Water Protection Zones—Impacts on Weed Vegetation of Arable Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Method of Vegetation Assessment
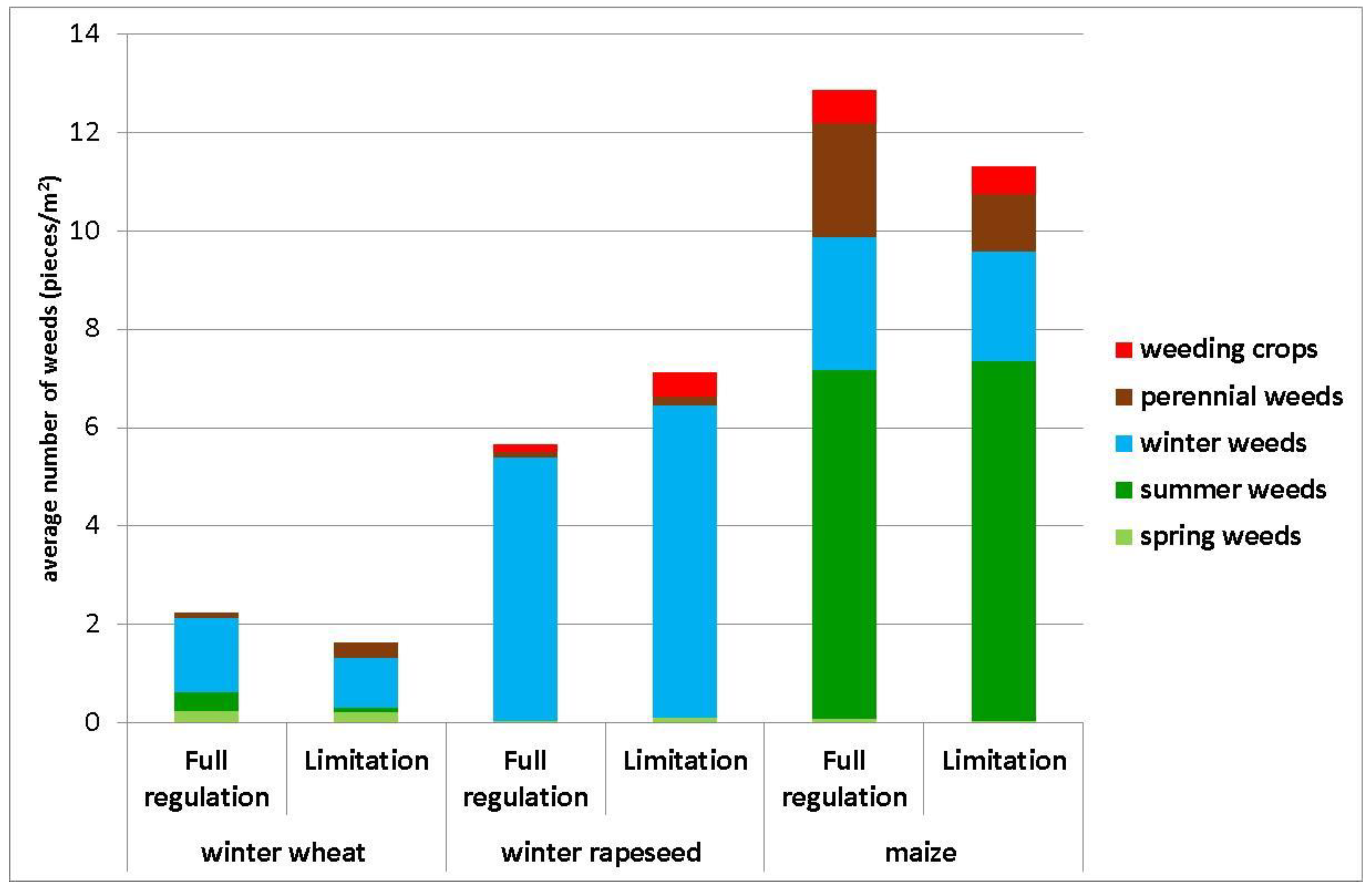
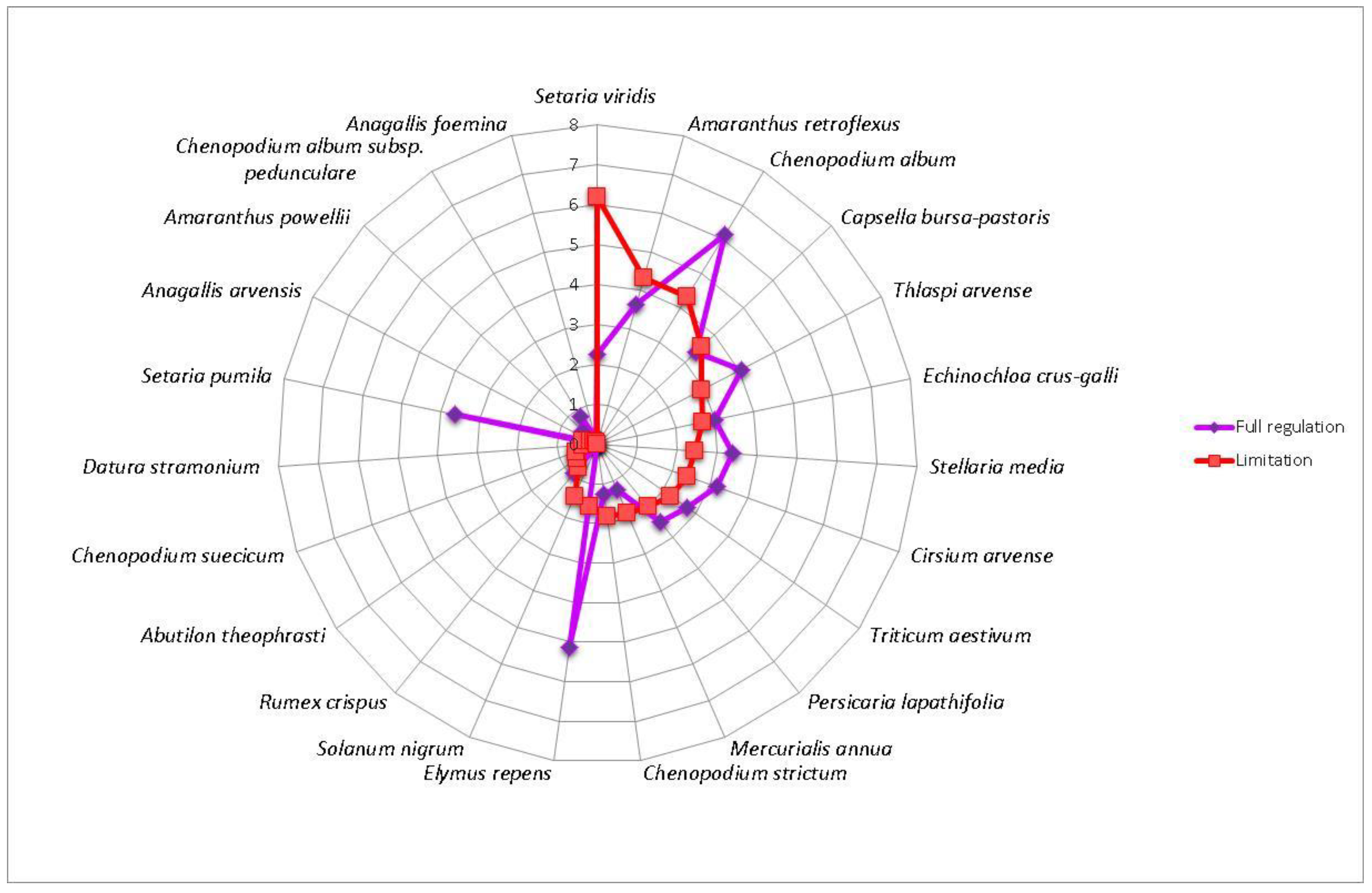
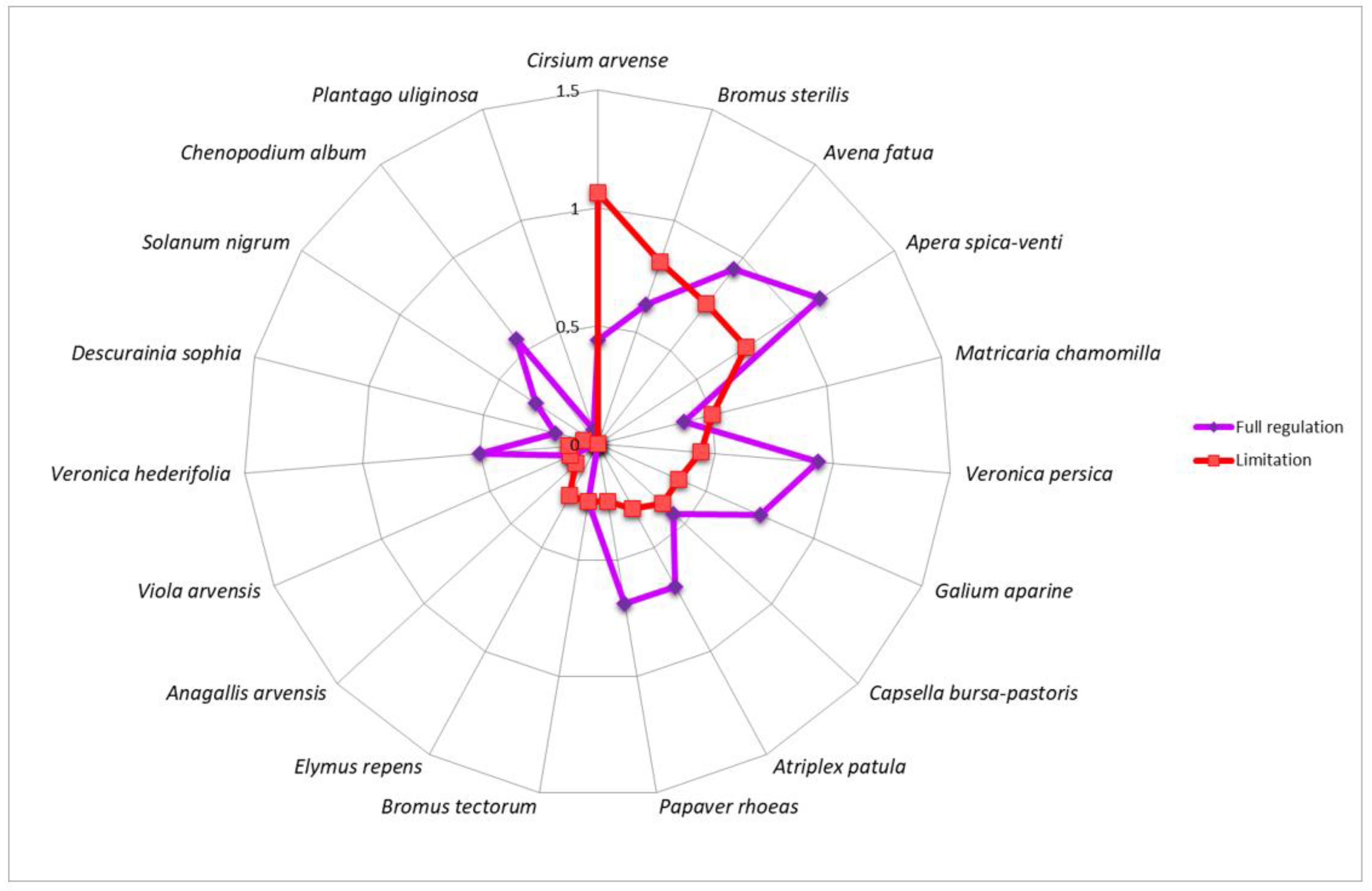
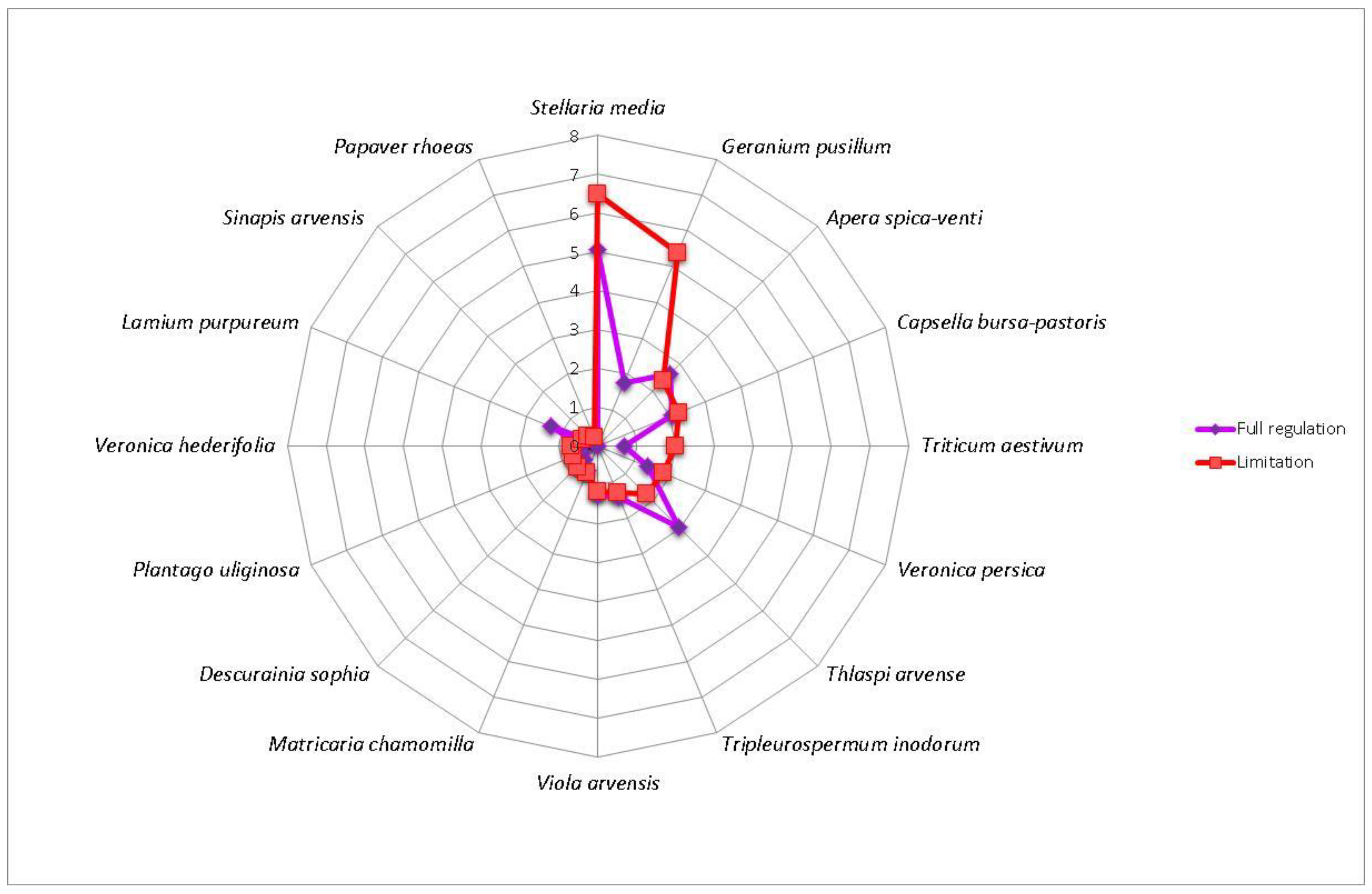
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Passariello, B.; Giuliano, V.; Quaresima, S.; Barbaro, M.; Caroli, S.; Forte, G.; Carelli, G.; Iavicoli, I. Evaluation of the Environmental Contamination at an Abandoned Mining Site. Microchem. J. 2002, 73, 245–250. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Hanifzadeh, M.; Nabati, Z.; Longka, P.; Malakul, P.; Apul, D.; Kim, D.S. Life cycle assessment of superheated steam drying technology as a novel cow manure management method. J. Environ. Manag. 2017, 199, 83–90. [Google Scholar] [CrossRef]
- Gilliom, R.J. Pesticides in U.S. streams and groundwater. Environ. Sci. Technol. 2007, 41, 3408–3414. [Google Scholar] [CrossRef]
- Blanchoud, H.; Moreau-Guigon, E.; Farrugia, F.; Chevreuil, M.; Mouchel, J.M. Contribution by urban and agricultural pesticide uses to water contamination at the scale of the Marne watershed. Sci. Total Environ. 2007, 375, 168–179. [Google Scholar] [CrossRef]
