Advances in Produced Water Treatment Technologies: An In-Depth Exploration with an Emphasis on Membrane-Based Systems and Future Perspectives
Abstract
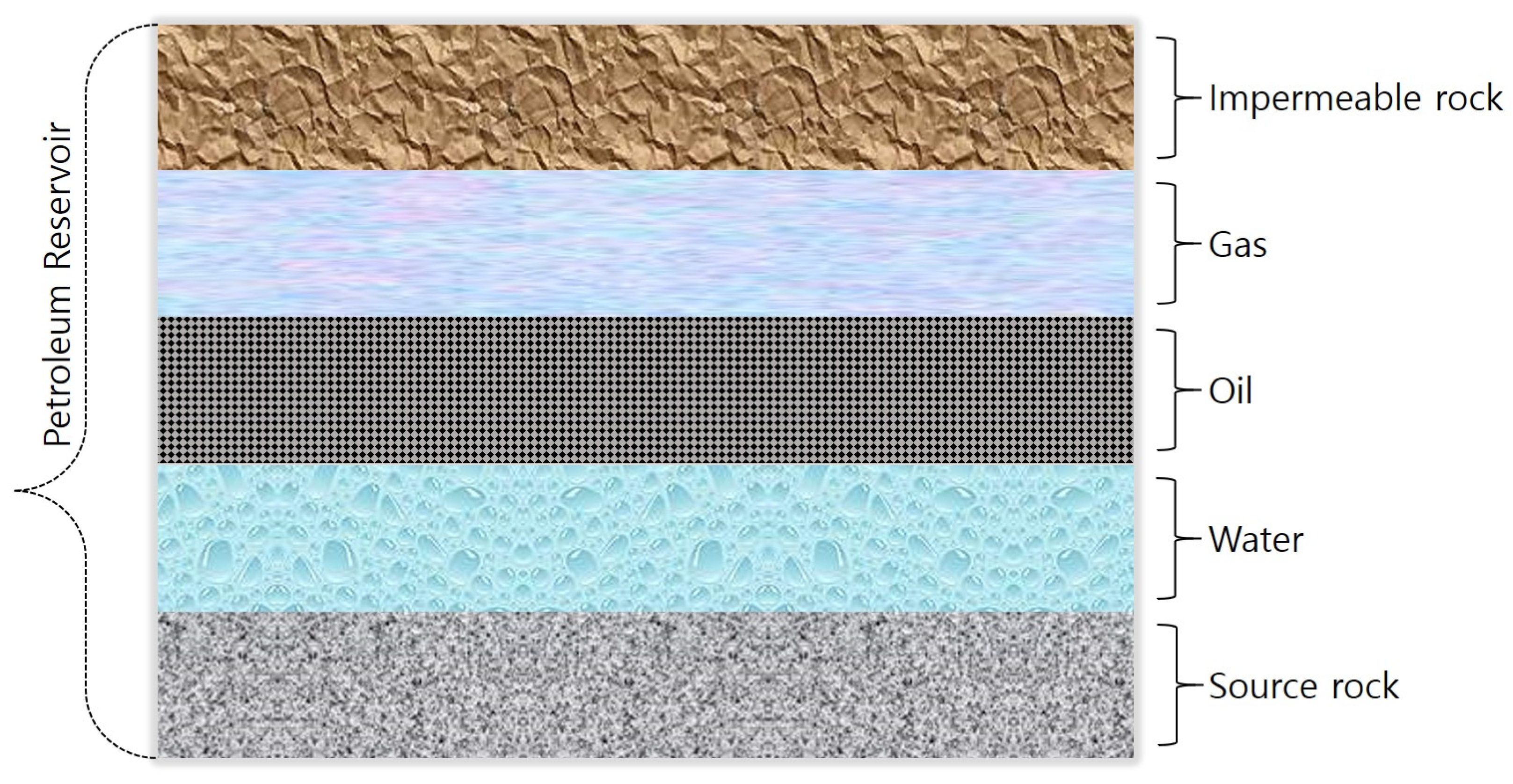
1. Introduction
| Parameters | Range | Unit |
|---|---|---|
| Conductivity | 4200–58,600 | (μS/cm) |
| pH | 4.3–10 | - |
| Density | 1014–1140 | (kg/m3) |
| Turbidity | 182 | (NTU) |
| Surface Tension | 43–78 | (dyne/cm) |
| COD | 1220 | (mg/L) |
| TOC | 0–1500 | (mg/L) |
| TSS | 1.2–1000 | (mg/L) |
| Total oil | 2–565 | (mg/L) |
| Volatiles | 0.39–35 | (BTEX; mg/L) |
| Petroleum hydrocarbon (total) | >20 | (TPH) |
| Non-volatile oil and grease (total) | 275 | (μg/L) |
| Bicarbonate | 77–3990 | (mg/L) |
| Chloride | 80–200,000 | (mg/L) |
| Sulfate | <2–1650 | (mg/L) |
| Volatile fatty acids (VFA’s) | 2–4900 | (mg/L) |
| Sodium | 132–97,000 | (mg/L) |
| Calcium | 13–25,800 | (mg/L) |
| Potassium | 24–4300 | (mg/L) |
| Lithium | 3–50 | (mg/L) |
| Iron | <0.1–100 | (mg/L) |
2. Treatment Technologies for Produced Water
2.1. Conventional Treatment Approaches
2.1.1. Adsorption
2.1.2. Cyclonic Separation
2.1.3. Sand Filtration
2.1.4. Dissolved Air Precipitation (DAP)
| Technology | Desalting | De-Oiling | Softening (Mg and Ca Removal) | Removal of Suspending Particles | Iron Removal | Trace/Soluble Organics Removal |
|---|---|---|---|---|---|---|
| Reverse osmosis (RO) | √ | √ | √ | √ | √ | √ |
| Nanofiltration (NF) | √ | √ | √ | √ | √ | √ |
| Ultrafiltration (UF) | √ | √ | √ | |||
| Electrodialysis (ED) | √ | √ | √ | |||
| Thermal desalination | √ | √ | √ | |||
| Chemical treatment processes | √ | √ | ||||
| Biological treatment processes | √ | |||||
| Activated carbon (AC) | √ | √ | √ | √ | ||
| Ion exchange process (IOP) | √ | √ | ||||
| Precipitation | √ | √ | ||||
| Aeration and sedimentation | √ | |||||
| Deep bed filter | √ | √ | ||||
| API separator | √ | √ | ||||
| Hydrocyclone | √ | √ |
2.1.5. Gravity Separator/Coalescing Filter
2.2. Thermal Treatment Processes
2.2.1. Evaporation
2.2.2. C-Tour
2.2.3. Freeze-Thaw Evaporation (FTE)
3. Advanced Thermal Separation Processes
3.1. Multi-Stage Flash (MSF) Distillation
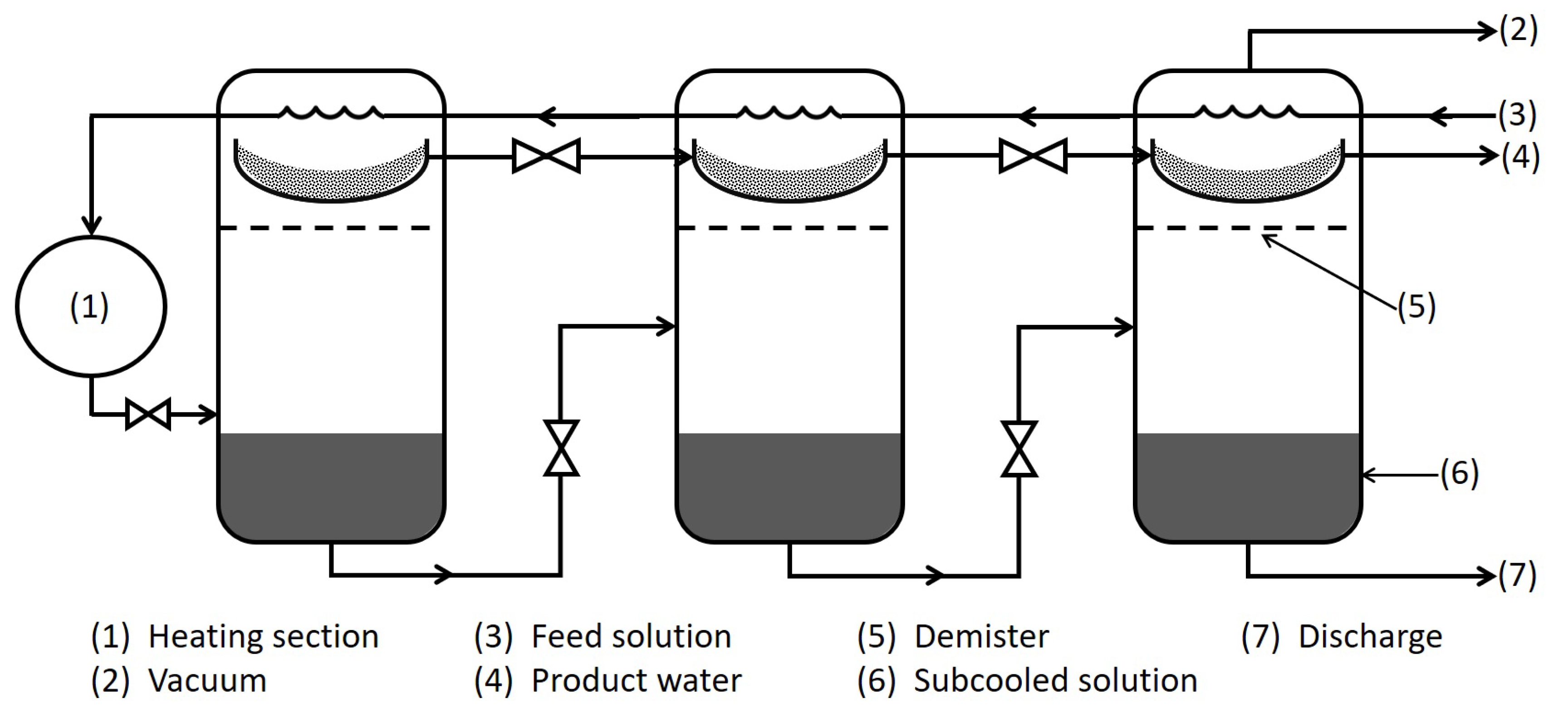
3.2. Multi-Effect Distillation (MED)
3.3. Vapor Compression Distillation (VCD)
3.4. MED–VCD Hybrid System
4. Membrane-Based Treatment Systems for Produced Water
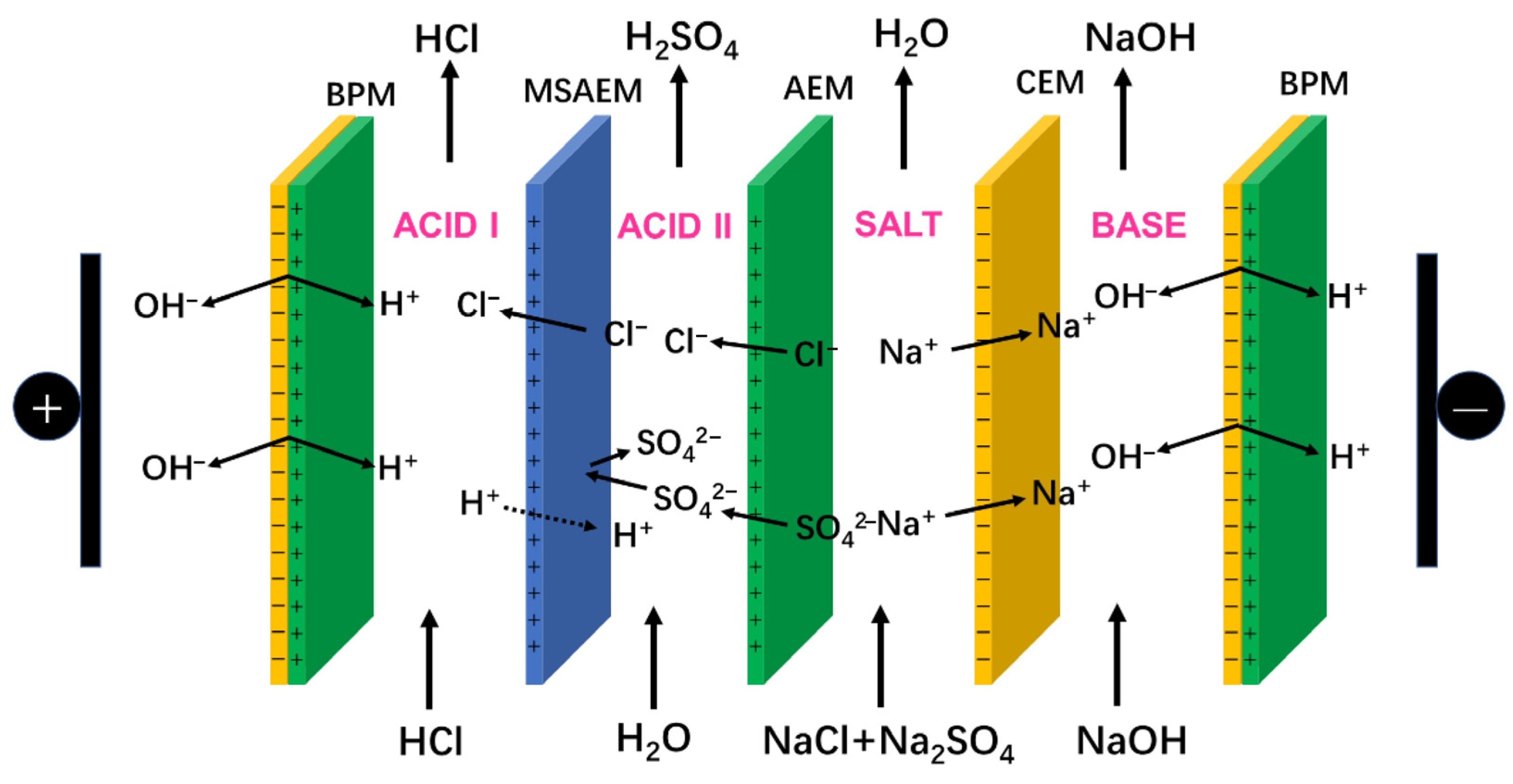
4.1. Electrodialysis (ED)
4.2. Membrane Bioreactors
5. Resource Recovery
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piccioli, M.; Aanesen, S.V.; Zhao, H.; Dudek, M.; Øye, G. Gas Flotation of Petroleum Produced Water: A Review on Status, Fundamental Aspects, and Perspectives. Energy Fuels 2020, 34, 15579–15592. [Google Scholar] [CrossRef]
- Al-salmi, M.; Laqbaqbi, M.; Al-obaidani, S.; Al-maamari, R.S. Application of Membrane Distillation for the Treatment of Oil Fi Eld Produced Water. Desalination 2020, 494, 114678. [Google Scholar] [CrossRef]
- Patel, C.V. Management of Produced Water in Oil and Gas Operations. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2005. [Google Scholar]
- Patni, H.; Ragunathan, B. Recycling and Re-Usage of Oilfield Produced Water—A Review. Mater. Today Proc. 2023, 77, 307–313. [Google Scholar] [CrossRef]
- Dayarathne, H.N.P.; Angove, M.J.; Aryal, R.; Abuel-Naga, H.; Mainali, B. Removal of Natural Organic Matter from Source Water: Review on Coagulants, Dual Coagulation, Alternative Coagulants, and Mechanisms. J. Water Process Eng. 2021, 40, 101820. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current Advances in Treatment Technologies for Removal of Emerging Contaminants from Water—A Critical Review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Nugraha, H.; Dharma, C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Hafiz, M.; Othman, D.; Rahman, M.A.; Natasha, N.; et al. A Review of Titanium Dioxide (TiO2) -Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef]
