Unveiling the Effects of Fennel (Foeniculum vulgare) Seed Essential Oil as a Diet Supplement on the Biochemical Parameters and Reproductive Function in Female Common Carps (Cyprinus carpio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparing Fennel Essential Oil and Determining the Amount of Effective Substance
2.2. Rearing Condition and Fish Feed
2.3. Design and Procedure
2.4. Sampling
2.5. Plasma Biochemical Indices
2.6. Oxidative Biomarkers
2.7. Measurement of Sexual Hormones
2.8. Statistical Analysis
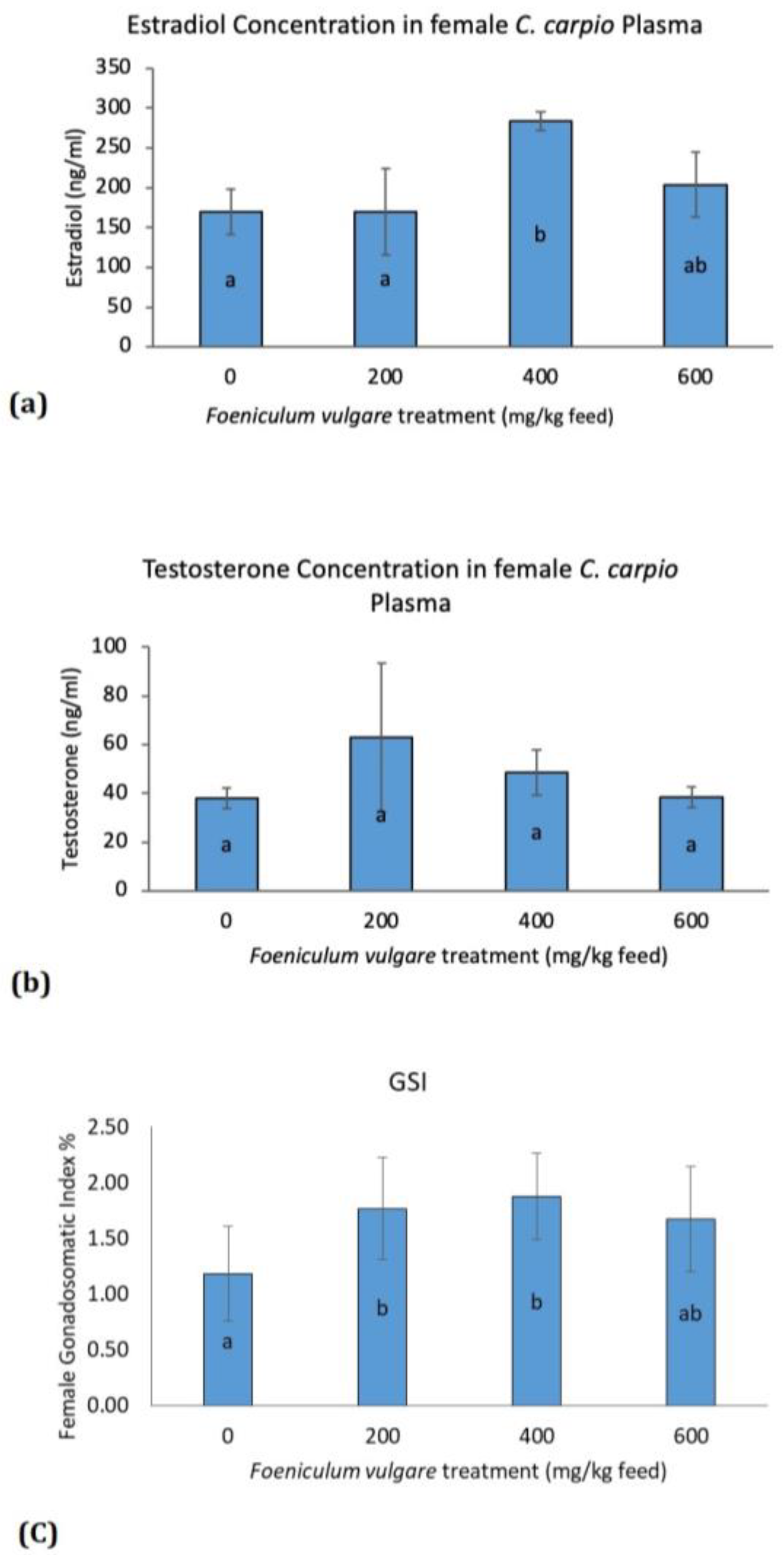
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bhat, R.A.; Saoca, C.; Cravana, C.; Fazio, F.; Guerrera, M.C.; Labh, S.N.; Kesbiç, O.S. Effects of heavy pollution in different water bodies on male rainbow trout (Oncorhynchus mykiss) reproductive health. Environ. Sci. Pollut. Res. 2023, 30, 23467–23479. [Google Scholar] [CrossRef] [PubMed]
- Chaube, R. An update on induced breeding methods in fish aquaculture and scope for new potential techniques. Front. Aquac. Biotechnol. 2023, 5, 55–68. [Google Scholar]
- Lange, A.; Katsu, Y.; Miyagawa, S.; Ogino, Y.; Urushitani, H.; Kobayashi, T.; Iguchi, T. Comparative responsiveness to natural and synthetic estrogens of fish species commonly used in the laboratory and field monitoring. Aquat. Toxicol. 2012, 109, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Clotfelter, E.D.; Rodriguez, A.C. Behavioral changes in fish exposed to phytoestrogens. Environ. Pollut. 2006, 144, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, G.; Mahboub, H.H.; Fahim, A.; Hefny, A.A.; Prokić, M.D.; Rainis, S.; Boldaji, J.T.; Faggio, C. Mooseer (Allium hirtifolium) boosts growth, general health status, and resistance of rainbow trout (Oncorhynchus mykiss) against Streptococcus iniae infection. Fish Shellfish Immunol. 2022, 120, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Jourdehi, A.Y.; Sudagar, M.; Bahmani, M.; Hosseini, S.A.; Dehghani, A.A.; Yazdani, M.A. Reproductive effects of dietary soy phytoestrogens, genistein and equol on farmed female beluga, Huso huso. Iran. J. Vet. Res. 2014, 15, 266–271. [Google Scholar]
- Naji, T.; Hossenzadeh Sahafi, H.; Saffari, M. The effects of phytoestrogens Matricaria recutita on growth, maturation of oocytes in the three spot gourami (Trichogaster trichopterus). Iran. Sci. Fish. J. 2014, 23, 85–94. [Google Scholar]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Muhammad, N.P.; Nirmal, T.; Prabhakaran, A.; Varghese, T. Phytoestrogens as Endocrine-Disrupting Agents in Aquaculture. In Xenobiotics in Aquatic Animals: Reproductive and Developmental Impacts; Rather, M.A., Amin, A., Hajam, Y.A., Jamwal, A., Ahmad, I., Eds.; Springer Nature: Singapore, 2023; pp. 213–231. [Google Scholar]
- Haji Begloo, A.; Aalaie, K.; Paknezhad, H.; Azizinezhad, F. A review of the use of plant compounds and haber phytoestrogens on reproductive sex reproduction and aquaculture. J. Ornam. Aquat. 2022, 9, 53–60. [Google Scholar]