- Ulrich, U.; Dietrich, A.; Fohrer, N. Herbicide transport via surface runoff during intermittent artificial rainfall: A laboratory plot scale study. Catena 2013, 101, 38–49. [Google Scholar] [CrossRef]
- Meyer, B.; Pailler, J.Y.; Guignard, C.; Hoffmann, L.; Krein, A. Concentrations of dissolved herbicides and pharmaceuticals in a small river in Luxembourg. Environ. Monit. Assess. 2011, 180, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Köck-Schulmeyer, M.; Ginebreda, A.; González, S.; Cortina, J.L.; de Alda, M.L.; Barceló, D. Analysis of the occurrence and risk assessment of polar pesticides in the Llobregat River Basin (NE Spain). Chemosphere 2012, 86, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Akerblom, N. Agricultural Pesticide Toxicity to Aquatic Organisms—A Literature Review, 1st ed.; Department of Environmental Assessment, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2004; p. 31. Available online: http://webstar.vatten.slu.se/IMA/Publikationer/internserie/2004-16.pdf (accessed on 10 February 2023).
- Wakabayashi, K.; Böger, P. Target sites for herbicides: Entering the 21st century. Pest Manag. Sci. Former. Pestic. Sci. 2002, 58, 1149–1154. [Google Scholar] [CrossRef]
- Délye, C.; Jasieniuk, M.; Le Corre, V. Deciphering the evolution of herbicide resistance in weeds. Trends Genet. 2013, 29, 649–658. [Google Scholar] [CrossRef]
