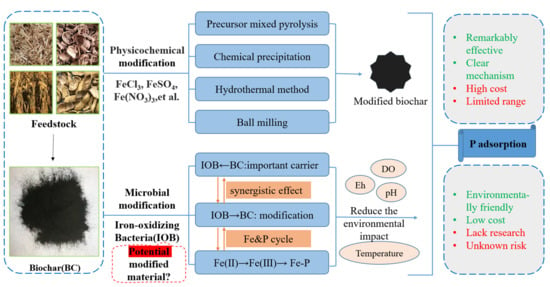
The Effect of Iron-Modified Biochar on Phosphorus Adsorption and the Prospect of Synergistic Adsorption between Biochar and Iron-Oxidizing Bacteria: A Review
Abstract
:1. Introduction
2. Mechanisms of Fe Oxidation and P Adsorption and the Influencing Factors
3. Research Progress on the Adsorption of P from Sediments by Fe-Modified Biochar through Physicochemical Pathways
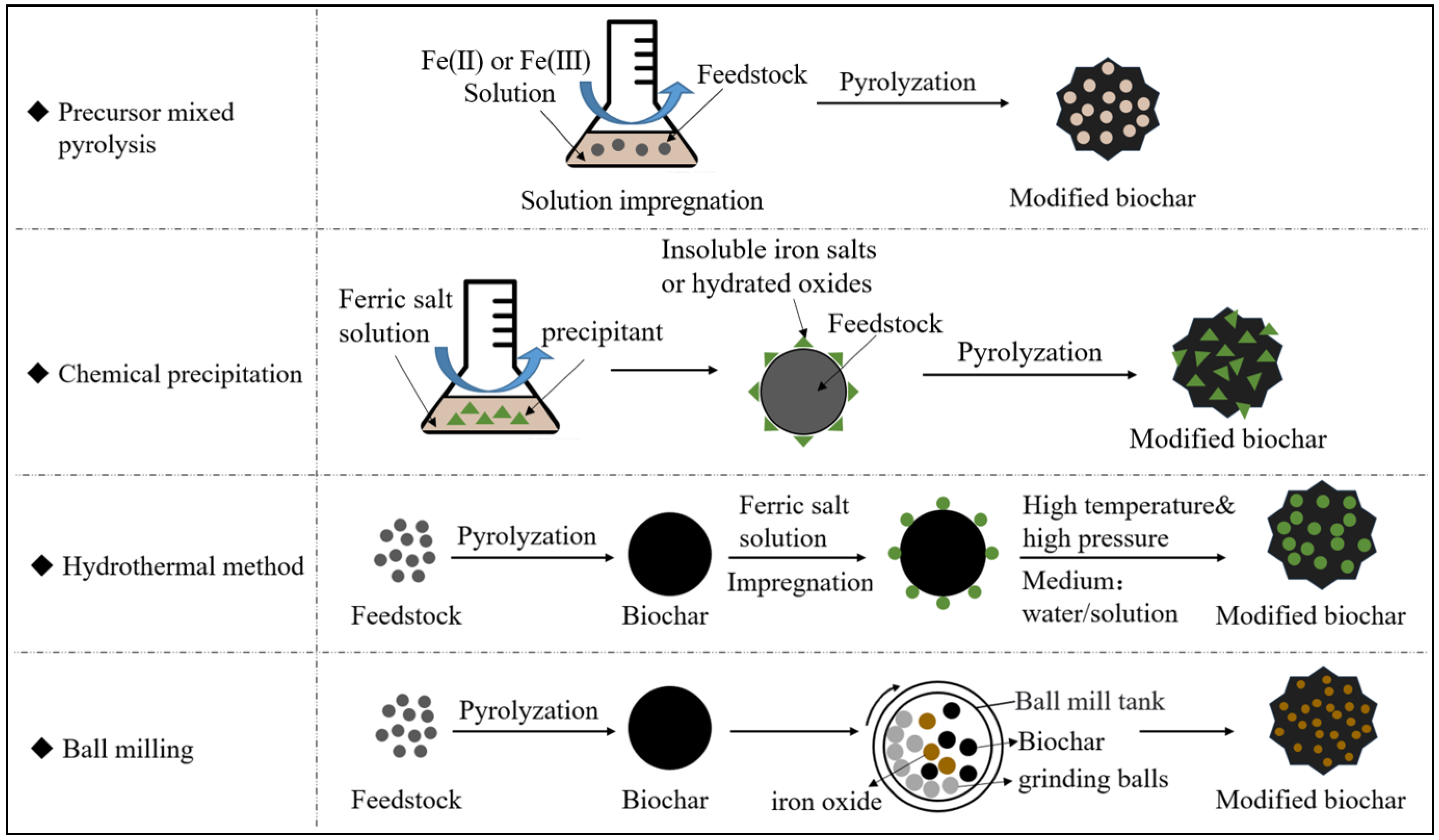
3.1. Preparation of Fe-Modified Biochar
3.2. Mechanism of Adsorption of P by Fe-Modified Biochar
3.3. Current Status and Existing Problems of the Adsorption Effect of Fe-Modified Biochar
| Feedstock | Modified Material | Modification Method | Initial Phosphate Concentration | Removal Rate | Adsorption Capacity | Mechanism of Biochar | Reference |
|---|---|---|---|---|---|---|---|
| Hemp root | FeCl3 | Impregnation | 0.795 mg/L | 50.10% | 71.00% | 1, 2 | [60] |
| Corn stalks | Sulfur-doped nano-zero-valent iron | Ball milling | 30 mg P/L | 84.40% | - | 2, 3, 4, 5 | [79] |
| Peanut shell | Sediment metals Ca2+, Fe3+ in the sediment | Capping reactor | - | Fe/Al-P: 70.9%, OP: 48.4% | 1.32 mg/g | 2, 3 | [80] |
| Reed straw | FeSO4·7H2O, NaBH4 | Impregnation | Total P in sediment is 376 mg/kg | SRP: 99% | - | 1, 2, 6, 7, 16 | [1] |
| Spent coffee grounds | FeCl3·6H2O, LaCl3·7H2O | Precursor mixed pyrolysis, chemical precipitation | 20 mg/L | 71.5–97.8% | - | 2, 3, 5, 16 | [81] |
| Walnut shell | Red mud, Fe2O3, calcite | Precursor mixed pyrolysis, chemical precipitation | 10 mg/L | 99.74% | 0.95 mg/g at 1 h | 2, 3, 4 | [82] |
| Spruce sawdust | FeCl3·6H2O | Impregnation, precursor mixed pyrolysis | 5 mg/L | 95% (1 g/L) | - | 6, 7 | [20] |
| Corn stalks | FeCl3, NaOH, NaH2PO4, NaNO3 | Impregnation | 2 mg/L | - | 1.78–2.19 mg/g | 4, 5, 6, 8 | [83] |
| Peanut shell | FeCl3, FeSO4·7H2O, Fe(NO3)3·9H2O | Impregnation | 5 mg/L | FS-BC: 99.6%, FN-BC: 95.0% | - | 2, 5 | [84] |
| Compress-ed sludge | Chitosan, ferrous sulfate, sodium sulfide | Impregnation | 100 mg/L | - | 49.32 mg/g | 2, 3, 4, 5, 10 | [85] |
| Peanut shell | FeSO4·7H2O | Impregnation | 5.0 mg/LKH2PO4 | 74% | 1.11 mg/g | 2, 3, 5, 11 | [86] |
| Corncob | FeCl3 | Impregnation | TP: 5.54 mg·P/L | 94.33% | - | 5, 9 | [87] |
| Rice hull | FeCl3·6H2O | Thermal activation | 100 mg/L | - | 69.92 mg/g | 3, 5, 8 | [88] |
| Wood and rice husks | Fe2(SO4)3·nH2O, n = 6–9, FeSO4 | Co-precipitation | 25–150 mg/L | - | 25–28 mg/g | 2, 3, 5 | [89] |
| Corn straw | FeCl3 | Impregnation | - | - | 26.14 mg/g | 1, 3, 12, 13, 14 | [90] |
| Corn stalk | FeCl3 | Impregnation | 10–50 mg/L | 85% | - | 1, 15, 16 | [91] |
| Waste-activated sludge | FeSO4·7H2O, FeCl3 | Chemical co-precipitation | 20 mg/L | 60% (after 5 successive recycles) | 111.0 mg/g | 1, 3, 5, 11 | [92] |
4. The Possibility of Adsorption of P by IOB in Combination with Biochar
4.1. The Mechanism of Biochar-Loading Microorganisms
4.2. Practical Application of Biochar-Supported Microorganisms in the P-Adsorption Process
4.3. Study of the Superiority of Biochar in Collaboration with IOB
5. Potential Risks to Sediment Environments from the Use of Modified Biochar
6. Conclusions and Outlook
- (1)
- First of all, it is necessary to ensure the safety of biochar itself, avoid other forms of pollution to water bodies and sediments, and not to threaten the microbial diversity of water bodies and sediments. Secondly, the tolerance level of microorganisms to the concentration of pollutants in water bodies should also be verified repeatedly through experiments, and the simulated experimental conditions should be adjusted to be infinitely close to the real environment, so as to prepare for better adaptations to different application scenarios;
- (2)
- Biochars created from different materials have different surface structures, and the characteristics of the microorganisms vary. Therefore, it is necessary to pay attention to whether the diameters match, in order to ensure that the absorption effect of biochar and the bacterial population are compatible;
- (3)
- As a carrier material, the recovery of biochar should become a key focus to prevent the accumulation of biomass or secondary pollution caused by its combination with other components in water and sediment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, L. Recovery of Phosphorus from Eutrophic Water Using Nano Zero-Valentiron-Modified Biochar and Its Utilization. Master’s Thesis, Qingdao University, Qingdao, China, 2021. [Google Scholar]