- Show, P.-L.; Thangalazhy-Gopakumar, S.; Foo, D. Sustainable Technologies for Waste Reduction and Pollutants Removals. Clean Technol. Environ. Policy 2021, 23, 1–2. [Google Scholar] [CrossRef]
- Hashim, E.; Thamer, K.; Mohammed, J.; Mirghaffari, N.; Dawood, A. Removal of Organic Pollutants from Produced Water by Batch Adsorption Treatment. Clean Technol. Environ. Policy 2022, 24, 713–720. [Google Scholar] [CrossRef]
- Kashif, A.; Rehman, R.; Fuwad, A.; Shahid, M.K.; Dayarathne, H.N.P.; Jamal, A.; Aftab, M.N.; Mainali, B.; Choi, Y. Current Advances in the Classification, Production, Properties and Applications of Microbial Biosurfactants—A Critical Review. Adv. Colloid Interface Sci. 2022, 306, 102718. [Google Scholar] [CrossRef]
- Guo, D.; Wang, H.; Fu, P.; Huang, Y.; Liu, Y.; Lv, W.; Wang, F. Diatomite Precoat Filtration for Wastewater Treatment: Filtration Performance and Pollution Mechanisms. Chem. Eng. Res. Des. 2018, 137, 403–411. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of Produced Water with Photocatalysis: Recent Advances, Affecting Factors and Future Research Prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Shen, W.; Mukherjee, D.; Koirala, N.; Hu, G.; Lee, K.; Zhao, M.; Li, J. Microbubble and Nanobubble-Based Gas Flotation for Oily Wastewater Treatment: A Review. Environ. Rev. 2022, 30, 359–379. [Google Scholar] [CrossRef]
- Shahid, M.K.; Dayarathne, H.N.P.; Mainali, B.; Lim, J.W.; Choi, Y. Ion Exchange Process for Removal of Microconstituents from Water and Wastewater. In Microconstituents in the Environment: Occurrence, Fate, Removal and Management; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 303–320. ISBN 9781119825289. [Google Scholar]
- Wu, M.; Zhai, M.; Li, X. Adsorptive Removal of Oil Drops from ASP Fl Ooding-Produced Water by Polyether Polysiloxane-Grafted ZIF-8. Powder Technol. 2021, 378, 76–84. [Google Scholar] [CrossRef]
- Shahid, M.K.; Pyo, M.; Choi, Y. Carbonate Scale Reduction in Reverse Osmosis Membrane by CO2 in Wastewater Reclamation. Membr. Water Treat. 2017, 8, 125–136. [Google Scholar] [CrossRef]
- Shahid, M.K.; Choi, Y. CO2 as an Alternative to Traditional Antiscalants in Pressure-Driven Membrane Processes: An Experimental Study of Lab-Scale Operation and Cleaning Strategies. Membranes 2022, 12, 918. [Google Scholar] [CrossRef]
- Klemz, A.C.; Weschenfelder, S.E.; de Carvalho Neto, S.L.; Damas, M.S.P.; Viviani, J.C.T.; Mazur, L.P.; Marinho, B.A.; Pereira, L.D.S.; da Silva, A.; Borges Valle, J.A.; et al. Oilfield Produced Water Treatment by Liquid-Liquid Extraction: A Review. J. Pet. Sci. Eng. 2021, 199, 108282. [Google Scholar] [CrossRef]
- Ahmadun, F.-R.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of Technologies for Oil and Gas Produced Water Treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Pichtel, J. Oil and Gas Production Wastewater: Soil Contamination and Pollution Prevention. Appl. Environ. Soil Sci. 2016, 2016, 2707989. [Google Scholar] [CrossRef]
- Tam, L.S.; Tang, T.W.; Lau, G.N.; Sharma, K.R.; Chen, G.H. A Pilot Study for Wastewater Reclamation and Reuse with MBR/RO and MF/RO Systems. Desalination 2007, 202, 106–113. [Google Scholar] [CrossRef]
- Attiogbe, F. Comparison of Membrane Bioreactor Technology and Conventional Activated Sludge System for Treating Bleached Kraft Mill Effluent. Afr. J. Environ. Sci. Technol. 2013, 7, 292–306. [Google Scholar]
