1. Introduction
Wastewater treatment plants (WWTPs) have faced growing demands to not only comply with effluent standards but also enhance energy efficiency, adopt resource recovery strategies, and actively monitor and mitigate greenhouse gas (GHG) emissions, and shift to Water Resource Recovery Facilities (WRRFs). Despite the advancements achieved in recent decades, a significant proportion of WWTPs still need to reach their full potential. Instrumentation, control, and automation (ICA) systems offer a viable opportunity to enhance the efficiency and environmental impact of current WWTPs.
In the search for more efficient, competitive, and eco-friendly technologies to replace conventional systems in WWTPs, anaerobic digestion (AD) and membrane filtration emerge as core processes of WRRFs. AD offers numerous advantages over aerobic processes, including low sludge production, reduced energy consumption, and organic matter valorization through methane production. Anaerobic membrane bioreactors (AnMBRs) are gaining recognition as they integrate AD with membrane filtration, providing additional benefits such as complete physical biomass retention, which allows for the decoupling of hydraulic retention time (HRT) and solids retention time (SRT), the efficient removal of organic matter and suspended solids, resource recovery potential, and lower energy costs [
1,
2,
3]. Moreover, the compactness of membrane modules contributes to minimizing the footprint required for wastewater treatment facilities, making it possible to retrofit existing treatment plants with a limited available space. The feasibility of AnMBR application for urban wastewater treatment has been verified by previous studies and numerous large-scale applications [
4,
5,
6,
7,
8], which have encompassed scale-up assessments [
9,
10,
11]. AnMBRs have also been applied for co-digesting several substrates. Co-digesting two or more substrates offers multiple advantages, including enhancements in the C/N ratio, system buffer capacity, dilution of inhibitory compounds, nutrient balance, digestion performance, and biogas yield [
12]. Cheng et al. [
13] reported an enhanced biogas yield through the co-digestion of sewage sludge and food waste in a high-solid AnMBR. Co-digestion holds particular significance when dealing with challenging-to-degrade substrates such as microalgae.
Within the context of wastewater treatment, microalgae-based approaches hold great promise, especially in terms of generating biofuels through AD. For instance, the nutrient-rich effluent from urban wastewater treatment obtained using AnMBR technology was proven effective for cultivating microalgae, and the subsequent AD of the cultivated microalgae yielded substantial methane production [
14]. This synergy underscores the potential of combining wastewater-based microalgae cultivation with AD, offering a mutually beneficial strategy that enables the simultaneous recovery of valuable nutrients and energy from wastewater sources. However, microalgae possess a cell wall that is highly resistant to degradation and a low C/N ratio that can lead to excessive ammonia levels and a decreased methane yield. These challenges can be addressed through the anaerobic co-digestion (AcoD) of microalgae with carbon-rich substrates such as sewage sludge and the integration of membrane filtration into the process. The ACoD of microalgae and primary sludge in AnMBRs has been demonstrated through pilot plant and laboratory-scale studies, resulting in higher methane yields [
15,
16]. Serna-García et al. [
17] reported that membrane installation in a continuous stirred tank reactor (CSTR) co-digesting microalgae and primary sludge promoted a robust microbial community able to degrade the recalcitrant microalgae cell wall. The authors also observed that membrane installation allowed for thermophilic AcoD, avoiding ammonia inhibition and improving methane yields.
Due to the nature of the emerging technology and the added complexity of operating under anaerobic conditions while optimizing membrane filtration, the design and operation of AnMBRs are not yet fully developed for all applications, and there are currently limited full-scale implementations. As a result, further research is needed to maximize the process efficiency and broaden its applicability [
18]. AnMBR operation can be enhanced with the implementation of a proper ICA system, which can minimize energy consumption, simplify system management, increase their robustness towards perturbations, and address the existing drawbacks [
19]. Different control systems have been applied to anaerobic processes, including simple on/off control or advanced control systems such as fuzzy logic or neural networks [
20,
21]. When designing control systems for anaerobic processes, it is crucial to consider key process parameters such as HRT, SRT, and the flow rate of recycled biogas [
22,
23,
24,
25]. However, there are a very limited number of references in the literature concerning the start-up and ICA implementation to ensure the proper operation of the AnMBR technology [
22]. Up to date, considerable efforts have been made to control membrane fouling in aerobic membrane technologies [
26], but few control strategies have been developed to optimize the performance of the filtration process in AnMBRs [
18,
22,
27].
In this work, an ICA system was developed to enhance energy efficiency and reduce manpower requirements while facilitating seamless system management in an AnMBR pilot plant co-digesting microalgae and primary sludge. The proposed ICA system aims to provide a valuable guide for the start-up and proper operation of an AnMBR co-digestion pilot plant. Its primary goal is to optimize the process performance of this emerging technology in terms of methanation and membrane filtration, ensuring its reliable and efficient long-term operation. By closely monitoring the process parameters that are sensitive to process disturbances, such as temperature, pH, biogas production rate, or oxidation-reduction potential (ORP), it effectively identifies the key process parameters essential for optimal operation and performance. Additionally, this study conducted a GHG emissions assessment to evaluate the economic and environmental feasibility of AnMBRs, considering various biological operating conditions (mesophilic and thermophilic) and biogas valorization scenarios.
2. Materials and Methods
An ICA system was proposed and validated in the long term to optimize the operation of an AnMBR co-digesting harvested microalgae and thickened primary sludge in proportions of 38% and 62%, respectively (according to [
16]) at a pilot scale. Based on previous works [
22], ICA was designed to manipulate the key operating variables for controlling the biological and physical separation processes: the daily feed volume, the daily sludge discharge volume, the permeate flow rate, the co-digester temperature and pressure, the biogas inflow to the membrane tank and the membrane operating stage control. AnMBR pilot plant operation, instrumentation, control, and monitoring are described in the following sections, providing information about all the data sources involved.