- Sun, C.; Wang, S.; Wang, H.; Hu, X.; Yang, F.; Tang, M.; Zhang, M.; Zhong, J. Internal Nitrogen and Phosphorus Loading in a Seasonally Stratified Reservoir: Implications for Eutrophication Management of Deep-Water Ecosystems. J. Environ. Manag. 2022, 319, 115681. [Google Scholar] [CrossRef]
- Zhang, M.; Wen, S.; Wu, T.; Wang, S.; Li, X.; Gong, W.; Wang, H.; Liu, C.; Zhong, J. Patterns of Internal Nitrogen and Phosphorus Loadings in a Cascade Reservoir with a Large Water Level Gradient: Effects of Reservoir Operation and Water Depth. J. Environ. Manag. 2022, 320, 115884. [Google Scholar] [CrossRef]
- Hooker, H.D. Liebig’s law of the minimum in relation to general biological problems. Science 1917, 46, 197–204. [Google Scholar] [CrossRef]
- Huang, L.; Fang, H.; Reible, D. Mathematical Model for Interactions and Transport of Phosphorus and Sediment in the Three Gorges Reservoir. Water Res. 2015, 85, 393–403. [Google Scholar] [CrossRef]
- Yang, C.; Tong, L.; Liu, X.; Tan, Q.; Liu, H. High-Resolution Imaging of Phosphorus Mobilization and Iron Redox Cycling in Sediments from Honghu Lake, China. J. Soils Sediments 2019, 19, 3856–3865. [Google Scholar] [CrossRef]
- Li, X.; Huang, L.; Reible, D.; Zeng, X.; Liu, S.; Fu, J.; Wang, K.; Fang, H. Inhibition of Sediment Erosion and Phosphorus Release by Remediation Strategy of Contaminated Sediment Backfilling. Water Res. 2023, 239, 120055. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Chen, M.; Dai, J.; Gu, W.; Wen, S.; Du, Y. Composition of Organic Matter-iron-phosphorus Associations in Sediments of Algae- and Macrophyte-Dominated Zones in Lake Taihu. Chem. Geol. 2023, 622, 121375. [Google Scholar] [CrossRef]
- Berbel, G.B.B.; Favaro, D.I.T.; Braga, E.S. Impact of Harbour, Industry and Sewage on the Phosphorus Geochemistry of a Subtropical Estuary in Brazil. Mar. Pollut. Bull. 2015, 93, 44–52. [Google Scholar] [CrossRef]
- Dugdug, A.A.; Chang, S.X.; Ok, Y.S.; Rajapaksha, A.U.; Anyia, A. Phosphorus Sorption Capacity of Biochars Varies with Biochar Type and Salinity Level. Environ. Sci. Pollut. Res. 2018, 25, 25799–25812. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, W.; Lu, L.; Yan, L.; Yu, D. Utilization of Biochar for the Removal of Nitrogen and Phosphorus. J. Clean. Prod. 2020, 257, 120573. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Chen, F.; Chen, Y.; Chen, W. Effect of activated carbon pore structure on the adsorption of phosphorus. Appl. Chem. Ind. 2022, 51, 1624–1628. [Google Scholar] [CrossRef]
- Tran, H.S.; Viet, N.T.T.; Duong, T.H.; Nguyen, L.H.; Kawamoto, K. Autoclaved Aerated Concrete Grains as Alternative Absorbent and Filter Media for Phosphorus Recovery from Municipal Wastewater: A Case Study in Hanoi, Vietnam. Environ. Technol. Innov. 2023, 31, 103175. [Google Scholar] [CrossRef]
- Ganesapillai, M.; Drewnowski, J.; Ranjan, S.; Selvaraj, T. Sustainable Recovery of Plant Essential Nitrogen and Phosphorus from Human Urine Using Industrial Coal Fly Ash. Environ. Technol. Innov. 2021, 24, 101985. [Google Scholar] [CrossRef]
- Ashraf, I.; Li, C.; Wang, T.; Li, R.; Chen, B. Phosphorus Removal from Oil and Aqueous Phases with a Multifunctional Adsorbent. Anal. Methods 2020, 12, 466–470. [Google Scholar] [CrossRef]
- Ding, S.; Sun, Q.; Chen, X.; Liu, Q.; Wang, D.; Lin, J.; Zhang, C.; Tsang, D.C.W. Synergistic Adsorption of Phosphorus by Iron in Lanthanum Modified Bentonite (Phoslock®): New Insight into Sediment Phosphorus Immobilization. Water Res. 2018, 134, 32–43. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Zhao, W.; Li, Z.; Zang, L. Facile Fabrication of Calcium-Doped Carbon for Efficient Phosphorus Adsorption. ACS Omega 2021, 6, 327–339. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Smith, R.L.; Fu, J.; Qi, X. High-Capacity Structured MgO-Co Adsorbent for Removal of Phosphorus from Aqueous Solutions. Chem. Eng. J. 2021, 426, 131381. [Google Scholar] [CrossRef]
- Zhao, D.; Luo, Y.; Feng, Y.; He, Q.; Zhang, L.; Zhang, K.; Wang, F. Enhanced Adsorption of Phosphorus in Soil by Lanthanum-Modified Biochar: Improving Phosphorus Retention and Storage Capacity. Environ. Sci. Pollut. Res. 2021, 28, 68996. [Google Scholar] [CrossRef] [PubMed]