- Kitanou, S.; Tahri, M.; Bachiri, B.; Mahi, M.; Hafsi, M.; Taky, M.; Elmidaoui, A. Comparative Study of Membrane Bioreactor (MBR) and Activated Sludge Processes in the Treatment of Moroccan Domestic Wastewater. Water Sci. Technol. 2018, 78, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Asante-Sackey, D.; Rathilal, S.; Tetteh, E.K.; Armah, E.K. Membrane Bioreactors for Produced Water Treatment: A Mini-Review. Membranes 2022, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.D.; Langhus, B.G.; Patel, C. Technical Summary of Oil & Gas Produced Water Treatment Technologies; All Consulting, LLC: Tulsa, OK, USA, 2005. [Google Scholar]
- Shahid, M.K.; Kim, Y.; Choi, Y.-G.G. Adsorption of Phosphate on Magnetite-Enriched Particles (MEP) Separated from the Mill Scale. Front. Environ. Sci. Eng. 2019, 13, 71. [Google Scholar] [CrossRef]
- Nonato, T.C.M.; Alves, A.A.D.A.; Sens, M.L.; Dalsasso, R.L. Produced Water from Oil—A Review of the Main Treatment Technologies. J. Environ. Chem. Toxicol. 2018, 2, 23–27. [Google Scholar]
- Apul, O.G.; Karanfil, T. Adsorption of Synthetic Organic Contaminants by Carbon Nanotubes: A Critical Review. Water Res. 2015, 68, 34–55. [Google Scholar] [CrossRef]
- Drewes, J.; Cath, T.; Xu, P.; Graydon, J. An Integrated Framework for Treatment and Management of Produced Water: Technical Assessment of Produced Water Treatment Technologies; Colorado School of Mines: Golden, CO, USA, 2009; pp. 1–157. [Google Scholar]
- Sharafi, M.M.; Bazgir, S.; Tamizifar, M.; Nemati, A. Adsorption of Petroleum Hydrocarbons on Organoclay. J. Appl. Chem. Res. 2010, 4, 19–23. [Google Scholar]
- Feng, C.; Khulbe, K.C.; Matsuura, T.; Farnood, R.; Ismail, A.F. Recent Progress in Zeolite/Zeotype Membranes. J. Membr. Sci. Res. 2015, 1, 49–72. [Google Scholar]
- Tajar, A.F.; Kaghazchi, T.; Soleimani, M. Adsorption of Cadmium from Aqueous Solutions on Sulfurized Activated Carbon Prepared from Nut Shells. J. Hazard. Mater. 2009, 165, 1159–1164. [Google Scholar] [CrossRef]
- Meidanchi, A.; Akhavan, O. Superparamagnetic Zinc Ferrite Spinel–Graphene Nanostructures for Fast Wastewater Purification. Carbon 2014, 69, 230–238. [Google Scholar] [CrossRef]
- Shahid, M.K.; Choi, Y. Characterization and Application of Magnetite Particles, Synthesized by Reverse Coprecipitation Method in Open Air from Mill Scale. J. Magn. Magn. Mater. 2020, 495, 165823. [Google Scholar] [CrossRef]
- Yong, J.; Huo, J.; Chen, F.; Yang, Q.; Hou, X. Oil/Water Separation Based on Natural Materials with Super-Wettability: Recent Advances. Phys. Chem. Chem. Phys. 2018, 20, 25140–25163. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.H.; Sabri, M.A.; Khamis, M.I.; Elsayed, Y.A.; Sara, Z.; Hafez, B. Produced Water Treatment Using Olive Leaves. Desalin. Water Treat. 2017, 60, 129–136. [Google Scholar] [CrossRef]
- Simões, A.; Macêdo-Júnior, R.; Santos, B.; Silva, L.; Silva, D.; Ruzene, D. Produced Water: An Overview of Treatment Technologies. Int. J. Innov. Educ. Res 2020, 8, 207–224. [Google Scholar] [CrossRef]
- Shi, M.; Guo, C.; Li, J.; Li, J.; Zhang, L.; Wang, X.; Ju, Y.; Zheng, J.; Li, X. Removal of Bromide from Water by Adsorption on Nanostructured δ-Bi2O3. J. Nanosci. Nanotechnol. 2017, 17, 6951–6956. [Google Scholar] [CrossRef]
- Akhlamadi, G.; Goharshadi, E.K. Sustainable and Superhydrophobic Cellulose Nanocrystal-Based Aerogel Derived from Waste Tissue Paper as a Sorbent for Efficient Oil/Water Separation. Process Saf. Environ. Prot. 2021, 154, 155–167. [Google Scholar] [CrossRef]
