Abstract
The use of treated wastewater (TWW) in irrigation has a positive impact by bringing fertilizers and organics. However, increases in the soil’s sodium adsorption ratio (SAR) creates a barrier to long-term TWW irrigation. Alternating well water with wastewater irrigation is one practical solution that could be used to address the problem. This work aims to study the effect of alternating two years of well water with two years of treated wastewater irrigation on the soil characteristics of a Koroneiki olive tree mesocosm. Urban and agri-food wastewater treated using various technologies, such as lagooning, activated sludge, multi-soil-layering, and constructed wetlands, were used for irrigation. The results showed that an increase in salinity (SAR and ESP) in soil and olive tree leaves are the main negative effects of continuous irrigation with TWW on soil and tree performance. Several chemical and biochemical parameters, such as SAR and Na+ concentration, demonstrated that alternating well water with treated wastewater irrigation can reverse these negative effects. This recovery effect occurs in a relatively short period of time, implying that such a management practice is viable. However, long-term well water application reduces soil fertility due to the leaching of organics and exchangeable ions.
1. Introduction
Agriculture is the most vulnerable domain to the effects of climate change. During summer, when evapotranspiration is high, temperatures rise, and precipitation frequency falls significantly, water is required for irrigation [1]. Reusing treated wastewater as a supplement to traditional irrigation represents a viable strategy for avoiding water crises during the warmest periods of the season, not only in arid and semi-arid environments, but also in areas with intermittent water shortages [1,2,3]. The use of treated wastewater (TWW) for irrigation has gained popularity in recent decades because it reduces well water pressure, alleviating concerns about water scarcity and saving money on fertilizer [1,4,5].
Furthermore, wastewater contains essential nutrients such as P, N, and K, as well as organic matter that aids plant growth [4], and has thus been identified as a valuable input resource for increased agricultural production at a lower cost. Several studies have been conducted on treated wastewater reuse in irrigation, with the goal of determining the effects of reuse on soil, plants/crops, water, and public health, as well as its economic viability [5,6,7,8]. The effects of pH, salinity, nutrient availability, heavy metal accumulation, and changes in soil structure and texture are all investigated in soil research.
Several studies have demonstrated the significant benefits of reusing treated wastewater on soil fertility and culture growth [7,9,10]. Obviously, the quality of wastewater after adequate treatment is improved, in a sanitary sense. Several wastewater treatment technologies integrating tertiary treatment could bring treated effluent up to WHO (World Health Organization) standards and allow for safe reuse.
Waste stabilization ponds, for example, ae a very appealing treatment process that, if well designed, provide very good quality TWW that is completely safe for irrigation. Furthermore, several studies have demonstrated that a hybrid (vertical and horizontal filter) constructed wetland (VF + HF) can produce high-quality microbial effluent (fecal indicators and helminth eggs) [11,12,13]. Other biological processes, such as activated sludge followed by disinfection, produced very interesting outflow quality that can be reused in irrigation, even for golf courses [14]. Furthermore, some studies on treated industrial wastewater have shown that it is of sufficient quality to be reused in agriculture [6,15]. Nonetheless, while some elements in wastewater, such as fertilizers and organics, may be beneficial to agriculture when used for irrigation, long-term impacts, such as concerns about salinity, sodicity, trace elements, and emerging pollutants, are among the most significant barriers to using such wastewater for irrigation [16]. The increase in soil sodium adsorption ratio (SAR) is an impediment to long-term irrigation with treated wastewater [17,18,19]. Soil sodicity, defined as exchangeable sodium percentage (ESP) in reclaimed wastewater, may degrade soil hydraulic conductivity, its water permeability, and soil structure [20], creating an unfavorable environment for root functioning and development [21,22]. Soil sodicity accumulates throughout the soil profile, especially in dry and semi-arid areas with high evaporative demand and limited natural precipitation [23]. Gallal et al. [24] discovered that salinity and sodicity are the most serious ecological issues that can arise when using treated wastewater for irrigation in arid areas. High levels of plant development-inhibitory ions such as sodium (Na) and chlorine (Cl) have been found in recycled wastewater (RWW) [25,26]. An increase in SAR can result in an increase in soil alkalinity, which can lead to significant permeability problems in TWW-irrigated soils [2,27].
One useful option suggested by several writers is alternating irrigation with well water and wastewater to reduce and ameliorate long-term salt problems related to wastewater irrigation [28]. According to them, this approach can counteract the effect of excessive salinity by washing soil when irrigation is applied at regular intervals with well water, or when irrigation is alternated with treated wastewater. Nonetheless, only a few previous studies have reported on the efficacy of such an approach to resolving the salinity problem [29]. An experiment carried out to investigate the effects of greywater irrigation on the growth of silver beet plants observed that alternating irrigation with potable water and greywater could lessen some of the dangers connected with the reuse of greywater. An investigation on grapefruit trees [21] found that alternating the irrigation water quality from TWW to FW had a significant impact on the soil’s electrical conductivity (EC), sodium adsorption ratio (SAR), exchangeable sodium percentage (ESP), and aggregate stability (AS). Furthermore, there are no results in the existing literature on alternating well water with various types of treated wastewater at the same time, and if there are, no comparisons between them have been investigated. Furthermore, there have so far been no outcomes that address switching between treated industrial wastewater and well water.
The purpose of this study is to look into the effect of alternating two years of wastewater irrigation with two years of well water irrigation on the soil and plant characteristics of olive tree mesocosms. Urban and agri-food wastewater were treated using various technologies such as lagooning, activated sludge, multi-soil-layering, and constructed wetlands that are used for irrigation.
2. Material and Methods
2.1. Experimental Device
Experiments were implemented on Olea europaea L.cv. Koroneiki during two different periods:
Period 1 (2017–2019): Olive tree mesocosms were watered during two years with crude or treated wastewater following the experimental design described in Figure 1.
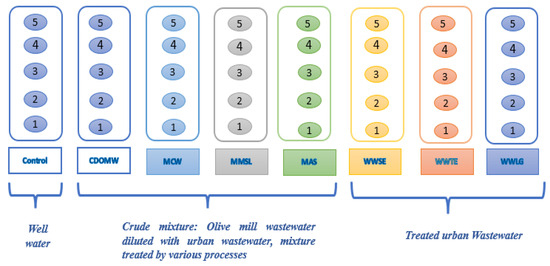
Figure 1.
Experimental design. CDOMW: crude mixture of olive mill wastewater plus urban wastewater; MCW: the mixture treated by constructed wetland; MMSL: the mixture treated by multi-soil-layering system; MAS: the mixture treated by activated sludge; WWSE: urban wastewater secondary effluent; WWTE: urban wastewater tertiary effluent; WWLG: urban wastewater treated by lagooning.
Seven types of irrigation water were studied: urban wastewater treated by lagooning (WWLG) or activated sludge (at secondary (WWSE) and tertiary (WWTE) levels), olive mill wastewater at crude status (OMW) and after treatment by activated sludge (OMWAS), constructed wetland (MCW), or multi-soil-layering (MMSL) pilots. As a control, well water (WW) was used.
Period 2: (2019–2022): Every wastewater irrigation was replaced with well water irrigation in order to compare the effects of alternating FW to TWW irrigation on soil salinity and fertility parameters, as well as agronomical parameters, of the olive trees (Olea europea L.cv. Koroneiki)).
