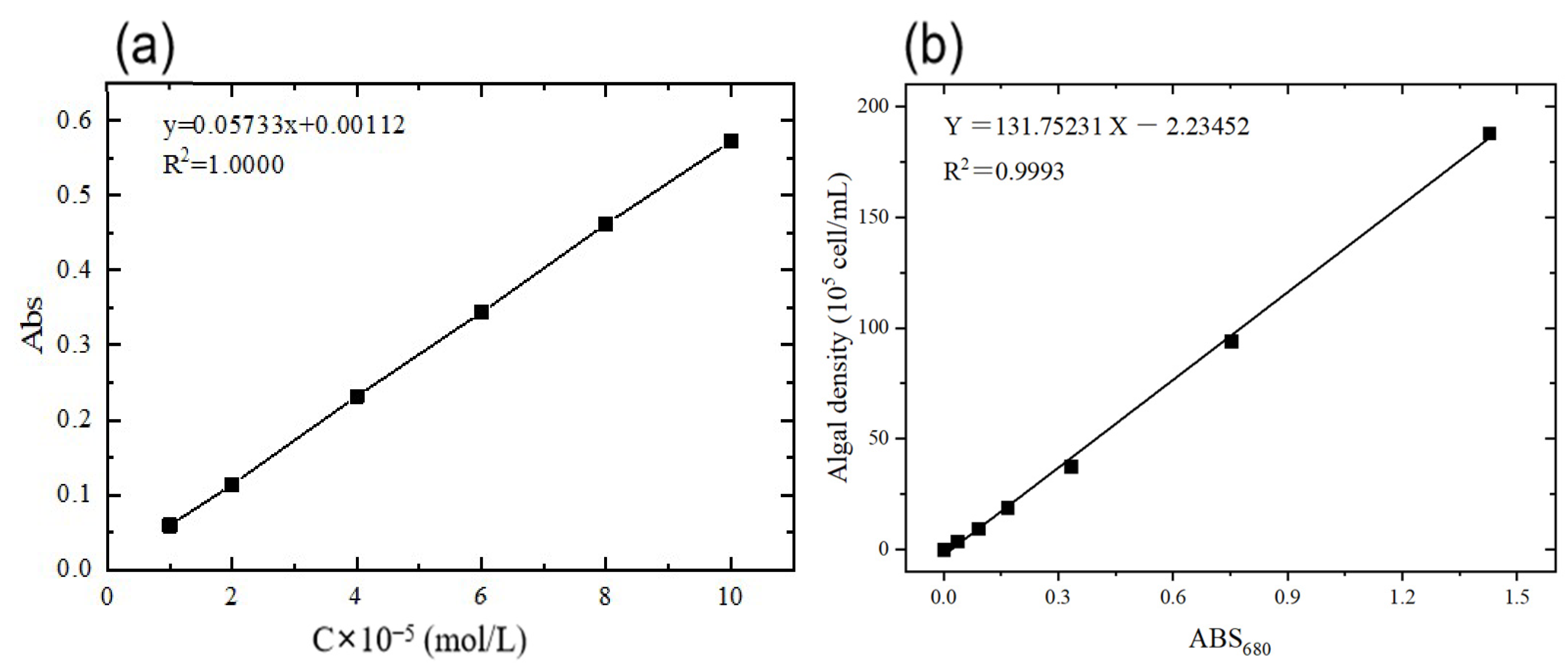
2.5. Cultivation and Determination of Algae
3. Results and Discussion
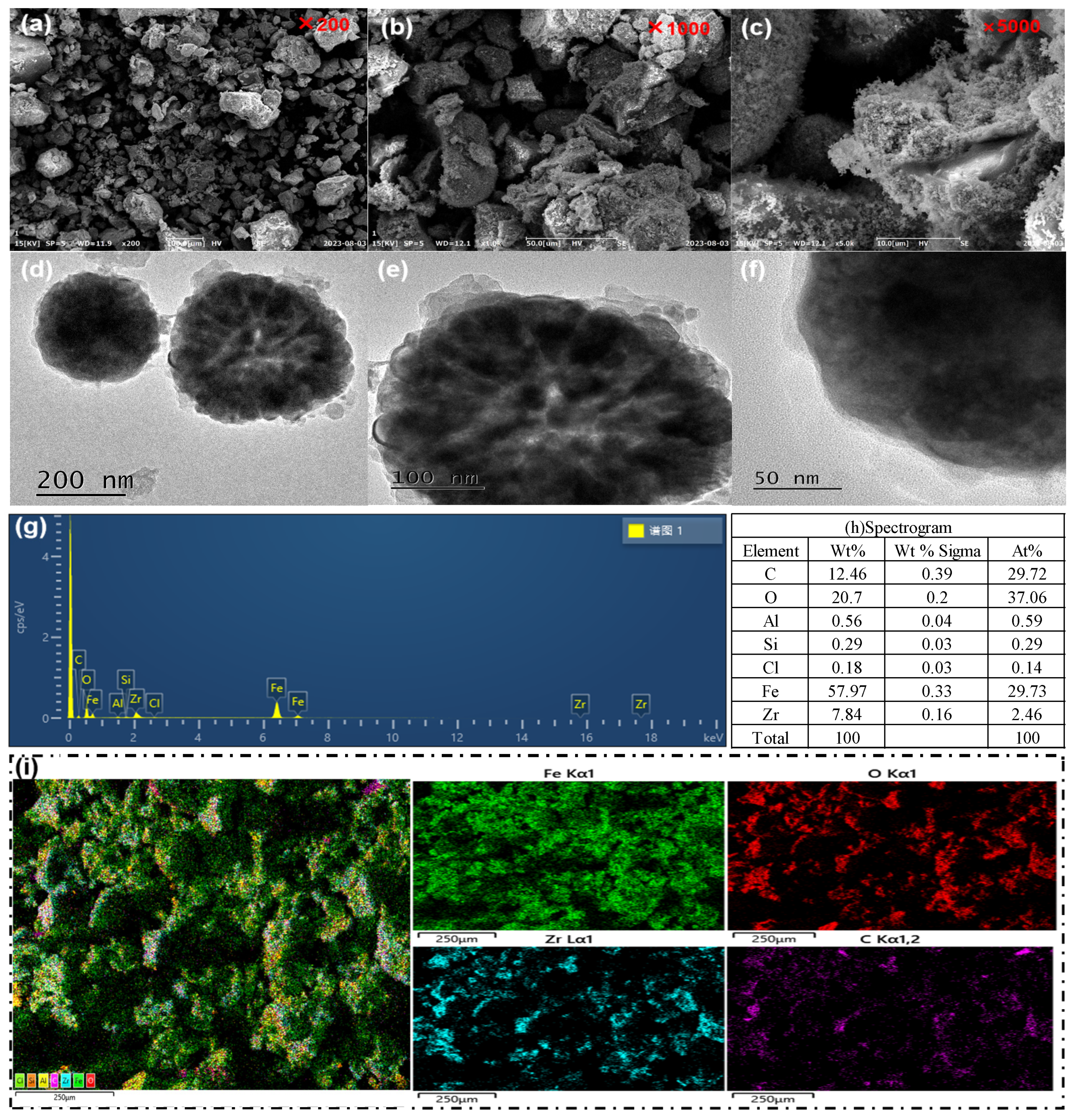
3.1. SEM, TEM and EDS Analysis
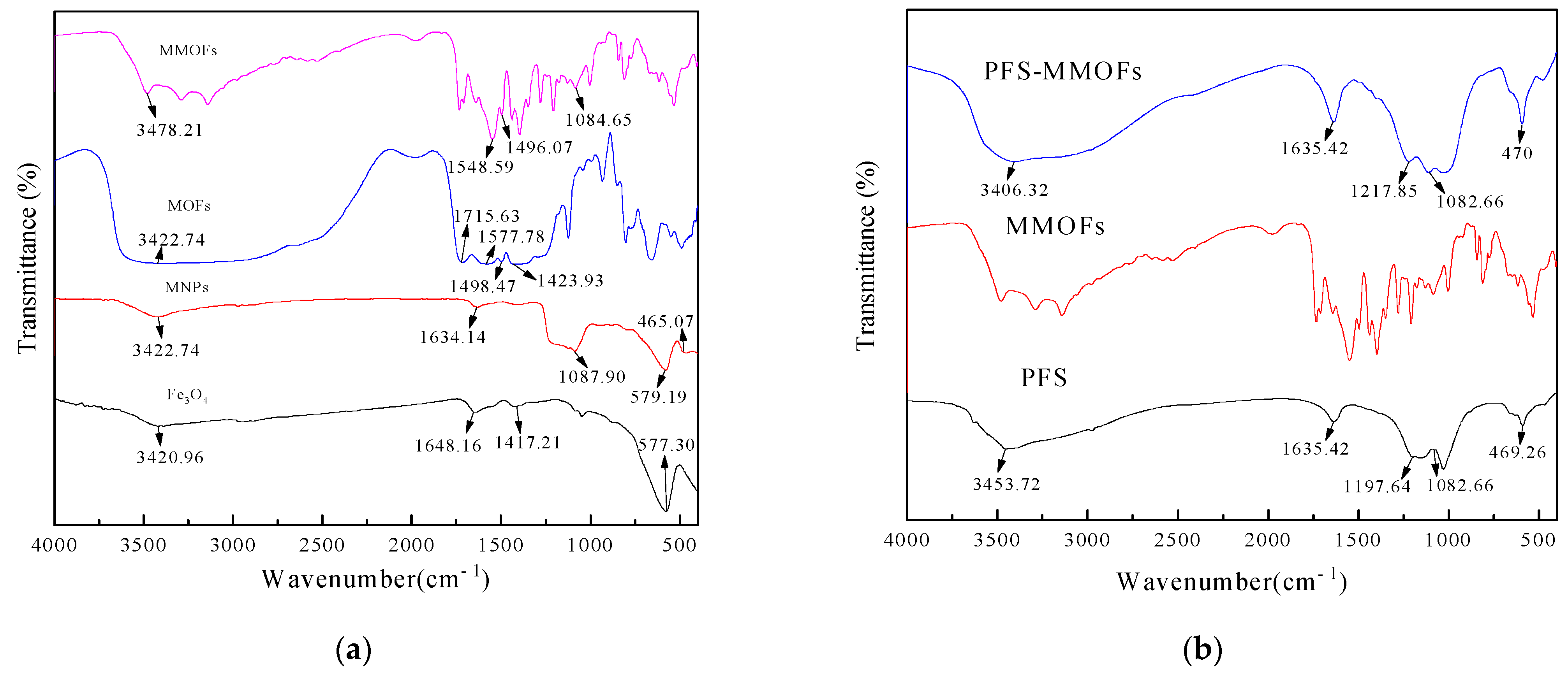
3.2. FT-IR Spectra Analysis
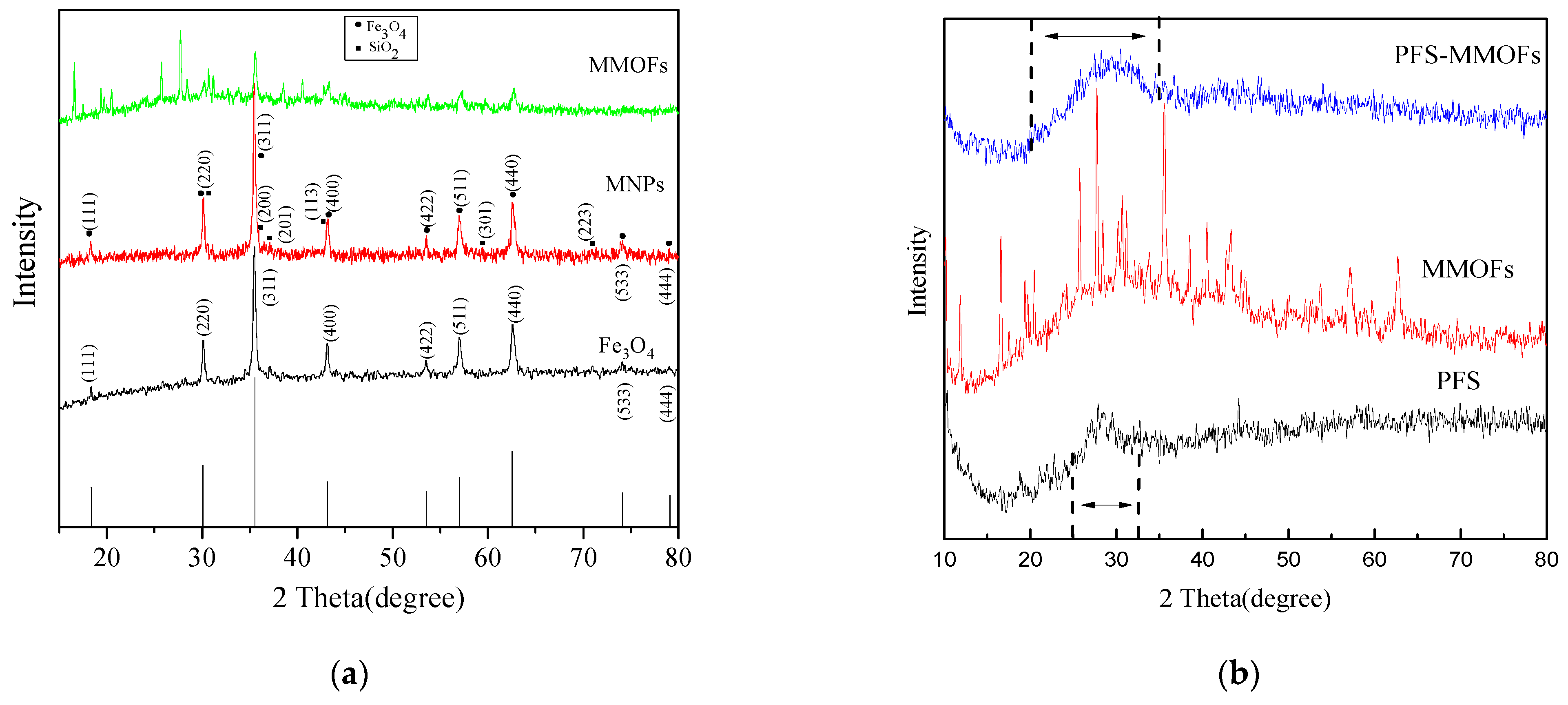
3.3. XRD Analysis
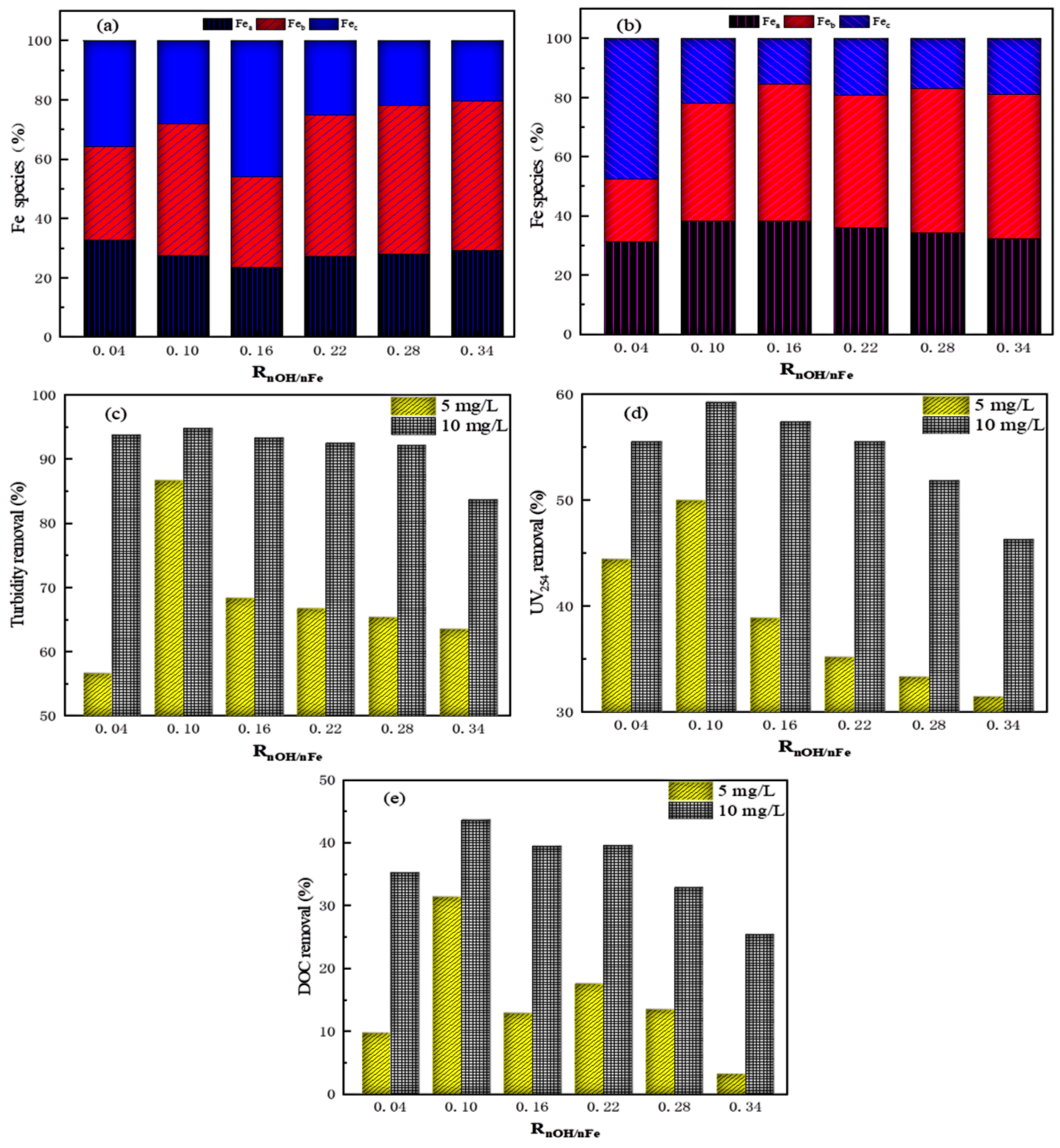
3.4. Iron Species Analysis
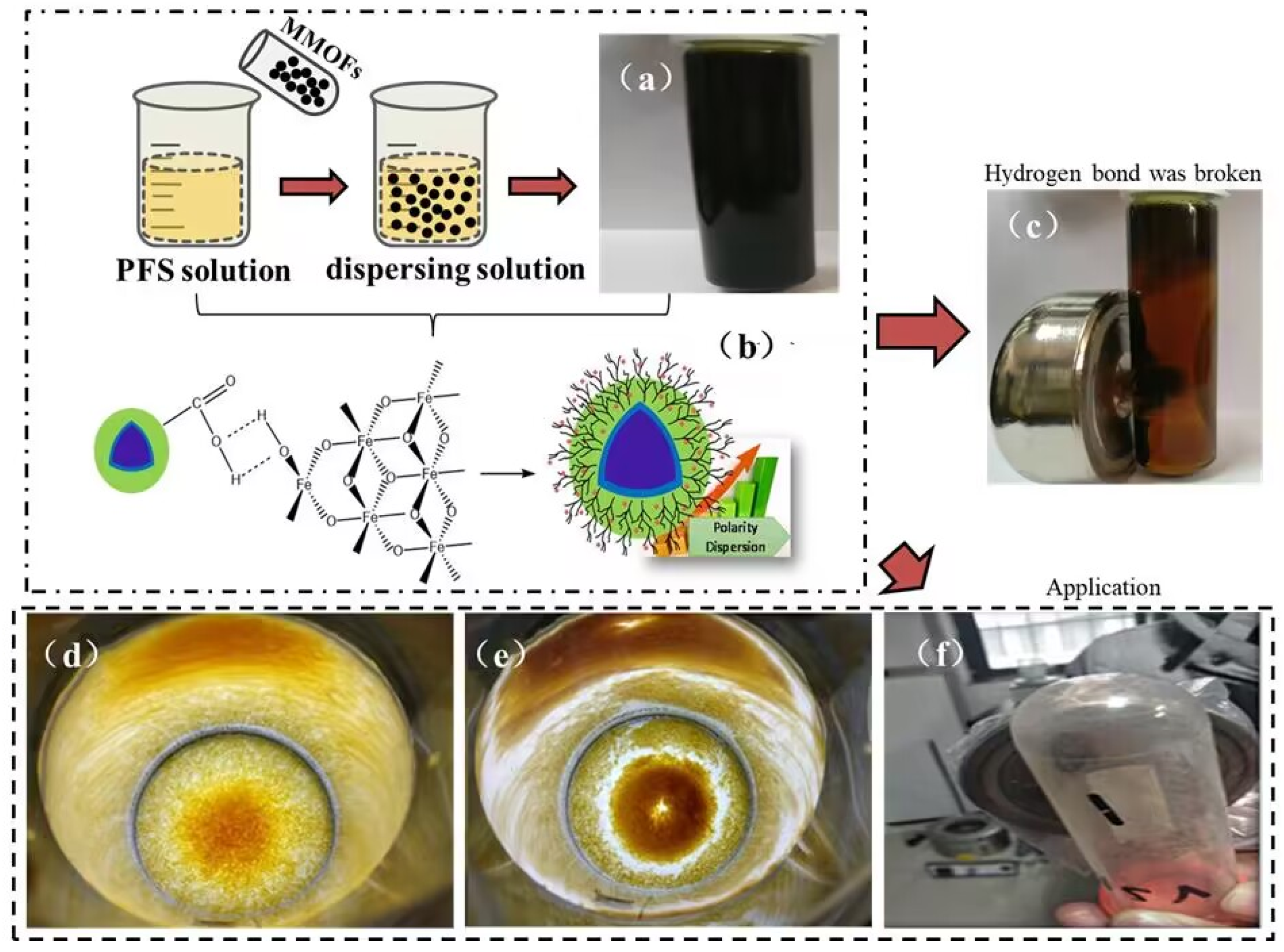
3.5. Discussion on Dispersion of MMOFs in PFS
3.6. Resistance to Hydraulic Factors
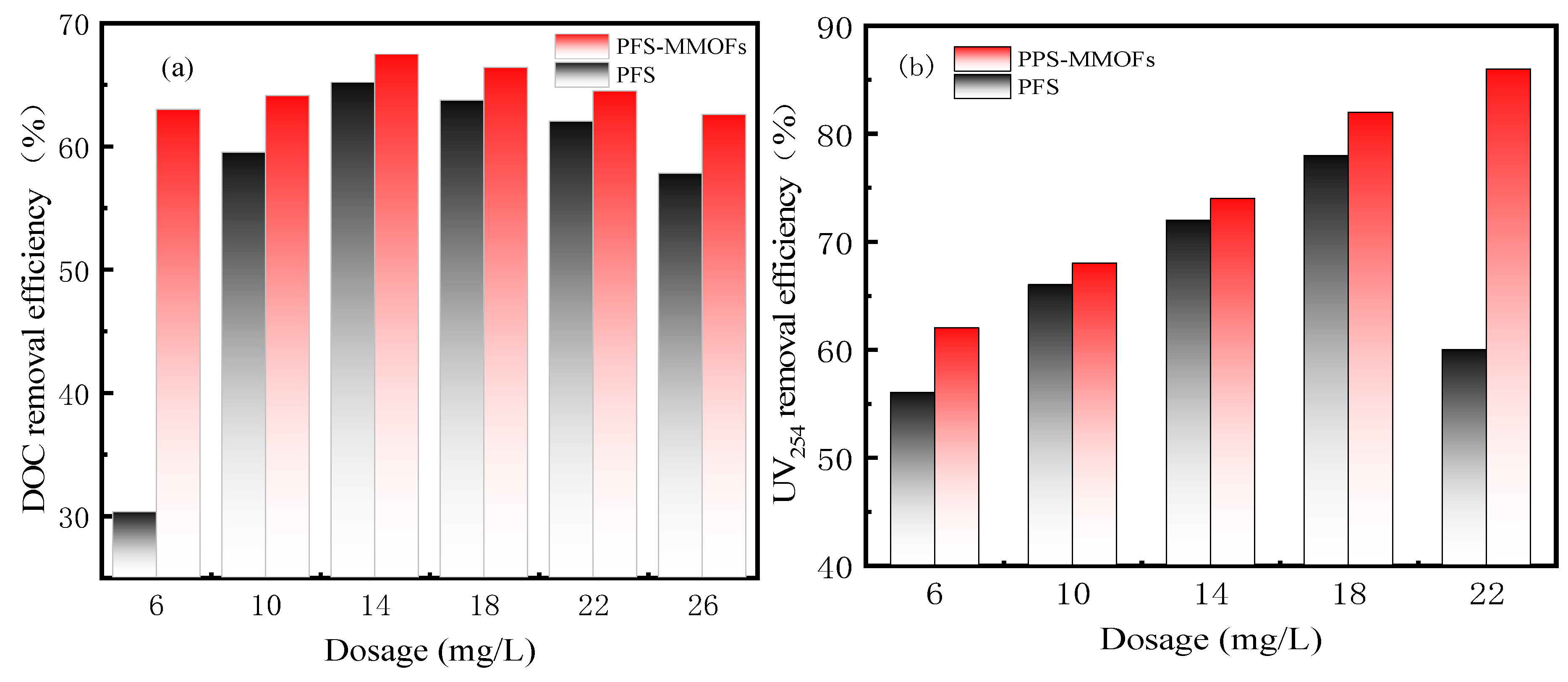
3.7. Comparison in Performance
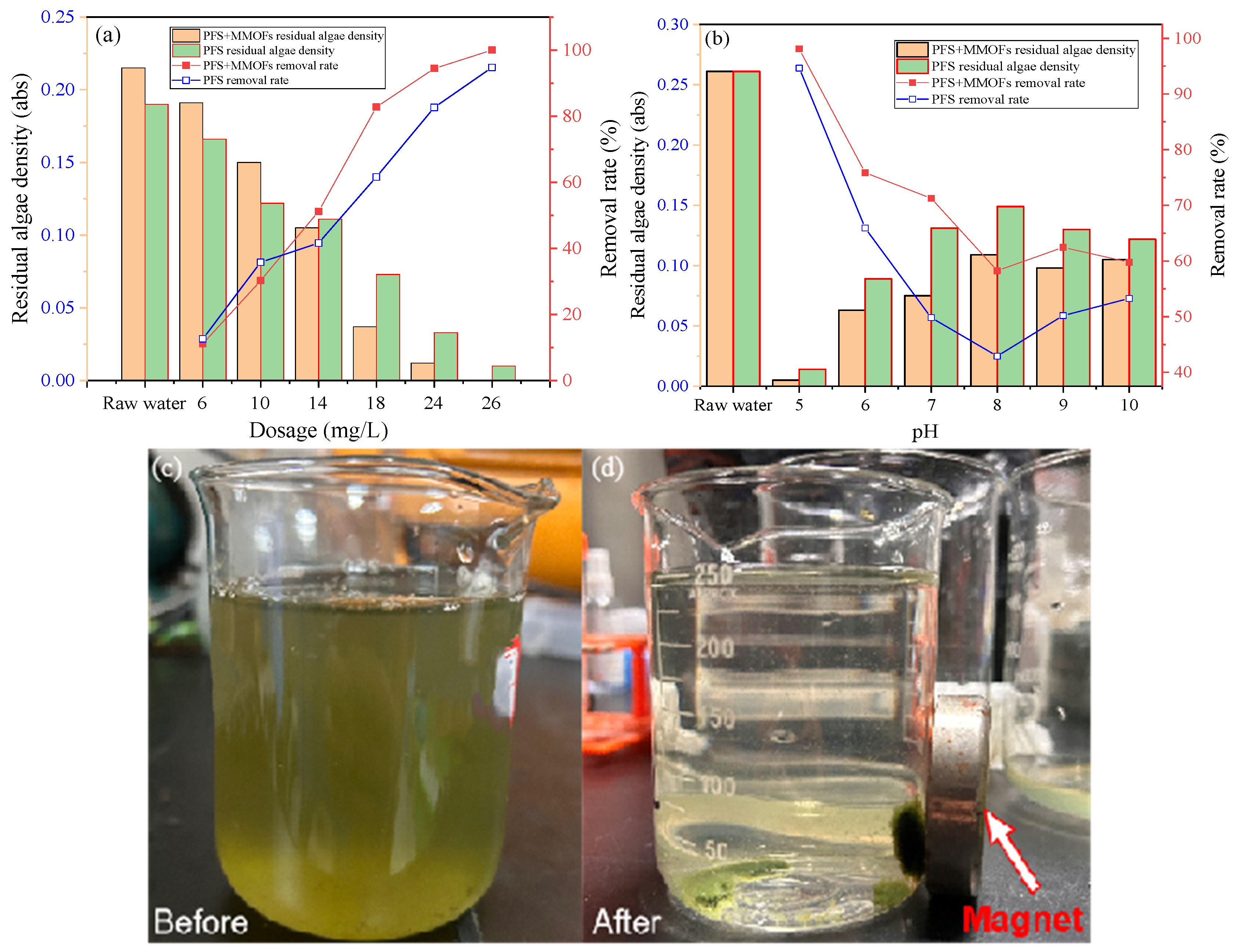
3.8. Application in Algae Water Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, H.-G.; Choi, K.; Lee, K.; Lee, S.; Jung, K.-W.; Choi, J.-W. Controlling the Structural Robustness of Zirconium-Based Metal Organic Frameworks for Efficient Adsorption on Tetracycline Antibiotics. Water 2021, 13, 1869. [Google Scholar] [CrossRef]
- Gu, S.; Jenkins, A.; Gao, S.-J.; Lu, Y.; Li, H.; Li, Y.; Ferrier, R.C.; Bailey, M.; Wang, Y.; Zhang, Y.; et al. Ensuring water resource security in China; the need for advances in evidence-based policy to support sustainable management. Environ. Sci. 2017, 75, 65–69. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, J.; Yang, H.; Sun, L.; Varis, O. Socioeconomic drivers of provincial-level changes in the blue and green water footprints in China. Resour. Conserv. Recycl. 2021, 175, 105834. [Google Scholar] [CrossRef]
- Chen, W.; Wu, S.; Lei, Y.; Li, S. China’s water footprint by province, and inter-provincial transfer of virtual water. J. Ecol. Indic. 2017, 74, 321–333. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, J.; Zhang, S.; Zhao, Z.; Luo, H.; Hursthouse, A.S.; Wan, P.; Fan, G. High removal of nitrogen and phosphorus from black-odorous water using a novel aeration-adsorption system. Environ. Chem. Lett. 2022, 20, 2243–2251. [Google Scholar] [CrossRef]
- Gleick, P.H.; Cooley, H. Freshwater Scarcity. Annu. Rev. Environ. Resour. 2021, 46, 319–348. [Google Scholar] [CrossRef]