- Nakamura, M.; Bhandari, R.K.; Higa, M. The role estrogens play in sex differentiation and sex changes of fish. Fish Physiol. Biochem. 2003, 28, 113–117. [Google Scholar] [CrossRef]
- Khalaj, H.; Labbafi, H.A.; Hasan, A.T.; Shaghaghi, J.; Hajiaghaee, R. A review on the botanical, ecological, agronomical and pharmacological properties of the fennel (Foeniculum vulgare Mill.). J. Med. Plants 2019, 18, 1–15. [Google Scholar]
- He, G.; Sun, H.; Liao, R.; Wei, Y.; Zhang, T.; Chen, Y.; Lin, S. Effects of herbal extracts (Foeniculum vulgare and Artemisia annua) on growth, liver antioxidant capacity, intestinal morphology and microorganism of juvenile largemouth bass, Micropterus salmoides. Aquac. Rep. 2022, 23, 101081. [Google Scholar] [CrossRef]
- Malini, T.; Vanithakumari, G.; Megala, N.; Anusya, S.; Devi, K.; Elango, V. Effect of Foeniculuai vulgare mill seed extract on the genital organs of male and female rats. Indian J. Physiol. Pharmacol. 1985, 29, 22–26. [Google Scholar]
- Aliakbari, F.; Mirsadeghi, M.N.; Hashemi, E.; Rahimi-Madiseh, M.; Mohammadi, B. Effects of combination therapy with Bunium persicum and Foeniculum vulgare extracts on patients with polycystic ovary syndrome. Adv. Biomed. Res. 2022, 11, 74. [Google Scholar] [PubMed]
- Sotoudeh, A.; Yeganeh, S. Effects of supplementary fennel (Foeniculum vulgare) essential oil in diet on growth and reproductive performance of the ornamental fish, Convict cichlid (Cichlasoma nigrofasciatum). Aquac. Res. 2017, 48, 4284–4291. [Google Scholar] [CrossRef]
- Nazari, A.; Roozbehani, S. Influence of fennel Foeniculum vulgar extract on fertility, growth rate and histology of 443 gonads on guppy Poecilia reticulata. Turk. J. Fish. Aquat. Sci. 2015, 15, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Dawood, M.A.; Gholamhosseini, A.; Sakhaie, F.; Banaee, M. Effect of the extract of lemon verbena (Aloysia citrodora) on the growth performance, digestive enzyme activities, and immune-related genes in Siberian sturgeon (Acipenser baerii). Aquaculture 2021, 541, 736797. [Google Scholar] [CrossRef]
- Banaee, M.; Impellitteri, F.; Evaz-Zadeh Samani, H.; Piccione, G.; Faggio, C. Dietary Arthrospira platensis in Rainbow Trout (Oncorhynchus mykiss): A Means to Reduce Threats Caused by CdCl2 Exposure? Toxics 2022, 10, 731. [Google Scholar] [CrossRef]
- Ekun, O.A.; Ogunyemi, G.A.; Azenabor, A.; Akinloye, O. A comparative analysis of glucose oxidase method and three point-of-care measuring devices for glucose determination. Ife J. Sci. 2018, 20, 43–49. [Google Scholar] [CrossRef]