2.1. Pilot Plant Description and Biological Process Operation
The pilot plant consisted of an anaerobic reactor (AnR) with a total volume of 1.01 m
3 (up to 0.91 m
3 working volume, rest head-space volume) connected to an external membrane tank (MT) with a total volume of 1 L (0.1 L head-space volume) (see [
16] for the whole description). The MT included one hollow-fiber (HF) ultrafiltration membrane unit (PURON
® KMS, Wilmington, MA, USA 0.03 µm pores). This module consisted of one HF bundle, 0.4 m long, giving a total membrane surface of 0.44 m
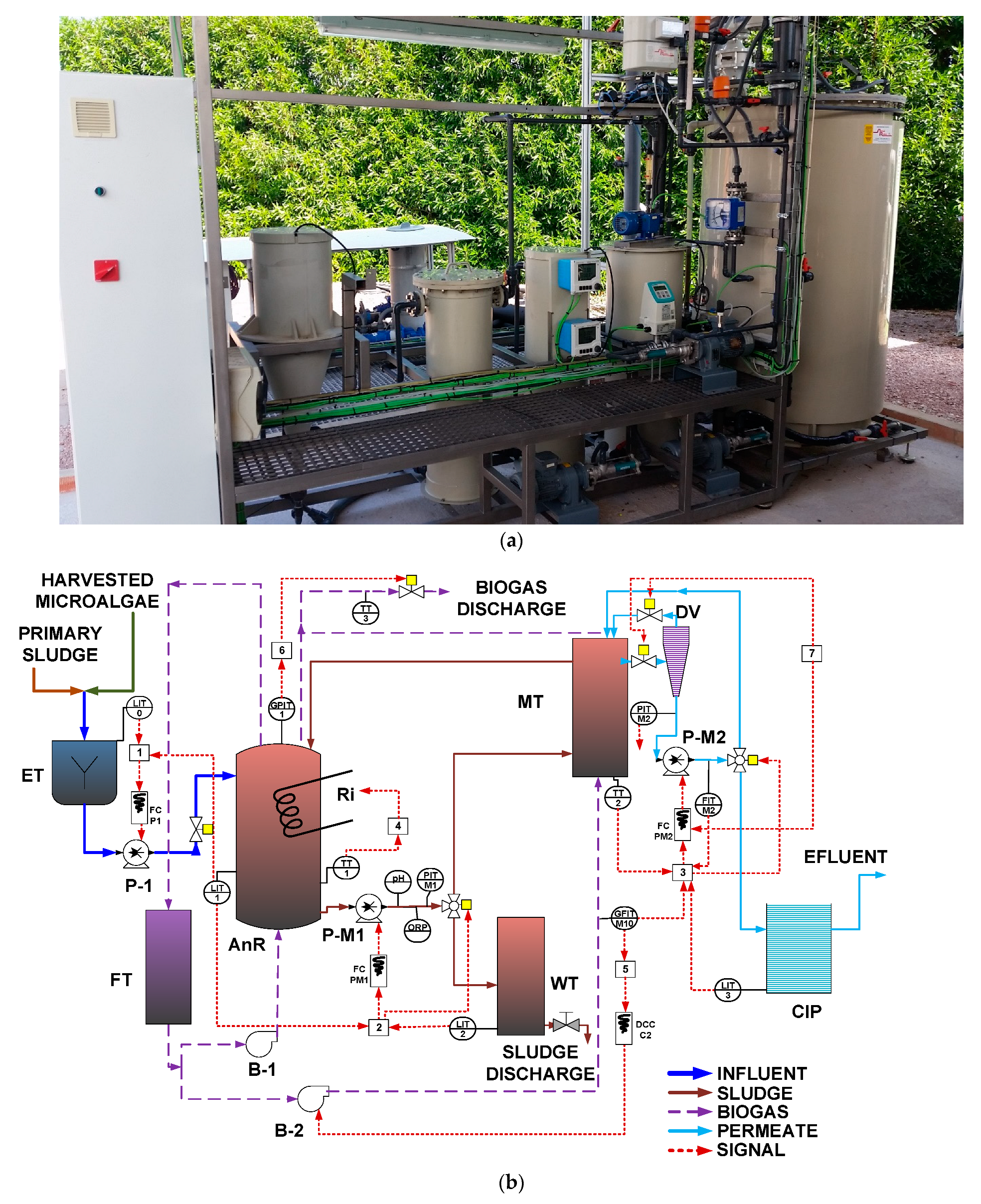
2. A 125 L equalization tank (ET), a 42 L clean-in-place (CIP) tank, a 70 L waste tank (WT), and a 70 L foam trap (FT) were also included as main elements of the plant. A real-time photograph of the AnMBR pilot plant is shown in
Figure 1a.
Figure 1b shows a simplified layout of the pilot plant, including lower-layer controllers. Primary sludge and microalgae were mixed in the ET and fed to the AnR. The primary sludge, sourced from the gravity thickener of the Carraixet WWTP (Alboraya, Spain), was sieved through a 0.5 mm aperture sieve before being fed to the ET. Microalgae were obtained from a membrane photobioreactor pilot plant that received the effluent of an AnMBR pilot plant treating wastewater from the Carraixet WWTP’s gravity-based primary clarifier (see [
28] for an in-depth description). The microalgal biomass, dominated by
Chlorella spp. and
Scenedesmus spp., was concentrated via filtration in an external cross-flow, ultrafiltration HF membrane unit (HF 5.0–43-PM500, PURON
® Koch Membrane Systems) before entering the ET. To improve the stirring conditions of the digester and to favor stripping of the produced gases from the liquid phase, a fraction of the produced biogas was recycled to the digester with a blower, and another fraction was recycled with a different blower to the external MT from the bottom of the fiber bundle to minimize cake-layer formation. Sludge was continuously recycled through the MT, where the effluent was obtained with vacuum filtration after passing through a degasification vessel (DV). The obtained permeate was stored in the CIP tank, where the effluent of the plant was obtained. Three external resistances were installed to control temperature. A fraction of the generated sludge was intermittently extracted from the co-digester throughout the day to achieve the desired SRT. The measurement of total biogas production was conducted using a biogas meter (BK-G4M, Elster, Raleigh, NC, USA). Gas volumes were normalized to standard conditions of 1 atm pressure and 0 °C temperature.
Two different experiments were conducted in the AnMBR co-digestion pilot plant. In Experiment 1, the digester was operated for 216 days (from Day 0 to 216) at thermophilic conditions (55.0 ± 0.9 °C), 69.7 ± 0.3 d SRT, 30.0 ± 0.3 d HRT, and 0.56 ± 0.05 gCOD·L
−1·d
−1 organic loading rate (OLR) [
29]. In Experiment 2, the reactor was operated for 360 days (from Day 217 to 576) at mesophilic conditions (35.2 ± 0.5 °C), 70.2 ± 4 d SRT, 30.1 ± 0.3 d HRT, and 0.58 ± 0.12 gCOD·L
−1·d
−1 OLR [
16].
2.2. Membrane Operation
Membrane operation was established based on the operation described in [
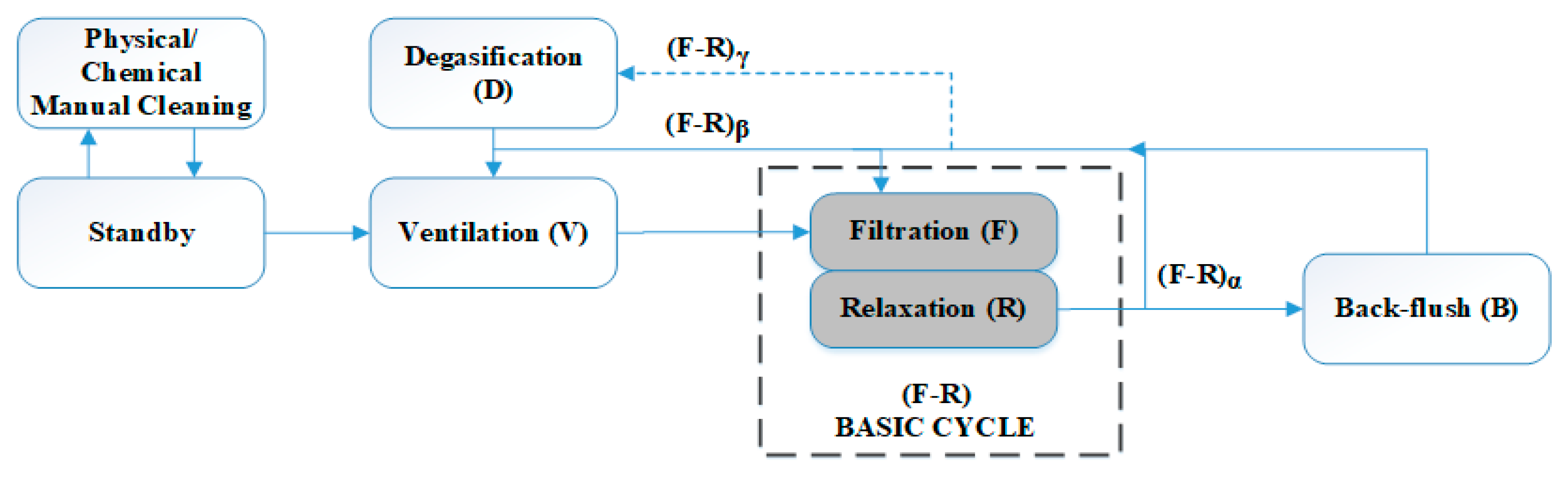
29] for a similar membrane filtration module in an AnMBR pilot plant. The classical operating stages were filtration, relaxation, and back flush. Two additional stages were considered to remove biogas accumulation on the top of the fibers (degasification) and inside the DV (ventilation). Degasification consisted of a short period of high-speed filtration to remove accumulated biogas and improve the efficiency of the filtration process. Ventilation was a stage similar to back flush, but with permeate being pumped to the MT through the DV.