The experiment was carried out at the Faculty of Sciences Semlalia, Cadi Ayyad University Garden (Marrakech, Morocco (3137′ N; 0802′ W)). The climate is typical Mediterranean arid, with a mean air temperature of 25 °C and an annual rainfall of about 240 mm. Each experiment was conducted in 0.11 m2 × 40 kg pots with five replications per treatment. Throughout the experiment, irrigation was applied twice a week to all pots in both periods (1 and 2) to maintain high moisture (80% of field capacity). A sandy-silt soil (52.2% sand, 37.8% silt, and 10% clay) was used. Olive trees (Olea europea L.cv. Koroneiki) (AGROMILLORA, furnisher, Larache, Morocco) were tested.
Greece gave rise to the well known olive tree variety known as Koroneiki. Due to their little size, Koroneiki olive trees are referred to as “dwarf trees” and are thought to be the smallest olive trees, making them an excellent option for research.
2.2. Chemical Characterization of Irrigation Water
Every week, the wastewater (Period 1) and well water (Period 2) used for irrigation was sampled and analyzed for a variety of physicochemical properties. A multi-parameter probe (HI 9829, HANNA, Cluj-Napoca, Romania) was used to measure pH, electrical conductivity (EC), and total dissolved solids (TDS). The dichromate open reflux method was used to measure chemical oxygen demand (COD) [30]. According to [30], the concentration of suspended solids (TSS) was determined using the filtration method; the concentration of NH4+ was determined using the indophenol method; the concentration of NO2− was determined using the diazotization method; and NO3− was determined using the same method, after its reduction to NO2− through a cadmium-copper column.
A flame photometer (AFP 100, Biotech Engineering Management Co., Ltd., London, UK) was used to measure Na and K. Titrimetric techniques were used to measure Ca, Mg, alkalinity, and chlorides in the presence of various colored indicators. The aforementioned parameters were investigated using standard methods [30].
2.3. Soil Sampling and Analysis
The soil was mixed with sand to ensure proper drainage in the pots (75% soil, 25% sand). At the end of each period, soil samples were collected from the rhizosphere zone in the top layer (0–10 cm) of each irrigated treatment. A total of approximately 100 g was collected, air-dried, and baked at 80 °C overnight before sieving in a 2 mm mesh sieve and storing for chemical analysis.
The pH and electrical conductivity were measured in soil solutions extracted with double-deionized water (soil: water ratio of 1:5), stirred for 1 h, and filtered through a Whatman paper filter of 0.45 µm for each sample using a multi-parameter probe (HI 9829, HANNA; Cluj-Napoca, Romania). Total organic carbon (TOC) was calculated by multiplying total carbon by 1.724, as described by [31]. Organic matter (OM) was calculated by multiplying total carbon by 1.724. The P-Olsen method was used to calculate available P, while the Kjeldahl method was used to calculate total N.
A Bernard Calcimeter was used to calculate the amount of CO2 emitted after solubilization of calcium carbonates (CaCO3) by acid attack (HCl N/2).
Na+ and K+ were detected using a flame photometer (AFP 100, Biotech Engineering Management Co., Ltd., London, UK). The SAR was calculated using the sodium-to-calcium-and-magnesium ratio following the equation:
2.4. Plant Analysis
At the end of every plant growth cycle, leaf samples were taken and washed twice with tap water, once with a 0.001 M HCl solution, and once with deionized water. Samples were oven-dried (65 °C) and milled overnight. A total of 250 mg of the samples were digested with 5 mL of 1 M HNO3. Deionized water was used to dilute the obtained solutions to 25 mL. The amounts of Na+, K+, and Ca2+ were determined using flame spectrophotometry (AFP 100, Biotech Engineering Management Co., Ltd., London, UK). A visible spectrophotometer, using the molybdate colorimetric method, was used to measure phosphorus (P).
Nitrogen was measured using the Kjeldahl method, which involves converting N to ammonium sulphate, then distilling the ammonium into boric acid and titrating it with 0.02 N H2SO4 solution in the presence of a Tashiro indicator [30].
2.5. Statistical Analysis
Principal component analysis (PCA) was used to compare the impact of changing treated wastewater by FW on soil characteristics and plant chemical properties. We applied the prcomp() function built into the R program to calculate the Principal Component Analysis (PCA), and the factoextra package to visualize the PCA.
3. Results
3.1. Characteristics of Irrigation Water
3.1.1. Treated Agrifood Wastewater Mixed with Urban Wastewater
All irrigation water used had pH values within the range of the Moroccan irrigation water norm (Table 1), except WWSE and WWTE, which slightly surpassed the standards. The CDOMW had the lowest pH values, followed by the mixture treated with activated sludge. The MCW and MMSL had the highest pH values, with values of 8.07 and 8.22, respectively.

Table 1.
Average physicochemical characteristics of types of water used for Koroneiki irrigation (mean ± standard deviation). CDOMW: crude mixture olive mill wastewater + urban wastewater, MCW: mixture treated by constructed wetland, MMSL: mixture treated by multi-soil-layering system, MAS: mixture treated by activated sludge, WWSE: urban WW after secondary treatment, WWTE: urban wastewater after tertiary treatment, WWLG: urban wastewater treated by lagooning.
For Electrical Conductivity (EC), all types of water met the standards (Table 1). However, we found that diluted agri-food wastewater by urban wastewater, whether in its raw state or after treatment, has a higher EC than other treated wastewater and the control (WW), particularly when treated by constructed wetland and lagooning.
The mixture treated by constructed wetland MCW had significantly higher salinity as electrical conductivity, with an EC of 7.89 mS·cm−1), followed by CDOMW with 4.44 mS·cm−1. MAS, on the other hand, had the lowest salinity, with 1.54 mS·cm−1 (Table 1).
In terms of Na+, MCW had the highest concentration, followed by CDOMW in second place. Compared to the other treated wastewater, where the sodium rate ranged between 138.4 and 168.79 mg·L−1, WW had only 90.56 mg·L−1.
The processed mixture of agri-food and urban wastewater, treated by MCW, MAS, and MMSL, had a higher sodium absorption ratio SAR than the other treated wastewater.
The lowest value was observed for urban wastewater treated by WWLG lagooning. Well water, on the other hand, had the lowest SAR value of only 6.63. The mineral composition of the various processed mixtures revealed that CDOMW had higher concentrations of total phosphorus, ammonium, and sulfate than MCW and MMSL, while the latter did have higher concentrations of nitrate and chlorides. The treatment processes may be responsible for the difference between CDOMW and treated MCW and MSL.
However, these treated wastewaters, in general, do not exceed the irrigation water standard, except for Na+ and Cl− for crude mixture or mixture treated by constructed wetland MCW (Table 1).
When the fertilizer value of the different types of water is considered, the CDOMW contains the most phosphate, ammonium, and potassium. These compounds decreased after the three treatments, but they remain high when compared to well water.
3.1.2. Treated Urban Wastewater
Urban wastewater after secondary treatment, and urban wastewater after tertiary treatment WWSE and WWTE, had the highest pH values, reaching 8.61 and 8.9, respectively, whereas WWLG had the lowest; it had the lowest pH when compared to well water. In general, all of these treated urban wastewaters were within the pH range of irrigation water guidelines.
In terms of salinity, WWSE and WWTE had lower values than WWLG, with the WW having the lowest value at 0.85 mS·cm−1. The salinity of all of these treated wastewaters, however, did not exceed the Moroccan guideline value.
All treated wastewater effluents contain a high concentration of minerals and micronutrients, but there was no significant difference in mineral content between the Secondary, Tertiary, and Lagooning effluents. They are, however, still beyond the well water.