- Tomin, O.; Vahala, R.; Yazdani, M.R. Tailoring Metal-Impregnated Biochars for Selective Removal of Natural Organic Matter and Dissolved Phosphorus from the Aqueous Phase. Micropor. Mesopor. Mater. 2021, 328, 111499. [Google Scholar] [CrossRef]
- Ahmad, A.; Priyadarshini, M.; Yadav, S.; Ghangrekar, M.M.; Surampalli, R.Y. The Potential of Biochar-Based Catalysts in Advanced Treatment Technologies for Efficacious Removal of Persistent Organic Pollutants from Wastewater: A Review. Chem. Eng. Res. Des. 2022, 187, 470–496. [Google Scholar] [CrossRef]
- Menya, E.; Storz, H.; Olupot, P.W. Editorial: Special Issue on Extended Application of Biomass-Based Activated Carbon in Water and Wastewater Treatment. Chem. Eng. Res. Des. 2023, 194, 242–244. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.; Yang, Y.; Lu, S.; Wang, G.; Qian, X. Interactions of Vallisneria Natans and Iron-Oxidizing Bacteria Enhance Iron-Bound Phosphorus Formation in Eutrophic Lake Sediments. Microorganisms 2022, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yu, Y.; Lin, J.; Wu, X.; Wang, Y.; Zhao, Y. Simultaneous Control of Nitrogen and Phosphorus Release from Sediments Using Iron-Modified Zeolite as Capping and Amendment Materials. J. Environ. Manag. 2019, 249, 109369. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Chen, P.; Liu, E.; Yu, J.; Tai, Z.; Li, Q.; Wang, H.; Cai, Y. Terrestrial Sources Regulate the Endogenous Phosphorus Load in Taihu Lake, China after Exogenous Controls: Evidence from a Representative Lake Watershed. J. Environ. Manag. 2023, 340, 118016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liao, Z.; Yao, D.; Yang, Z.; Wu, Y.; Tang, C. Phosphorus Immobilization in Water and Sediment Using Iron-Based Materials: A Review. Sci. Total Environ. 2021, 767, 144246. [Google Scholar] [CrossRef]
- van Dael, T.; De Cooman, T.; Verbeeck, M.; Smolders, E. Sediment Respiration Contributes to Phosphate Release in Lowland Surface Waters. Water Res. 2020, 168, 115168. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yan, P.; Liu, Z.; Zhang, M.; Yan, W.; Liu, Y.; Wu, Z.; Zhang, Y. Spatial Distribution of Phosphorus Forms and the Release Risk of Sediments Phosphorus in West Lake, Hangzhou, China. Ecol. Eng. 2021, 173, 106421. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Ding, S.; Guo, J.; Christopher, D.; Dai, Z.; Yang, H. Effects of Seasonal Hypoxia on the Release of Phosphorus from Sediments in Deep-Water Ecosystem: A Case Study in Hongfeng Reservoir, Southwest China. Environ. Pollut. 2016, 219, 858–865. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.; Xu, K.; Hao, J.; Li, Y.-Y. Phosphorus Release from Coprecipitants Formed during Orthophosphate Removal with Fe(III) Salt Coagulation: Effects of PH, Eh, Temperature and Aging Time. J. Environ. Chem. Eng. 2016, 4, 3322–3329. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, C. Discussion on the Role of Oxidation-reduction Potential in the Purification of Sewage and Surface Water. Energ. Conserv. Environ. Prot. 2020, 10, 29–30. [Google Scholar]
- Li, Y.; Liu, Y.; Wang, H.; Zuo, Z.; Yan, Z.; Wang, L.; Wang, D.; Liu, C.; Yu, D. In Situ Remediation Mechanism of Internal Nitrogen and Phosphorus Regeneration and Release in Shallow Eutrophic Lakes by Combining Multiple Remediation Techniques. Water Res. 2022, 229, 119394. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Yuan, R.; Salam, M.; Zhang, L.; Wei, Y.; Tang, B.; Yuan, X.; Liu, B.; Yu, X.; Li, H.; et al. Achieving Simultaneous Removal of Nitrogen and Phosphorus in Sediment via Combined Adsorption and Oxygen Supplement. Chem. Eng. J. 2022, 441, 136056. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, R.; Xia, C.; Xu, J. Effects of oxidation-reduction potential and microorganism on the release of phosphorus from sediments. Environ. Chem. 2014, 33, 930–936. [Google Scholar]
- Du, H.; Cao, Y.; Li, Z.; Li, L.; Xu, H. Formation and Mechanisms of Hydroxyl Radicals during the Oxygenation of Sediments in Lake Poyang, China. Water Res. 2021, 202, 117442. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Wei, Q.; Qi, X.; Yin, Q.; Liu, B.; He, K. Response and Synergistic Effect of Microbial Community to Submerged Macrophyte in Restoring Urban Black and Smelly Water Bodies. J. Water Process. Eng. 2023, 53, 103906. [Google Scholar] [CrossRef]