- Azad, P.; Raut, S.; Vaish, R. Candle Soot-Coated Egg Carton Material for Oil Water Separation and Detergent Adsorption. Bull. Mater. Sci. 2019, 43, 7. [Google Scholar] [CrossRef]
- Yu, C.; Lin, W.; Jiang, J.; Jing, Z.; Hong, P.; Li, Y. Preparation of a Porous Superhydrophobic Foam from Waste Plastic and Its Application for Oil Spill Cleanup. RSC Adv. 2019, 9, 37759–37767. [Google Scholar] [CrossRef]
- Jaji, K.T. Treatment of Oilfield Produced Water with Dissolved Air Floatation. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, 2012. [Google Scholar]
- Yeganeh, M.M.; Kaghazchi, T.; Soleimani, M. Effect of Raw Materials on Properties of Activated Carbons. Chem. Eng. Technol. 2006, 29, 1247–1251. [Google Scholar] [CrossRef]
- Mehrabi, N.; Soleimani, M.; Yeganeh, M.M.; Sharififard, H. Parameter Optimization for Nitrate Removal from Water Using Activated Carbon and Composite of Activated Carbon and Fe2O3 Nanoparticles. RSC Adv. 2015, 5, 51470–51482. [Google Scholar] [CrossRef]
- Doyle, D.H.; Brown, A.B. Produced Water Treatment and Hydrocarbon Removal with Organoclay. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 1–4 October 2000; OnePetro: Richardson, TX, USA, 2000. [Google Scholar]
- Plebon, M.J.; Saad, M.; Fraser, S. Further Advances in Produced Ater De-Oiling Utilizing a Technology That Removes and Recovers Dispersed Oil in Produced Water 2 Micron and Larger. In Proceedings of the 12th International Petroleum Environmental Conference, Houston, TX, USA, 8–11 November 2005; Citeseer: Princeton, NJ, USA, 2005; pp. 8–11. [Google Scholar]
- Chasib, M.I.; Qasim, R.F. Designing and Studying Operational Parameters of Hydrocyclone for Oil-Water Separation. Assoc. Arab Univ. J. Eng. Sci. 2019, 26, 41–51. [Google Scholar] [CrossRef]
- Igunnu, E.T.; Chen, G.Z. Produced Water Treatment Technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef]
- Nasiri, M.; Jafari, I. Produced Water from Oil-Gas Plants: A Short Review on Challenges and Opportunities. Period. Polytech. Chem. Eng. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Souza, J.S.; Paiva, M.K.N.; Farias, F.P.M.; Neto, S.R.F.; Lima, A.G.B. Hydrocyclone Applications in Produced Water: A Steady-State Numerical Analysis. Braz. J. Pet. Gas 2012, 6, 133–143. [Google Scholar] [CrossRef]
- Al-Kayiem, H.H.; Osei, H.; Hashim, F.M.; Hamza, J.E. Flow Structures and Their Impact on Single and Dual Inlets Hydrocyclone Performance for Oil-Water Separation. J. Pet. Explor. Prod. Technol. 2019, 9, 2943–2952. [Google Scholar] [CrossRef]
- Ku Ishak, K.E.H.; Abdalla Ayoub, M. Performance of Liquid-Liquid Hydrocyclone (LLHC) for Treating Produced Water from Surfactant Flooding Produced Water. World J. Eng. 2020, 17, 215–222. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Li, Y.; Xu, H.; Pan, Z.; Dai, P.; Wang, H.; Yang, Q. A Review of Treatment Technologies for Produced Water in Offshore Oil and Gas Fields. Sci. Total Environ. 2021, 775, 145485. [Google Scholar] [CrossRef]
- Geankoplis, C.J. Transport Processes and Separation Process Principles: Includes Unit Operations; Prentice Hall Professional Technical Reference: Hoboken, NJ, USA, 2003; ISBN 9780131013674. [Google Scholar]
- Ramadan, M. Efficiency of New Miswak, Titanium Dioxide and Sand Filters in Reducing Pollutants from Wastewater. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 47–51. [Google Scholar] [CrossRef][Green Version]
- Simate, G.S. The Treatment of Brewery Wastewater for Reuse by Integration of Coagulation/Flocculation and Sedimentation with Carbon Nanotubes ‘Sandwiched’ in a Granular Filter Bed. J. Ind. Eng. Chem. 2015, 21, 1277–1285. [Google Scholar] [CrossRef]