Sodium concentration varied between the three effluents, with the WWSE and WWTE having higher concentrations of 168.79 mg·L−1 and 167.71 mg·L−1, respectively, while the WWLG had a lower concentration of 146.67 mg·L−1. The WWSE and WWTE had higher sodium absorption ratios (SAR), while the WWLG had a lower SAR of 9.66. This disparity is most likely due to the treatment processes. In general, at the opposite of all parameters the Na+ contents of these wastewaters were higher than well water and exceed the limits of irrigation norms (Table 1).
3.2. Effect of Irrigation by Treated Wastewater on the Soil
3.2.1. Case of Treated Mixture of Agrifood Plus Urban Wastewater
The composition of the soil solution and exchangeable cations extracted from the topsoil at the end of period 1 are given in Table 2.

Table 2.
Main physicochemical characteristics of the soil before and after treated wastewater irrigation. CDOMW: crude mixture olive mill wastewater plus urban wastewater, MCW: mixture treated by constructed wetland, MSL: mixture treated by multi-soil-layering system, MAS: mixture treated by activated sludge, WWSE: urban wastewater after secondary treatment, WWTE: urban wastewater after tertiary treatment, WWLG: urban wastewater treated by lagooning.
The samples from the irrigated plots with agrifood plus urban wastewater have higher salinity, a higher sodium content, and thus higher SAR and ESP values than the samples from the reference soils. In comparison to other treatments and the initial state of the soil, MCW irrigated soils had the highest EC value of 0.659 mS·cm−1 and and an SAR of 25.81 (Table 2). These findings are consistent with the chemical properties of the wastewater and suggest that wastewater irrigation increased soil salinization. In terms of soil fertility, MAS irrigated soil samples had higher levels of OP, OC, and TN than other treatments, while CDOMW soils had higher levels of K+.
3.2.2. Case of Treated Urban Wastewater
Treated urban wastewater-irrigated soils exhibited a high salinity effect, a slight increase in EC, significant sodium amounts, and thus higher SAR when compared to the initial soil. The WWLG had the highest EC value of 0.41 mS·cm−1 and the highest SAR of 23.92 for the WWSE (Table 2). These findings are consistent with the fact that the wastewater is highly charged with ions that control salinity. The effect of treated urban wastewater irrigation water on soil fertility was found to be significant for OC, OP, TP, and K+. When compared to other treatments and the initial soil, WWSE had higher TP (1270 mg·kg−1), K+ (3605 mg·kg−1), and OC (2.78%) levels.
3.3. Effect of Alternating Well Water to Wastewater Irrigation on the Soil Characteristics
Figure 2 and Figure 3 illustrate the effect of well water irrigation alternation on the soils in the second period, in every mesocosm.
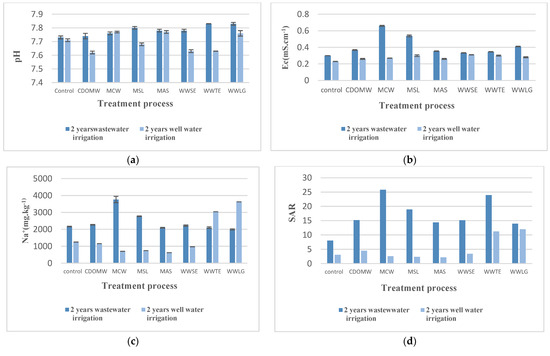
Figure 2.
Effect of alternating well water to wastewater irrigation on soil pH and salinity. (a) Effect on pH; (b) Effect on electrical conductivity; (c) Effect on sodium; (d) Effect on SAR. CDOMW: crude mixture olive mill wastewater plus urban wastewater, MCW: mixture treated by constructed wetland, MSL: mixture treated by multi-soil-layering system, MAS: mixture treated by activated sludge, WWSE: urban wastewater after secondary treatment, WWTE: urban wastewater after tertiary treatment, WWLG: urban wastewater treated by lagooning.
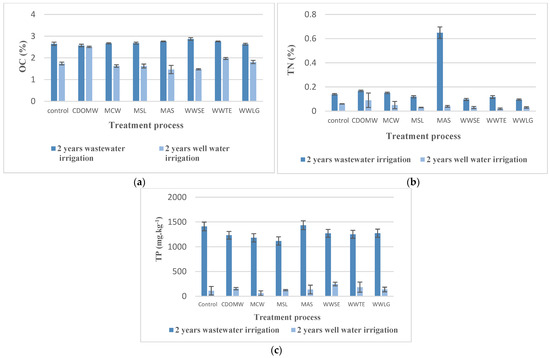
Figure 3.
Effect of alternating well water to treated wastewater irrigation on the soil fertility. (a) Effect on organic carbon; (b) Effect on total nitrogen; (c) Effect on total phosphorus. CDOMW: crude mixture olive mill wastewater plus urban wastewater, MCW: mixture treated by constructed wetland, MSL: mixture treated by multi-soil-layering system, MAS: mixture treated by activated sludge, WWSE: urban wastewater after secondary treatment, WWTE: urban wastewater after tertiary treatment, WWLG: urban wastewater treated by lagooning.
- Soil pH;
In comparison to the soil in the first period, pH in the soil irrigated by CDOMW and in the processed soil decreased after two years of well water irrigation. The pH values in the CDOMW and MSL irrigated soils fell by 1.5%, from 7.74 and 7.8 to 7.62 and 7.68, respectively. Simultaneously, the soils irrigated by MCW and MAS showed no significant variation after switching to well water irrigation (Figure 2a).
In terms of soils irrigated by wastewater effluents, the WWTE irrigated soils had the highest decrease, followed by the WWSE irrigated ones and then the WWLG.
- Soil Salinity;
After switching to well water irrigation, electrical conductivity in soils irrigated by CDOMW and by the treated crude mixture decreased (Figure 2b). After two years, we noticed a significant decrease in EC in the MCW-irrigated soils. This increased by more than 50%, from 0.659 to 0.27 mS·cm−1. The second range included MSL irrigated soils, where the EC had decreased by 44%. The lowest reduction rate was observed in irrigated CDOMW and MAS soils, where the reduction did not exceed 29%. However, no significant difference in reduction was found between the CDOMW and the MAS. In both soils, the EC was 0.26 mS·cm−1.
There was no significant difference in electrical conductivity between soils irrigated by various treated urban wastewater effluents. The EC in the WWSE and WWTE irrigated soils decreased by about 0.023 and 0.047 mS·cm−1, respectively. In contrast, the EC value in WWLG irrigated soils fell by 31%. Despite the different treatment processes, the EC in the various soils ranged between 0.26 and 0.3 mS·cm−1.
It was also discovered that switching to well water irrigation had a significant impact on sodium levels (Figure 2c). Na concentration in the MCW decreased by 81% in the CDOMW and the treated crude mixture-irrigated soils, falling from 3763.33 to 704.25 mg·kg1.
MSL- and MAS-irrigated soils were second, with reductions of 73 and 70%, respectively. The CM-irrigated soils had the lowest percentage decline, with Na levels reduced by only 50%. Na concentration decreased by 56% in WWSE-irrigated soils compared to those irrigated with wastewater effluents. Sodium levels in WWTE and WWLG soils, on the other hand, increased by 45 and 82%, respectively.
The sodium absorption ratio SAR and ESP decreased significantly in almost all treatments except the WWLG, where the SAR barely decreased (Figure 2d). After two years of well water application, the SAR was reduced by more than 50% in all treatments. For the same application and time period, SAR decreased by approximately 14% with the WWLG treatment. For all soil samples, SAR after replacing wastewater with FW was less than 12.
- Soil fertility parameters;
Figure 3a shows that the organic carbon (OC) content in the treated CM irrigated soils decreased. After well water application, it decreased by nearly 40%, 47%, and 47% for MCW, MSL, and MAS, respectively. The OC and OM content of the CDOMW-irrigated soils did not show any significant differences.