- Liebman, M.; Mohler, C.; Staver, C. Ecological Management of Agricultural Weeds; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar] [CrossRef]
- Merotto, A., Jr.; Gazziero, D.L.; Oliveira, M.C.; Scursoni, J.; Garcia, M.A.; Figueroa, R.; Turra, G. Herbicide use history and perspective in South America. Adv. Weed Sci. 2022, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chauvel, B.; Tschudy, C.; Munier-Jolain, N. Gestion intégrée de la flore adventice dans les systèmes de culture sans labour. Cah. Agric. 2011, 20, 194–203. [Google Scholar] [CrossRef]
- Thompson, M.; Chauhan, B.S. History and perspective of herbicide use in Australia and New Zealand. Adv. Weed Sci. 2022, 40, 1–12. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Wu, J.; Zhai, Q. The dual impacts of specialized agricultural services on pesticide application intensity: Evidence from China. Pest Management Science 2023, 79, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Dugon, J.; Favre, G.; Zimmermann, A.; Charles, R. Pratiques phytosanitaires dans un réseau d’exploitations de grandes cultures de 1992 a 2004. Rech. Agron. Suisse 2010, 1, 416–423. (In French). Available online: https://www.agrarforschungschweiz.ch/wp-content/uploads/pdf_archive/2010_1112_f_1615.pdf (accessed on 10 February 2023).