- Grace, M.A.; Healy, M.G.; Clifford, E. Performance and Surface Clogging in Intermittently Loaded and Slow Sand Filters Containing Novel Media. J. Environ. Manag. 2016, 180, 102–110. [Google Scholar] [CrossRef]
- Keller, J.; Bliesner, R.D. Sprinkle and Trickle Irrigation; Springer: Berlin/Heidelberg, Germany, 1990; Volume 3. [Google Scholar]
- Zheng, X.; Mehrez, R.; Jekel, M.; Ernst, M. Effect of Slow Sand Filtration of Treated Wastewater as Pre-Treatment to UF. Desalination 2009, 249, 591–595. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, G.; Deng, L.; Liu, Y.; Yang, H.; Wang, L.; Song, L.; Pu, X.; Wang, Z.; Zhang, Y. Startup Strategy for Partial Nitritation Treatment of Anaerobically Digested Effluent of Swine Wastewater in a Sand Filter. Ecol. Eng. 2016, 93, 13–17. [Google Scholar] [CrossRef]
- Cha, Z.; Lin, C.-F.; Cheng, C.-J.; Hong, P.K.A. Removal of Oil and Oil Sheen from Produced Water by Pressure-Assisted Ozonation and Sand Filtration. Chemosphere 2010, 78, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, R.T.; Beni, A.H.; Henni, A. State of the Art Treatment of Produced Water. In Water Treatment; IntechOpen: London, UK, 2013; Volume 199. [Google Scholar]
- Thoma, G.J.; Bowen, M.L.; Hollensworth, D. Dissolved Air Precipitation/Solvent Sublation for Oil-Field Produced Water Treatment. Sep. Purif. Technol. 1999, 16, 101–107. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Zhang, X.; Tang, J.; Wei, B.; Liu, J. Studies on the Mechanism of Indigo Carmine Removal by Solvent Sublation. J. Colloid Interface Sci. 2005, 292, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Bayati, F.; Shayegan, J.; Noorjahan, A. Treatment of Oilfield Produced Water by Dissolved Air Precipitation/Solvent Sublation. J. Pet. Sci. Eng. 2011, 80, 26–31. [Google Scholar] [CrossRef]
- Dawoud, H.D.; Saleem, H.; Alnuaimi, N.A.; Zaidi, S.J. Characterization and Treatment Technologies Applied for Produced Water in Qatar. Water 2021, 13, 3573. [Google Scholar] [CrossRef]
- Multon, L.M.; Viraraghavan, T. Removal of Oil from Produced Water by Coalescence/Filtration in a Granular Bed. Environ. Technol. 2006, 27, 529–544. [Google Scholar] [CrossRef]
- Shirazi, M.J.A.; Bazgir, S.; Shirazi, M.M.A. Edible Oil Mill Effluent; a Low-Cost Source for Economizing Biodiesel Production: Electrospun Nanofibrous Coalescing Filtration Approach. Biofuel Res. J. 2014, 1, 39–42. [Google Scholar] [CrossRef]
- Shirazi, M.J.A.; Bazgir, S.; Shirazi, M.M.A.; Ramakrishna, S. Coalescing Filtration of Oily Wastewaters: Characterization and Application of Thermal Treated, Electrospun Polystyrene Filters. Desalin. Water Treat. 2013, 51, 5974–5986. [Google Scholar] [CrossRef]
- Rommel, W.; Blass, E.; Meon, W. Plate Separators for Dispersed Liquid-Liquid Systems: The Role of Partial Coalescence. Chem. Eng. Sci. 1993, 48, 1735–1743. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, J.; Jiang, J.; Peng, H.; Luo, Y.; Zhang, L. Numerical Simulation and Experimental Study of a Multistage Multiphase Separation System. Separations 2022, 9, 405. [Google Scholar] [CrossRef]
- Heins, W.; Peterson, D. Use of Evaporation for Heavy Oil Produced Water Treatment. J. Can. Pet. Technol. 2005, 44, PETSOC-05-01-01. [Google Scholar] [CrossRef]
- Lefebvre, O.; Moletta, R. Treatment of Organic Pollution in Industrial Saline Wastewater: A Literature Review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef]
- Becker, R.F. Produced and Process Water Recycling Using Two Highly Efficient Systems to Make Distilled Water. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, Texas, USA, 1–4 October 2000; OnePetro: Richardson, TX, USA, 2000. [Google Scholar]