A similar pattern was observed in soils irrigated by wastewater effluents. OC decreased by nearly 50% in WWSE- and WWTE-irrigated soils, from 2.76 and 2.87% to 1.47 and 1.97%, respectively, while WWLG-irrigated soils showed the lowest reduction, with a percentage of around 30%.
The total nitrogen content of the CDOMW- and CM-irrigated soils was significantly reduced (Figure 3b). The greatest reduction was observed in MAS-irrigated soils, where Total Nitrogen decreased by more than 90% from 0.65 to 0.04%. A similar trend was observed in the irrigated soils of CDOMW, MCW, and MSL, with reductions of 52.66 and 74%, respectively.
According to (Figure 3c), MCW-irrigated soils had a significant drop in TP concentration by more than 90%, from 1180 to 60.33 mg·kg−1. Second place goes to MAS-irrigated soils, where the TP concentration dropped by 90%, while CDOMW- and MSL-irrigated soils showed a drop that did not exceed 88%. In terms of soils irrigated by treated urban wastewater effluents, WWLT-irrigated soils had the highest TP reduction rate of 89%. Meanwhile, the TP decline in WWSE and WWTE was less than 85%, from 1270 to 248.07 mg·kg−1 and from 1249.66 to 183.44 mg·kg−1, respectively.
3.4. Effect of Alternating Wastewater Irrigation by Well Water on the Leaf Mineral Content of Koroneiki Plant
Well water irrigation resulted in a significant decrease in phosphorus amounts at the leaf level (Figure 4b). P content in leaves decreased by 79% in plants irrigated with CDOMW, reaching 433 mg·kg−1, the lowest value. The reduction was 59% for the MCW- and MSL-irrigated plants, with P levels of 659.88 and 469.6 mg·kg−1, respectively. The MAS-irrigated plants had the lowest reduction rate, with P concentration dropping by less than half, 43%, to 690 mg·kg−1.
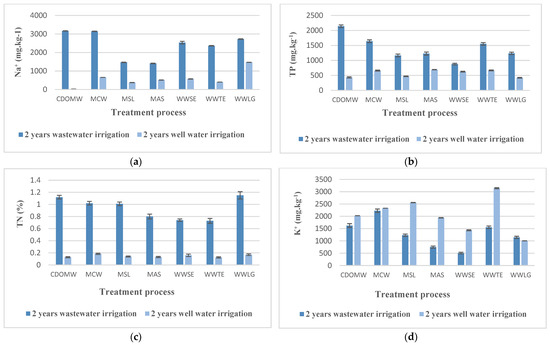
Figure 4.
Effect of alternating well water to wastewater irrigation on leaves mineral content (a) sodium, (b) total phosphorus, (c) total nitrogen, (d) potassium. CDOMW: crude mixture olive mill wastewater plus urban wastewater, MCW: mixture treated by constructed wetland, MSL: mixture treated by multi-soil-layering soil system, MAS: mixture treated by activated sludge, WWSE: urban wastewater after secondary treatment, WWSE: urban wastewater after secondary treatment.
A similar pattern was observed for plants that were irrigated with wastewater effluents. The greatest reduction percentage was observed in the WWTE- and WWLG-irrigated plants, with a drop of 57% and 65%, respectively, to 666.21 and 420.33 mg·kg−1, respectively. The smallest reduction was observed in WWSE irrigated plants, where P content decreased by only 28%, from 877 to 624.18 mg·kg−1.
Regardless of the treatment processes, the P concentration ranged from 420 to 690 mg·kg−1.
Figure 4a shows the Na+ content of the leaves after switching to well water irrigation. CDOMW-irrigated plants had the lowest Na+ at leaf level, with 30.12 mg·kg−1, a 99% reduction. The MCW and MSL showed a reduction of 79 and 74%, with 657 and 377.43 mg·kg−1, respectively. Na+ concentration decreased by 63% in MAS-irrigated plants, reaching 512.37 mg·kg−1.
The plants irrigated with wastewater effluents followed a similar pattern. WWTE-irrigated plants reduced the most, from 2366.66 to 405.5 mg·kg−1. Then there were the WWSE plants, where the reduction was 82%. Meanwhile, the Na+ level in plants irrigated with WWLG was reduced by only 46%, from 2726.66 to 1470.37 mg·kg−1.
Following replacement with well water irrigation, the TN% in the various plant leaves decreased (Figure 4c). The reduction rate ranged from 78 to 88%. As a result, N in leaves ranged between 0.126 and 0.183.
Compare this to the K+ amount, which, regardless of treatment process, increased by more than half (Figure 4d).
4. Statistical Analysis
4.1. PCA on Irrigation Waters Samples
The correlation matrix derived from the log-transformed data set of all physico-chemical characteristics of used irrigation water in the two different periods was then subjected to a principal component analysis (PCA) to determine the effects of water quality variation in the analyzed samples. The correlation matrix retained two major components that accounted for 76.8% of the variance.
Figure 5 clearly shows that the water used in Period 1 was strongly correlated with salinity and fertility variables (Na+, EC, OM, K+, AP, TP). These findings are explained by the fact that wastewater contains a high concentration of ions and organic matter. Water consumption in period 2 is, on the other hand, negatively correlated with those variables. According to our findings, well water contains far fewer of these components than wastewater.
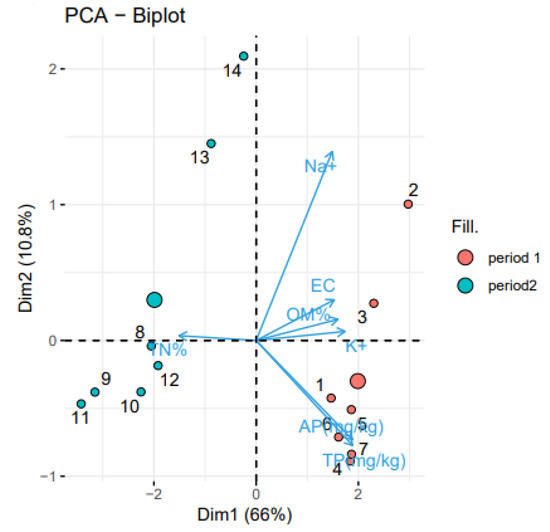
Figure 5.
PCA results of the used irrigation water during Periods 1 (numbers 1 to 7) and period 2 (numbers 8 to 13). Dim1: Dimension 1, Dim 2: Dimension 2.
4.2. PCA of Soil Characteristics
To determine the effects of two water qualities on the total variation in component concentrations in the analyzed samples, a principal component analysis (PCA) and a correlation analysis were performed using a data set of all physico-chemical characteristics of soil and water samples taken from the two different periods. Dim1 (66%) and Dim2 (10.8%) were used to present our data set, accounting for 76.8% of the variance in the correlation matrix. These components were interpreted to represent soil salinity and fertility status rather than irrigation water type (Periods 1 and 2).
Figure 6a,b shows that the various irrigated Soils in Period 1 were highly correlated with the variables responsible for salinity and fertility (Na+, K+, TP, AP). However, in Period 2, these variables had a negative correlation with soils. The PCA Cos squared plot (Figure 6) shows the mean difference in variability of the distribution of Na+, AP, TP, and K+ between Period 1 and Period 2 soils. It was discovered that Na+, AP, TP, and K+ have high cos2 values, ranging from 0.8 to 0.9, indicating that they are significant variables influenced by the wastewater used in Period 1. However, the variable least affected by irrigation water type was TN% (cos2 0.6).