- Arancibia, F.; Motta, R.C.; Clausing, P. The neglected burden of agricultural intensification: A contribution to the debate on land-use change. J. Land Use Sci. 2020, 15, 235–251. [Google Scholar] [CrossRef]
- Blettler, D.; Manresa, J.A.B.; Fagúndez, G.A. A review of the effects of agricultural intensification and the use of pesticides on honey bees and their products and possible palliatives. Span. J. Agric. Res. 2022, 20, e03R02. [Google Scholar] [CrossRef]
- Mitra, A.; Chatterjee, C.; Mandal, F.B. Synthetic chemical pesticides and theireffects on birds. Res. J. Environ. Toxicol. 2011, 5, 81–96. [Google Scholar] [CrossRef]
- Hole, D.G.; Perkins, A.J.; Wilson, J.D.; Alexander, I.H.; Grice, P.V.; Evans, A.D. Does organic farming benefit biodiversity? Biol. Conserv. 2005, 122, 113–130. [Google Scholar] [CrossRef]
- Bruggisser, O.T.; Schmidt-Entling, M.H.; Bacher, S. Effects of vineyardmanagement on biodiversity at three trophic levels. Biol. Conserv. 2010, 143, 1521–1528. [Google Scholar] [CrossRef]
- Serrão, J.E.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, J.C. Side-effects of pesticides on non-target insects in agriculture: A mini-review. Sci. Nat. 2022, 109, 17. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure routes and health risks associated with pesticide application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Chiron, F.; Chargé, R.; Julliard, R.; Jiguet, F.; Muratet, A. Pesticide doses, landscape structure and their relative effects on farmland birds. Agric. Ecosyst. Environ. 2014, 185, 153–160. [Google Scholar] [CrossRef]
- Kleijn, D.; Kohler, F.; Báldi, A.; Batáry, P.; Concepción, E.D.; Clough, Y.; Díaz, M.; Gabriel, D.; Holzschuh, A.; Knop, E.; et al. On the relationship between farmland biodiversity and land-use intensityin Europe. Proc. R. Soc. 2009, 276, 903–909. [Google Scholar] [CrossRef]
- Guerrero, I.; Morales, M.B.; Oñate, J.J.; Geiger, F.; Berendse, F.; de Snoo, G.; Eggers, S.; Pärt, T.; Bengtsson, J.; Clement, L.W.; et al. Response of ground-nesting farmland birds to agricultural intensificationacross Europe: Landscape and field level management factors. Biol. Conserv. 2012, 152, 74–80. [Google Scholar] [CrossRef]
- de Montaigu, C.T.; Goulson, D. Habitat quality, urbanisation & pesticides influence bird abundance and richness in gardens. Sci. Total Environ. 2023, 870, 161916. [Google Scholar] [CrossRef]
- Leu, C.; Singer, H.; Stamm, C.; Müller, S.R.; Schwarzenbach, R.P. Simultaneous assessment of sources, processes, and factors influencing herbicide losses to surface waters in a small agricultural catchment. Environ. Sci. Technol. 2004, 38, 3827–3834. [Google Scholar] [CrossRef]
- Liess, M.; Von der Ohe, P.C. Analyzing effects of pesticides on invertebrate communities in streams. Environ. Toxicol. Chem. 2005, 24, 954–965. [Google Scholar] [CrossRef]
- Dugan, S.T.; Muhammetoglu, A.; Uslu, A. A combined approach for the estimation of groundwater leaching potential and environmental impacts of pesticides for agricultural lands. Sci. Total Environ. 2023, 901, 165892. [Google Scholar] [CrossRef]
- Nahar, K.; Baillie, J.; Zulkarnain, N.A. Herbicide Fate and Transport in the Great Barrier Reef: A Review of Critical Parameters. Water 2023, 15, 237. [Google Scholar] [CrossRef]
- EEA. European Waters—Assessment of Status and Pressures 2018; European Environmental Agency: Luxembourg, 2018; p. 90. [Google Scholar] [CrossRef]
- Wuijts, S.; Claessens, J.; Farrow, L.; Doody, D.G.; Klages, S.; Christophoridis, C.; Cvejić, R.; Glavan, M.; Nesheim, I.; Platjouw, F.; et al. Protection of drinking water resources from agricultural pressures: Effectiveness of EU regulations in the context of local realities. J. Environ. Manag. 2021, 287, 112270. [Google Scholar] [CrossRef] [PubMed]
- EEC. Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. 1991. Available online: https://eur-lex.europa.eu/eli/dir/1991/676/oj (accessed on 10 February 2023).
- EC. Council Directive 98/83/EC on the quality of water intended for human consumption. 1998. Available online: https://eur-lex.europa.eu/eli/dir/1998/83/oj (accessed on 10 February 2023).
- EC. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. 2000. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32000L0060 (accessed on 10 February 2023).
- Act No. 254/2001 Coll. Water Act [Zákon č. 254/2001 Sb., vodní zákon]. Available online: https://eagri.cz/public/web/mze/legislativa/pravni-predpisy-mze/tematicky-prehled/Legislativa-MZe_uplna-zneni_zakon-2001-254-viceoblasti.html (accessed on 8 June 2023). (In Czech).
- OECD. OECD INVENTORY Water Governance Indicators and Measurement Frameworks; OECD: Paris, France, 2015; p. 44. Available online: https://www.oecd.org/cfe/regionaldevelopment/Inventory_Indicators.pdf (accessed on 10 February 2023).
- OECD. OECD Principles on Water Governance (Daegu Declaration); OECD: Paris, France, 2015. [Google Scholar]
- Wuijts, S.; Driessen, P.P.J.; Van Rijswick, H.F.M.W. Towards More Effective Water Quality Governance: A Review of Social-Economic, Legal and Ecological Perspectives and Their Interactions. Sustainability 2018, 10, 914. [Google Scholar] [CrossRef]
- Jørgensen, W.L.N.; Kudsk, P.; Ørum, J.E. Links between pesticide use pattern and crop production in Denmark with special reference to winter heat. Crop Prot. 2019, 119, 147–157. [Google Scholar] [CrossRef]
- Devi, P.I.; Manjula, M.; Bhavani, R.V. Agrochemicals, environment, and human health. Annu. Rev. Environ. Resour. 2022, 47, 399–421. [Google Scholar] [CrossRef]
- Adedibu, P.A. Ecological problems of agriculture: Impacts and sustainable solutions. ScienceOpen 2023. preprints. [Google Scholar] [CrossRef]
- Zahoor, I.; Mushtaq, A. Water Pollution from Agricultural Activities: A Critical Global Review. Int. J. Chem. Biochem. Sci. 2023, 23, 164–176. [Google Scholar]
- Islam, M.A.; Amin, S.N.; Rahman, M.A.; Juraimi, A.S.; Uddin, M.K.; Brown, C.L.; Arshad, A. Chronic effects of organic pesticides on the aquatic environment and human health: A review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100740. [Google Scholar] [CrossRef]
- Le Gal, A.S.; Priol, P.; Georges, J.Y.; Verneau, O. Population structure and dynamics of the Mediterranean Pond Turtle Mauremys leprosa (Schweigger, 1812) in contrasted polluted aquatic environments. Environ. Pollut. 2023, 330, 121746. [Google Scholar] [CrossRef]
- Culek, M. (Ed.) Biogeographical Division of the Czech Republic (Biogeografické Členění České Republiky), 1st ed.; Enigma: Prague, Czech Republic, 1996; p. 347. (In Czech) [Google Scholar]
- CGS. Geological Map of the Czech Republic, 1:50 000; Czech Geological Society: Prague, Czech Republic, 2018; Available online: https://mapy.geology.cz/geocr50/ (accessed on 8 November 2022).