- Wu, H.; Hao, B.; Cai, Y.; Liu, G.; Xing, W. Effects of Submerged Vegetation on Sediment Nitrogen-Cycling Bacterial Communities in Honghu Lake (China). Sci. Total Environ. 2021, 755, 142541. [Google Scholar] [CrossRef]
- Ma, K.; Xie, J.; Wei, L.; Jiang, X.; Gao, L. Effect of organic phosphate-solubilizing bacteria on phosphorus release from sediments in Swan Lagoon. J. Agro-Environ. Sci. 2023, 1–14. Available online: http://kns.cnki.net/kcms/detail/12.1347.S.20221230.1624.001.html (accessed on 3 January 2023).
- Ma, K. Phosphate Solubilizing Characteristics of Organic Phosphate-Solubilizing Bacteria in Sediments from Swan Lake and Their Effect on Phosphorus Release. Master’s Thesis, Yantai University, Yantai, China, 2023. [Google Scholar]
- Wang, X.; Gao, L.; Ma, K.; Wei, L.; Pi, Y. Isolation of Inorganic-Phosphate-Solubilizing Bacteria and Analysis of Their Effect on Phosphorus Release from Lagoon Sediments. Geomicrobiol. J. 2023, 40, 46–59. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Q.; Huang, S.; Wang, Z.; Li, D. Cyanobacterial Organic Matter (COM) Positive Feedback Aggravates Lake Eutrophication by Changing the Phosphorus Release Characteristics of Sediments. Sci. Total Environ. 2023, 892, 164540. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, Q.; Sheng, Y.; Chen, C.; Yu, G.; Kappler, A. Coupled Iron Cycling and Organic Matter Transformation across Redox Interfaces. Nat. Rev. Earth Environ. 2023, 4, 659–673. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Lee, C. A Review of the Effects of Iron Compounds on Methanogenesis in Anaerobic Environments. Renew. Sustain. Energy Rev. 2019, 113, 109282. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 130, pp. 1–140. [Google Scholar]
- Sheng, Z.; Zuo, J.; Mao, W.; Shen, Y.; He, F. Performance and mechanisms of phosphate adsorption in aqueous solution by acid/alkali-modified cattail biochar. Acta Sci. Circumstantiae 2022, 42, 195–203. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; Feng, Q.; Chen, M.; Zhang, X.; Zhao, R. Recovery of Nitrogen and Phosphorus in Wastewater by Red Mud-Modified Biochar and Its Potential Application. Sci. Total Environ. 2023, 860, 160289. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, W.; Yin, S.; Liu, X.; Zhang, M.; Lou, X.; Li, Z. Adsorption characteristics and mechanism of phosphate in wastewater by iron-zirconium modified biochar. Ind. Water Treat. 2022, 42, 153–161. [Google Scholar] [CrossRef]
- Zhai, Z.; Deng, J.; Li, G. Research progress of phosphorus removal by adsorption. Technol. Inno. Appl. 2019, 5, 117–119. [Google Scholar]
- Fang, J.; Zhang, X.; Jing, H. Adsorption of Phosphorus in Water by Magnetized Modified Sludge-based Biochar. Technol. Water Treat. 2021, 47, 37–41. [Google Scholar] [CrossRef]
- Wang, W.; Cai, Y.; Su, K.; Ma, C.; Song, X.; Cao, X. Effect of Nitrogen and Phosphorus Removal by Simulated Vertical Flow Constructed Wetland with Biochar of Procambarus clarkii Shell and Sponge Zero-valent Iron. Wetland Sci. 2021, 19, 743–752. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Li, H.; Westholm, L.J.; Carvalho, L.; Thorin, E.; Yu, Z.; Yu, X.; Skreiberg, Ø. A Critical Review on Production, Modification and Utilization of Biochar. J. Anal. Appl. Pyrolysis 2021, 161, 105405. [Google Scholar] [CrossRef]
- Li, G.; Zeng, S.; Sun, S.; Xu, K.; Bian, D. Preparation of biochar supported iron oxides composites and its application in water treatment. Chem. Ind. Eng. Prog. 2021, 40, 917–931. [Google Scholar] [CrossRef]
- Shen, Y. Carbothermal Synthesis of Metal-Functionalized Nanostructures for Energy and Environmental Applications. J. Mater. Chem. A 2015, 3, 13114–13188. [Google Scholar] [CrossRef]
- Su, H.; Wu, J.; Wang, Z.; Cheng, X. Research progress of metal-modified biochar and its removal of nitrogenand phosphorus in water. Ind. Water Treat. 2022, 42, 46–55. [Google Scholar] [CrossRef]
- Ullah, M.; Ali, M.E.; Hamid, S.B.A. Structure-Controlled Nanomaterial Synthesis Using Surfactant-Assisted Ball Milling- A Review. Curr. Nanosci. 2014, 10, 344–354. [Google Scholar] [CrossRef]
- Užarević, K.; Halasz, I.; Friščić, T. Real-Time and In Situ Monitoring of Mechanochemical Reactions: A New Playground for All Chemists. J. Phys. Chem. Lett. 2015, 6, 4129–4140. [Google Scholar] [CrossRef] [PubMed]