Figure 2 shows the possible modes in which the membrane module can be operated, based on the methodology described in [
4]. A basic filtration–relaxation cycle was established and taken as the basis to schedule the frequency of the remaining stages. Different back-flush and relaxation frequencies and durations were studied to evaluate the effect on membrane operation.
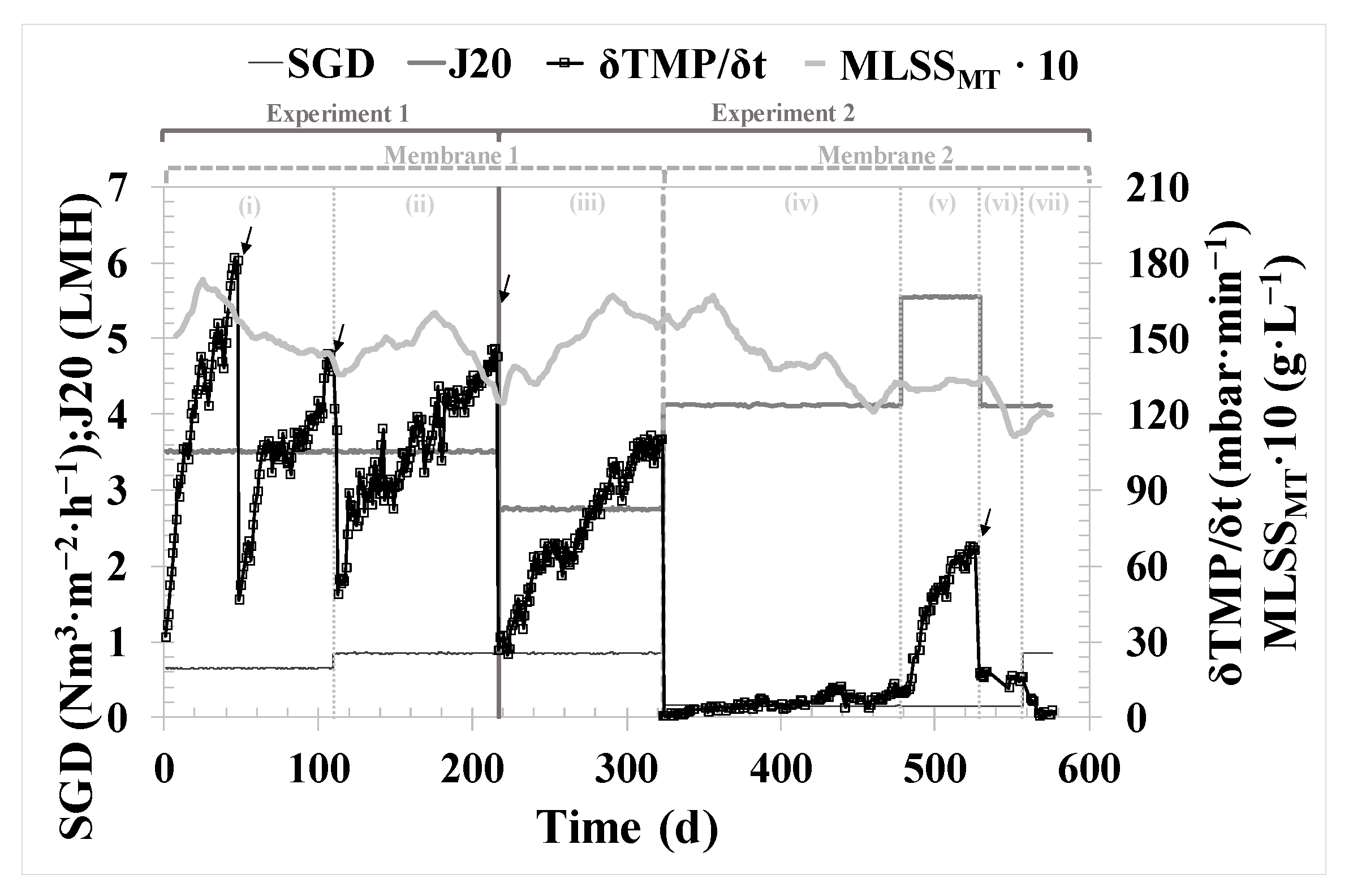
During Experiment 1 (thermophilic conditions of AnR), the filtration was performed using a membrane previously used in other experiments, i.e., with a non-negligible contribution of irreversible fouling. The membrane was cleaned chemically just before starting Experiment 1. The operating mode was α = 11; β = 13; γ = 50 (see
Figure 2), which means a back flush was performed every eleven basic F-R cycles, ventilation was carried out every thirteen F-R cycles, and a double degasification stage followed by ventilation was conducted every fifty F-R cycles. Filtration was conducted with a filtration time of 150 s. The duration of the remaining stages was as follows: relaxation, 100 s; back-flush, 50 s; ventilation, 150 s; and degasification, 50 s. There were two operating periods, (i) and (ii), with different conditions of 20 °C-normalized permeate flux (J
20), specific gas demand (SGD), and mixed liquor suspended solids in the membrane tank (MLSS
MT), as shown in
Table 1.
Experiment 2 (mesophilic conditions of AnR) started its operation with the same fouled membrane until Day 324 of operation (period iii), maintaining the same operating mode. On Day 325, a new membrane was installed, and short-term experiments were conducted to determine the critical flux (J
CW). For the rest of the experimental time (from Day 325 to 576), the operating mode was α = 2; β = 13; γ = 50. Filtration was carried out with a filtration time of 200 s. The durations of the remaining stages were as follows: relaxation, 30 s; back-flush, 45 s; ventilation, 50 s and degasification, 30 s. There were four more operating periods, (iv) to (vii), with different conditions of J
20, SGD, and MLSS
MT, as can be observed in
Table 1.
