Glyphosate Removal from Water Using Biochar Based Coffee Husk Loaded Fe3O4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
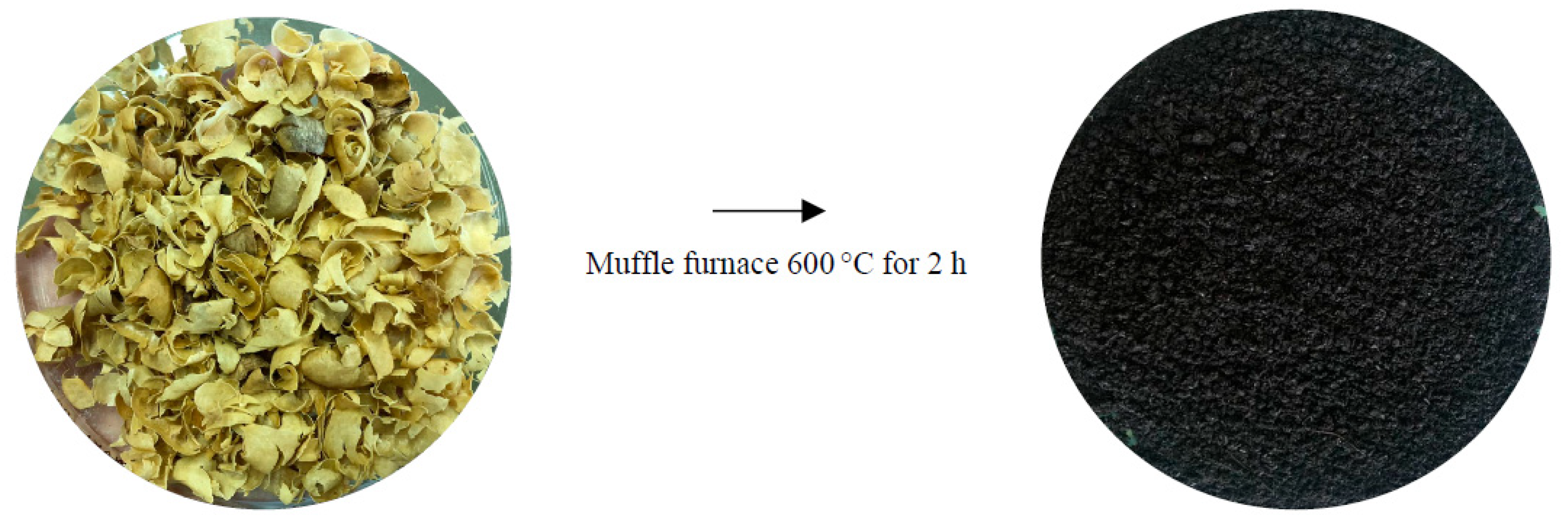
2.2. Preparation of Coffee Husk Biochar (CHB)
2.3. Preparation of Coffee-Husk-Biochar-Loaded Fe3O4 (CHB-Fe3O4)
2.4. Characterization of Adsorbent
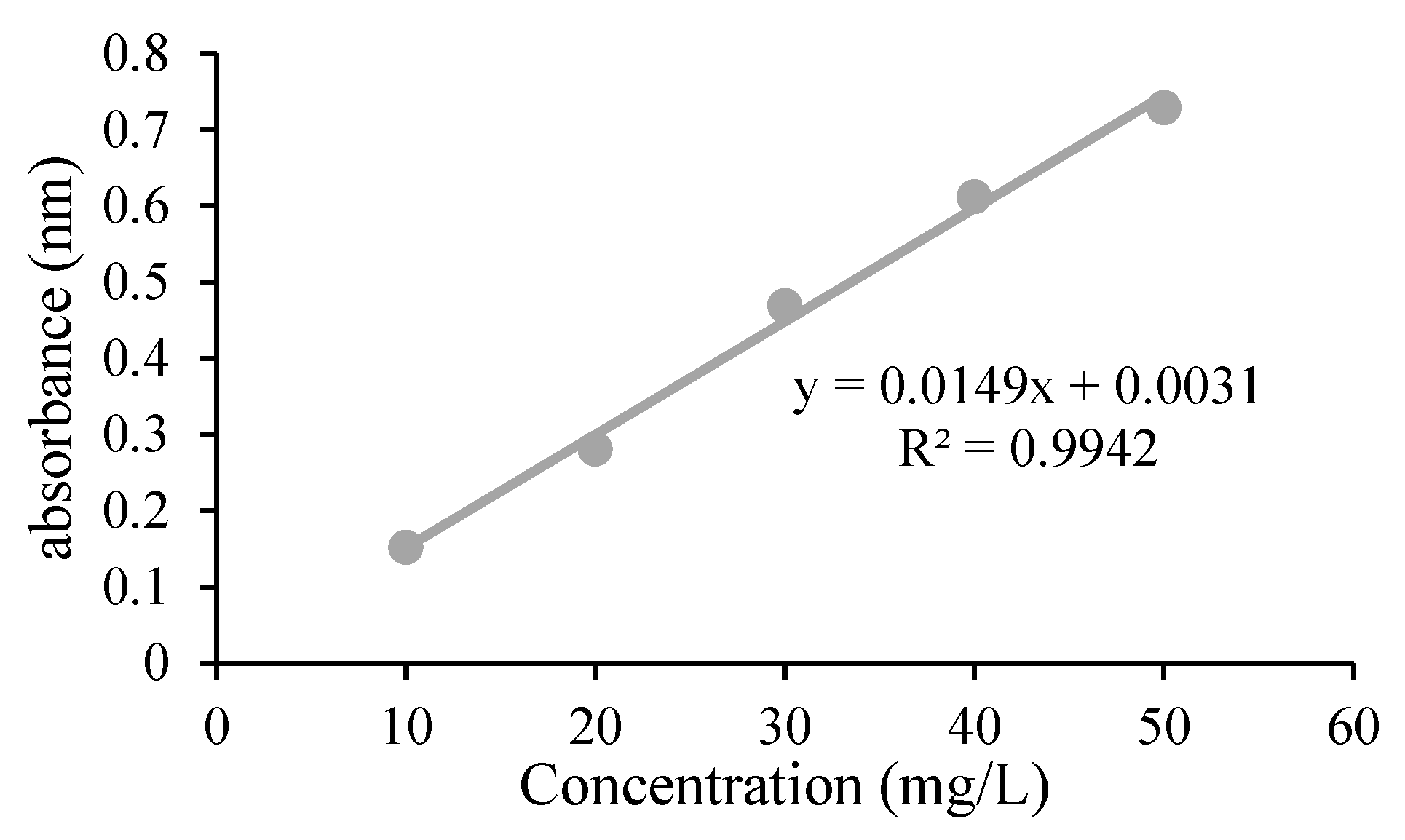
2.5. Detection of Glyphosate and Calibration Curve
2.6. Batch Adsorption and Desorption Experiments
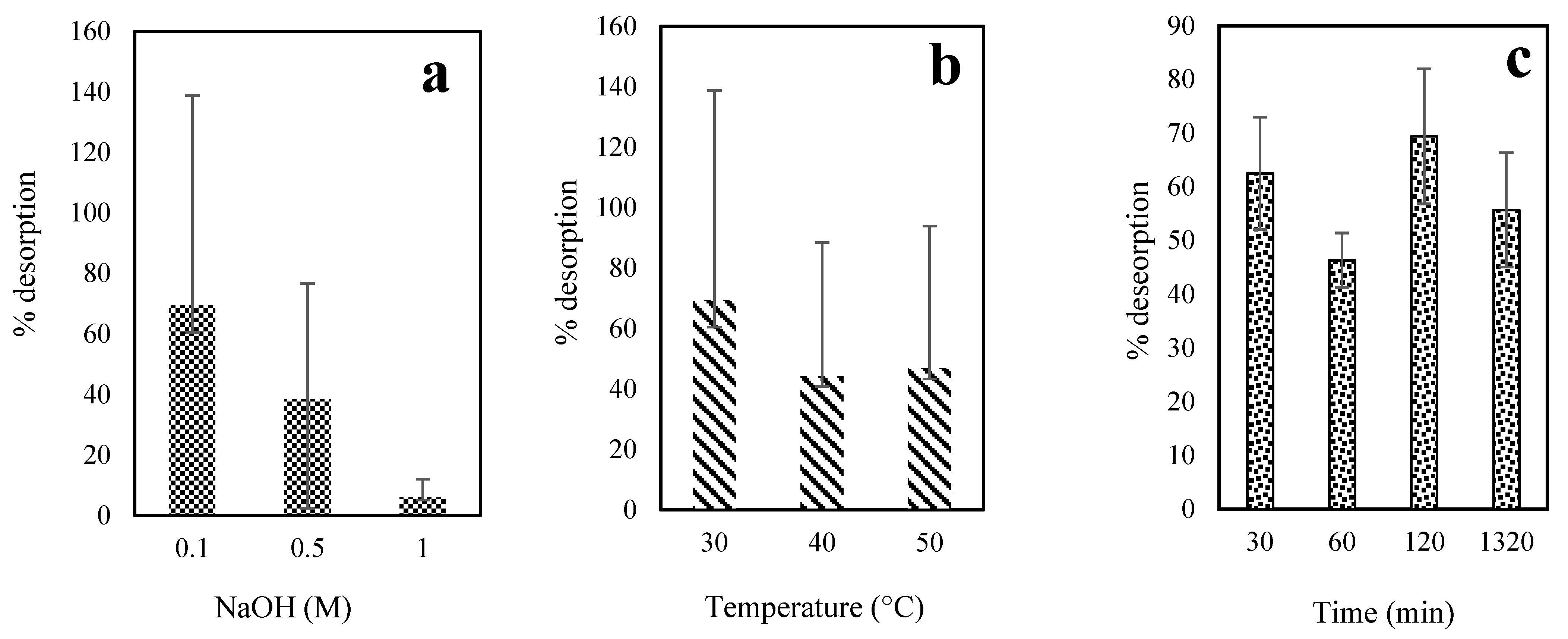
2.7. Desorption Studies
3. Results and Discussion
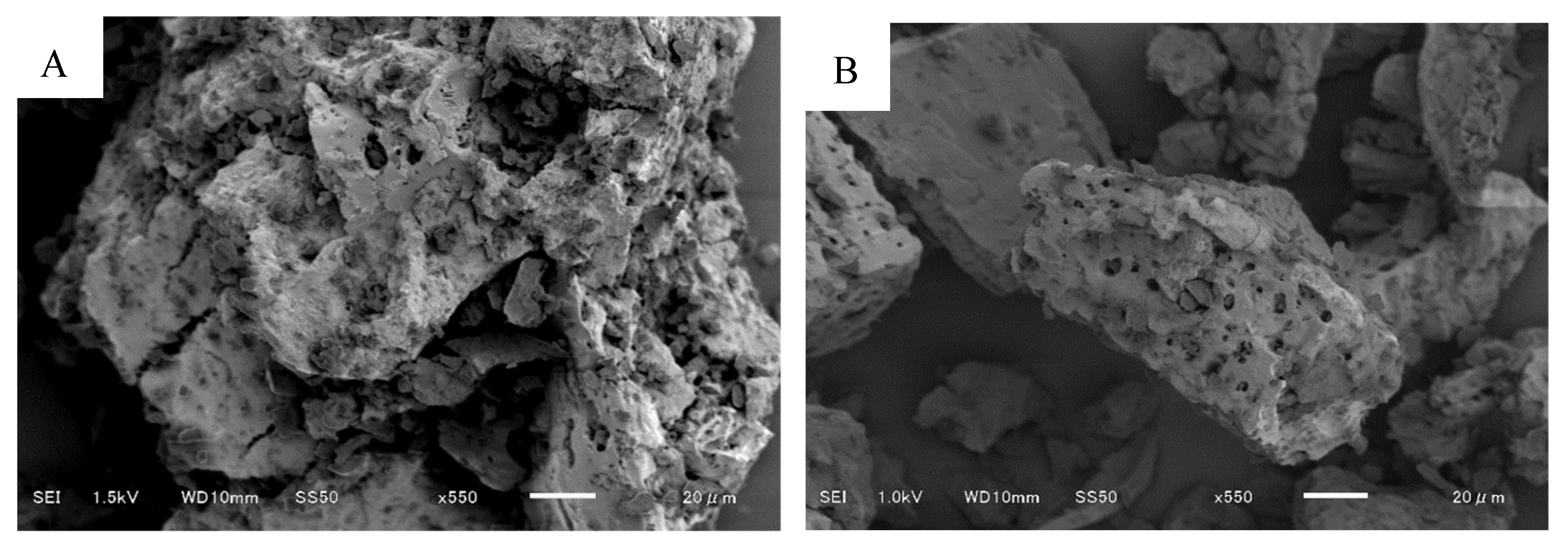
3.1. SEM Results of CHB-Fe3O4
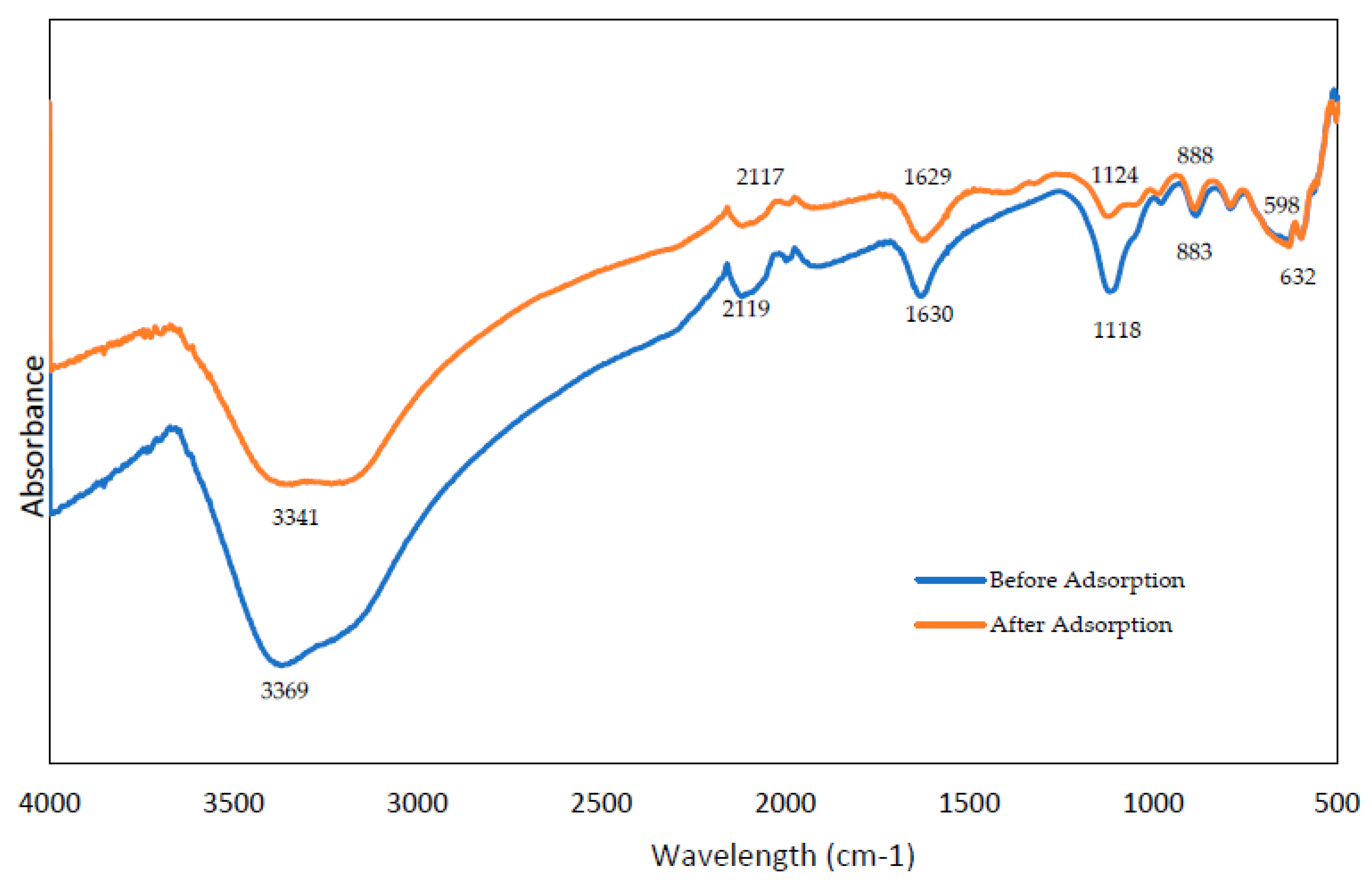
3.2. FTIR Spectra of Adsorbent before and after Glyphosate Adsorption
3.3. Interaction Glyphosate with CHB-Fe3O4
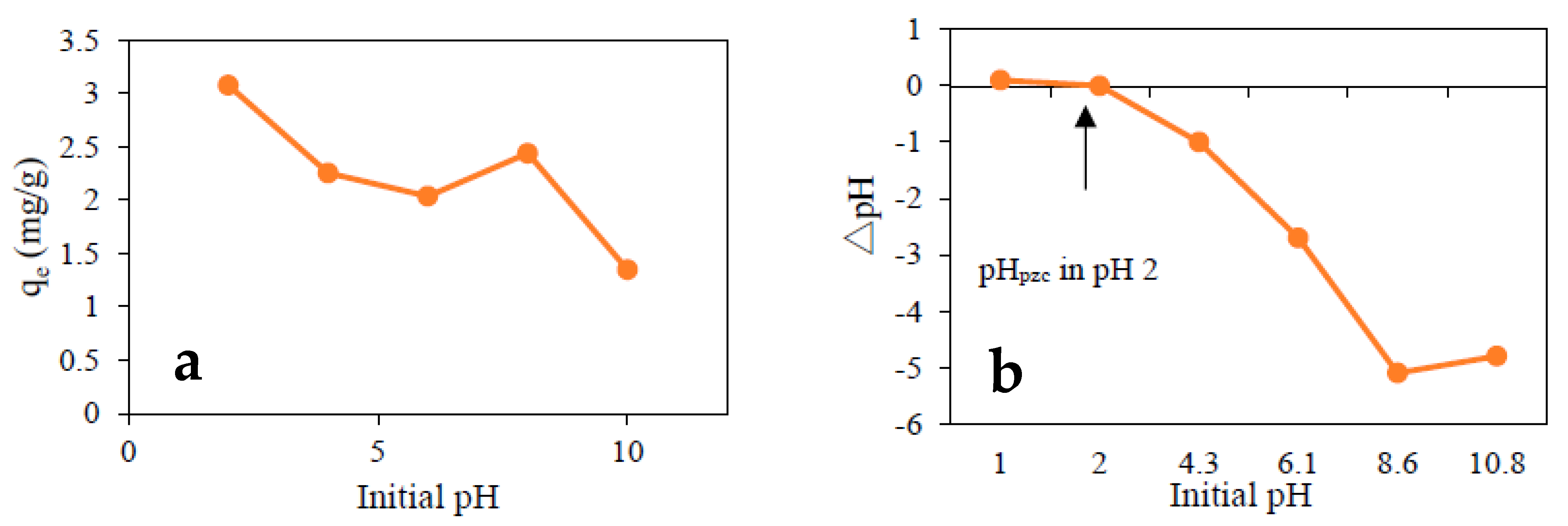
3.4. Initial pH Effects
3.5. Initial Concentration Effects
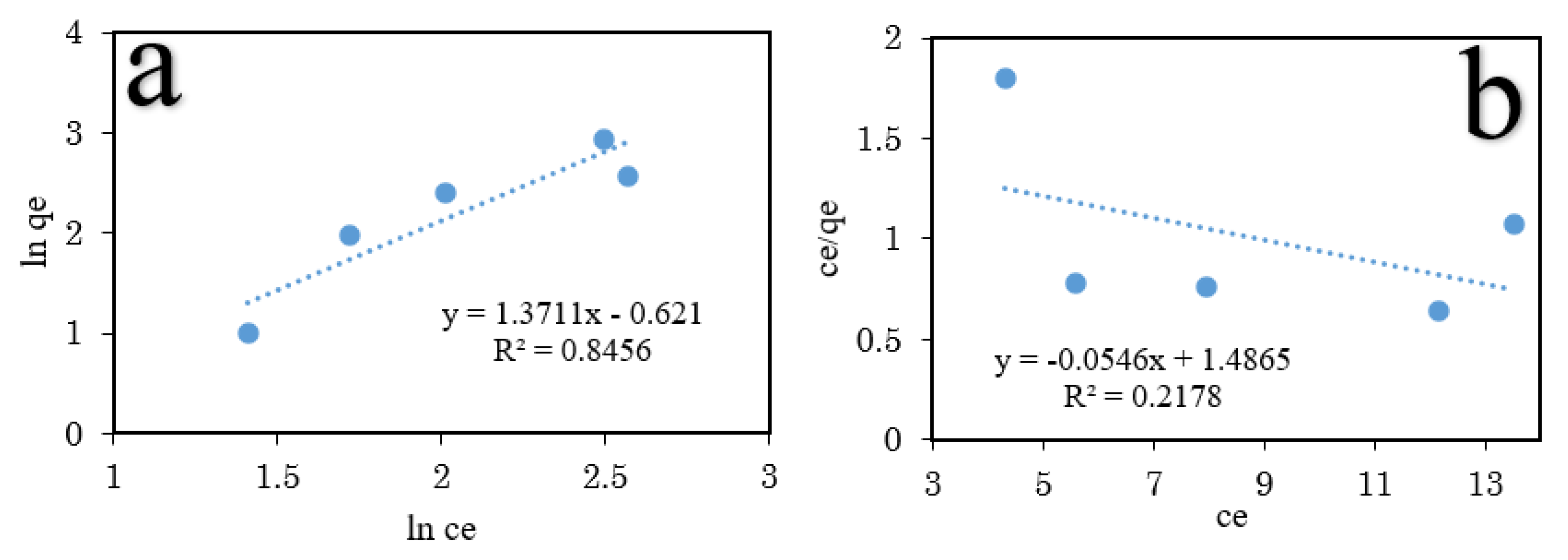
3.6. Adsorption Isotherm Studies
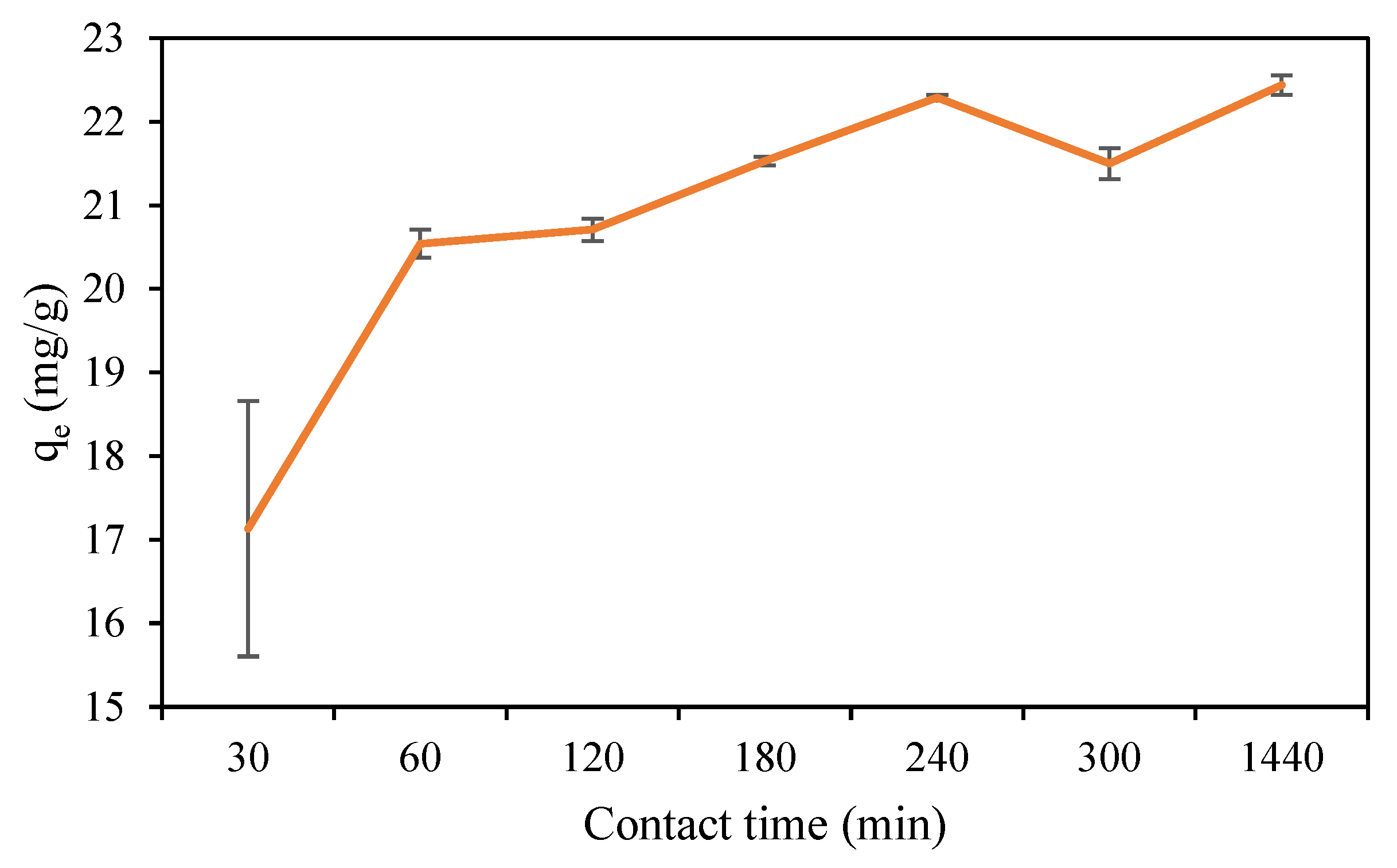
3.7. Adsorption Kinetic Studies
3.8. Desorption Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.S.; Zhao, Y.Q.; Sorohan, B. Removal of glyphosate from aqueous environment by adsorption using water industrial residual. Desalination 2011, 271, 150–156. [Google Scholar] [CrossRef]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health 2016, 15, 19. [Google Scholar] [CrossRef] [Green Version]
- Meftaul, I.; Venkateswarlu, K.; Dharmarajan, R. Controversies over human health and ecological impacts of glyphosate: Is it to be banned in modern agriculture? Environ. Pollut. 2020, 263, 114372. [Google Scholar] [CrossRef]