- Ghodszad, L.; Reyhanitabar, A.; Maghsoodi, M.R.; Asgari Lajayer, B.; Chang, S.X. Biochar Affects the Fate of Phosphorus in Soil and Water: A Critical Review. Chemosphere 2021, 283, 131176. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Yin, T.; Liu, H.; Fan, Z.; Fan, S. Co-removal of Nitrogen and Phosphate from Water by Modified Biochar: Modification Methods and Adsorption Mechanisms. Chem. Reag. 2023, 45, 119–127. [Google Scholar] [CrossRef]
- Law, X.N.; Cheah, W.Y.; Chew, K.W.; Ibrahim, M.F.; Park, Y.-K.; Ho, S.-H.; Show, P.L. Microalgal-Based Biochar in Wastewater Remediation: Its Synthesis, Characterization and Applications. Environ. Res. 2022, 204, 111966. [Google Scholar] [CrossRef]
- Wang, N.; Wang, J.; Li, Y.; Li, J. A study on adsorption characteristics of iron-modified hemp rootbiochar for phosphate in water. J. Dalian Ocean Univ. 2023, 38, 464–473. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, Y.; Sheng, G. Progress in the preparation of modified biochar and its removal of pollutants in water. J. Funct. Mater. 2022, 53, 12073–12084. [Google Scholar]
- Kamran, M.A.; Bibi, S.; Chen, B.; Jiang, J.; Xu, R.-K. Elucidating the Mechanisms Determining the Availability of Phosphate by Application of Biochars from Different Parent Materials. Environ. Geochem. Health 2022, 44, 4191–4200. [Google Scholar] [CrossRef]
- Ji, M.; Wang, X.; Usman, M.; Liu, F.; Dan, Y.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W. Effects of Different Feedstocks-Based Biochar on Soil Remediation: A Review. Environ. Pollut. 2022, 294, 118655. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of Feedstock Source and Pyrolysis Temperature on Biochar Bulk and Surface Properties. Biomass Bioenerg. 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Zeng, Q.; Liang, Z.; Ye, X.; Lv, Y.; Liu, M. Preparation of Eucommia Ulmoides Lignin-Based High-Performance Biochar Containing Sulfonic Group: Synergistic Pyrolysis Mechanism and Tetracycline Hydrochloride Adsorption. Bioresour. Technol. 2021, 329, 124856. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Yan, L.; Li, J.; Yang, Y.; Shan, L.; Meng, X.; Li, X.; Zhao, Y. Adsorption Performance and Mechanistic Study of Heavy Metals by Facile Synthesized Magnetic Layered Double Oxide/Carbon Composite from Spent Adsorbent. Chem. Eng. J. 2020, 384, 123331. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lamb, D.; Kunhikrishnan, A.; Rahman, M.M. Kinetics, Isotherms and Adsorption–Desorption Behavior of Phosphorus from Aqueous Solution Using Zirconium–Iron and Iron Modified Biosolid Biochars. Water 2021, 13, 3320. [Google Scholar] [CrossRef]
- Sun, T.; Gao, F.; Lin, L.; Li, R.; Dong, L. Adsorption of Low-Concentration Phosphorus from Water by Composite Metal Modified Biochar. Environ. Sci. 2020, 41, 784–791. [Google Scholar]
- Almanassra, I.W.; Mckay, G.; Kochkodan, V.; Ali Atieh, M.; Al-Ansari, T. A State of the Art Review on Phosphate Removal from Water by Biochars. Chem. Eng. J. 2021, 409, 128211. [Google Scholar] [CrossRef]
- Hou, L.; Liang, Q.; Wang, F. Mechanisms That Control the Adsorption–Desorption Behavior of Phosphate on Magnetite Nanoparticles: The Role of Particle Size and Surface Chemistry Characteristics. RSC Adv. 2020, 10, 2378–2388. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Huang, H.; Zhao, N.; Zhang, M.; Cao, L. Investigation into Lanthanum-Coated Biochar Obtained from Urban Dewatered Sewage Sludge for Enhanced Phosphate Adsorption. Sci. Total Environ. 2020, 714, 136839. [Google Scholar] [CrossRef]
- Ou, W.; Lan, X.; Guo, J.; Cai, A.; Liu, P.; Liu, N.; Liu, Y.; Lei, Y. Preparation of Iron/Calcium-Modified Biochar for Phosphate Removal from Industrial Wastewater. J. Clean. Prod. 2023, 383, 135468. [Google Scholar] [CrossRef]
- Liang, W.; Guo, P.; Shi, S.; Liang, H. The Progress of Phosphate Adsorption by Modified Biochar from Wastewater. Shandong Chem. Ind. 2022, 51, 137–138+145. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, J.; Yin, Y.; Shang, J.; Chen, C.; Zhai, T. Application of Goethite Modified Biochar for Arsenic Removal from Aqueous Solution. Environ. Sci. 2019, 40, 2773–2782. [Google Scholar] [CrossRef]
- Lu, Y.; Li, B.; Li, X.; Liu, H.; Liu, H.; Chen, J. Research progress on preparation of waste straw biochar and itsapplication in waste water treatment. New Chem. Mater. 2023, 1–13. Available online: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C45S0n9fL2suRadTyEVl2pW9UrhTDCdPD642JQ5UnStQItWCjTSQvumg0hebTfnFIfrZ6m8AdmCFOlww7nGtaSzN&uniplatform=NZKPT (accessed on 12 June 2023).