To assess the performance of the filtration process, the online recorded data of flow rate (J), TMP, and MT temperature were considered. Due to the significant impact of permeated viscosity on filtration performance, a temperature correction factor (f
T) was included in the transmembrane flux control (Equation (1)), which was used to determine J
20 (Equation (2)). Additionally, the evolution and performance of the filtration process were evaluated using the fouling rate (FR) (Equation (3)).
J
CW was determined by applying a modified flux-step method based on the methodology proposed by [
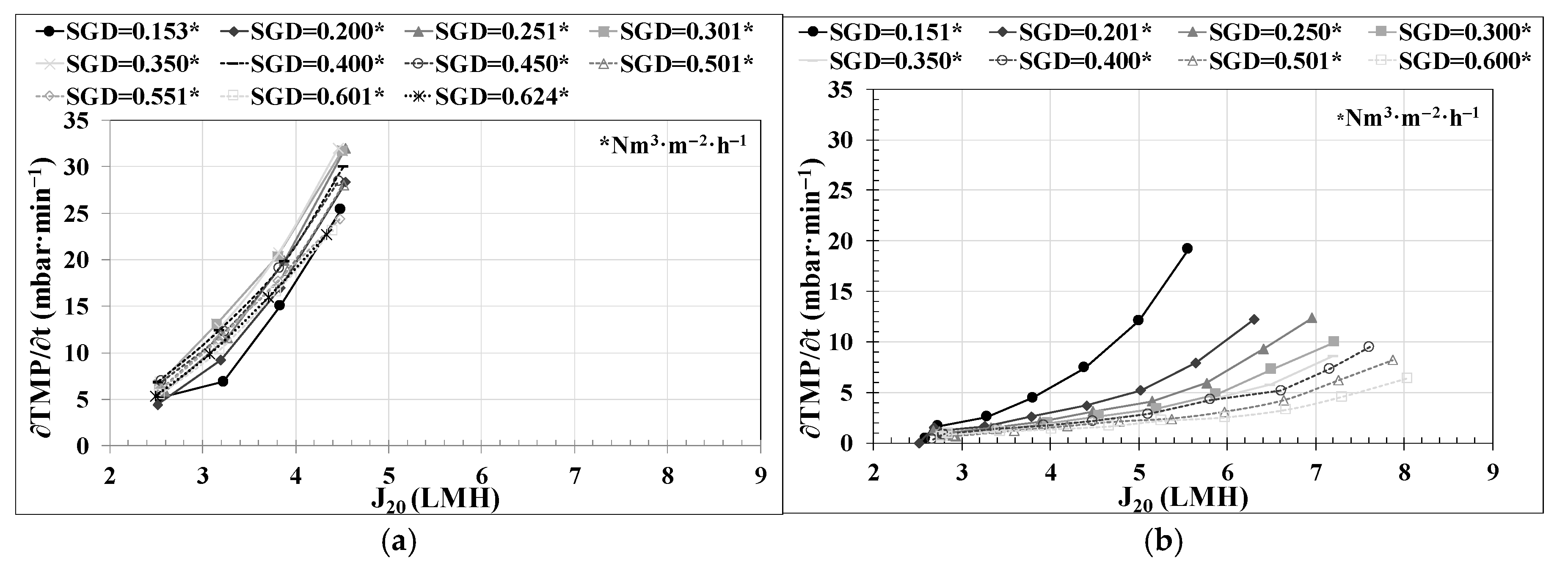
29]. Successive flux steps were established, conducting the trials just in the upward direction. The filtration stage duration was set to 10 min. The flux-step size was fixed at a J
20 of 0.65 LMH. To minimize the reversible fouling effect on J
CW calculations, a relaxation stage of 30 s followed by a back-flush stage of 1 min was executed between each flux step. The relaxation stages were conducted using the same SGD as in the filtration stages. In this study, eleven flux-step tests were performed at an SGD of 0.15, 0.2, 0.25, 0.3, 0.35, 0.4, 0.45, 0.5, 0.55, 0.6, and 0.625 Nm
3·h
−1·m
−2.
Following the approach suggested by [
29], data processing of the flux-step method results used the average values of TMP for each flux step (j
n) from Equation (4). The fouling rate was calculated based on Equation (3).
To evaluate the technical-economic optimum of the membrane module after critical flow testing, the total annualized equivalent cost (TAEC) was calculated by adding the annualized cost of capital expenditure (CAPEX) to the annual cost of operating expenditure (OPEX) for different operating conditions, employing the methodology of [
30].
2.3. Pilot Plant Instrumentation
Several online sensors and actuators were installed to achieve the control and automation of the plant operation. The online sensors installed were a pH transmitter; an ORP transmitter; four-level transmitters (LIT), one for each tank except for the FT; a liquid-flow transmitter (FIT) in the MT permeate; a gas-flow transmitter (GFIT) for biogas inflow to the MT; two liquid pressure transmitters (PIT), one to determine the pressure in the MT and the other for permeate; a biogas pressure transmitter (GPIT) in the AnR head space; and three temperature transmitters (TT) for monitoring the AnR, the MT and the biogas discharge temperatures. Additionally, a rotameter was installed in the biogas inflow to the AnR.
The group of actuators included three frequency converters (FC) responsible for controlling the rotating speed of three pumps (feed pump, sludge recycling pump, and permeate pump); one direct current converter (DCC) that controlled the voltage of the MT blower; two three-way valves that controlled sludge wastage and permeate recirculation to the AnR; and four on–off valves used to control the feeding to the AnR, biogas discharges, and the different membrane operating stages.
2.4. Pilot Plant Automation
Anaerobic processes and membrane technology in general, and AnMBR systems in particular, present a high level of complexity, so a complete network system was needed to ensure a safe and robust performance against disturbances. The instrumentation was connected to a network system that included several transmitters, a programmable logic controller (VIPA S7-317-DP PLC), a PC to perform multi-parameter control and data acquisition, and a SCADA. This system showed a high degree of process autonomy and allowed for minimal human supervision.
2.5. Pilot Plant Control
The pilot plant included several control loops organized in lower-layer controllers (see
Figure 1b).