- Ahmad, S.; Almehmadi, M.; Janjuhah, H.T.; Kontakiotis, G.; Abdulaziz, O.; Saeed, K.; Ahmad, H.; Allahyani, M.; Aljuaid, A.; Alsaiari, A.A.; et al. The Effect of Mineral Ions Present in Tap Water on Photodegradation of Organic Pollutants: Future Perspectives. Water 2023, 15, 175. [Google Scholar] [CrossRef]
- Fadaei, A.; Dehghani, M.; Mahvi, A.; Nasseri, S.; Rastkari, N.; Shayeghi, M. Degradation of organophosphorus pesticides in water during UV/Htreatment: Role of sulphate and bicarbonate ions. J. Chem. 2012, 9, 2015–2022. [Google Scholar] [CrossRef] [Green Version]
- Herath, I.; Kumarathilaka, P.; Al-Wabel, M.I.; Abduljabbar, A.; Ahmad, M.; Usman, A.R.; Vithanage, M. Mechanistic modeling of glyphosate interaction with rice husk derived engineered biochar. Microporous Mesoporous Mater. 2016, 225, 280–288. [Google Scholar] [CrossRef]
- Jiang, Y.; Ni, D.; Ding, Q.; Chen, B.; Chen, X.; Kan, Y.; Dong, S. Synthesis and characterization of nano-crystalized HfC based on an aqueous solution-derived precursor. RSC Adv. 2018, 8, 39284–39290. [Google Scholar] [CrossRef] [PubMed]
- Mayakaduwa, S.; Kumarathilaka, P.; Herath, I.; Ahmad, M.; Al-Wabel, M.; Ok, Y.S.; Usman, A.; Abduljabbar, A.; Vithanage, M. Equilibrium and kinetic mechanisms of woody biochar on aqueous glyphosate removal. Chemosphere 2016, 144, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Serra-Clusellas, A.; De Angelis, L.; Beltramo, M.; Bava, M.; De Frankenberg, J.; Vigliarolo, J.; Di Giovanni, N.; Stripeikis, J.D.; Rengifo-Herrera, J.A.; de Cortalezzi, M.M.F. Glyphosate and AMPA removal from water by solar induced processes using low Fe(III) or Fe(II) concentrations. Environ. Sci. Water Res. Technol. 2019, 5, 1932–1942. [Google Scholar] [CrossRef]
- Valle, A.L.; Mello, F.C.C.; Alves-Balvedi, R.P.; Rodrigues, L.P.; Goulart, L.R. Glyphosate detection: Methods, needs and challenges. Environ. Chem. Lett. 2019, 17, 291–317. [Google Scholar] [CrossRef]
