Multivariate Statistical Analysis and Geospatial Mapping for Assessing Groundwater Quality in West El Minia District, Egypt
Abstract
:1. Introduction
2. Description of the Study Area
2.1. Geographic Location
2.2. Geomorphological and Geological Settings
2.3. Hydrogeological Settings
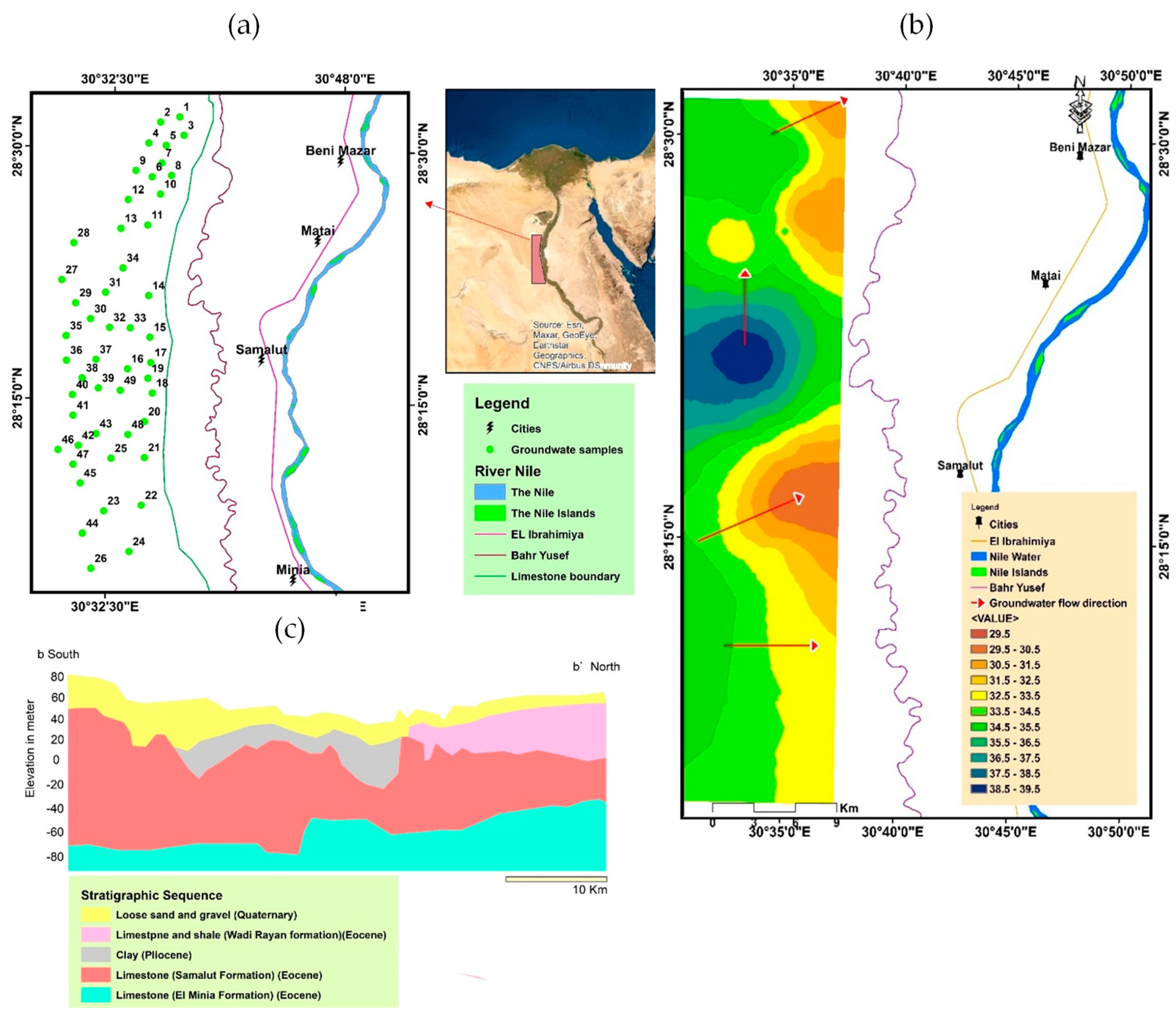