- Janardhana Raju, N.; Shukla, U.; Ram, P. Hydrogeochemistry for the assessment of groundwater quality in Varanasi: A fast-urbanizing center in Uttar Pradesh, India. Environ. Monit. Assess. 2011, 173, 279–300. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.; Sun, W.; Shah, K.J.; Xu, Y.; Zheng, H. Efficient cationic flocculant MHCS-g-P(AM-DAC) synthesized by UV-induced polymerization for algae removal. Sep. Purif. Technol. 2019, 210, 10–19. [Google Scholar] [CrossRef]
- Ma, J.; Xia, W.; Fu, X.; Ding, L.; Kong, Y.; Zhang, H.; Fu, K. Magnetic flocculation of algae-laden raw water and removal of extracellular organic matter by using composite flocculant of Fe3O4/cationic polyacrylamide. J. Clean. Prod. 2020, 248, 119276. [Google Scholar] [CrossRef]
- Moosova, Z.; Pekarova, M.; Sindlerova, L.S.; Vasicek, O.; Kubala, L.; Blaha, L.; Adamovsky, O. Immunomodulatory effects of cyanobacterial toxin cylindrospermopsin on innate immune cells. Chemosphere 2019, 226, 439–446. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Wei, Q.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef]
- Alam, G.; Ihsanullah, I.; Naushad, M.; Sillanpää, M. Applications of artificial intelligence in water treatment for optimization and automation of adsorption processes: Recent advances and prospects. Chem. Eng. J. 2022, 427, 130011. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, J.; Fang, H.; Yuan, F.; Li, X.; Yuan, C.; Hursthouse, A.S. A flocculation tensor to monitor water quality using a deep learning model. Environ. Chem. Lett. 2022, 20, 3405–3414. [Google Scholar] [CrossRef]
- Ihsanullah, I. MXenes (two-dimensional metal carbides) as emerging nanomaterials for water purification: Progress, challenges and prospects. Chem. Eng. J. 2020, 388, 124340. [Google Scholar] [CrossRef]
- Zubair, M.; Ihsanullah, I.; Aziz, H.A.; Ahmad, M.A.; Al-Harthi, M.A. Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook. Bioresour. Technol. 2021, 319, 124128. [Google Scholar] [CrossRef]
- Naushad, M.; Sharma, G.; Alothman, Z.A. Photodegradation of toxic dye using Gum Arabic-crosslinked-poly (acrylamide)/Ni (OH) 2/FeOOH nanocomposites hydrogel. J. Clean. Prod. 2019, 241, 118263. [Google Scholar] [CrossRef]