- Banaee, M.; Sureda, A.; Faggio, C. Protective effect of protexin concentrate in reducing the toxicity of chlorpyrifos in common carp (Cyprinus carpio). Environ. Toxicol. Pharmacol. 2022, 94, 103918. [Google Scholar] [CrossRef]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Gholamhosseini, A.; Banaee, M.; Sureda, A.; Timar, N.; Zeidi, A.; Faggio, C. Physiological response of freshwater crayfish, Astacus leptodactylus exposed to polyethylene microplastics at different temperature. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 267, 109581. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Kubitza, F.; Salem, S.M.; Hanson, T.R.; Davis, D.A. Comparison of organic and inorganic microminerals in all plant diets for Nile tilapia Oreochromis niloticus. Aquaculture 2019, 498, 297–304. [Google Scholar] [CrossRef]
- Shahsavari, M.; Mohammadabadi, M.; Khezri, A.; Asadi Fozi, M.; Babenko, O.; Kalashnyk, O.; Olrshko, V.; Tkachenko, S. Correlation between insulin-like growth factor 1 gene expression and fennel (Foeniculum vulgare) seed powder consumption in muscle of sheep. Anim. Biotechnol. 2021, 34, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Ahmadniaye Motlagh, H.; Rokhnareh, Z.; Safari, O.; Selahvarzi, Y. Growth performance and intestinal microbial changes of Carassius auratus in response to pomegranate (Punica granatum) peel extract-supplemented diets. J. World Aquac. Soc. 2021, 52, 820–828. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Adorian, T.J.; Ferrigolo, F.R.G.; Raissy, M.; van Doan, H. The effects of combined inclusion of Malvae sylvestris, Origanum vulgare, and Allium hirtifolium boiss for common carp (Cyprinus carpio) diet: Growth performance, antioxidant defense, and immunological parameters. Fish Shellfish Immunol. 2021, 119, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Kesbiç, O.S.; Parrino, V.; Acar, Ü.; Yilmaz, S.; Paro, G.L.; Fazio, F. Effects of Monterey cypress (Cupressus macrocarpa Hartw) leaf essential oil as a dietary supplement on growth performance and haematological and biochemical parameters of common carp (Cyprinus carpio L.). Ann. Anim. Sci. 2020, 20, 1411–1426. [Google Scholar] [CrossRef]
- Abd El Hakim, N.F.; Ahmad, M.H.; Azab, E.S.; Lashien, M.S.; Baghdady, E.S. Response of Nile tilapia, Oreochromis niloticus to diets supplemented with different levels of fennel seeds meal (Foeniculum vulgare). Abbassa Int. J. Aquac. 2010, 3, 215–230. [Google Scholar]
- Kesbiç, O.S.; Acar, Ü.; Yilmaz, S.; Aydin, Ö.D. Effects of bergamot (Citrus bergamia) peel oil-supplemented diets on growth performance, haematology and serum biochemical parameters of Nile tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2020, 46, 103–110. [Google Scholar] [CrossRef]
- Acar, Ü.; Kesbiç, O.S.; İnanan, B.E.; Yılmaz, S. Effects of dietary Bergamot (Citrus bergamia) peel oil on growth, haematology and immune response of European sea bass (Dicentrarchus labrax) juveniles. Aquac. Res. 2019, 50, 3305–3312. [Google Scholar] [CrossRef]