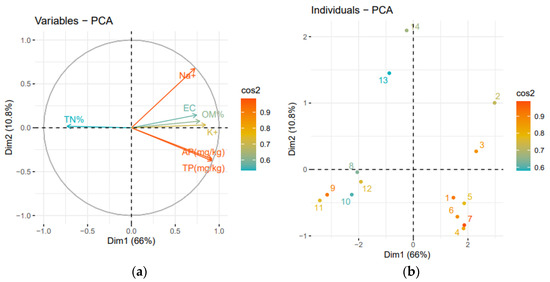
Figure 6.
PCA results on the effect of replacing wastewater by well water on soil properties. (a): variable Plot, (b) individual Plot, Dim1: Dimension 1, Dim: Dimension 2. Period 1 (numbers 1 to 7); Period (numbers 8 to 13).
These findings can be explained by the higher concentrations of these components in wastewater (Period 1) than in well water (Period 2). This clearly shows the opposite effect of the two types of irrigation water, an enrichment of salinity and fertility with treated wastewater irrigation and restoration of soil salinity during well water irrigation due to the leaching mechanism after the alternation of application.
5. Discussion
5.1. Physicochemical Parameters
Regarding the pH, after wastewater irrigation pH seems to slightly decrease in comparison with the control soil. A similar result was observed by [32], who reported that soils with UWW irrigation showed lower pH values, and that this is probably due to the high organic matter content of the irrigation water. The soil pH decrease with TWW was probably due to: (i) the absorption of ammonia ions by the plant or the nitrification of ammonium, and (ii) the leaching of alkaline cations [33]. This slight pH change can also be attributed to the release of exchangeable cations during the mineralization of organic matter [34].
After replacing wastewater by FW irrigation, the pH values showed a slight decrease. These results are in good agreement with [35], who reported that the pH in the soil decreased for soil irrigated with groundwater. This is likely due to the decomposition of organic matter and production of organic acids in soils, which is in line with the findings of [36]. Another explanation for this reduction in pH may be the nitrification of NH4+ from the wastewater, as observed by [37] and reported by [38].
5.2. Soil Fertility Parameters: OC, OM, TN, TP
OC and OM contents in soil increased after the application of different treated wastewaters. Identical results were observed in a study of four years of treated-wastewater irrigation of an olive orchard, where [17] reported a significant increase of OM. Some studies have shown that high OM values were temporary, since soil microorganisms rapidly mineralize the organic material [39,40], thus OC increases. Bedbabis et al. [17] stated that the short-term sharp OM increase detected in TWW irrigation can be explained by the composition of this water, which presented high values of BOD and COD.
The organic matter and total N level of the soils with Urban wastewater was higher than those of the other irrigated soils. Organic matter and N levels of the soils decreased sharply with treated wastewater compared to the non-wastewater-irrigated soil. According to Kiziloglu et al [32], the organic matter content was greater at UWWI (Untreated Wastewater), PLTWI (Preliminary Treated Wastewater), and PTW (Primary Treated Wastewater), respectively, in the upper 30 cm soil layer of cauliflower soil. The same author noticed that N content of the same soils showed similarities with organic matter behavior.
With the switch to well water irrigation, both contents significantly decreased. Our findings are consistent with research done in the arid and semi-arid climates of California and Arizona, USA, which showed that conventional well water gravity irrigation of sandy loam and clay loam calcareous soils over 90 years resulted in a 56–62% reduction of the organic carbon content (OC) in the 0–30 cm soil layer [41,42].
According to [43], sodium ions (Na+) greatly accelerated the leaching of OC and OM. The solubility of soil carbonates was substantially associated with the amount of OC and OM leaching.
In the semi-arid region of Russia with annual precipitation of 300–650 mm, the effects of long-term well water gravity and sprinkling irrigation on chernozem (rich in organic Matter) and castanozem (rich in carbonate) soils were also investigated. According to the findings, 20 years of well water irrigation on chernozem and castanozem soils reduced their respective OM contents from roughly 6.7 and 2.7 to 5.9 and 2.3% [44].
Adejumobi et al. [45] found that the soil’s TN was low, which they attributed to the soil’s low solubility of organic matter (SOM). SOM is highly correlated with TN, according to the same author, because Soluble OM is a source of TN when mineralized by microorganisms. Furthermore, well water used for irrigation in this study had very low amounts of fertility elements and no fertilizers were applied.
Following wastewater irrigation, the available phosphorus rate sharply rose compared to initial soil phosphorus content. Available P concentrations were larger in the soils with untreated wastewater irrigation, and this is probably due to high organic matter supplied with the wastewater [32]. Consequently, replacing wastewater by FW has led to an important diminishment in P content. According to [46], the availability of phosphorus decreases when pH decreases, particularly between pH values of 5 and 6. This has to do with both the amount of organic matter in the soil as well as how soluble phosphates are in relation to pH. As stated by [47], the effect of well water irrigation on soil fertility is a relatively sluggish process that takes tens of years. Existing literature has observed that well water irrigation increases soil fertility or at the very least preserves it [48]. The fundamental qualities of irrigated soils can, however, gradually deteriorate over time, and their fertility can decline, according to certain publications that discuss long-term well water irrigation under various climatic situations.
Changes in the historical conditions of soil formation are the principal cause of the loss of OM and total soil fertility during long-term well water irrigation. Intensification of microbiological activity, increased solubility and movability of organic compounds, intensification of OM formation and its mineralization, intensification of deep soil water percolation below the root zone, leaching of mineral and organic substances, etc., are all caused by an increase in soil moisture content [43].
5.3. Soil Salinity Parameters: Na+, EC and SAR
Na and K cation concentration in the soil of the mesocosms was low before wastewater irrigation. According to [32], wastewater irrigation caused an increase in the exchangeable cations Na and K.
The increased salinity of the soil may be caused by the wastewater’s high electrical conductivity. According to [49,50], the rise was directly attributed to (i) the high K content in the TWW, which is consistent with prior observations by various authors, and (ii) to the adsorption of organic matter.
The antagonistic activity of either K+ or NH4+, which inhibited the adsorption of Na on exchangeable complexes, and the high calcium supply, which improved the selectivity for the uptake and transport of K over Na, can both be used to explain the high Na concentration in soil solution [17].
Furthermore, an increase in EC and SAR was also noticed after wastewater irrigation.
According to [17], the large rise in SAR in TWW irrigated soil was likely caused by Na accumulations and a decreased water infiltration rate, both of which had a detrimental impact on the Na transport to the bottom layers.
Switching to FW irrigation resulted in a considerable decrease in Na concentration during Period 2. Additionally, following extensive TWW irrigation, [51] reported EC, SAR levels, and Na concentrations in the 0–20 cm layer of the FW plots. For SAR, EC, and Na concentrations, the ranges were approximately 4.4 meq·L−1, to 3 meq·L−1, 3. 6, to 2. 8, and 15. 2, to up to 9. 8 mg·L−1, respectively.
Changes in pH and SAR, as reported by [52], were quite substantial. After converting from TWW irrigation to FW irrigation, the pH of the soil dramatically increased from an acidified pH of 5.7 to 7.2. The same author discovered that after applying FW, the SAR value, which was highest for TWW watered plots, significantly decreased. It showed a small decrease in EC values from TWW to FW irrigation. This could be explained by well water aiding in the growth of soluble Ca2+ in the soil, which on its own favors infiltration and leaching, as well as the dissolution and solubilization of native soil calcite. Soil will consequently release more Na+ than Ca2+. SAR and EC drop as a result of salt and exchangeable cations leaching, in contrast to wastewater, which retains large levels of Na+ and increases CaCo3 precipitation and salt accumulation.