- Bian, Y.; Xiong, N.; Zhu, G. Technology for the remediation of water pollution: A review on the fabrication of metal organic frameworks. Processes 2018, 6, 122. [Google Scholar] [CrossRef]
- Li, S.; Zhu, G.; Li, X.; Wan, P.; Yuan, F.; Xu, S.; Hursthouse, A.S. Ecosystem-inspired model and artificial intelligence predicts pollutant consumption capacity by coagulation in drinking water treatment. Environ. Chem. Lett. 2023, 21, 2499–2508. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, J.; Ma, J.; Hursthouse, A.S. Interference of the polyacrylamide coagulant in the fluorescence analysis of dissolved organic matter during water treatment. Environ. Chem. Lett. 2020, 18, 1603–1621. [Google Scholar] [CrossRef]
- Zaman, N.K.; Rohani, R.; Yusoff, I.I.; Kamsol, M.A.; Basiron, S.A.; Rashid, A.I.A. Eco-Friendly Coagulant versus Industrially Used Coagulants: Identification of Their Coagulation Performance, Mechanism and Optimization in Water Treatment Process. Int. J. Environ. Res. Public Health 2021, 18, 9164. [Google Scholar] [CrossRef]
- Sun, F.; Pei, H.-Y.; Hu, W.-R.; Ma, C.-X. The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes. Chem. Eng. J. 2012, 193–194, 196–202. [Google Scholar] [CrossRef]
- Jin, Y.; Pei, H.; Hu, W.; Zhu, Y.; Xu, H.; Ma, C.; Sun, J.; Li, H. A promising application of chitosan quaternary ammonium salt to removal of Microcystis aeruginosa cells from drinking water. Sci. Total Environ. 2017, 583, 496–504. [Google Scholar] [CrossRef]
- Ma, J.; Fu, X.; Jiang, L.; Zhu, G.; Shi, J. Magnetic flocculants synthesized by Fe3O4 coated with cationic polyacrylamide for high turbid water flocculation. Environ. Sci. Pollut. Res. 2018, 25, 25955–25966. [Google Scholar] [CrossRef]
- Nithya, R.; Thirunavukkarasu, A.; Sathya, A.B.; Sivashankar, R. Magnetic materials and magnetic separation of dyes from aqueous solutions: A review. Environ. Chem. Lett. 2021, 19, 1275–1294. [Google Scholar] [CrossRef]
- Yu, B.; Chang, H.; Wei, W.; Yu, H.; Chen, Z.; Cheng, X.; Chen, D.; Jin, Y.; Han, D.; Xu, W. Highly Effective Removal of Ciprofloxacin Antibiotic from Water by Magnetic Metal–Organic Framework. Water 2023, 15, 2531. [Google Scholar]