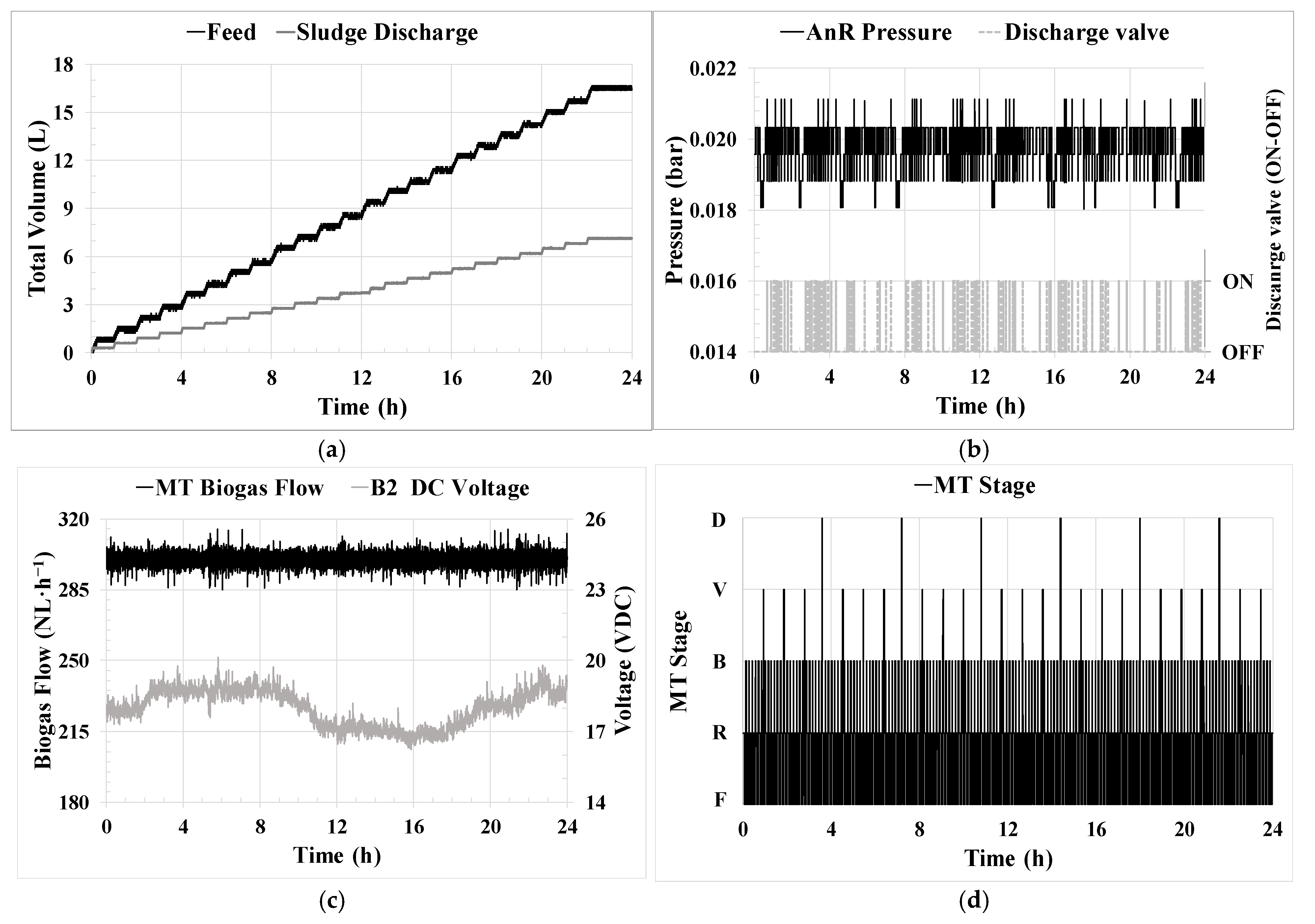
HRT control [
1] was achieved by operating the feed pump (P-1) at a fixed frequency. The controller allowed for the total daily feed volume to be reached, which was distributed in several cycles per day. These cycles were determined based on LIT-0 variation, allowing precise HRT control.
SRT control [
2] was accomplished by operating the recycling sludge pump (P-M1) at a fixed frequency. The controller allowed for the total daily wasting volume to be reached, which was distributed in several cycles per day, opening a three-way valve to the waste tank. These cycles were determined by monitoring LIT-2 variation and allowing effective SRT control.
Permeate flow/flux control [
3] was achieved through the utilization of a permeate pump (P-M2) controlled by a PID controller. The controller manipulated the pump’s frequency to maintain the desired transmembrane flow rate, which was determined based on the flow obtained from the FIT-M2 sensor. This controller was also able to operate in a ‘J
20 mode’. In this mode, the controller maintained a specific J
20 set point considering the temperature measured by the TT-2 sensor instead of directly controlling the flow rate.
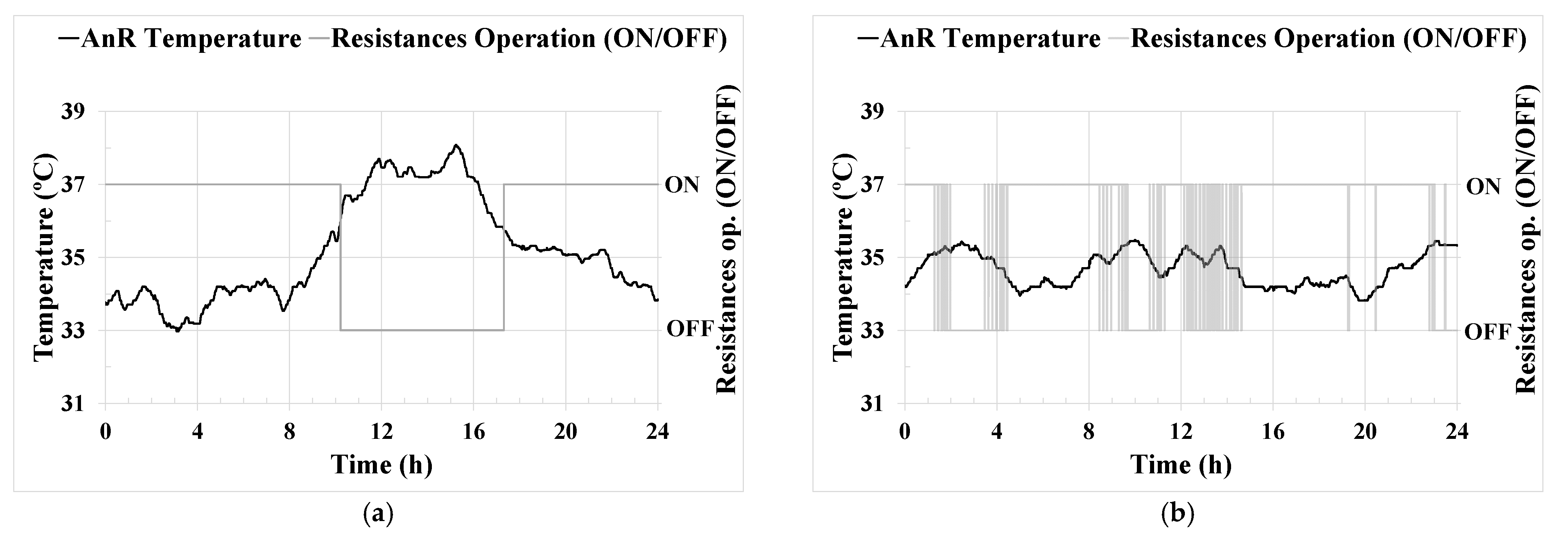
AnR temperature [
4] was controlled by adjusting the operation time of three resistances. Two different controllers were tested: an on–off controller and a PID controller.
Biogas MT flow [
5] was achieved through a PID controller. The controller was used to ensure a set point for the biogas inflow to the MT, measured by GFIT-M10. The independent variable was the blower B-2 voltage through DCC.
Pressure control [
6] was achieved by maintaining an operating pressure with hysteresis in the AnR and MT head space. An on–off controller was used to control the V-3 valve.
MT operating stage control [
7] consisted of one on–off controller determining the membrane operating stage. This control was achieved by changing both the position of the corresponding on–off valves and the flux direction of the P-M2.