- CGS. Map of Soil Types of the Czech Republic, 1:50 000; Czech Geological Society: Prague, Czech Republic, 2017; Available online: https://mapy.geology.cz/pudy/ (accessed on 8 November 2022).
- Kaplan, Z.; Danihelka, J.; Chrtek, J.; Kirschner, J.; Kubát, K.; Štech, M.; Štěpánek, J. (Eds.) Key to the Flora of the Czech Republic [Klíč ke Květeně České Republiky], 2nd ed.; Academia: Prague, Czech Republic, 2019; p. 1168. (In Czech) [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination; Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialistspecies: Towards a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Kolářová, M.; Piskáčková, T.A.R.; Tyšer, L.; Hoová, T.T. Characterisation of Czech arable weed communities according to management and production area considering the prevalence of herbicide-resistant species. Weed Res. 2023, 63, 57–67. [Google Scholar] [CrossRef]
- Winkler, J.; Dvořák, J.; Hosa, J.; Martínez Barroso, P.; Vaverková, M.D. Impact of Conservation Tillage Technologies on the Biological Relevance of Weeds. Land 2023, 12, 121. [Google Scholar] [CrossRef]
- Winkler, J.; Vaverková, M.D.; Havel, L. Anthropogenic life strategy of plants. Anthr. Rev. 2023, 10, 455–462. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent negative effects of pesticides onbiodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Chiron, F.; Filippi-Codaccioni, O.; Jiguet, F.; Devictor, V. Effects of non croppedlandscape diversity on spatial dynamics of farmland birds in intensive farmingsystems. Biol. Conserv. 2010, 43, 2609–2616. [Google Scholar] [CrossRef]
- Frelih-Larsen, A.; Chivers, C.A.; Herb, I.; Mills, J.; Reed, M. The role of public consultations in decision-making on future agricultural pesticide use: Insights from European Union’s Farm to Fork Strategy public consultation. J. Environ. Policy Plan. 2023, 25, 476–492. [Google Scholar] [CrossRef]
- FAO; ITPS; GSBI; SCBD; EC. State of Knowledge of Soil Biodiversity—Status, Challenges and Potentialities, 1st ed.; FAO: Rome, Italy, 2020; p. 618. [Google Scholar] [CrossRef]
- Phillips, H.R.P.; Cameron, E.K.; Ferlian, O.; Türke, M.; Winter, M.; Eisenhauer, N. Red list of a black box. Nat. Ecol. Evol. 2017, 1, 103. [Google Scholar] [CrossRef]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, E.S.; Rosi, E.J.; Gessner, M.O. Synthetic chemicals as agents of global change. Front. Ecol. Environ. 2017, 15, 84–90. [Google Scholar] [CrossRef]
- Pelosi, C.; Bertrand, C.; Daniele, G.; Coeurdassier, M.; Benoit, P.; Nélieu, S.; Lafay, F.; Bretagnolle, V.; Gaba, S.; Vulliet, E.; et al. Residues of currently used pesticides in soils and earthworms: A silent threat. Agric. Ecosyst. Environ. 2021, 305, 107167. [Google Scholar] [CrossRef]
- Wang, Z.; Walker, G.W.; Muir, D.C.G.; Nagatani-Yoshida, K. Toward a global understanding of chemical pollution: A first comprehensive analysis of national and regional chemical inventories. Environ. Sci. Technol. 2020, 54, 2575–2584. [Google Scholar] [CrossRef]
- Gunstone, T.; Cornelisse, T.; Klein, K.; Dubey, A.; Donley, N. Pesticides and soil invertebrates: A Hazard assessment. Front. Environ. Sci. 2021, 9, 643847. [Google Scholar] [CrossRef]
- Beaumelle, L.; Tison, L.; Eisenhauer, N.; Hines, J.; Malladi, S.; Pelosi, C.; Thouvenot, L.; Phillips, H.R.P. Pesticide effects on soil fauna communities—A meta-analysis. J. Appl. Ecol. 2023, 60, 1239–1253. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Bonn, A.; Guerra, C.A. Recognizing the quiet extinction of invertebrates. Nat. Commun. 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.E.; Robinson, R.A.; Pearce-Higgins, J.W. Collation of a century of soil invertebrate abundance data suggests long-term declines in earthworms but not tipulids. PLoS ONE 2023, 18, e0282069. [Google Scholar] [CrossRef]
- Scherber, C.; Eisenhauer, N.; Weisser, W.W.; Schmid, B.; Voigt, W.; Fischer, M.; Schulze, E.D.; Roscher, C.; Weigelt, A.; Allan, E.; et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 2010, 468, 553–556. [Google Scholar] [CrossRef]
- Schuldt, A.; Assmann, T.; Brezzi, M.; Buscot, F.; Eichenberg, D.; Gutknecht, J.; Härdtle, W.; He, J.S.; Klein, A.M.; Kühn, P.; et al. Biodiversity across trophic levels drives multifunctionality in highly diverse forests. Nat. Commun. 2018, 9, 2989. [Google Scholar] [CrossRef] [PubMed]
- Soliveres, S.; Van Der Plas, F.; Manning, P.; Prati, D.; Gossner, M.M.; Renner, S.C.; Alt, F.; Arndt, H.; Baumgartner, V.; Binkenstein, J.; et al. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 2016, 536, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Bender, S.F.; Widmer, F.; Heijden, M.G. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2016, 111, 5266–5270. [Google Scholar] [CrossRef]
- Schiesari, L.; Saito, V.; Ferreira, J.; Freitas, L.S.; Goebbels, A.J.; Leite, J.P.C.B.; Oliveira, J.C.; Pelinson, R.M.; Querido, B.B.; Carmo, J.; et al. Community reorganization stabilizes freshwater ecosystems in intensively managed agricultural fields. J. Appl. Ecol. 2023, 60, 1327–1339. [Google Scholar] [CrossRef]
- Braga, L.; Furia, E.; Buldrini, F.; Mercuri, A.M. Pollen and Flora as Bioindicators in Assessing the Status of Polluted Sites: The Case Study of the Mantua Lakes (SIN “Laghi di Mantova e Polo Chimico”; N Italy). Sustainability 2023, 15, 9414. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Dawson, W.; Maurel, N. Characteristics of Successful Alien Plants. Mol. Ecol. 2015, 24, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- Newig, J.; Fritsch, O. Environmental governance: Participatory, multi-level—And effective? Environ. Policy Gov. 2009, 19, 18. [Google Scholar] [CrossRef]
- Wuijts, S.; Driessen, P.P.J.; Van Rijswick, H.F.M.W. Governance Conditions for Improving Quality Drinking Water Resources: The Need for Enhancing Connectivity. Water Resour. Manag. 2018, 32, 1245–1260. [Google Scholar] [CrossRef]
- Melander, B.; Rasmussen, I.; Bàrberi, P. Integrating physical and cultural methods of weed control—Examples from European research. Weed Sci. 2005, 53, 369–381. [Google Scholar] [CrossRef]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Mykrä, H.; Annala, M.; Hilli, A.; Hotanen, J.P.; Hokajärvi, R.; Jokikokko, P.; Karttunen, K.; Kesälä, M.; Kuoppala, M.; Leinonen, A.; et al. GIS-based planning of buffer zones for protection of boreal streams and their riparian forests. For. Ecol. Manag. 2023, 528, 120639. [Google Scholar] [CrossRef]
- Arora, K.; Mickelson, S.K.; Baker, J.L.; Tierney, D.P.; Peters, C.J. Herbicide retention by vegetative buffer strips from runoff under natural rainfall. Trans. ASAE 1996, 39, 2155–2162. [Google Scholar] [CrossRef]
- Decamps, H.; Pinay, G.; Naiman, R.J.; Petts, G.E.; McClain, M.E.; Hillbricht-Ilkowska, A.H.T.A.; Hanley, T.A.; Holmes, R.M.; Quinn, J.; Gibert, J.; et al. Riparian zones: Where biogeochemistry meets biodiversity in management practice. Pol. J. Ecol. 2004, 52, 3–18. Available online: https://miiz.waw.pl/pliki/article/ar52_1_01.pdf (accessed on 10 February 2023).