- Zhu, G.; Bian, Y.; Hursthouse, A.S.; Xu, S.; Xiong, N.; Wan, P. The role of magnetic MOFs nanoparticles in enhanced iron coagulation of aquatic dissolved organic matter. Chemosphere 2020, 247, 125921. [Google Scholar] [CrossRef]
- Mohamed Noor, M.; Wong, S.; Ngadi, N.; Mohammed Inuwa, I.; Opotu, L. Assessing the effectiveness of magnetic nanoparticles coagulation/flocculation in water treatment: A systematic literature review. Int. J. Environ. Sci. Technol. 2021, 19, 6935–6956. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, C.; Xu, H.; Hao, R.; Zhang, J.; Li, D. Preparation of Fe3O4@ C with water treatment residuals and its potential in the magnetic coagulation process. J. Clean. Prod. 2022, 362, 132327. [Google Scholar] [CrossRef]
- Kumar, P.; Bansal, V.; Kim, K.-H.; Kwon, E.E. Metal-organic frameworks (MOFs) as futuristic options for wastewater treatment. J. Ind. Eng. Chem. 2018, 62, 130–145. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Zhu, Z.; He, M.; Zhou, P. Effective Removal of Humic Acid by Zr-MOFs with Surface Modification. Water 2022, 14, 1800. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–organic framework nanocarriers for drug delivery in biomedical applications. Nano-Micro Lett. 2020, 12, 102. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal–organic frameworks for drug delivery: A design perspective. Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, X.; Zhang, Y.; Wu, D.; Yang, W. Applications of metal-organic framework (MOF)-based sensors for food safety: Enhancing mechanisms and recent advances. Trends Food Sci. Technol. 2021, 112, 268–282. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; Geh, S.T.; Caruso, R.A.; Doherty, C.M. Metal–organic frameworks for chemical sensing devices. Mater. Horiz. 2021, 8, 2387–2419. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lee, L.Y.S. Metal–organic frameworks for electrocatalysis: Catalyst or precatalyst? ACS Energy Lett. 2021, 6, 2838–2843. [Google Scholar] [CrossRef]