Scherer et al. [53] stated that the combination of SAR, EC, and pH showed that the following parameters were satisfied for the soil to be classified normal, with no salinity, and sodicity hazard, which is in good agreement with our findings: SAR 13, EC 4, and pH 8.5.
In accordance with [54], using saline-sodic water under a management strategy that includes alternative irrigations with well water may be done without having a negative impact on the quality of the soil or crop production (over a 3-year period).
Well water’s capacity to reduce some parameters, such as OC, OM, TN, TP, pH, exchangeable cations, and salt accumulation (EC, SAR), by mineralization of some and leaching of others is responsible for these changes in soil chemical characteristics.
5.4. Leaf Mineral Content: Na+, K+, TP, TN
In all mesocosms, the amounts of N, P, and Na in the leaves have significantly decreased since wastewater irrigation was replaced with well water irrigation for a period of two years.
In the diverse plant leaves, N% has fallen. These elements’ decline may be explained by their increased mobility during the growth of reproductive (high yield phase) and vegetative (low yield period) structures. Furthermore, well water used for irrigation has less of those substances than wastewater, and no fertilizer was utilized. The substantial drop in accessible phosphorus that was seen in the soil after switching to well water irrigation may be responsible for the P decline.
The Na+ in the leaves has significantly decreased by watering with well water. Most olive cultivars show a salt exclusion ability at low and intermediate salinities [55,56]. According to [55], the exclusion mechanism is successful for low and intermediate salinities.
Limiting soil Na+ absorption, reducing Na+ xylem transport, storing Na+ in the lower portion of plant leaves, such as the sheath in cereal crops, isolating Na+ into the vacuole, and cycling Na+ from plant shoots to roots are some of the mechanisms used to reduce Na+ toxicity in leaves [57].
Relying on our data, this decrease could be attributed to the fact that well water used for irrigation is less loaded in Na ions. Furthermore, according to our findings, the soil’s salinity has greatly decreased following well water treatment, therefore the Na content in leaves would certainly decline.
On other hand, the levels of K+ seem to be higher after switching to well water irrigation.
The K+ concentration of Leccino’s leaves, shoots, thick roots, and thin roots dropped in comparison to levels in the control plants at the greatest salinity level, 12 dS·m−1 [58]. According to [59], the Na+ and K+ concentrations of cotton leaves are inversely related.
One of the defining characteristics of the salt-tolerance system is the equilibrium between Na+ and K+ in plant tissues. According to [59], this is caused by the selective distribution of Na+, Cl−, and K+, with the partial exclusion of Na+ from developing tissues and the transport of K+ in meristematic cells and leaf mesophyll cells. According to [56,60], the effectiveness of a salt-exclusion/retention system functioning at the root level that inhibits Na+ buildup into actively growing shoots while maintaining significant K+ transport rates is the major factor in Olea europea’s ability to tolerate salt.
The majority of olive cultivars possess this sodium exclusion capacity, which promotes K+ assimilation by removing Na+ from the leaves. Regarding the ion selectivity response, olive cultivars have the capacity to reduce the uptake of particular cations, such as Na+, as well as other advantageous cations, such as K+ (favoring K+ over Na+ due to its high affinity to the plant growth).
6. Conclusions
In semi-arid and arid areas, treated wastewater is a valuable source for irrigation, since it gives crops water and some nutrients (N, P, and K). Long-term TWW irrigation, however, may have a deleterious impact on the qualities of the soil and plants. The current study demonstrated that increased soil sodicity (SAR and ESP) and deterioration of soil characteristics were related to the main adverse impacts of continuous irrigation with TWW, as opposed to FW, on the performance of the olive trees. The buildup of Na in olive tree soil is another adverse effect of irrigation with TWW. Chemical indicators, such as SAR and Na+, however, demonstrated that after several years of irrigation with TWW, switching to FW can reverse the detrimental effects on the trees. Our results show that the benefits of alternating TWW with FW occur over a short period of time, between a few months and two years, promoting the sustainability of this management strategy. On the other hand, under long term well-water application, soil fertility declines due to the leaching of organic matter and exchangeable ions.
Author Contributions
Conceptualization N.O. and J.F.; Methodology J.F., A.A. and N.O.; Formal analysis A.E.A.E.F.; Investigation J.F. and A.A.; Writing original draft preparation J.F. and N.O.; Writing-review and editing N.O. and L.M.; Funding acquisition X.Z. and N.O. All authors have read and agreed to the published version of the manuscript.
Funding
National Key R&D Program of China (2021YFB4001502), the National Natural Science Foundation of China (No. 22075231), and the Sichuan Science and Technology Program (No. 2021YFSY0022). This project has also received funding from the Horizon 2020 research and innovation program under grant agreement N° 862555. The project “SECUREFOOD2050” was carried out under the ERA-Net Co-fund FOSC (Grant N° 862555), built upon and supported by experience from the Joint Programming Initiative on Agriculture, Food Security and Climate change (FACCE-JPI), and the ERA-Net Co-fund LEAP-Agri.
Data Availability Statement
Data are not available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perulli, G.D.; Bresilla, K.; Manfrini, L.; Boini, A.; Sorrenti, G.; Grappadelli, L.C.; Morandi, B. Beneficial Effect of Secondary Treated Wastewater Irrigation on Nectarine Tree Physiology. Agric. Water Manag. 2019, 221, 120–130. [Google Scholar] [CrossRef]
- Ofori, S.; Puškáčová, A.; Růžičková, I.; Wanner, J. Treated Wastewater Reuse for Irrigation: Pros and Cons. Sci. Total Environ. 2021, 760, 144026. [Google Scholar] [CrossRef]
- Mahjoub, O.; Mauffret, A.; Michel, C.; Chmingui, W. Use of Groundwater and Reclaimed Water for Agricultural Irrigation: Farmers’ Practices and Attitudes and Related Environmental and Health Risks. Chemosphere 2022, 295, 133945. [Google Scholar] [CrossRef] [PubMed]