- Velmurugan, V.; Srithar, K. Prospects and Scopes of Solar Pond: A Detailed Review. Renew. Sustain. Energy Rev. 2008, 12, 2253–2263. [Google Scholar] [CrossRef]
- Grini, P.G.; Hjeldsvold, M.; Johnsen, S. Choosing Produced Water Treatment Technologies Based on Environmental Impact Reduction. In Proceedings of the HSE Conference, Kuala Lumpur, Malaysia, 22 March 2002; Society of Petroleum Engineers: Richardson, TX, USA, 2002. [Google Scholar]
- Descousse, A.; Mönig, K.; Voldum, K. Evaluation Study of Various Produced-Water Treatment Technologies to Remove Dissolved Aromatic Components. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004; OnePetro: Richardson, TX, USA, 2004. [Google Scholar]
- Ekins, P.; Vanner, R.; Firebrace, J. Zero Emissions of Oil in Water from Offshore Oil and Gas Installations: Economic and Environmental Implications. J. Clean. Prod. 2007, 15, 1302–1315. [Google Scholar] [CrossRef]
- Knudsen, B.L.; Hjelsvold, M.; Frost, T.K.; Svarstad, M.B.E.; Grini, P.G.; Willumsen, C.F.; Torvik, H. Meeting the Zero Discharge Challenge for Produced Water. In Proceedings of the SPE International Conference on Health, Safety, and Environment in Oil and Gas Exploration and Production, Houston, TX, USA, 26–29 September 2004; OnePetro: Richardson, TX, USA, 2004. [Google Scholar]
- Sorensen, J.A.; Boysen, J.; Boysen, D.; Larson, T. Field Application of the Freeze/Thaw Evaporation (FTE®) Process for the Treatment of Natural Gas Produced Water in Wyoming; National Technical Information Service: Springfield, VA, USA, 2002. [Google Scholar]
- Boysen, J.E.; Harju, J.A.; Shaw, B.; Fosdick, M.; Grisanti, A.; Sorensen, J.A. The Current Status of Commercial Deployment of the Freeze Thaw Evaporation Treatment of Produced Water. In Proceedings of the SPE/EPA Exploration and Production Environmental Conference, Austin, TX, USA, 1–3 March 1999; OnePetro: Richardson, TX, USA, 1999. [Google Scholar]
- Boysen, J.; Boysen, D. The Freeze-Thaw/Evaporation (FTE) Process for Produced Water Treatment, Disposal and Beneficial Uses. In Proceedings of the 14th Annual International Petroleum Environmental Conference, Houston, TX, USA, 5–9 November 2007; Volume 5. [Google Scholar]
- Hu, G.; Li, J.; Hou, H. A Combination of Solvent Extraction and Freeze Thaw for Oil Recovery from Petroleum Refinery Wastewater Treatment Pond Sludge. J. Hazard. Mater. 2015, 283, 832–840. [Google Scholar] [CrossRef]
- Kargari, A.; Shirazi, M.M.A. Water Desalination: Solar-Assisted Membrane Distillation. In Encyclopedia of Energy Engineering and Technology-Four Volume Set (Print); CRC Press: Boca Raton, FL, USA, 2014; pp. 2095–2109. [Google Scholar]
- Hamed, O.A. Evolutionary Developments of Thermal Desalination Plants in the Arab Gulf Region. In Proceedings of the 2004 Beirut Conference, Beirut, Lebanon, 21–23 September 2004. [Google Scholar]
- Khawaji, A.D.; Kutubkhanah, I.K.; Wie, J.-M. Advances in Seawater Desalination Technologies. Desalination 2008, 221, 47–69. [Google Scholar] [CrossRef]
- Ettouney, H.M.; El-Dessouky, H.T.; Faibish, R.S.; Gowin, P.J. Evaluating the Economics of Desalination. Chem. Eng. Prog. 2002, 98, 32–39. [Google Scholar]
- Darwish, M.A.; Al Asfour, F.; Al-Najem, N. Energy Consumption in Equivalent Work by Different Desalting Methods: Case Study for Kuwait. Desalination 2003, 152, 83–92. [Google Scholar] [CrossRef]
- Riley, J.D.; Johnson, D.L. System for Decontaminating Water and Generating Water Vapor. Canada Patent CA2726911C, 17 May 2017. [Google Scholar]
- Katz Water Tech, LLC. Apparatus System and Method to Extract Minerals and Metals from Water. U.S. Patent US11034605B2, 15 June 2021.