- Song, J.; Li, X.-M.; Figoli, A.; Huang, H.; Pan, C.; He, T.; Jiang, B. Composite hollow fiber nanofiltration membranes for recovery of glyphosate from saline wastewater. Water Res. 2013, 47, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Sun, Y.; Lin, Y.; Wang, N.; Wang, P. Study on preparation of microwave absorbing MnOx/Al2O3 adsorbent and degradation of adsorbed glyphosate in MW–UV system. Chem. Eng. J. 2016, 298, 68–74. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, Q.; Yan, W.; Jing, C.; Zhang, Y. Comparative study of glyphosate removal on goethite and magnetite: Adsorption and photo-degradation. Chem. Eng. J. 2018, 352, 581–589. [Google Scholar] [CrossRef]
- Nourouzi, M.M.; Chuah, T.G.; Choong, T.S.Y.; Rabiei, F. Modeling biodegradation and kinetics of glyphosate by artificial neural network. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2012, 47, 455–465. [Google Scholar] [CrossRef]
- Hidayat, E.; Yoshino, T.; Yonemura, S.; Mitoma, Y.; Harada, H. A Carbonized Zeolite/Chitosan Composite as an Adsorbent for Copper (II) and Chromium (VI) Removal from Water. Materials 2023, 16, 2532. [Google Scholar] [CrossRef]
- Hidayat, S.E.; Khaekhum, S.; Yonemura, Y.M.; Harada, H. Biosorption of Eriochrome Black T Using Exserohilum rostratum NMS1.5 Mycelia Biomass. J 2022, 5, 427–434. [Google Scholar] [CrossRef]
- Hidayat, E.; Yoshino, T.; Yonemura, S.; Mitoma, Y.; Harada, H. Synthesis, Adsorption Isotherm and Kinetic Study of Alkaline- Treated Zeolite/Chitosan/Fe3+ Composites for Nitrate Removal from Aqueous Solution—Anion and Dye Effects. Gels 2022, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zeng, G.; Wu, H.; Zhang, C.; Liang, J.; Dai, J.; Liu, Z.; Xiong, W.; Wan, J.; Xu, P.; et al. Co-occurrence and interactions of pollutants, and their impacts on soil remediation—A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1528–1553. [Google Scholar] [CrossRef]