3. Materials and Methods
3.1. Sampling and Analytical Procedures
3.2. Statistical Investigation Approaches
3.3. Spatial Mapping
4. Results and Discussion
4.1. Hydrochemical Characteristics
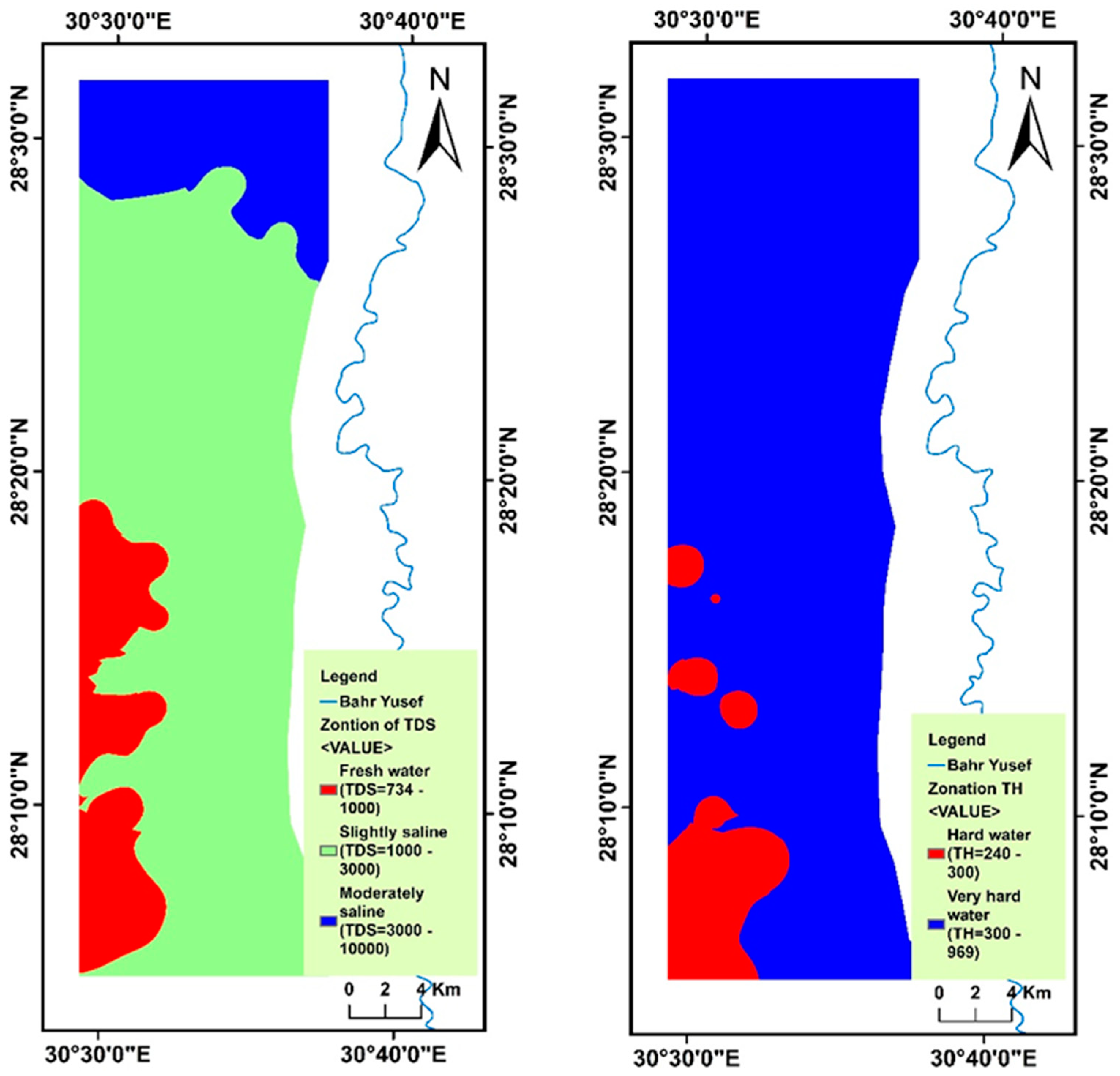
4.2. Spatial Variability of Groundwater Parameters
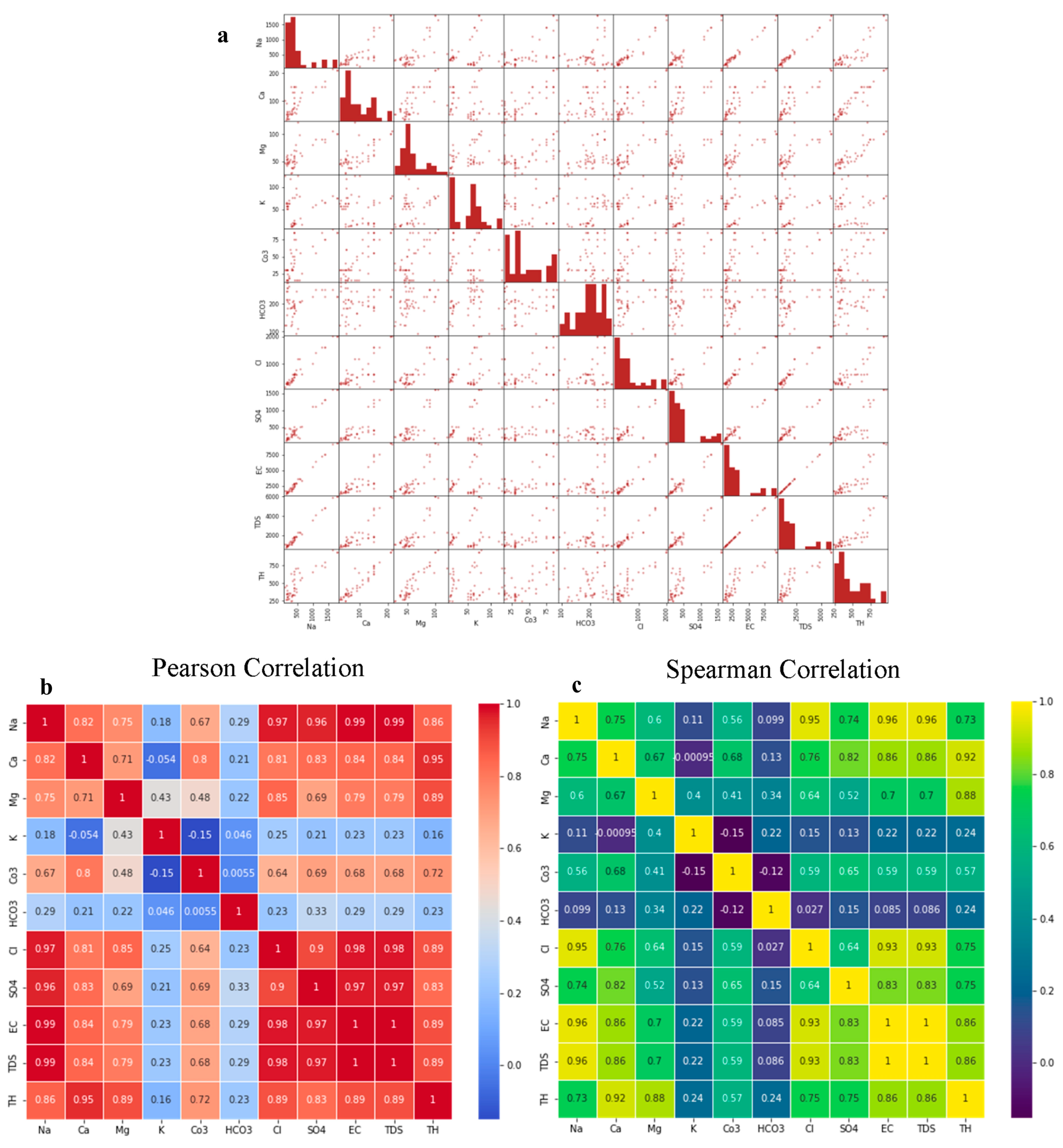