- Gufrankhan, M.; Daniel, G.; Konjit, M.; Thomas, A.; Eyasu, S.S.; Awoke, G. Impact of Textile Waste Water on Seed Germination and Some Physiological Parameters in Pea (Pisum Sativum L.), Lentil (Lens Esculentum L.) and Gram (Cicer Arietinum L.). Asian J. Plant Sci. 2011, 10, 269–273. [Google Scholar]
- Urbaniak, M.; Wyrwicka, A.; Tołoczko, W.; Serwecińska, L.; Zieliński, M. The Effect of Sewage Sludge Application on Soil Properties and Willow (Salix Sp.) Cultivation. Sci. Total Environ. 2017, 586, 66–75. [Google Scholar] [CrossRef]
- Ahmali, A.; Mandi, L.; Loutfi, K.; El Ghadraoui, A.; El Mansour, T.E.; El Kerroumi, A.; Hejjaj, A.; Del Bubba, M.; Ouazzani, N. Agro-Physiological Responses of Koroneiki Olive Trees (Olea Europaea L.) Irrigated by Crude and Treated Mixture of Olive Mill and Urban Wastewaters. Sci. Hortic. 2020, 263, 109101. [Google Scholar] [CrossRef]
- Leonel, L.P.; Tonetti, A.L. Wastewater Reuse for Crop Irrigation: Crop Yield, Soil and Human Health Implications Based on Giardiasis Epidemiology. Sci. Total Environ. 2021, 775, 145833. [Google Scholar] [CrossRef]
- Liu, C.; Cui, B.; Wang, J.; Hu, C.; Huang, P.; Shen, X.; Gao, F.; Li, Z. Does Short-Term Combined Irrigation Using Brackish-Reclaimed Water Cause the Risk of Soil Secondary Salinization? Plants 2022, 11, 2552. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Moustakas, K.; Skrzypczak, D.; Mikula, K.; Loizidou, M. A Transition from Conventional Irrigation to Fertigation with Reclaimed Wastewater: Prospects and Challenges. Renew. Sustain. Energy Rev. 2020, 130. [Google Scholar] [CrossRef]
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment Technologies for Olive Mill Wastewater with Impacts on Plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef]
- Elfanssi, S.; Ouazzani, N.; Mandi, L. Soil Properties and Agro-Physiological Responses of Alfalfa (Medicago Sativa L.) Irrigated by Treated Domestic Wastewater. Agric. Water Manag. 2018, 202, 231–240. [Google Scholar] [CrossRef]
- Kataki, S.; Chatterjee, S.; Vairale, M.G.; Dwivedi, S.K.; Gupta, D.K. Constructed Wetland, an Eco-Technology for Wastewater Treatment: A Review on Types of Wastewater Treated and Components of the Technology (Macrophyte, Biolfilm and Substrate). J. Environ. Manag. 2021, 283, 111986. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J.; Zhao, Y.; Mander, Ü. Recent Research Challenges in Constructed Wetlands for Wastewater Treatment: A Review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Hajji, S.; Alla, A.A.; Noureddine, S.; Haddad, M.B.; Moukrim, A. Study of Physicochemical and Bacteriological Quality of Treated Wastewater by the New Aourir Plant (Southwestern of Morocco) Using Activated Sludge Technology in a Semi-Arid Region. J. Ecol. Eng. 2021, 22, 83–98. [Google Scholar] [CrossRef]
- Elhanafi, L.; Houhou, M.; Rais, C.; Mansouri, I.; Elghadraoui, L.; Greche, H. Impact of Excessive Nitrogen Fertilization on the Biochemical Quality, Phenolic Compounds, and Antioxidant Power of Sesamum Indicum L. Seeds. J. Food Qual. 2019, 2019, 9428092. [Google Scholar] [CrossRef]
- Pedrero, F.; Grattan, S.R.; Ben-gal, A.; Vivaldi, G.A. Opportunities for Expanding the Use of Wastewaters for Irrigation of Olives. Agric. Water Manag. 2020, 241, 106333. [Google Scholar] [CrossRef]
- Bedbabis, S.; Ben Rouina, B.; Boukhris, M.; Ferrara, G. Effect of Irrigation with Treated Wastewater on Soil Chemical Properties and Infiltration Rate. J. Environ. Manag. 2014, 133, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.M.; Gonzalez-Rubio, A.; Suarez, D.L. Impact of Treated Wastewater for Irrigation on Soil Microbial Communities. Sci. Total Environ. 2018, 622–623, 1603–1610. [Google Scholar] [CrossRef]
- Singh, P.K.; Deshbhratar, P.B.; Ramteke, D.S. Effects of Sewage Wastewater Irrigation on Soil Properties, Crop Yield and Environment. Agric. Water Manag. 2012, 103, 100–104. [Google Scholar] [CrossRef]
- SouDakouré, M.Y.; Mermoud, A.; Yacouba, H.; Boivin, P. Impacts of Irrigation with Industrial Treated Wastewater on Soil Properties. Geoderma 2013, 200–201, 31–39. [Google Scholar] [CrossRef]
- Paudel, I.; Cohen, S.; Shaviv, A.; Bar-Tal, A.; Bernstein, N.; Heuer, B.; Ephrath, J. Impact of Treated Wastewater on Growth, Respiration and Hydraulic Conductivity of Citrus Root Systems in Light and Heavy Soils. Tree Physiol. 2016, 36, 770–785. [Google Scholar] [CrossRef] [PubMed]
- Yalin, D.; Schwartz, A.; Assouline, S.; Narkis, K.; Eshel, A.; Levin, A.G.; Lowengart-Aycicegi, A.; Tarchitzky, J.; Shenker, M. Insights from “The Hidden Half”: The Impact of Root-Zone Oxygen and Redox Dynamics on the Response of Avocado to Long-Term Irrigation with Treated Wastewater in Clayey Soil. Isr. J. Plant Sci. 2017, 64, 3–4. [Google Scholar] [CrossRef]
- Muyen, Z.; Moore, G.A.; Wrigley, R.J. Soil Salinity and Sodicity Effects of Wastewater Irrigation in South East Australia. Agric. Water Manag. 2011, 99, 33–41. [Google Scholar] [CrossRef]
- Elgallal, M.; Fletcher, L.; Evans, B. Assessment of Potential Risks Associated with Chemicals in Wastewater Used for Irrigation in Arid and Semiarid Zones: A Review. Agric. Water Manag. 2016, 177, 419–431. [Google Scholar] [CrossRef]
- Beiersdorf, I.; Yermiyahu, U.; Soda, N.; Presnov, E.; Zipori, I.; Crisostomo, R.R.; Dag, A. Response of Young Bearing Olive Trees to Irrigation—Induced Salinity. Irrig. Sci. 2017, 35, 99–109. [Google Scholar] [CrossRef]
- Raveh, E.; Ben-Gal, A. Irrigation with Water Containing Salts: Evidence from a Macro-Data National Case Study in Israel. Agric. Water Manag. 2016, 170, 176–179. [Google Scholar] [CrossRef]
- Erel, R.; Eppel, A.; Yermiyahu, U.; Ben-Gal, A.; Levy, G.; Zipori, I.; Schaumann, G.E.; Mayer, O.; Dag, A. Long-Term Irrigation with Reclaimed Wastewater: Implications on Nutrient Management, Soil Chemistry and Olive (Olea Europaea L.) Performance. Agric. Water Manag. 2019, 213, 324–335. [Google Scholar] [CrossRef]
- Hashem, M.S.; Qi, X. Bin Treated Wastewater Irrigation-a Review. Water 2021, 13. [Google Scholar] [CrossRef]
- Pinto, U.; Maheshwari, B.L.; Grewal, H.S. Effects of Greywater Irrigation on Plant Growth, Water Use and Soil Properties. Resour. Conserv. Recycl. 2010, 54, 429–435. [Google Scholar] [CrossRef]
- L’analyse de l’eau_Rodier 9eme Édition, Dunod. 1994. Available online: https://books.google.com.hk/books/about/L_analyse_de_l_eau_9e_%C3%A9d.html?id=qUEGsUBZkL0C&redir_esc=y (accessed on 15 April 2023).