- Lin, Q.; Tan, X.; Almatrafi, E.; Yang, Y.; Wang, W.; Luo, H.; Qin, F.; Zhou, C.; Zeng, G.; Zhang, C. Effects of Biochar-Based Materials on the Bioavailability of Soil Organic Pollutants and Their Biological Impacts. Sci. Total Environ. 2022, 826, 153956. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Creamer, A.E.; He, F. Adsorptive Removal of Arsenate from Aqueous Solutions by Biochar Supported Zero-Valent Iron Nanocomposite: Batch and Continuous Flow Tests. J. Hazard. Mater. 2016, 322, 172–181. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Liu, S.; Liu, H.; Zeng, G.; Tan, X.; Yang, C.; Ding, Y.; Yan, Z.; Cai, X. Sorption Performance and Mechanisms of Arsenic(V) Removal by Magnetic Gelatin-Modified Biochar. Chem. Eng. J. 2016, 314, 223–231. [Google Scholar] [CrossRef]
- Ai, D.; Wei, T.; Meng, Y.; Chen, X.; Wang, B. Ball Milling Sulfur-Doped Nano Zero-Valent Iron @biochar Composite for the Efficient Removal of Phosphorus from Water: Performance and Mechanisms. Bioresour. Technol. 2022, 357, 127316. [Google Scholar] [CrossRef]
- Gao, C.; Fan, J.; Zhang, X.; Gong, Z.; Tan, Z. Sediment Metals Adhering to Biochar Enhanced Phosphorus Adsorption in Sediment Capping. Water Sci. Technol. 2021, 84, 2057–2067. [Google Scholar] [CrossRef]
- Akindolie, M.S.; Choi, H.J. Fe12LaO19 Fabricated Biochar for Removal of Phosphorus in Water and Exploration of Its Adsorption Mechanism. J. Environ. Manag. 2023, 329, 117053. [Google Scholar] [CrossRef]
- Yang, J.; Ma, X.; Xiong, Q.; Zhou, X.; Wu, H.; Yan, S.; Zhang, Z. Functional Biochar Fabricated from Red Mud and Walnut Shell for Phosphorus Wastewater Treatment: Role of Minerals. Environ. Res. 2023, 232, 116348. [Google Scholar] [CrossRef]
- He, K.; Jiang, Y.; Zhou, L.; Han, M.; Jin, J. Study on Adsorption of Nitrogen and Phosphorus from lnitial Rain by lron-modified Biochar. Environ. Sci. Technol. 2022, 45, 78–86. [Google Scholar] [CrossRef]
- Zhang, B. Performance and Mechanism on the Phosphorus Adsorption by Iron Modified Peanut Shell Biochar. Master’ Thesis, China University of Geosciences, Wuhan, China, 2018. [Google Scholar]
- Sang, Q.; Wang, F.; Zhao, Y.; Zhou, Q.; Cai, Y.; Deng, Y.; Tian, W.; Chen, Y.; Ma, J. Application of lron and Sulfate-Modified Biochar in Phosphorus Removal from Water. J. Environ. Sci. 2021, 42, 2313–2323. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zhang, Y.; Zhang, H.; Rao, Z. Adsorption Properties of Phosphorus by Modified Peanut Shell Biochar. Environ. Sci. Technol. 2022, 45, 21–26. [Google Scholar] [CrossRef]
- Xiao, S. Modification of Biochar and Its Adsorption Behavior for Phosphate. Master’s Thesis, Southeast University, Nanjing, China, 2021. [Google Scholar]
- Zeng, F.; Sun, F.; Sun, E.; Du, J.; Huang, H.; Qu, P.; Yong, C.; Xu, Y. Fabrication of Fe2O3/PC functionalized biochar composites for phosphate removal from wastewater: Adsorption properties and mechanism. Acta Sci. Circumstantiae 2021, 41, 3487–3496. [Google Scholar] [CrossRef]
- Ajmal, Z.; Muhmood, A.; Dong, R.; Wu, S. Probing the Efficiency of Magnetically Modified Biomass-Derived Biochar for Effective Phosphate Removal. J. Environ. Manag. 2020, 253, 109730. [Google Scholar] [CrossRef]
- Li, B.; Jing, F.; Hu, Z.; Liu, Y.; Xiao, B.; Guo, D. Simultaneous Recovery of Nitrogen and Phosphorus from Biogas Slurry by Fe-Modified Biochar. J. Saudi Chem. Soc. 2021, 25, 101213. [Google Scholar] [CrossRef]
- Min, L.; Zhongsheng, Z.; Zhe, L.; Haitao, W. Removal of Nitrogen and Phosphorus Pollutants from Water by FeCl3- Impregnated Biochar. Ecol. Eng. 2020, 149, 105792. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and Mechanisms of Phosphate Adsorption on Iron-Modified Biochars Derived from Waste Activated Sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef]
- Liu, J.; Pan, D.; Wu, X.; Chen, H.; Cao, H.; Li, Q.X.; Hua, R. Enhanced Degradation of Prometryn and Other S-Triazine Herbicides in Pure Cultures and Wastewater by Polyvinyl Alcohol-Sodium Alginate Immobilized Leucobacter Sp. JW-1. Sci. Total Environ. 2017, 615, 78–86. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Niu, A.; Cheng, N.; Chen, M.; Zhang, X.; Yu, Z.; Wang, S. Application of Biochar Immobilized Microorganisms for Pollutants Removal from Wastewater: A Review. Sci. Total Environ. 2022, 837, 155563. [Google Scholar] [CrossRef]
- Gong, Y.-Z.; Niu, Q.-Y.; Liu, Y.-G.; Dong, J.; Xia, M.-M. Development of Multifarious Carrier Materials and Impact Conditions of Immobilised Microbial Technology for Environmental Remediation: A Review. Environ. Pollut. 2022, 314, 120232. [Google Scholar] [CrossRef]