- Shet, S.P.; Priya, S.S.; Sudhakar, K.; Tahir, M. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int. J. Hydrog. Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Jaramillo, D.E.; Jiang, H.Z.; Evans, H.A.; Chakraborty, R.; Furukawa, H.; Brown, C.M.; Head-Gordon, M.; Long, J.R. Ambient-temperature hydrogen storage via vanadium (II)-dihydrogen complexation in a metal–organic framework. J. Am. Chem. Soc. 2021, 143, 6248–6256. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, B.; Salari, D.; Zarei, M. Synthesis and characterization of magnetic Fe3O4@ SiO2-MIL-53 (Fe) metal-organic framework and its application for efficient removal of arsenate from surface and groundwater. J. Environ. Chem. Eng. 2022, 10, 107144. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, P.; Zhou, H.-C.; Sharma, V.K. Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: A review. Chemosphere 2018, 209, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Luan, J.; Wu, C. Metal-organic frameworks for aquatic arsenic removal. Water Res. 2019, 158, 370–382. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, J.; Habimana, F.; Ji, S. Preparation of magnetically recyclable MIL-53(Al)@SiO2@Fe3O4 catalysts and their catalytic performance for Friedel–Crafts acylation reaction. Catal. Today 2016, 264, 83–90. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Wei, M.; Li, F.; Hou, P.; Zhang, C. Magnetic flocculation treatment of coal mine water and a comparison of water quality prediction algorithms. Mine Water Environ. 2019, 38, 391–401. [Google Scholar] [CrossRef]
- Li, S.; Kang, Y. Polymerization mechanism of polyferric aluminum phosphatic sulfate (PFAPS) and its flocculation effect on simulated dye wastewater. Korean J. Chem. Eng. 2022, 39, 1831–1838. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, X.; Huang, H.; Su, Q.; Ren, B.; Zhang, Z.; Shi, X. Fluorescence and molecular weight dependence of disinfection by-products formation from extracellular organic matter after ultrasound irradiation. Chemosphere 2023, 323, 138279. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Z.; Kong, Y.; Li, Y.; Zhou, Y.; Shi, X.; Shi, X. Effects of ultrasound on Microcystis aeruginosa cell destruction and release of intracellular organic matter. Ultrason. Sonochem. 2020, 63, 104909. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, F.; Zhang, S.; Li, J.-Y.; Lian, H.-Z. Ti(IV) immobilized bisphosphate fructose-modified magnetic Zr metal organic framework (MOF) for specific enrichment of phosphopeptides. Sep. Purif. Technol. 2023, 305, 122426. [Google Scholar] [CrossRef]