- Soares, D.; Silva, L.; Duarte, S.; Pena, A.; Pereira, A. Glyphosate use, toxicity and occurrence in food. Foods 2021, 10, 2785. [Google Scholar] [CrossRef]
- Odoemelam, S.A.; Oji, E.O.; Okon, N.; Rajni, E. Zinc oxide nanoparticles adsorb emerging pollutants (glyphosate pesticide) from aqueous solutions. Environ. Monit. Assess. 2023, 195, 1–19. [Google Scholar] [CrossRef]
- Hottes, E.; da Silva, C.O.; Bauerfeldt, G.F.; Castro, R.N.; de Lima, J.H.C.; Camargo, L.P.; Dall’antonia, L.H.; Herbst, M.H. Efficient removal of glyphosate from aqueous solutions by adsorption on Mg–Al-layered double oxides: Thermodynamic, kinetic, and mechanistic investigation. Environ. Sci. Pollut. Res. 2022, 29, 83698–83710. [Google Scholar] [CrossRef]
- Rallet, D.; Paltahe, A.; Tsamo, C.; Loura, B. Synthesis of clay-biochar composite for glyphosate removal from aqueous solution. Heliyon 2022, 8, e09112. [Google Scholar] [CrossRef]
- Junior, E.G.S.; Perez, V.H.; de Paula, S.C.S.E.; Silveira, T.d.C.; Olivares, F.L.; Justo, O.R. Coffee Husks Valorization for Levoglucosan Production and Other Pyrolytic Products through Thermochemical Conversion by Fast Pyrolysis. Energies 2023, 16, 2835. [Google Scholar] [CrossRef]
- Oosterkamp, W.J. Use of Volatile Solids from Biomass for Energy Production; Elsevier: Amsterdam, The Netherlnads, 2014. [Google Scholar] [CrossRef]
- Hidayat, E.; Halem, H.I.A.; Mitoma, Y.; Harada, H. Recovery of Biomass Incinerated as Struvite-K Precipitates Followed Aluminium Removal. Green Sustain. Chem. 2021, 11, 96–106. [Google Scholar] [CrossRef]