- Enayat Gholampour, T.; Fadaei Raieni, R.; Pouladi, M.; Larijani, M.; Pagano, M.; Faggio, C. The dietary effect of Vitex agnus-castus hydroalcoholic extract on growth performance, blood biochemical parameters, carcass quality, sex ratio and gonad histology in zebrafish (Danio rerio). Appl. Sci. 2020, 10, 1402. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and Western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, C.; Cates, P.S.; Brooks, A.N.; Swanson, I.A.; Milligan, S.R.; Coen, C.W.; O’Byrne, K.T. Phytoestrogens and gonadotropin-releasing hormone pulse generator activity and pituitary luteinizing hormone release in the rat. Endocrinology 2001, 142, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, P.; Subramanian, P. Influence of Tribulus terrestris on testicular enzyme in fresh water ornamental fish Poecilia latipinna. Fish Physiol. Biochem. 2011, 37, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Gharaei, A.; Ebrahimi Jorjani, H.; Mirdar Harijani, J.; Kolangi Miandare, H. Effects of Tribullus terrestris extract on masculinization, growth indices, sex deteminationreversal and steroid hormones level in Zebra fish (Danio rerio). Int. Aquat. Res. 2020, 12, 22–29. [Google Scholar]
- Ahmadniaye Motlagh, H.; Paolucci, M.; Lashkarizadeh Bami, M.; Safari, O. Sexual parameters, digestive enzyme activities, and growth performance of guppy (Poecilia reticulata) fed garlic (Allium sativum) extract supplemented diets. J. World Aquac. Soc. 2020, 51, 1087–1097. [Google Scholar] [CrossRef]
- Gabriel, N.N.; Qiang, J.; Ma, X.Y.; He, J.; Xu, P.; Omoregie, E. Sex-reversal effect of dietary Aloe vera (Liliaceae) on genetically improved farmed Nile tilapia fry. North Am. J. Aquac. 2017, 79, 100–105. [Google Scholar] [CrossRef]
- Kurzer, M.S.; Xu, X. Dietary phytoestrogens. Annu. Rev. Nutr. 1997, 17, 353–381. [Google Scholar] [CrossRef]
- Dehghani, F.; Panjehshahin, M.R.; Mirzaee, Z.; Mehrabani, D. Effect of Foeniculum vulgare organic extract on blood sex hormones and reproductive tissues of male rats. J. Appl. Anim. Res. 2005, 27, 17–20. [Google Scholar] [CrossRef]
- Nair, S.; Rocha-Ferreira, E.; Fleiss, B.; Nijboer, C.H.; Gressens, P.; Mallard, C.; Hagberg, H. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: Role of mitochondria, inflammation, and reactive oxygen species. J. Neurochem. 2021, 158, 59–73. [Google Scholar] [CrossRef]
- Yanai, N.; Shiotani, S.; Hagiwara, S.; Nabetani, H.; Nakajima, M. Antioxidant combination inhibits reactive oxygen species mediated damage. Biosci. Biotechnol. Biochem. 2008, 72, 3100–3106. [Google Scholar] [CrossRef] [PubMed]
- Oktay, M.; Gülçin, İ.; Küfrevioğlu, Ö.İ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Catalkaya, G.; Capanoglu, E.; Karbancioglu-Guler, F. Antioxidant and antimicrobial activities of fennel, ginger, oregano and thyme essential oils. Food Front. 2021, 2, 508–518. [Google Scholar] [CrossRef]