- Mankin, K.; Daniel, R.; Ngandu, M.; Barden, C.J.; Hutchinson, S.L.; Geyer, W.A. Grass-shrub riparian buffer removal of sediment, phosphorus, and nitrogen from simulated runoff. JAWRA 2007, 43, 1108–1116. [Google Scholar] [CrossRef]
- Sieczka, A.; Bujakowski, F.; Falkowski, T.; Koda, E. Morphogenesis of a Floodplain as a Criterion for Assessing the Susceptibility to Water Pollution in an Agriculturally Rich Valley of a Lowland River. Water 2018, 10, 399. [Google Scholar] [CrossRef]
- Mander, Ü.; Kuusemets, V.; Hayakawa, Y. Purification processes, ecological functions, planning and design of riparian buffer zones in agricultural watersheds. Ecol. Eng. 2005, 24, 21–432. [Google Scholar] [CrossRef]
- Dosskey, M.G.; Helmers, M.J.; Eisenhauer, D.E. An approach for using soil surveys to guide the placement of water quality buffers. J. Soil Water Conserv. 2006, 61, 344–354. Available online: https://www.srs.fs.usda.gov/pubs/ja/ja_dosskey003.pdf (accessed on 10 February 2023).
- Lam, Q.D.; Schmalz, B.; Fohrer, N. The impact of agricultural Best Management Practices on water quality in a North German lowland catchment. Environ. Monit Assess. 2011, 183, 351–379. [Google Scholar] [CrossRef]
- Winkler, J.; Jeznach, J.; Koda, E.; Sas, W.; Mazur, Ł.; Vaverková, M.D. Promoting Biodiversity: Vegetation in a Model Small Park Located in the Research and Educational Centre. J. Ecol. Eng. 2022, 23, 146–157. [Google Scholar] [CrossRef]
- Renouf, K.; Harding, J.S. Characterizing riparian buffer zones of an agriculturally modified landscape. New Zealand J. Mar. Freshw. Res. 2015, 49, 323–332. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Zhang, M. Major factors influencing the efficacy of vegetated buffers on sediment trapping: A review and analysis. J. Environ. Qual. 2008, 37, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Unger, I.M.; Goyne, K.W.; Kremer, R.J.; Kennedy, A.C. Microbial community diversity in agroforestry and grass vegetative filter strips. Agrofor. Syst. 2013, 87, 395–402. [Google Scholar] [CrossRef]