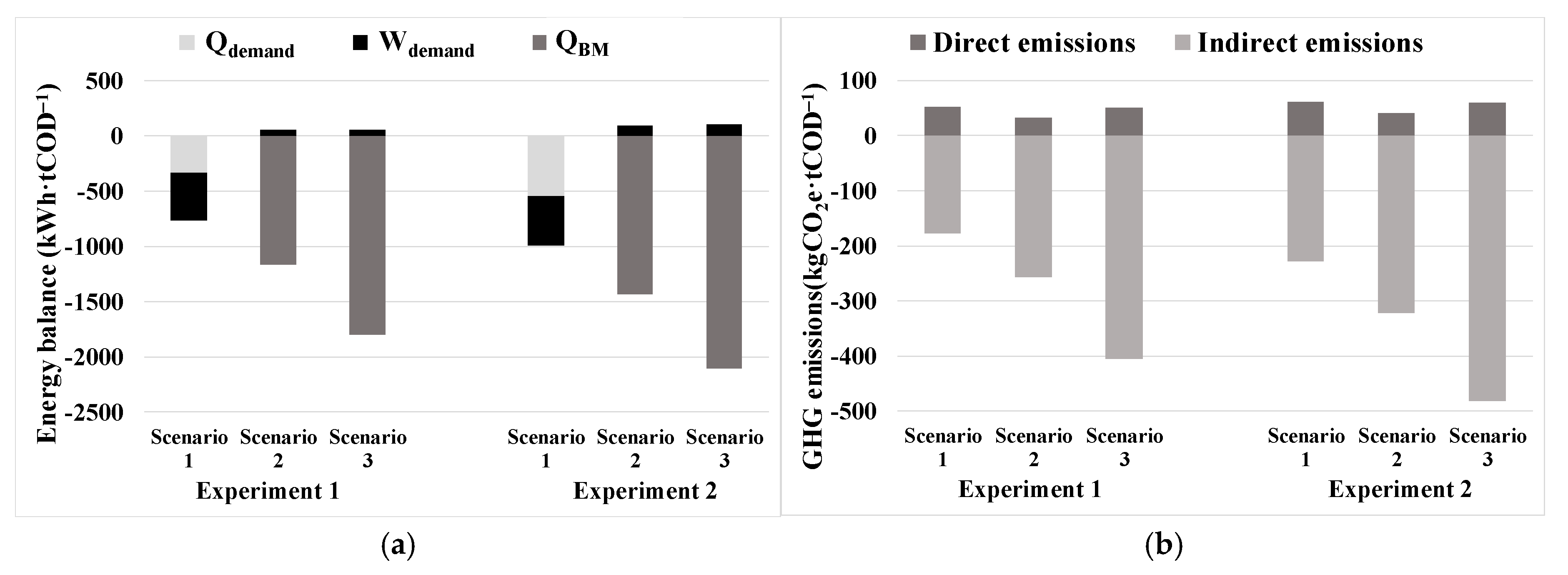
2.6. GHG Emissions Assessment
To determine the GHG emissions generated in the AnMBR pilot plant, the methodology proposed by [
31] was considered. This methodology consists of estimating direct and indirect emissions for different combinations of the AnMBR co-digestion pilot plant operation, considering mesophilic or thermophilic conditions, and the various biogas valorization systems proposed. Three scenarios were established for biogas valorization: (i) Scenario 1, which involved the use of high-efficiency co-generation technology with a combined heat and power (CHP) system; (ii) Scenario 2, in which biogas was upgraded to biomethane using conventional membrane technology and subsequently injected into the grid; and (iii) Scenario 3, in which biogas was upgraded to biomethane using microalgae with high CO
2 tolerance in a specialized reactor to absorb the CO
2 contained in the biogas. The upgraded biomethane was then injected into the grid. Notably, this process led to the absorption of CO
2 into the algal biomass, consequently increasing methane production during the AD stage.
Direct emissions (GHGdirect) are defined as methane losses, which vary depending on the valorization system. As for indirect emissions (GHGindirect), it is necessary to perform an energy assessment to calculate the total consumption and recovery of electrical energy, the consumption and recovery of thermal energy, and, in scenarios that include it, the thermal energy contained in the biomethane injected into the grid. To determine the avoided GHGindirect, it was considered that co-generated electrical energy avoids the use of electricity from the grid. In the case of thermal energy and biomethane injected into the grid, it was considered that the use of fossil natural gas was being avoided.
The lower specific heat of natural gas and pure methane values were taken from [
31]. The specific emission factor for fossil natural gas from the grid (EF
natural_gas) was 0.238 kg CO
2e/kWh and was taken from [
32]. The specific emission factor of European power companies (EF
electricity) was 0.224 kg CO
2e/kWh and was taken from [
33].
GHG
direct, (Equation (5) for Scenario 1 and Equation (6) for Scenarios 2 and 3), GHG
indirect (Equation (7) for Scenario 1 and Equation (8) for Scenarios 2 and 3), and total emissions, GHG
total, (Equation (9)) were calculated according to the following equations.
where Q
BG is the registered gross production of raw biogas;
is the methane loss emission factor; Q
BM is the biomethane production; Q
demand is the total thermal energy demand balance; W
demand is the total electrical energy demand balance; EF
natural_gas is the specific emission factor for fossil natural gas from the grid; and EF
electricity is the specific emission factor of European power companies.
To calculate GHG
indirect, the [
4,
29] methodologies were employed to determine energy consumption. The consumption ratios in upgrading and grid injection systems were obtained from [
34], while the CHP efficiencies were obtained from [
35]. The total thermal energy demand balance (Q
demand) and the total electrical energy demand balance (W
demand) were calculated according to Equation (10) and Equation (11), respectively. Biomethane production (Q
BM) was calculated for both Scenarios 2 and 3 using Equation (12), considering that raw biogas was utilized to meet the Q
demand, and the remaining fraction was upgraded for injection into the natural gas grid. A net efficiency of the upgrading system (φ
upgrading) of 99.5% was considered for Scenario 2 [
36] and 97.6% for Scenario 3 [
37]. In both scenarios, normalization was carried out, considering a 97% methane content for injection into the natural gas grid. Net energy assessment (E) was calculated for Scenario 1 using Equation (13) and for Scenarios 2 and 3 using Equation (14). The results indicated a net energy consumption if the value was positive and a net energy recovery if the value was negative for each respective stage.
where Q
TOT is the total heat required by the AnR process; Q
recovered is the recovered heat in the CHP for Scenario 1 or the heat recovered in the upgrading stage for scenario 2; W
TOT is the result of adding all the electrical consumptions of the plant equipment; W
recovered is the electricity recovered in the CHP stage; Q
BG is the registered gross production of raw biogas; Q
demand is the total thermal energy demand balance; W
demand is the total electrical energy demand balance; φ
upgrading is the net efficiency of the upgrading system; and Q
BM is the biomethane production.
To calculate GHG
direct, methane losses were considered and expressed as an emission factor (
). An
of a 1.5% loss of CH
4 burnt in CHP was considered for Scenario 1, while an
of a 1% loss of upgraded CH
4 was considered for Scenarios 2 and 3 [
31].