- Karagiannis, I.C.; Soldatos, P.G. Water Desalination Cost Literature: Review and Assessment. Desalination 2008, 223, 448–456. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, Y.; Wang, J.; Wang, D. Feasibility Study of Multi-Effect Distillation Dealing with High-Salinity Organic RO Concentrates: Experiment and Theoretical Analysis. Desalination 2021, 505, 115007. [Google Scholar] [CrossRef]
- Norouzi, M.; Rashidi, F.; Noorollahi, Y.; Qom, H.F. CuO/Water and Al2O3/Water Nanofluids as Working Fluid in an Abandoned Oil Well to Improve Thermal Performance in the Seawater Desalination Process. J. Taiwan Inst. Chem. Eng. 2023, 144, 104754. [Google Scholar] [CrossRef]
- Onishi, V.C.; Carrero-Parreño, A.; Reyes-Labarta, J.A.; Fraga, E.S.; Caballero, J.A. Desalination of Shale Gas Produced Water: A Rigorous Design Approach for Zero-Liquid Discharge Evaporation Systems. J. Clean. Prod. 2017, 140, 1399–1414. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the Art of Produced Water Treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef]
- McGhee, T.J. Treatment of Brackish and Saline Waters. In Water Supply Sewerage, 6th ed.; McGraw-Hill Inc.: New York, NY, USA, 1991. [Google Scholar]
- Heins, W.F.; McNeill, R.; Albion, S. World’s First SAGD Facility Using Evaporators, Drum Boilers, and Zero Discharge Crystallizers to Treat Produced Water. J. Can. Pet. Technol. 2006, 45. [Google Scholar] [CrossRef]
- Heins, W.F.; McNeill, R. Vertical-Tube Evaporator System Provides SAGD-Quality Feed Water. World Oil 2007, 228, 135–144. [Google Scholar]
- Tamunokuro, K.; Ramirez, A.; Molinari, M. Review of Oilfield Produced Water Treatment Technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Rout, P.R.; Aslam, M.; Fuwad, A.; Choi, Y.; Park, J.H.; Kumar, G. A Brief Review of Anaerobic Membrane Bioreactors Emphasizing Recent Advancements, Fouling Issues and Future Perspectives. J. Environ. Manag. 2020, 270, 110909. [Google Scholar] [CrossRef]
- Bolto, B.; Zhang, J.; Wu, X.; Xie, Z. A Review on Current Development of Membranes for Oil Removal from Wastewaters. Membranes 2020, 10, 65. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent Advances on the Treatment Technology of Oil and Gas Produced Water for Sustainable Energy Industry-Mechanistic Aspects and Process Chemistry Perspectives. Chem. Eng. J. Adv. 2020, 4, 100049. [Google Scholar] [CrossRef]
- Fakhraee, M.; Akhavan, O. Ultrahigh Permeable C2N-Inspired Graphene Nanomesh Membranes versus Highly Strained C2N for Reverse Osmosis Desalination. J. Phys. Chem. B 2019, 123, 8740–8752. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, E.K.; Rathilal, S.; Asante-Sackey, D.; Chollom, M.N. Prospects of Synthesized Magnetic TiO2-Based Membranes for Wastewater Treatment: A Review. Materials 2021, 14, 3524. [Google Scholar] [CrossRef] [PubMed]
- Rahighi, R.; Hosseini-Hosseinabad, S.M.; Zeraati, A.S.; Suwaileh, W.; Norouzi, A.; Panahi, M.; Gholipour, S.; Karaman, C.; Akhavan, O.; Khollari, M.A.R.; et al. Two-Dimensional Materials in Enhancement of Membrane-Based Lithium Recovery from Metallic-Ions-Rich Wastewaters: A Review. Desalination 2022, 543, 116096. [Google Scholar] [CrossRef]
- Siagian, U.W.R.; Widodo, S.; Wardani, A.K.; Wenten, I.G. Oilfield Produced Water Reuse and Reinjection with Membrane. In Proceedings of the MATEC Web of Conferences, Semarang, Indonesia, 15–16 November 2017; EDP Sciences: Les Ulis, France, 2018; Volume 156, p. 8005. [Google Scholar]
- Shahid, M.K.; Mainali, B.; Rout, P.R.; Lim, J.W.; Aslam, M.; Al-Rawajfeh, A.E.; Choi, Y. A Review of Membrane-Based Desalination Systems Powered by Renewable Energy Sources. Water 2023, 15, 534. [Google Scholar] [CrossRef]
- Salas, B.V.; Wiener, M.S. Desalination, Trends and Technologies. Desalin. Water Treat. 2012, 42, 347–348. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.; Zhou, Y.; Irfan, M.; Wang, Y.; Jiang, C.; Xu, T. In-Situ Combination of Bipolar Membrane Electrodialysis with Monovalent Selective Anion-Exchange Membrane for the Valorization of Mixed Salts into Relatively High-Purity Monoprotic and Diprotic Acids. Membranes 2020, 10, 135. [Google Scholar] [CrossRef]
- Dallbauman, L.; Sirivedhin, T. Reclamation of Produced Water for Beneficial Use. Sep. Sci. Technol. 2005, 40, 185–200. [Google Scholar] [CrossRef]
- Sirivedhin, T.; McCue, J.; Dallbauman, L. Reclaiming Produced Water for Beneficial Use: Salt Removal by Electrodialysis. J. Membr. Sci. 2004, 243, 335–343. [Google Scholar] [CrossRef]
- Eddy, M.; Abu-Orf, M.; Bowden, G.; Burton, F.L.; Pfrang, W.; Stensel, H.D.; Tchobanoglous, G.; Tsuchihashi, R.; Firm, A. Wastewater Engineering: Treatment and Resource Recovery; McGraw Hill Education: New York, NY, USA, 2014; ISBN 1259010791. [Google Scholar]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Olsson, G. Application of Membrane Bioreactor Technology in Treating High Strength Industrial Wastewater: A Performance Review. Desalination 2012, 305, 1–11. [Google Scholar] [CrossRef]
- Melin, T.; Jefferson, B.; Bixio, D.; Thoeye, C.; De Wilde, W.; De Koning, J.; van der Graaf, J.; Wintgens, T. Membrane Bioreactor Technology for Wastewater Treatment and Reuse. Desalination 2006, 187, 271–282. [Google Scholar] [CrossRef]