- Hamed, H.S.; Ismal, S.M.; Faggio, C. Effect of allicin on antioxidant defense system, and immune response after carbofuran exposure in Nile tilapia, Oreochromis niloticus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 240, 108919. [Google Scholar] [CrossRef] [PubMed]
- Gulec, A.K.; Danabas, D.; Ural, M.; Seker, E.; Arslan, A.; Serdar, O. Effect of mixed use of thyme and fennel oils on biochemical properties and electrolytes in rainbow trout as a response to Yersinia ruckeri infection. Acta Vet. Brno 2013, 82, 297–302. [Google Scholar] [CrossRef]
- Salazar, J.H. Overview of urea and creatinine. Lab. Med. 2014, 45, e19–e20. [Google Scholar] [CrossRef]
- Abdel-Rahman, T.; Ali, D.; Abo-hagger, A.; Ahmed, M. Efficacy of banana peel in reduction of aflatoxin toxicity in rats. J. Agric. Chem. Biotechnol. 2017, 8, 251–259. [Google Scholar] [CrossRef]
- Stevenson, L.M.; Brown, A.C.; Montgomery, T.M.; Clotfelter, E.D. Reproductive consequences of exposure to waterborne phytoestrogens in male fighting fish Betta splendens. Arch. Environ. Contam. Toxicol. 2011, 60, 501–510. [Google Scholar] [CrossRef]
- Clotfelter, E.D.; McNitt, M.M.; Carpenter, R.E.; Summers, C.H. Modulation of monoamine neurotransmitters in fighting fish Betta splendens exposed to waterborne phytoestrogens. Fish Physiol. Biochem. 2010, 36, 933–943. [Google Scholar] [CrossRef]
| Compounds | Essential Oil (%) | KI |
|---|---|---|
| ɑ-Pinene | 1.96 | 914 |
| β-Pinen | 0.95 | 969 |
| Limonen | 7.31 | 1036 |
| 1,8 Cineol | 7.54 | 1042 |
| Trepinen | 0.81 | 1093 |
| Fenchone | 7.05 | 1101 |
| Camphor | 0.65 | 1152 |
| 4-Terpineol | 0.33 | 1168 |
| ɑ-Terpineol | 7.78 | 1168 |
| Estragloe | 13.25 | 1242 |
| E-Anethole | 33.4 | 1276 |
| 2,4-Decadienal | 18.12 | 1326 |
| Germacrene | 0.85 | 1493 |
| Feed Items | Percentage (%) | |||
|---|---|---|---|---|
| Basic Diet | Experimental Diet 1 | Experimental Diet 2 | Experimental Diet 3 | |
| Fish meal | 33 | 33 | 33 | 33 |
| Meat meal | 9 | 9 | 9 | 9 |
| Wheat gluten | 10 | 10 | 10 | 10 |
| Hydrolyzed yeast | 4 | 4 | 4 | 4 |
| Wheat flour | 34 | 34 | 34 | 34 |
| Rice bran | 3.5 | 3.5 | 3.5 | 3.5 |
| Fish oil | 2 | 2 | 2 | 2 |
| Mineral supplement | 1.5 | 1.5 | 1.5 | 1.5 |
| Vitamin supplement | 1.5 | 1.5 | 1.5 | 1.5 |
| Bentonite | 0.5 | 0.48 | 0.46 | 0.44 |
| Sodium chloride | 0.5 | 0.5 | 0.5 | 0.5 |
| Antifungal | 0.5 | 0.5 | 0.5 | 0.5 |
| Fennel extract | 0 | 0.02 | 0.04 | 0.06 |
| Chemical Composition (% of Dry Matter) | ||||
| Dry matter | 94.5 | 94.68 | 94.78 | 94.87 |
| Crude protein | 32.5 | 32.56 | 32.63 | 32.59 |
| Crude fat | 6.5 | 6.51 | 6.52 | 6.52 |
| Ash | 4.5 | 4.49 | 4.5 | 4.49 |
| Crude fiber | 7.5 | 7.48 | 7.53 | 7.54 |
| Nitrogen free extract | 49 | 49 | 49 | 49 |
| Gross energy (kcal/kg) | 3656 | 3663 | 3670 | 3667 |
| F. vulgare Essence Levels (mg/kg Feed) | ||||
|---|---|---|---|---|
| 0 | 200 | 400 | 600 | |