- Khan, M.A.; Mahmood-Ur-Rahman; Ramzani, P.M.A.; Zubair, M.; Rasool, B.; Khan, M.K.; Ahmed, A.; Khan, S.A.; Turan, V.; Iqbal, M. Associative Effects of Lignin-Derived Biochar and Arbuscular Mycorrhizal Fungi Applied to Soil Polluted from Pb-Acid Batteries Effluents on Barley Grain Safety. Sci. Total Environ. 2019, 710, 136294. [Google Scholar] [CrossRef] [PubMed]
- Batool, M.; Rahman, S.U.; Ali, M.; Nadeem, F.; Ashraf, M.N.; Harris, M.; Du, Z.; Khan, W.-U.-D. Microbial-Assisted Soil Chromium Immobilization through Zinc and Iron-Enriched Rice Husk Biochar. Front. Microbiol. 2022, 13, 990329. [Google Scholar] [CrossRef]
- Lu, L.; Li, A.; Ji, X.; Yang, C.; He, S. Removal of Acenaphthene from Water by Triton X-100-Facilitated Biochar-Immobilized Pseudomonas Aeruginosa. RSC Adv. 2018, 8, 23426–23432. [Google Scholar] [CrossRef]
- Sun, T.; Miao, J.; Saleem, M.; Zhang, H.; Yang, Y.; Zhang, Q. Bacterial Compatibility and Immobilization with Biochar Improved Tebuconazole Degradation, Soil Microbiome Composition and Functioning. J. Hazard. Mater. 2020, 398, 122941. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhi, D.; Yao, B.; Zhou, Y.; Yang, Y.; Zhou, Y. Immobilization of Microbes on Biochar for Water and Soil Remediation: A Review. Environ. Res. 2022, 212, 113226. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, F.; Xu, K.; Zhang, J.; Li, D. Optimization and Regeneration of Chitosan-Alginate Hybrid Adsorbent Embedding Iron-Manganese Sludge for Arsenic Removal. Colloids Surf. A Physicochem. Eng. Aspects 2020, 607, 125500. [Google Scholar] [CrossRef]
- Liu, C.; Yu, D.; Wang, Y.; Chen, G.; Tang, P.; Huang, S. A Novel Control Strategy for the Partial Nitrification and Anammox Process (PN/A) of Immobilized Particles: Using Salinity as a Factor. Bioresour. Technol. 2020, 302, 122864. [Google Scholar] [CrossRef]
- Karel, S.F.; Libicki, S.B.; Robertson, C.R. The Immobilization of Whole Cells: Engineering Principles. Chem. Eng. Sci. 1985, 40, 1321–1354. [Google Scholar] [CrossRef]
- Li, R.; Huang, H.; Wang, J.J.; Liang, W.; Gao, P.; Zhang, Z.; Xiao, R.; Zhou, B.; Zhang, X. Conversion of Cu(II)-Polluted Biomass into an Environmentally Benign Cu Nanoparticles-Embedded Biochar Composite and Its Potential Use on Cyanobacteria Inhibition. J. Clean. Prod. 2019, 216, 25–32. [Google Scholar] [CrossRef]
- Mu, H.; Hu, K.; Zhu, H.; Peng, Y.; Wang, Q.; Wang, Y.; Li, J. Research progress on microbial immobilization technology and its enhanced biological nitrogen removal. Ind. Water Treat. 2023, 43, 28–35. [Google Scholar] [CrossRef]
- Mahadevan, H.; Krishnan, K.A.; Pillai, R.R.; Sudhakaran, S. Assessment of Urban River Water Quality and Developing Strategies for Phosphate Removal from Water and Wastewaters: Integrated Monitoring and Mitigation Studies. SN Appl. Sci. 2020, 2, 772. [Google Scholar] [CrossRef]
- Vasilieva, S.; Lobakova, E.; Grigoriev, T.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Gotovtsev, P.; Antipova, C.; Zagoskin, Y.; Scherbakov, P.; et al. Bio-Inspired Materials for Nutrient Biocapture from Wastewater: Microalgal Cells Immobilized on Chitosan-Based Carriers. J. Water Process. Eng. 2020, 40, 101774. [Google Scholar] [CrossRef]
- Wang, J. Study on the Diversity and Community Structure of PAOs in Eutrophication of Reservoir Sediment. Master’s Thesis, Fujian Normal University, Fuzhou, China, 2017. [Google Scholar]
- Yang, B.; Wang, W.; Bi, L.; Zeng, Q.; Zhou, X.; Liu, A. Isolation, identification and phosphororus-removal characterization of phosphate accumulating organisms collected from eutrophic lake sediment. In Proceedings of the 2019 Science and Technology Annual Conference of the Chinese Society for Environmental Sciences–Environmental Engineering Technology Innovation and Application Sub-Forum (3), Shaanxi, China, 23 August 2019; Environmental Engineering Branch, Chinese Society of Environmental Sciences: Beijing, China, 2019; pp. 342–348+411. [Google Scholar]
- Shao, Y.; Zhong, H.; Mao, X.; Zhang, H. Biochar-Immobilized Sphingomonas Sp. and Acinetobacter Sp. Isolates to Enhance Nutrient Removal: Potential Application in Crab Aquaculture. Aquac. Environ. Interact. 2020, 12, 251–262. [Google Scholar] [CrossRef]
- Wang, F.; Bai, Y.; Yang, F.; Zhu, Q.; Zhao, Q.; Zhang, X.; Wei, Y.; Liao, H. Degradation of Nitrogen, Phosphorus, and Organic Matter in Urban River Sediments by Adding Microorganisms. Sustainability 2021, 13, 2580. [Google Scholar] [CrossRef]
- Lin, M.; Li, F.; Li, X.; Rong, X.; Oh, K. Biochar-Clay, Biochar-Microorganism and Biochar-Enzyme Composites for Environmental Remediation: A Review. Environ. Chem. Lett. 2023, 21, 1837–1862. [Google Scholar] [CrossRef]