4.3. Correlation and Multivariate Analysis
4.3.1. Scatter Matrix and Correlation Analysis
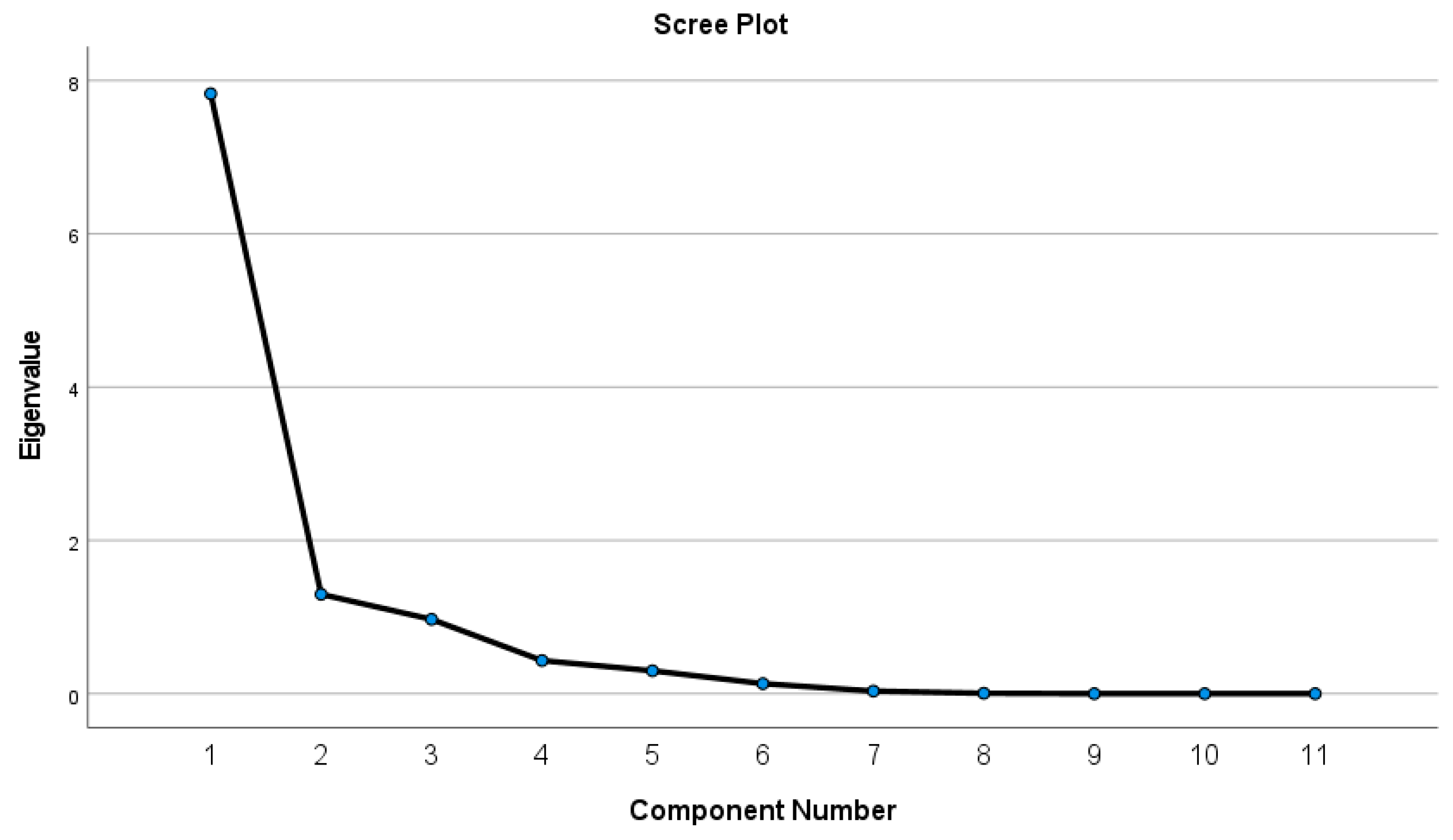
4.3.2. Principal Component Analysis (PCA)