| Initial weight | 79.25 ± 12.34 | 77.20 ± 12.08 | 78.25 ± 12.80 | 79.93 ± 14.32 |
| Final weight | 89.20 ± 16.32 a | 93.81 ± 18.20 c | 91.10 ± 15.71 b | 90.85 ± 10.4 b |
| Weight gain | 09.87 ± 0.21 a | 16.40 ± 0.54 c | 12.84 ± 1.96 b | 10.92 ± 1.24 b |
| FCR | 1.86 ± 0.11 bc | 1.43 ± 0.25 a | 1.55 ± 1.14 ab | 2.00 ± 0.07 c |
| SGR | 0.25 ± 0.17 a | 0.48 ± 0.05 b | 0.41 ± 0.10 ab | 0.29 ± 0.05 ab |
| F. vulgare Essence Levels (mg/kg Feed) | ||||
|---|---|---|---|---|
| 0 | 200 | 400 | 600 | |
| AST (U/L) | 48.57 ± 6.14 a | 53.22 ± 4.65 ab | 55.71 ± 2.71 b | 58.86 ± 3.75 b |
| ALT (U/L) | 15.92 ± 1.15 ab | 13.16 ± 0.55 a | 14.92 ± 0.98 b | 16.39 ± 0.94 c |
| LDH (U/L) | 416.68 ± 19.64 a | 441.14 ± 39.76 ab | 462.78 ± 78.47 ab | 484.22 ± 27.27 b |
| ALP (U/L) | 184.71 ± 8.35 | 181.35 ± 10.71 | 193.42 ± 14.48 | 190.48 ± 13.89 |
| Glucose (mg/dL) | 64.94 ± 4.33 | 65.03 ± 10.72 | 60.99 ± 5.59 | 67.11 ± 10.21 |
| Total protein (g/dL) | 2.71 ± 0.14 a | 2.23 ± 0.50 a | 3.45 ± 0.60 b | 3.53 ± 0.64 b |
| Albumin | 2.00 ± 0.15 b | 1.24 ± 0.19 a | 1.86 ± 0.08 b | 2.48 ± 0.46 c |
| Globulin (g/dL) | 0.71 ± 0.11 a | 1.00 ± 0.39 a | 1.59 ± 0.60 b | 1.05 ± 0.53 a |
| Cholesterol (mg/dL) | 73.80 ± 4.87 ab | 80.18 ± 5.55 b | 67.57 ± 8.41 a | 69.80 ± 6.05 a |
| Triglycerides (mg/dL) | 185.9 ± 17.25 | 173.75 ± 12.06 | 168.34 ± 15.52 | 171.33 ± 14.43 |
| Creatinine (mg/dL) | 0.31 ± 0.04 a | 0.36 ± 0.11 a | 0.40 ± 0.11 a | 0.53 ± 0.10 b |
| F. vulgare Essence Levels (mg/kg Feed) | ||||
|---|---|---|---|---|
| 0 | 200 | 400 | 600 | |
| CAT (KU/mg protein) | 0.15 ± 0.01 | 0.15 ± 0.02 | 0.14 ± 0.03 | 0.14 ± 0.03 |
| SOD (U/mg protein) | 0.78 ± 0.25 b | 0.90 ± 0.24 b | 0.70 ± 0.32 b | 0.36 ± 0.07 a |
| GPx (U/mg protein) | 7.80 ± 1.06 c | 4.45 ± 1.45 b | 2.51 ± 0.75 a | 2.66 ± 0.46 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadniaye Motlagh, H.; Horie, Y.; Rashid, H.; Banaee, M.; Multisanti, C.R.; Faggio, C. Unveiling the Effects of Fennel (Foeniculum vulgare) Seed Essential Oil as a Diet Supplement on the Biochemical Parameters and Reproductive Function in Female Common Carps (Cyprinus carpio). Water 2023, 15, 2978. https://doi.org/10.3390/w15162978
Ahmadniaye Motlagh H, Horie Y, Rashid H, Banaee M, Multisanti CR, Faggio C. Unveiling the Effects of Fennel (Foeniculum vulgare) Seed Essential Oil as a Diet Supplement on the Biochemical Parameters and Reproductive Function in Female Common Carps (Cyprinus carpio). Water. 2023; 15(16):2978. https://doi.org/10.3390/w15162978
Chicago/Turabian StyleAhmadniaye Motlagh, Hamidreza, Yoshifumi Horie, Hediye Rashid, Mahdi Banaee, Cristiana Roberta Multisanti, and Caterina Faggio. 2023. "Unveiling the Effects of Fennel (Foeniculum vulgare) Seed Essential Oil as a Diet Supplement on the Biochemical Parameters and Reproductive Function in Female Common Carps (Cyprinus carpio)" Water 15, no. 16: 2978. https://doi.org/10.3390/w15162978