- Ma, J.; Li, S.; Wu, G.; Arabi, M.; Tan, F.; Guan, Y.; Li, J.; Chen, L. Preparation of magnetic metal-organic frameworks with high binding capacity for removal of two fungicides from aqueous environments. J. Ind. Eng. Chem. 2020, 90, 178–189. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, J.; Bian, Y. Evaluation of cationic polyacrylamide-based hybrid coagulation for the removal of dissolved organic nitrogen. Environ. Sci. Pollut. Res. 2018, 25, 14447–14459. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.; Xing, H.; Zhao, J.; Li, X.; Huang, Y.; Liu, H. Synthesis of polyethylenimine-functionalized poly(glycidyl methacrylate) magnetic microspheres and their excellent Cr(VI) ion removal properties. Chem. Eng. J. 2013, 234, 338–345. [Google Scholar] [CrossRef]
- Xu, W.; Yue, Q.; Gao, B.; Du, B. Impacts of organic coagulant aid on purification performance and membrane fouling of coagulation/ultrafiltration hybrid process with different Al-based coagulants. Desalination 2015, 363, 126–133. [Google Scholar] [CrossRef]
- Wang, X.; Gan, Y.; Zhang, S. Improved resistance to organic matter load by compositing a cationic flocculant into the titanium xerogel coagulant. Sep. Purif. Technol. 2019, 211, 715–722. [Google Scholar] [CrossRef]
- Wang, C.; Alpatova, A.; McPhedran, K.N.; El-Din, M.G. Coagulation/flocculation process with polyaluminum chloride for the remediation of oil sands process-affected water: Performance and mechanism study. J. Environ. Manag. 2015, 160, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wan, F. Immobilization of polyoxometalate-based sulfonated ionic liquids on UiO-66-2COOH metal-organic frameworks for biodiesel production via one-pot transesterification-esterification of acidic vegetable oils. Chem. Eng. J. 2019, 365, 40–50. [Google Scholar] [CrossRef]
- Tomljenovic, L. Aluminum and Alzheimer’s disease: After a century of controversy, is there a plausible link? J. Alzheimer’s Dis. 2011, 23, 567–598. [Google Scholar] [CrossRef]