- Latham, M.; Quantin, P.; Aubert, G. Etude des sols de la Nouvelle-Calédonie; Centre de Nouméa, Carte d’aptitudes Culturale et Forestière Des Sols de La Nouvelle-Calédonie, ORSTOM: Paris, France, 1978; ISBN 2709905191. [Google Scholar]
- Kiziloglu, F.M.; Turan, M.; Sahin, U.; Kuslu, Y.; Dursun, A. Effects of Untreated and Treated Wastewater Irrigation on Some Chemical Properties of Cauliflower (Brassica Olerecea L. Var. Botrytis) and Red Cabbage (Brassica Olerecea L. Var. Rubra) Grown on Calcareous Soil in Turkey. Agric. Water Manag. 2008, 95, 716–724. [Google Scholar] [CrossRef]
- Tarchouna, L.G.; Merdy, P.; Raynaud, M.; Pfeifer, H.R.; Lucas, Y. Effects of Long-Term Irrigation with Treated Wastewater. Part I: Evolution of Soil Physico-Chemical Properties. Appl. Geochem. 2010, 25, 1703–1710. [Google Scholar] [CrossRef]
- Woomer, P.L.; Martin, A.; Albrecht, A.; Resck, D.V.S.; Scharpenseel, H.W. The Importance and Management of Soil Organic Matter in the Tropics. In The Biological Management of Tropical Soil Fertility; John Wiley & Sons: Hoboken, NJ, USA, 1994; pp. 47–80. [Google Scholar]
- Abegunrin, A. Effect of Kitchen Wastewater Irrigation on Soil Properties and Growth of Cucumber (Cucumis Sativus). J. Soil Sci. Environ. Manag. 2013, 4, 139–145. [Google Scholar] [CrossRef]
- Khai, N.M.; Tuan, P.T.; Vinh, N.C.; Oborn, I. Peri-Urban Agricultural Systems Effects of Using Wastewater as Nutrient Sources on Soil Chemical Properties in Peri—Urban Agricultural Systems. VNU J. Sci. Earth Environ. Sci. 2008, 24, 87–95. [Google Scholar]
- Stamatiadis, S.; Doran, J.W.; Kettler, T. Field and Laboratory Evaluation of Soil Quality Changes Resulting from Injection of Liquid Sewage Sludge. Appl. Soil Ecol. 1999, 12, 263–272. [Google Scholar] [CrossRef]
- Hussein, A.H.A. Impact of Sewage Sludge as Organic Manure on Some Soil Properties, Growth, Yield and Nutrient Contents of Cucumber Crop. J. Appl. Sci. 2009, 9, 1401–1411. [Google Scholar] [CrossRef]
- Mechri, B.; Mariem, F.B.; Baham, M.; Elhadj, S.B.; Hammami, M. Change in Soil Properties and the Soil Microbial Community Following Land Spreading of Olive Mill Wastewater Affects Olive Trees Key Physiological Parameters and the Abundance of Arbuscular Mycorrhizal Fungi. Soil Biol. Biochem. 2008, 40, 152–161. [Google Scholar] [CrossRef]
- Di Serio, M.G.; Lanza, B.; Mucciarella, M.R.; Russi, F.; Iannucci, E.; Marfisi, P.; Madeo, A. Effects of Olive Mill Wastewater Spreading on the Physico-Chemical and Microbiological Characteristics of Soil. Int. Biodeterior. Biodegrad. 2008, 62, 403–407. [Google Scholar] [CrossRef]
- Richter, D.D.B.; Hofmockel, M.; Callaham, M.A.; Powlson, D.S.; Smith, P. Long-Term Soil Experiments: Keys to Managing Earth’s Rapidly Changing Ecosystems. Soil Sci. Soc. Am. J. 2007, 71, 266–279. [Google Scholar] [CrossRef]
- Artiola, J.F.; Walworth, J.L. Irrigation Water Quality Effects on Soil Carbon Fractionation and Organic Carbon Dissolution and Leaching in a Semiarid Calcareous Soil. Soil.Sci. 2009, 174, 365–371. [Google Scholar] [CrossRef]
- Nikolskii, Y.N.; Aidarov, I.P.; Landeros-Sanchez, C.; Pchyolkin, V.V. Impact of Long-Term Freshwater Irrigation on Soil Fertility. Irrig. Drain. 2019, 68, 993–1001. [Google Scholar] [CrossRef]
- Aidarov, I.P. Regulation of the Water, Salt and Nutrients Regimes of Irrigated Lands; Agropromizdat: Moscow, Russia, 1985; pp. 42–234. [Google Scholar]
- Adejumobi, M.A.; Awe, G.O.; Abegunrin, T.P.; Oyetunji, O.M.; Kareem, T.S. Effect of Irrigation on Soil Health: A Case Study of the Ikere Irrigation Project in Oyo State, Southwest Nigeria. Environ. Monit. Assess. 2016, 188, 696. [Google Scholar] [CrossRef] [PubMed]
- Nikolskii-Gavrilov, I.; Aidarov, I.P.; Landeros-Sanchez, C.; Herrera-Gomez, S.; Bakhlaeva-Egorova, O. Evaluation of Soil Fertility Indices of Freshwater Irrigated Soils in Mexico Across Different Climatic Regions. J. Agric. Sci. 2014, 6, p98. [Google Scholar] [CrossRef][Green Version]
- Arnold, R.W.; Szabolcs, I.; Targulian, V.O. Global Soil Change; International lnstitute for Applied Systems Analysis: Laxenburg, Austria, 1990. [Google Scholar]
- Molden, D. Water for Food Water for Life: A Comprehensive Assessment of Water Management in Agriculture; Routledge: Abingdon, UK, 2007; ISBN 9781849773799. [Google Scholar]
- Emongor, V.E.; Ramolemana, G.M. Treated Sewage Effluent (Water) Potential to Be Used for Horticultural Production in Botswana. Phys. Chem. Earth 2004, 29, 1101–1108. [Google Scholar] [CrossRef]
- Heidarpour, M.; Mostafazadeh-Fard, B.; Abedi Koupai, J.; Malekian, R. The Effects of Treated Wastewater on Soil Chemical Properties Using Subsurface and Surface Irrigation Methods. Agric. Water Manag. 2007, 90, 87–94. [Google Scholar] [CrossRef]
- Rahav, M.; Brindt, N.; Yermiyahu, U. Water Resources Research. J. Am. Water Resour. Assoc. 2017, 19, 2. [Google Scholar] [CrossRef]
- Leuther, F.; Schlüter, S.; Wallach, R.; Vogel, H.J. Structure and Hydraulic Properties in Soils under Long-Term Irrigation with Treated Wastewater. Geoderma 2019, 333, 90–98. [Google Scholar] [CrossRef]
- Scherer, T.F.; Franzen, D.; Cihacek, L. Soil, Water and Plant Characteristics Important to Irrigation; North Dakota State University Extension Services: Fargo, ND, USA, 1996; Volume 1675, pp. 1–16. [Google Scholar]
- Murtaza, G.; Ghafoor, A.; Qadir, M. Irrigation and Soil Management Strategies for Using Saline-Sodic Water in a Cotton-Wheat Rotation. Agric. Water Manag. 2006, 81, 98–114. [Google Scholar] [CrossRef]
- Chartzoulakis, K.S. Salinity and Olive: Growth, Salt Tolerance, Photosynthesis and Yield. Agric. Water Manag. 2005, 78, 108–121. [Google Scholar] [CrossRef]
- Tattini, M.; Bertoni, P.; Caselli, S. Genotipic Responses of Olive Plants to Sodium Chloride. J. Plant Nutr. 1992, 15, 1467–1485. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, J.; Wu, X.; Dong, L. Na+/K+ Balance and Transport Regulatory Mechanisms in Weedy and Cultivated Rice (Oryza Sativa L.) Under Salt Stress. BMC Plant Biol. 2018, 18, 375 . [Google Scholar] [CrossRef]
- Demiral, M.A. Comparative Response of Two Olive (Olea Europaea L.) Cultivars to Salinity. Turk. J. Agric. For. 2005, 29, 267–274. [Google Scholar]
- Ashraf, M. Salt Tolerance of Cotton: Some New Advances. CRC Crit. Rev. Plant Sci. 2002, 21, 1–30. [Google Scholar] [CrossRef]
- Cresti, M.; Ciampolini, F.; Tattini, M.; Cimato, A. Effect of Salinity on Productivity and Oil Quality of Olive (Olea Europaea L.). Plants 1994, 8, 1000–1005. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).