- Frey, M.P.; Schneider, M.K.; Dietzel, A.; Reichert, P.; Stamm, C. Predicting critical source areas for diffuse herbicide losses to surface waters: Role of connectivity and boundary conditions. J. Hydrol. 2009, 365, 23–36. [Google Scholar] [CrossRef]
- Lind, L.; Hasselquist, E.M.; Laudon, H. Towards ecologically functional riparian zones: A meta-analysis to develop guidelines for protecting ecosystem functions and biodiversity in agricultural landscapes. J. Environ. Manag. 2019, 249, 109391. [Google Scholar] [CrossRef]
| Water Protection Zone | Crop | Surface Area (ha) | GPS |
|---|---|---|---|
| No herbicide limitations (full regulation) | Maize | 7.12 | 48.9282369 N, 17.3842381 E |
| Winter wheat | 11.11 | 48.9334244 N, 17.3749683 E | |
| Rapeseed | 9.08 | 48.9294775 N, 17.3726725 E | |
| Herbicide limitations (limitation) | Maize | 7.07 | 48.9418253 N, 17.3554419 E |
| Winter wheat | 15.95 | 48.9441083 N, 17.3509789 E | |
| Rapeseed | 8.05 | 48.9502931 N, 17.3555419 E |
| Occurence in Crop Stand | Groups of Weeds | ||
|---|---|---|---|
| Species Preferring Herbicide Limitation | Species without Any Preference, Affected by Other Factors | Species Preferring Full Herbicide Regulation | |
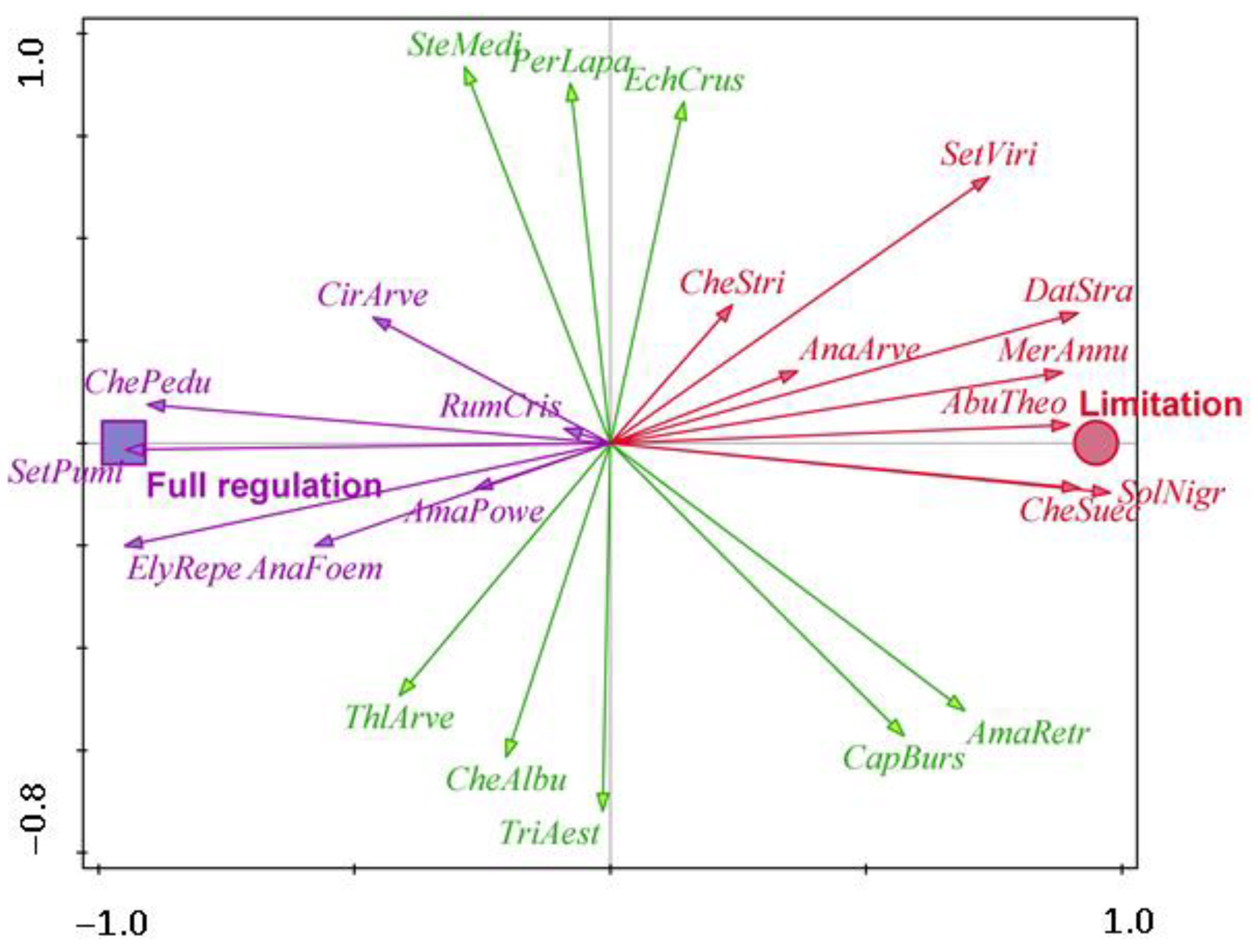
| Flint maize | AbuTheo—Abutilon theophrasti, AnaArve—Anagallis arvensis, DatStra—Datura stramonium, CheStri—Chenopodium strictum, CheSuec—Chenopodium suecicum, MerAnnu—Mercurialis annua, SetViri—Setaria viridis, SolNigr—Solanum nigrum | AmaRetr—Amaranthus retroflexus, CapBurs—Capsella bursa-pastoris, EchCrus—Echinochloa crus-galli, CheAlbu—Chenopodium album, PerLapa—Persicaria lapathifolia, SteMedi—Stellaria media, ThlArve—Thlaspi arvense, TriAest—Triticum aestivum | AmaPowe—Amaranthus powellii, AnaFoem—Anagallis foemina, CirArve—Cirsium arvense, ElyRepe—Elymus repens, ChePedu—Chenopodium album subsp. Pedunculare, RumCris—Rumex crispus, SetPumi—Setaria pumila |
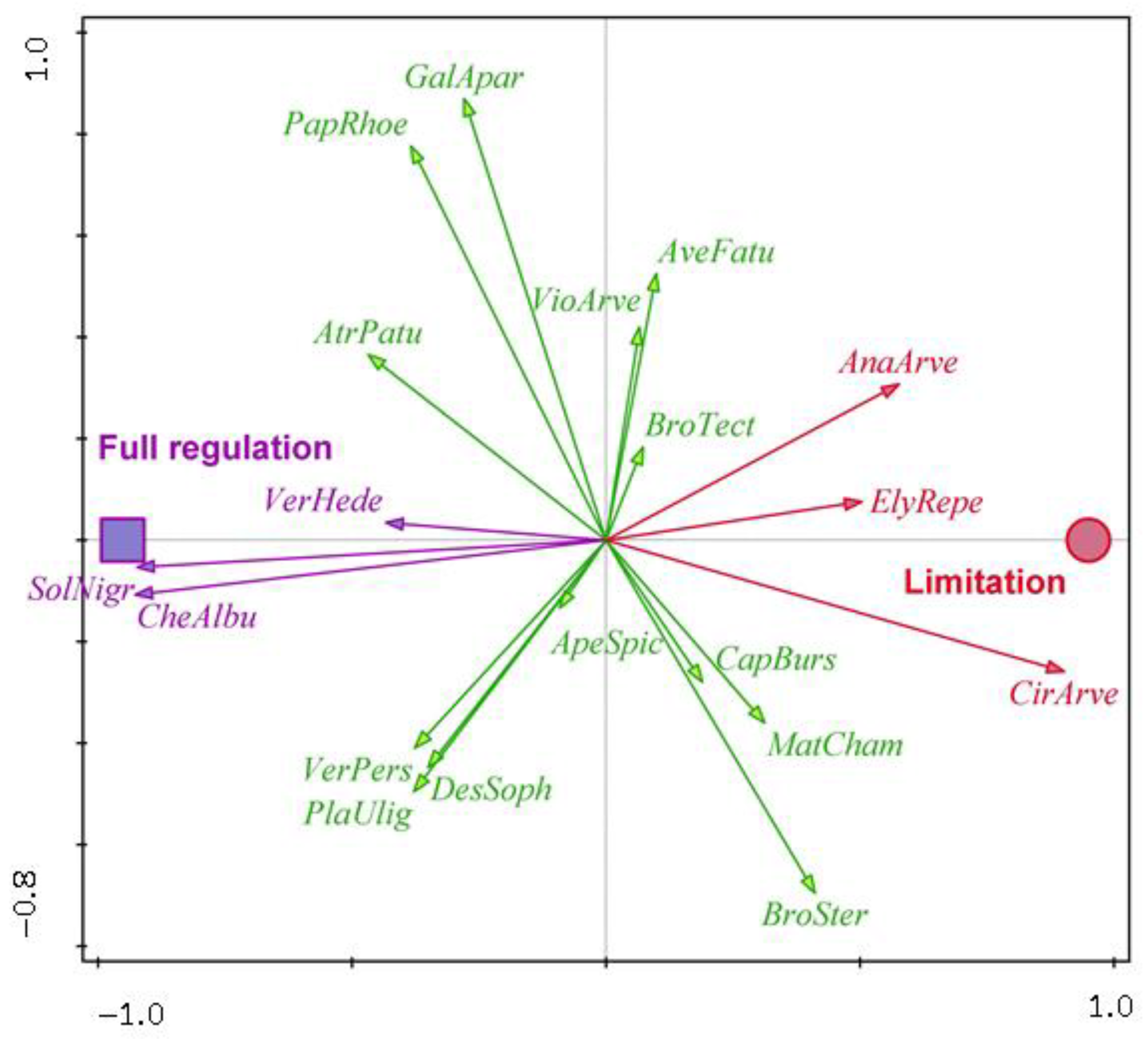