4. Discussion
The results derived from monitoring and controlling the AnMBR pilot plant for microalgae and primary sludge AcoD and utilizing the implemented ICA system exhibit remarkable stability during long-term operation. Mesophilic and thermophilic conditions were evaluated, both yielding high methane production and demonstrating the effective AD of microalgae biomass despite its challenging biodegradability. The co-digestion of microalgae with primary sludge and the integration of the ultrafiltration membrane into the system further enhanced the microalgae’s degradability [
12,
18]. In addition, the employed harvesting system (cross-flow ultrafiltration membranes) has previously shown a significant pretreatment effect in enhancing microalgae biodegradability [
39].
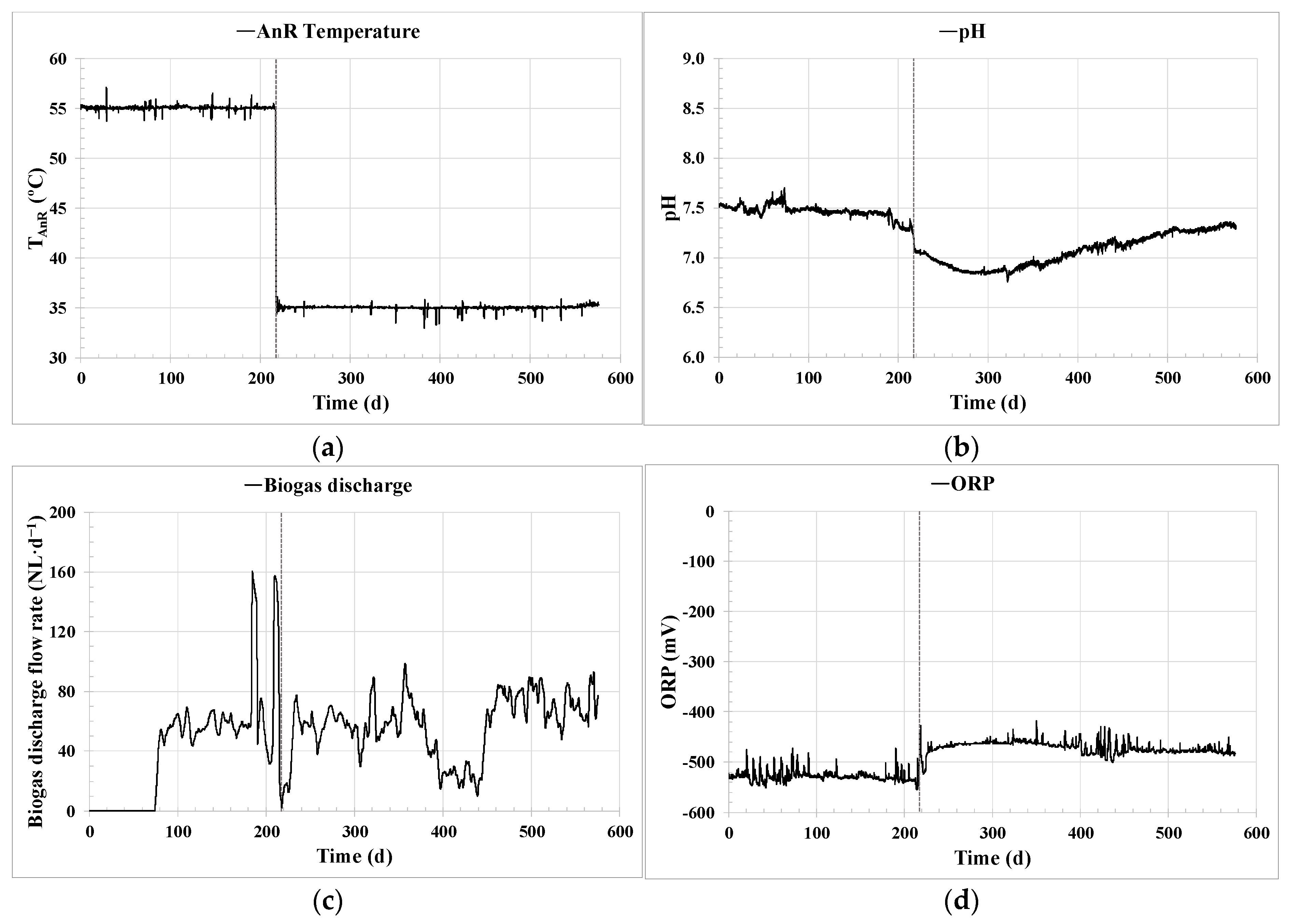
Effective key parameter control was achieved through the implemented ICA system. Regarding the control of the AnR temperature,
Figure 3b shows the significant improvement in temperature stability achieved around the set point established by the PID controller. The temperature maintained in the pilot plant was in the optimum operational range found in the literature [
21], and the PID controller performance was similar to other comparable monitoring processes [
40], reaching a standard deviation of only 0.5%. This steady temperature control corresponds to the consistent long-term operation demonstrated in
Figure 6a. Likewise, optimizing energy consumption to maintain the AnR process temperature prevents unnecessary oscillations and peaks. Temperature control is vital for maintaining a stable microbial community in the digester, as previously reported in [
17]. In this study [
17], an important effect on the microbial community was observed at a peak of 30 °C during the operation of a thermophilic anaerobic co-digester at the lab scale.
Similarly, the control philosophy implemented in the ICA for the SRT and HRT parameters (
Figure 5a) ensured the regulation of feed and waste flows by distributing them in various cycles throughout the day. This also contributes to the microbiological stability of the process by avoiding load peaks that can induce a redistribution in the microbial community [
41,
42], and it enables efficient pump operation through the use of frequency converters.
The steadiness and optimization achieved in the process using ICA are also reflected in the observed stability in maintaining the AnR head pressure, as shown in
Figure 5b, as well as the highly robust values observed throughout Experiments 1 and 2 for the pH (
Figure 6b) and ORP (
Figure 6d), showing a similar trend to other monitoring processes found in the literature [
21]. ORP proved to be of great importance for the early detection and immediate repair of possible leaks in the biogas line, which is one of the major issues that can occur in anaerobic systems.