- Ayalew, A.A.; Aragaw, T.A. Utilization of treated coffee husk as low-cost bio-sorbent for adsorption of methylene blue. Adsorpt. Sci. Technol. 2020, 38, 205–222. [Google Scholar] [CrossRef]
- Quyen, V.T.; Pham, T.H.; Kim, J.; Thanh, D.M.; Thang, P.Q.; Van Le, Q.; Jung, S.H.; Kim, T. Biosorbent derived from coffee husk for efficient removal of toxic heavy metals from wastewater. Chemosphere 2021, 284, 131312. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, P.; Bhaskara, B.L. Sensitive spectrophotometric assessment of carbofuran using dapsone as a new chromogenic reagent in formulations and environmental samples. Eclética Química 2006, 31, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Harada, H.; Hidayat, E.; Uemoto, S.; Fujita, K. Extraction of phosphorous from thermally treated sludge and separation of aluminum by adsorption. J. Mater. Cycles Waste Manag. 2021, 23, 2112–2119. [Google Scholar] [CrossRef]
- Hidayat, E.; Harada, H.; Mitoma, Y.; Yonemura, S.; Halem, H.I.A. Rapid Removal of Acid Red 88 by Zeolite/Chitosan Hydrogel in Aqueous Solution. Polymers 2022, 14, 893. [Google Scholar] [CrossRef]
- Hien, T.T.; Vu, N.T.; Thien, P.H.; Thanh, N.D.; Tuan, P.D. Synthesis of Novel Magnetic Adsorbents from Coffee Husks By Hydrothermal Carbonization. Vietnam. J. Sci. Technol. 2017, 55, 526–533. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of Slow Pyrolysis Biochars: Eff ects of Feedstocks and Pyrolysis Temperature on Biochar Properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Xie, J.; Lin, R.; Liang, Z.; Zhao, Z.; Yang, C.; Cui, F. Effect of cations on the enhanced adsorption of cationic dye in Fe3O4-loaded biochar and mechanism. J. Environ. Chem. Eng. 2021, 9, 105744. [Google Scholar] [CrossRef]