| Winter wheat | AnaArve—Anagallis arvensis, CirArve—Cirsium arvense, ElyRepe—Elymus repens | CheAlbu—Chenopodium album, SolNigr—Solanum nigrum, VerHede—Veronica hederifolia | ApeSpic—Apera spica-venti, AtrPatu—Atriplex patula, AveFatu—Avena fatua, BroSter—Bromus sterilis, BroTect—Bromus tectorum, CapBurs—Capsella bursa-pastoris, DesSoph—Descurainia Sophia, GalApar—Galium aparine, MatCham—Matricaria chamomilla, PapRhoe—Papaver rhoeas, PlaUlig—Plantago uliginosa, VerPers—Veronica persica, VioArve—Viola arvensis |
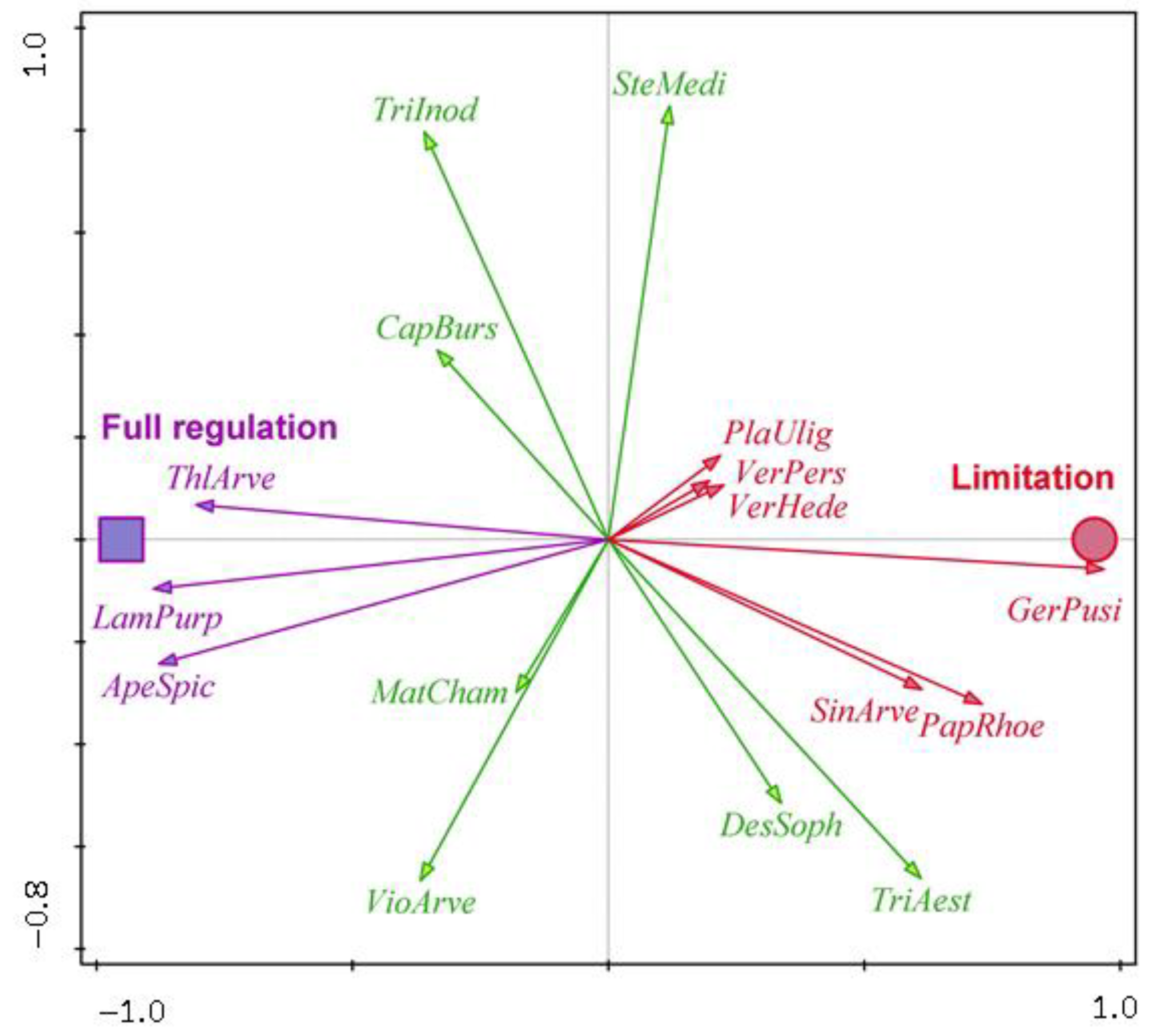
| Winter rapeseed | GerPusi—Geranium pusillum, PapRhoe—Papaver rhoeas, PlaUlig—Plantago uliginosa, SinArve—Sinapis arvensis, VerHede—Veronica hederifolia, VerPers—Veronica persica | CapBurs—Capsella bursa-pastoris, DesSoph—Descurainia Sophia, MatCham—Matricaria chamomilla, SteMedi—Stellaria media, TriAest—Triticum aestivum, TriInod—Tripleurospermum inodorum, VioArve—Viola arvensis | ApeSpic—Apera spica-venti, LamPurp—Lamium purpureum, ThlArve—Thlaspi arvense |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkler, J.; Řičica, T.; Hubačíková, V.; Koda, E.; Vaverková, M.D.; Havel, L.; Żółtowski, M. Water Protection Zones—Impacts on Weed Vegetation of Arable Soil. Water 2023, 15, 3161. https://doi.org/10.3390/w15173161
Winkler J, Řičica T, Hubačíková V, Koda E, Vaverková MD, Havel L, Żółtowski M. Water Protection Zones—Impacts on Weed Vegetation of Arable Soil. Water. 2023; 15(17):3161. https://doi.org/10.3390/w15173161
Chicago/Turabian StyleWinkler, Jan, Tomáš Řičica, Věra Hubačíková, Eugeniusz Koda, Magdalena Daria Vaverková, Ladislav Havel, and Mariusz Żółtowski. 2023. "Water Protection Zones—Impacts on Weed Vegetation of Arable Soil" Water 15, no. 17: 3161. https://doi.org/10.3390/w15173161
APA StyleWinkler, J., Řičica, T., Hubačíková, V., Koda, E., Vaverková, M. D., Havel, L., & Żółtowski, M. (2023). Water Protection Zones—Impacts on Weed Vegetation of Arable Soil. Water, 15(17), 3161. https://doi.org/10.3390/w15173161