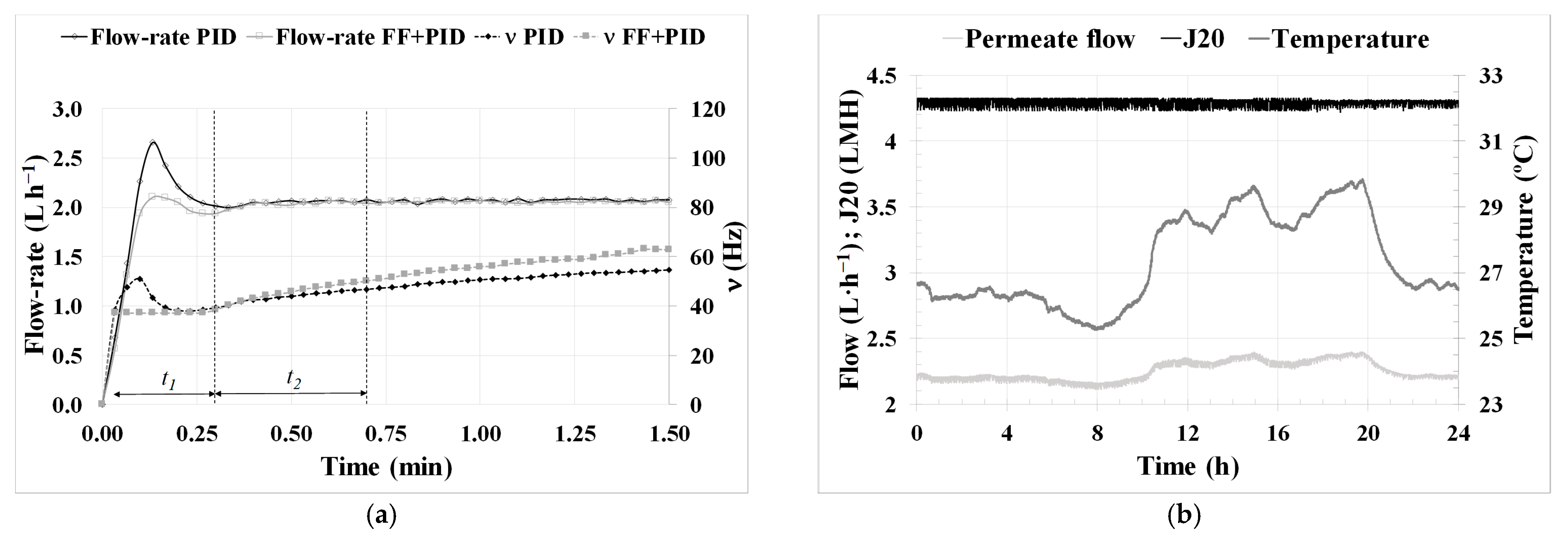
Regarding the lower-layer controllers related to permeate flow, the use of an FF in the PID control and the use of a ‘J
20 mode’ enabled the reliable execution of critical flow tests and the determination of the optimal technical and economic conditions for filtration in an AnMBR co-digestion pilot plant, as can be observed in
Figure 4a.
The performance of the filtration process was assessed over the entire experimental period while employing two different membranes, as detailed in
Section 2.2. The fouled membrane used during periods (i) to (iii) (
Table 1) enabled a good filtration performance, conducting only physical cleanings when necessary. However, it exhibited a 33% higher fouling rate compared to periods when a new membrane was employed (from (iv) to (vii)). This increase in fouling rate, aside from using a previously fouled membrane, could also be attributed to the J
20, which averaged 23% higher in periods from (i) to (iii).
The validation of the results obtained during critical flux testing over the 154 days of long-term operation in period (v) highlights the remarkable filtration performance. The system was operated under subcritical conditions, even reducing the SGD to the lowest possible level. Building on this success, an experiment (vi) was conducted to test an increase in flux up to supercritical conditions. However, this led to a significant increase in the fouling rate, which could negatively impact filtration performance if sustained over time. From the analysis of the performance of the new membrane throughout Experiment 2, it can be confirmed that operating it with a back flush every two basic filtration-relaxation cycles, maintaining subcritical flow conditions, and keeping SGD low ensure optimal technical and economic operating conditions that guarantee proper long-term operation.
The energy and GHG assessments revealed the significant superiority of Scenarios 2 and 3, which included biogas upgrading to biomethane and its injection into the natural gas grid, over Scenario 1, which included co-generation. The mesophilic conditions (Experiment 2) also outperformed the thermophilic ones (Experiment 1), showcasing promising results. In terms of energy recovery, the values for the different scenarios of Experiment 2 would be 22–34% higher than in Experiment 1, and in terms of GHG emissions, the total avoided emissions would be 19–34% higher in Experiment 2. Scenario 2 appears to be a good alternative, as it involves the separation of CO2 from biogas using membranes. This separation results in two streams: one containing biomethane and another containing biogenic CO2. Both streams could be valorized productively, contributing to resource recovery. Nevertheless, Scenario 3 emerges as the most favorable option concerning both energy recovery and GHG emission avoidance, as microalgae would capture the CO2 from biogas in their biomass during the upgrading stage, resulting in higher CH4 production in the AnR.
The significance of GHG emission reduction is highlighted by the comparison of results between Scenario 3 of Experiment 2, the most favorable case, and Scenario 1 of Experiment 1, the least favorable case. Scenario 3 in Experiment 2 would produce a recovery of 2001 kWh·tCOD
−1 treated in the AnMBR pilot plant, a remarkable 161% higher than Scenario 1 in Experiment 1. Moreover, in terms of GHG emissions, Scenario 3 in Experiment 2 would avoid the emission of 423 kgCO
2e·tCOD
−1 treated in the AnMBR pilot plant, a substantial 238% higher reduction compared to the least favorable Scenario 1 of Experiment 1. This value corresponds to 513 kgCO
2e·tDM
−1, which is consistent with the order of magnitude seen in other similar experiments [
43]. These findings emphasize the crucial importance of reducing GHG emissions in AnMBR technology for environmental sustainability and its potential impact on the circular economy by maximizing energy recovery and minimizing GHG.
AnMBR systems combine two complex processes: AD and membrane filtration. Establishing the most suitable operating parameters for both the start-up and long-term operation of these facilities is challenging. The definition of the ICA system presented in this work, coupled with the analysis and validation of the appropriate conditions for long-term ultrafiltration membrane operation, as well as the GHG assessment to determine the most favorable biogas valorization scenario, serve as valuable guidelines for the implementation of such technological solutions. The guidelines provided in this work for AnMBRs’ long-term operation play a crucial role in effectively introducing circularity into the wastewater treatment sector, enabling the integration of AnMBR technology and promoting sustainable practices for resource recovery in WRRFs.
5. Conclusions
A robust ICA system for AnMBR co-digestion pilot plants is presented in this paper. It has been implemented and tested in a continuously operated pilot plant for almost two years. Several lower-layer control loops were implemented and described, allowing for suitable, stable, and optimized process performance. The improved permeate flow-rate controller reduced the IAE by a 23% with respect to the regular PID, and the AnR temperature controller reached a standard deviation of only 0.5%. The high level of flexibility provided by the developed ICA system enabled the optimization of the AnMBR performance, the rapid detection of any process issues, and allowed operators to take early corrective actions. The remarkable methane yield of 215 NmLCH4·gCODinf−1 achieved in Experiment 2 could not have been obtained without the implemented ICA. Similarly, the ICA system ensured optimal and reliable membrane performance, facilitating a technical-economic assessment through critical flow tests in terms of TAEC and showing a competitive cost of 0.0016 EUR/m3. These operating conditions that were established as optimal in the critical flow tests were validated in the long term, demonstrating that the operation was viable and could be sustained over time for a J20 of around 4.2 LMH, an SGD of 0.15 Nm3·m−2·h−1, and the performance of a back-flush stage every two basic cycles of filtration-relaxation. However, further analyses are required to optimize the functioning of the membranes. Finally, different scenarios were established for the net energy and GHG assessment. The results confirmed that the most favorable scenario is the combination of the AnMBR co-digestion pilot plant under mesophilic conditions (35 °C) with microbiological upgrading of biogas to biomethane based on microalgae technology, followed by its injection into the natural gas grid. This scenario would avoid the emission of 423 kgCO2e·tCOD−1. The presented optimal scenario, along with the key guidelines provided throughout the manuscript for AnMBR operation and ICA implementation, offers a promising approach to maximize energy recovery and reduce GHG, highlighting the potential of the emerging AnMBR technology for promoting sustainable practices in the circular economy context of the wastewater sector.