- Li, Y.; Tsend, N.; Li, T.; Liu, H.; Yang, R.; Gai, X.; Wang, H.; Shan, S. Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals. Bioresour. Technol. 2019, 273, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Filley, T.R.; McCormick, M.K.; Crow, S.E.; Szlavecz, K.; Whigham, D.F.; Johnston, C.T.; Heuvel, R.N.v.D. Comparison of the chemical alteration trajectory of Liriodendron tulipifera L. leaf litter among forests with different earthworm abundance. J. Geophys. Res. Biogeosciences 2008, 113, G01027. [Google Scholar] [CrossRef]
- Nalbandian, M.J.; Zhang, M.; Sanchez, J.; Nam, J.; Cwiertny, D.M.; Myung, N.V. Mesoporous θ-Alumina/Hematite (θ-Al2O3/Fe2O3) composite nanofibers for heavy metal removal. Sci. Adv. Mater. 2017, 9, 22–29. [Google Scholar] [CrossRef]
- Samuel, L.; Wang, R.; Dubois, G.; Allen, R.; Wojtecki, R.; La, Y.H. Amine-functionalized, multi-arm star polymers: A novel platform for removing glyphosate from aqueous media. Chemosphere 2017, 169, 437–442. [Google Scholar] [CrossRef]
- Herath, G.A.D.; Poh, L.S.; Ng, W.J. Statistical optimization of glyphosate adsorption by biochar and activated carbon with response surface methodology. Chemosphere 2019, 227, 533–540. [Google Scholar] [CrossRef]
- Hosseini, N.; Toosi, M.R. Removal of 2,4-D, glyphosate, trifluralin, and butachlor herbicides from water by polysulfone membranes mixed by graphene oxide/TiO2 nanocomposite: Study of filtration and batch adsorption. J. Environ. Health Sci. Eng. 2019, 17, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Barikbin, B. Biosorption of chromium(VI) from industrial wastewater onto pistachio hull waste biomass. Chem. Eng. J. 2010, 162, 893–900. [Google Scholar] [CrossRef]