- Ayala-Muñoz, D.; Macalady, J.L.; Sánchez-España, J.; Falagán, C.; Couradeau, E.; Burgos, W.D. Microbial Carbon, Sulfur, Iron, and Nitrogen Cycling Linked to the Potential Remediation of a Meromictic Acidic Pit Lake. ISME J. 2022, 16, 2666–2679. [Google Scholar] [CrossRef]
- Schulz-Vogt, H.N.; Pollehne, F.; Jürgens, K.; Arz, H.W.; Beier, S.; Bahlo, R.; Dellwig, O.; Henkel, J.V.; Herlemann, D.P.R.; Krüger, S.; et al. Effect of Large Magnetotactic Bacteria with Polyphosphate Inclusions on the Phosphate Profile of the Suboxic Zone in the Black Sea. ISME J. 2019, 13, 1198–1208. [Google Scholar] [CrossRef]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms Pumping Iron: Anaerobic Microbial Iron Oxidation and Reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef]
- Chen, R.; Liu, H.; Zhang, P.; Ma, J.; Jin, M. Co-Response of Fe-Reducing/Oxidizing Bacteria and Fe Species to the Dynamic Redox Cycles of Natural Sediment. Sci. Total Environ. 2022, 815, 152953. [Google Scholar] [CrossRef]
- Druschel, G.K.; Emerson, D.; Sutka, R.; Suchecki, P.; Luther, G.W. Low-Oxygen and Chemical Kinetic Constraints on the Geochemical Niche of Neutrophilic Iron(II) Oxidizing Microorganisms. Geochim. Cosmochim. Acta 2008, 72, 3358–3370. [Google Scholar] [CrossRef]
- Fan, X.; Xing, X.; Ding, S. Enhancing the Retention of Phosphorus through Bacterial Oxidation of Iron or Sulfide in the Eutrophic Sediments of Lake Taihu. Sci. Total Environ. 2021, 791, 148039. [Google Scholar] [CrossRef]
- Sun, P.; Zhou, L. Recent Progress on Mechanism and Effect of Removing Arsenic from Water by Iron(II) -oxidizing Bacteria. J. Hunan Ecol. Sci. 2022, 9, 89–96. [Google Scholar]
- Field, H.R.; Whitaker, A.H.; Henson, J.A.; Duckworth, O.W. Sorption of Copper and Phosphate to Diverse Biogenic Iron (Oxyhydr) Oxide Deposits. Sci. Total Environ. 2019, 697, 134111. [Google Scholar] [CrossRef]
- Buliauskaite, R.; Wilfert, P.; Kumar, P.S.; de Vet, W.W.J.M.; Witkamp, G.-J.; Korving, L.; van Loosdrecht, M.C.M. Biogenic Iron Oxides for Phosphate Removal. Environ. Technol. 2020, 41, 260–266. [Google Scholar] [CrossRef]
- Chen, J.; Wang, C.; Pan, Y.; Farzana, S.S.; Tam, N.F.-Y. Biochar Accelerates Microbial Reductive Debromination of 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47) in Anaerobic Mangrove Sediments. J. Hazard. Mater. 2018, 341, 177–186. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, H.; Bolan, N.; Sarkar, B.; Wang, H.; Chen, C. Both Sides of Coin: Benefits and Potential Negative Consequences of Biochar in Sediment Remediation. Rev. Environ. Contam. Toxicol. 2023, 261, 4. [Google Scholar] [CrossRef]
- Huang, D.; Liu, L.; Zeng, G.; Xu, P.; Huang, C.; Deng, L.; Wang, R.; Wan, J. The Effects of Rice Straw Biochar on Indigenous Microbial Community and Enzymes Activity in Heavy Metal-Contaminated Sediment. Chemosphere 2017, 174, 545–553. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Ding, H.; Fu, H.; Liu, J.; Chen, Y.; Dai, T.; Lou, Q.; Zhong, X.; Fan, H.; et al. Low-Dose Biochar Added to Sediment Improves Water Quality and Promotes the Growth of Submerged Macrophytes. Sci. Total Environ. 2020, 742, 140602. [Google Scholar] [CrossRef]
- Ojeda, G.; Patrício, J.; Mattana, S.; Sobral, A.J.F.N. Effects of Biochar Addition to Estuarine Sediments. J. Soils Sediments 2016, 16, 2482–2491. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; He, N.; Borham, A.; Zhang, S.; Xie, R.; Zhao, C.; Hu, J.; Wang, J. The Effect of Iron-Modified Biochar on Phosphorus Adsorption and the Prospect of Synergistic Adsorption between Biochar and Iron-Oxidizing Bacteria: A Review. Water 2023, 15, 3315. https://doi.org/10.3390/w15183315
Liu L, He N, Borham A, Zhang S, Xie R, Zhao C, Hu J, Wang J. The Effect of Iron-Modified Biochar on Phosphorus Adsorption and the Prospect of Synergistic Adsorption between Biochar and Iron-Oxidizing Bacteria: A Review. Water. 2023; 15(18):3315. https://doi.org/10.3390/w15183315
Chicago/Turabian StyleLiu, Lei, Nannan He, Ali Borham, Siwen Zhang, Ruqing Xie, Chen Zhao, Jiawei Hu, and Juanjuan Wang. 2023. "The Effect of Iron-Modified Biochar on Phosphorus Adsorption and the Prospect of Synergistic Adsorption between Biochar and Iron-Oxidizing Bacteria: A Review" Water 15, no. 18: 3315. https://doi.org/10.3390/w15183315
APA StyleLiu, L., He, N., Borham, A., Zhang, S., Xie, R., Zhao, C., Hu, J., & Wang, J. (2023). The Effect of Iron-Modified Biochar on Phosphorus Adsorption and the Prospect of Synergistic Adsorption between Biochar and Iron-Oxidizing Bacteria: A Review. Water, 15(18), 3315. https://doi.org/10.3390/w15183315