- Frank, V.B.; Regnery, J.; Chan, K.E.; Ramey, D.F.; Spear, J.R.; Cath, T.Y. Co-Treatment of Residential and Oil and Gas Production Wastewater with a Hybrid Sequencing Batch Reactor-Membrane Bioreactor Process. J. Water Process Eng. 2017, 17, 82–94. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abidin, Z.Z.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S. Application of Membrane-Coupled Sequencing Batch Reactor for Oilfield Produced Water Recycle and Beneficial Re-Use. Bioresour. Technol. 2010, 101, 6942–6949. [Google Scholar] [CrossRef] [PubMed]
- Pendashteh, A.R.; Abdullah, L.C.; Fakhru’l-Razi, A.; Madaeni, S.S.; Abidin, Z.Z.; Biak, D.R.A. Evaluation of Membrane Bioreactor for Hypersaline Oily Wastewater Treatment. Process Saf. Environ. Prot. 2012, 90, 45–55. [Google Scholar] [CrossRef]
- Sharghi, E.A.; Bonakdarpour, B.; Pakzadeh, M. Treatment of Hypersaline Produced Water Employing a Moderately Halophilic Bacterial Consortium in a Membrane Bioreactor: Effect of Salt Concentration on Organic Removal Performance, Mixed Liquor Characteristics and Membrane Fouling. Bioresour. Technol. 2014, 164, 203–213. [Google Scholar] [CrossRef]
- Sharghi, E.A.; Bonakdarpour, B.; Roustazade, P.; Amoozegar, M.A.; Rabbani, A.R. The Biological Treatment of High Salinity Synthetic Oilfield Produced Water in a Submerged Membrane Bioreactor Using a Halophilic Bacterial Consortium. J. Chem. Technol. Biotechnol. 2013, 88, 2016–2026. [Google Scholar] [CrossRef]
- Fulazzaky, M.; Setiadi, T.; Fulazzaky, M.A. An Evaluation of the Oilfield-Produced Water Treatment by the Membrane Bioreactor. J. Environ. Chem. Eng. 2020, 8, 104417. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Lin, H.; Zhao, L.; Shen, L.; Li, R.; Xu, Y.; Hong, H.; He, Y. Membrane Fouling Caused by Biological Foams in a Submerged Membrane Bioreactor: Mechanism Insights. Water Res. 2020, 181, 115932. [Google Scholar] [CrossRef]
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.M.; Boels, L.; Lammertink, R.G.H.; de Vos, W.M. Produced Water Treatment by Membranes: A Review from a Colloidal Perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ahmad, R.; Yasin, M.; Khan, A.L.; Shahid, M.K.; Hossain, S.; Khan, Z.; Jamil, F.; Rafiq, S.; Bilad, M.R.; et al. Anaerobic Membrane Bioreactors for Biohydrogen Production: Recent Developments, Challenges and Perspectives. Bioresour. Technol. 2018, 269, 452–464. [Google Scholar] [CrossRef]
- Amin, N.; Aslam, M.; Yasin, M.; Hossain, S.; Shahid, M.K.; Inayat, A.; Samir, A.; Ahmad, R.; Murshed, M.N.; Khurram, M.S. Municipal Solid Waste Treatment for Bioenergy and Resource Production: Potential Technologies, Techno-Economic-Environmental Aspects and Implications of Membrane-Based Recovery. Chemosphere 2023, 323, 138196. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Truong, M.V.; Nguyen, A.Q.; Johir, M.A.H.; Commault, A.S.; Ralph, P.J.; Semblante, G.U.; Nghiem, L.D. A Sequential Membrane Bioreactor Followed by a Membrane Microalgal Reactor for Nutrient Removal and Algal Biomass Production. Environ. Sci. Water Res. Technol. 2020, 6, 189–196. [Google Scholar] [CrossRef]
- Miranda, M.A.; Ghosh, A.; Mahmodi, G.; Xie, S.; Shaw, M.; Kim, S.; Krzmarzick, M.J.; Lampert, D.J.; Aichele, C.P. Treatment and Recovery of High-Value Elements from Produced Water. Water 2022, 14, 880. [Google Scholar] [CrossRef]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium Market Research–Global Supply, Future Demand and Price Development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Swain, B. Recovery and Recycling of Lithium: A Review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Jiang, W.; Lin, L.; Xu, X.; Wang, H.; Xu, P. Analysis of Regulatory Framework for Produced Water Management and Reuse in Major Oil- and Gas-Producing Regions in the United States. Water 2022, 14, 2162. [Google Scholar] [CrossRef]
- Zhong, C.; Zolfaghari, A.; Hou, D.; Goss, G.G.; Lanoil, B.D.; Gehman, J.; Tsang, D.C.W.; He, Y.; Alessi, D.S. Comparison of the Hydraulic Fracturing Water Cycle in China and North America: A Critical Review. Environ. Sci. Technol. 2021, 55, 7167–7185. [Google Scholar] [CrossRef]
- Danforth, C.; Chiu, W.A.; Rusyn, I.; Schultz, K.; Bolden, A.; Kwiatkowski, C.; Craft, E. An Integrative Method for Identification and Prioritization of Constituents of Concern in Produced Water from Onshore Oil and Gas Extraction. Environ. Int. 2020, 134, 105280. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.; Nawaz, M.H.; Rout, P.R.; Lim, J.-W.; Mainali, B.; Shahid, M.K. Advances in Produced Water Treatment Technologies: An In-Depth Exploration with an Emphasis on Membrane-Based Systems and Future Perspectives. Water 2023, 15, 2980. https://doi.org/10.3390/w15162980
Ibrahim M, Nawaz MH, Rout PR, Lim J-W, Mainali B, Shahid MK. Advances in Produced Water Treatment Technologies: An In-Depth Exploration with an Emphasis on Membrane-Based Systems and Future Perspectives. Water. 2023; 15(16):2980. https://doi.org/10.3390/w15162980
Chicago/Turabian StyleIbrahim, Muhammad, Muhammad Haq Nawaz, Prangya Ranjan Rout, Jun-Wei Lim, Bandita Mainali, and Muhammad Kashif Shahid. 2023. "Advances in Produced Water Treatment Technologies: An In-Depth Exploration with an Emphasis on Membrane-Based Systems and Future Perspectives" Water 15, no. 16: 2980. https://doi.org/10.3390/w15162980
APA StyleIbrahim, M., Nawaz, M. H., Rout, P. R., Lim, J.-W., Mainali, B., & Shahid, M. K. (2023). Advances in Produced Water Treatment Technologies: An In-Depth Exploration with an Emphasis on Membrane-Based Systems and Future Perspectives. Water, 15(16), 2980. https://doi.org/10.3390/w15162980