- Sen, K.; Mondal, N.K.; Chattoraj, S.; Datta, J.K. Statistical optimization study of adsorption parameters for the removal of glyphosate on forest soil using the response surface methodology. Environ. Earth Sci. 2017, 76, 22. [Google Scholar] [CrossRef]
- Jiang, X.; Ouyang, Z.; Zhang, Z.; Yang, C.; Li, X.; Dang, Z.; Wu, P. Mechanism of glyphosate removal by biochar supported nano-zero-valent iron in aqueous solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 547, 64–72. [Google Scholar] [CrossRef]
- Nourouzi, M.M.; Chuah, T.G.; Choong, T.S.Y. Adsorption of glyphosate onto activated carbon derived from waste newspaper. Desalin. Water Treat. 2010, 24, 321–326. [Google Scholar] [CrossRef]
- Zhou, C.; Jia, D.; Liu, M.; Liu, X.; Li, C. Removal of Glyphosate from Aqueous Solution Using Nanosized Copper Hydroxide Modified Resin: Equilibrium Isotherms and Kinetics. J. Chem. Eng. Data 2017, 62, 3585–3592. [Google Scholar] [CrossRef]
- Diel, J.C.; Franco, D.S.; Igansi, A.V.; Cadaval, T.R.; Pereira, H.A.; Nunes, I.d.S.; Basso, C.W.; Alves, M.D.C.M.; Morais, J.; Pinto, D.; et al. Green synthesis of carbon nanotubes impregnated with metallic nanoparticles: Characterization and application in glyphosate adsorption. Chemosphere 2021, 283, 131193. [Google Scholar] [CrossRef]
- Zavareh, S.; Farrokhzad, Z.; Darvishi, F. Modification of zeolite 4A for use as an adsorbent for glyphosate and as an antibacterial agent for water. Ecotoxicol. Environ. Saf. 2018, 155, 1–8. [Google Scholar] [CrossRef]



| Model | Equation | Supporting Equation |
|---|---|---|
| Langmuir | ||
| Freundlich |
| Adsorption Isotherm | Isotherm Constant | Glyphosate |
|---|---|---|