- Zhu, G.; Zheng, H.; Zhang, Z.; Tshukudu, T.; Zhang, P.; Xiang, X. Characterization and coagulation–flocculation behavior of polymeric aluminum ferric sulfate (PAFS). Chem. Eng. J. 2011, 178, 50–59. [Google Scholar] [CrossRef]
- Liu, J. Study on the Preparation of Organic-Inorganic Composite Coagulant and Removal of Dissolved Organic Nitrogen/Carbon in Water; Hunan University of Science and Technology: Xiangtan, China, 2017. [Google Scholar]
- Patricia, M.F.; Budihardjo, M.A. Dose of Biocoagulant-Mixing Rate Combinations for Optimum Reduction of COD in Wastewater. E3S Web Conf. 2018, 31, 03018. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, H.L.; Zhao, C.; Xue, W.W.; Cai, N.; Yang, Q.Q.; Feng, L. Study on the Preparation of A New Composit Coagulant: Poly-Ferric-Titanium-Sulfate and Analysis of FTIR Spectrum and UV-Vis Spectrum. Spectrosc. Spectr. Anal. 2016, 36, 1038–1043. [Google Scholar]
- Costa, A.F.S.; Albuquerque, C.D.C.; Salgueiro, A.A.; Sarubbo, L.A. Color removal from industrial dyeing and laundry effluent by microbial consortium and coagulant agents. Process Saf. Environ. Prot. 2018, 118, 203–210. [Google Scholar] [CrossRef]
- Lee, K.E.; Morad, N.; Teng, T.T.; Poh, B.T. Development, characterization and the application of hybrid materials in coagulation/flocculation of wastewater: A review. Chem. Eng. J. 2012, 203, 370–386. [Google Scholar] [CrossRef]
- Yang, W.; Qian, J.; Shen, Z. A novel flocculant of Al (OH)3–polyacrylamide ionic hybrid. J. Colloid Interface Sci. 2004, 273, 400–405. [Google Scholar] [CrossRef]
- Bian, Y. Study on Preparation of a Novel Magnetic MOFs/Polymeric-Iron and Its Coagulation for Dissolved Organic Matter (DOM); Hunan University of Science and Technology: Xiangtan, China, 2019. [Google Scholar]
- Zhu, G.; Wang, Q.; Yin, J.; Li, Z.; Zhang, P.; Ren, B.; Fan, G.; Wan, P. Toward a better understanding of coagulation for dissolved organic nitrogen using polymeric zinc-iron-phosphate coagulant. Water Res. 2016, 100, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, C.; Liu, X.; Dong, X.; Zhang, P.; Yin, J.; Ren, B.; Wang, Z. Preparation and characterization of polymeric phosphate-aluminum sulphate for source water treatment. Water Sci. Technol. Water Supply 2016, 16, 1138–1148. [Google Scholar] [CrossRef]