| Langmuir | qmax | 18.315 |
| KL | 0.6727 | |
| RL | 0.0146 | |
| R2 | 0.2178 | |
| Freundlich | KF | 4.1783 |
| 1/n | 1.3711 | |
| R2 | 0.8456 |
| Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|
| qe (mg/g) | K1 (min −1) | R2 | qe (mg/g) | K2 (g mg−1 min−1) | R2 |
| 1.1904 | 1.38889−7 | 0.0686 | 22.5733 | 0.0050 | 0.9999 |
| Adsorbents | qe (mg/g) | Reference |
|---|---|---|
| Woody Biochar Zinc oxide nanoparticles | 44 82.97 | [9] [21] |
| Synthesis of clay-biochar | 37.06 | [23] |
| Multi-walled carbon nanotubes (MWCNTs) | 21.17 | [47] |
| Cu-zeolite 4A | 112.7 | [48] |
| Nanosized Copper Hydroxide | 140 | [46] |
| CHB-Fe3O4 | 22.44 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lita, A.L.; Hidayat, E.; Mohamad Sarbani, N.M.; Harada, H.; Yonemura, S.; Mitoma, Y.; Herviyanti; Gusmini. Glyphosate Removal from Water Using Biochar Based Coffee Husk Loaded Fe3O4. Water 2023, 15, 2945. https://doi.org/10.3390/w15162945
Lita AL, Hidayat E, Mohamad Sarbani NM, Harada H, Yonemura S, Mitoma Y, Herviyanti, Gusmini. Glyphosate Removal from Water Using Biochar Based Coffee Husk Loaded Fe3O4. Water. 2023; 15(16):2945. https://doi.org/10.3390/w15162945
Chicago/Turabian StyleLita, Arestha Leo, Endar Hidayat, Nur Maisarah Mohamad Sarbani, Hiroyuki Harada, Seiichiro Yonemura, Yoshiharu Mitoma, Herviyanti, and Gusmini. 2023. "Glyphosate Removal from Water Using Biochar Based Coffee Husk Loaded Fe3O4" Water 15, no. 16: 2945. https://doi.org/10.3390/w15162945
APA StyleLita, A. L., Hidayat, E., Mohamad Sarbani, N. M., Harada, H., Yonemura, S., Mitoma, Y., Herviyanti, & Gusmini. (2023). Glyphosate Removal from Water Using Biochar Based Coffee Husk Loaded Fe3O4. Water, 15(16), 2945. https://doi.org/10.3390/w15162945