- Bidin, N.; Azni, S.R.; Islam, S.; Abdullah, M.; Ahmad, M.F.S.; Krishnan, G.; Johari, A.R.; Bakar, M.A.A.; Sahidan, N.S.; Musa, N.; et al. The effect of magnetic and optic field in water electrolysis. Int. J. Hydrogen Energy 2017, 42, 16325–16332. [Google Scholar] [CrossRef]
- Tai, C.; Li, Y.; Yin, Y.; Cai, Y.; Jiang, G. Free Radical Photochemistry of Dissolved Organic Matter in Natural Water. Prog. Chem. 2012, 24, 1388–1397. [Google Scholar]
- Jiao, Y.; Liu, L.; Zhang, Q.; Zhou, M.; Zhang, Y. Treatment of reverse osmosis concentrate from industrial coal wastewater using an electro-peroxone process with a natural air diffusion electrode. Sep. Purif. Technol. 2021, 279, 119667. [Google Scholar] [CrossRef]
- Tan, X.; Duan, Z.; Duan, P.; Parajuli, K.; Newman, J.; Shu, X.; Zhang, D.; Gao, L.; Li, M. Flocculation of Microcystis unicells induced by pH regulation: Mechanism and potential application. Chemosphere 2021, 263, 127708. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Tao, Y.; Zhang, Y.; Li, A.; Li, T.; Sang, M.; Zhang, C. Freshwater microalgae harvested via flocculation induced by pH decrease. Biotechnol. Biofuels 2013, 6, 98. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, Y.; Li, S.; Luo, H.; Lv, L.; Zan, S.; Ren, B.; Zhu, G. Magnetic Metal–Organic Framework Enhanced Inorganic Coagulation for Water Purification. Water 2023, 15, 3391. https://doi.org/10.3390/w15193391
Bian Y, Li S, Luo H, Lv L, Zan S, Ren B, Zhu G. Magnetic Metal–Organic Framework Enhanced Inorganic Coagulation for Water Purification. Water. 2023; 15(19):3391. https://doi.org/10.3390/w15193391
Chicago/Turabian StyleBian, Yongning, Si Li, Huihao Luo, Longjiao Lv, Shubin Zan, Bozhi Ren, and Guocheng Zhu. 2023. "Magnetic Metal–Organic Framework Enhanced Inorganic Coagulation for Water Purification" Water 15, no. 19: 3391. https://doi.org/10.3390/w15193391
APA StyleBian, Y., Li, S., Luo, H., Lv, L., Zan, S., Ren, B., & Zhu, G. (2023). Magnetic Metal–Organic Framework Enhanced Inorganic Coagulation for Water Purification. Water, 15(19), 3391. https://doi.org/10.3390/w15193391