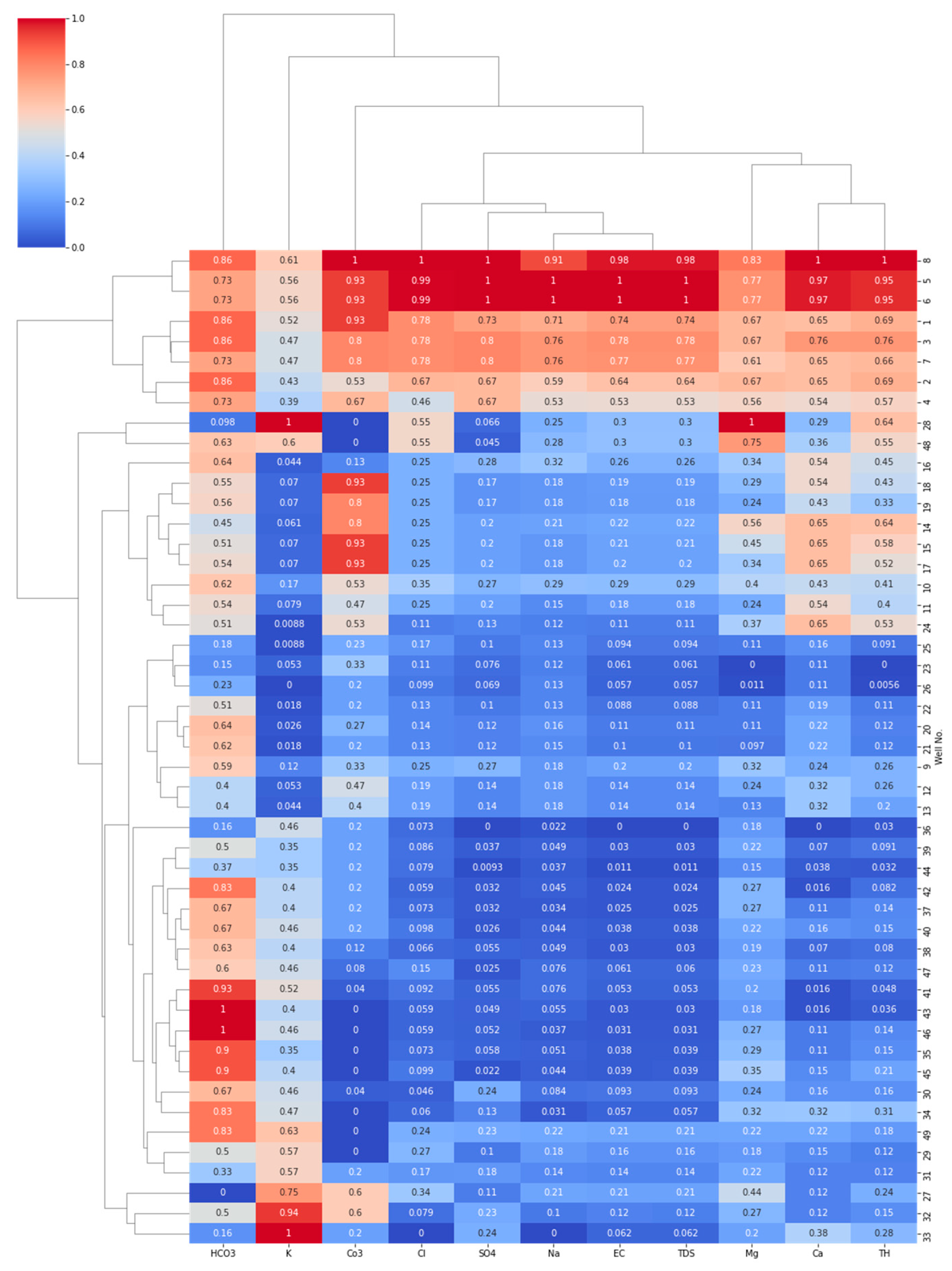
4.3.3. Hierarchical Cluster Analysis (HCA)
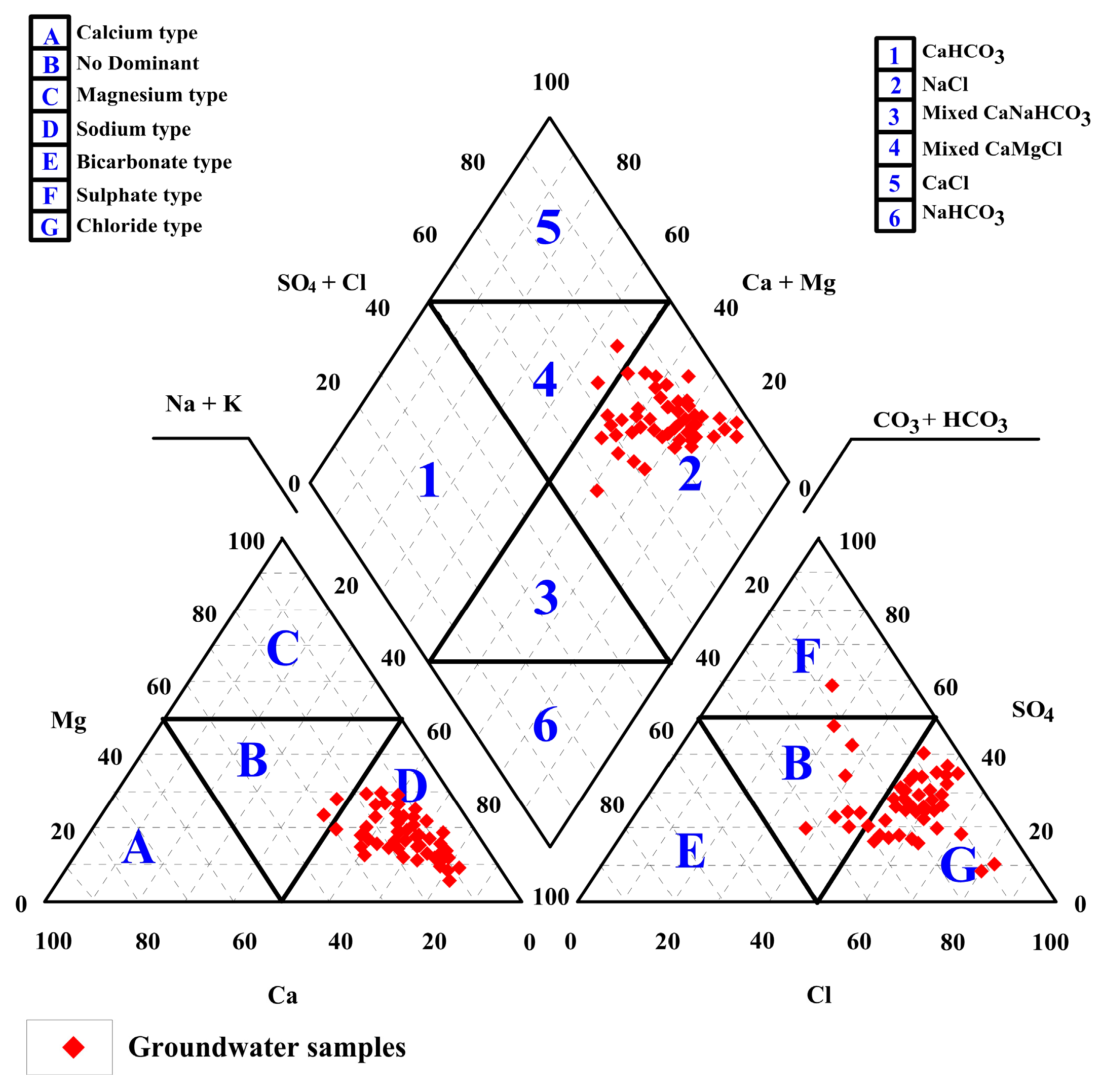
4.4. Hydrochemical Analysis
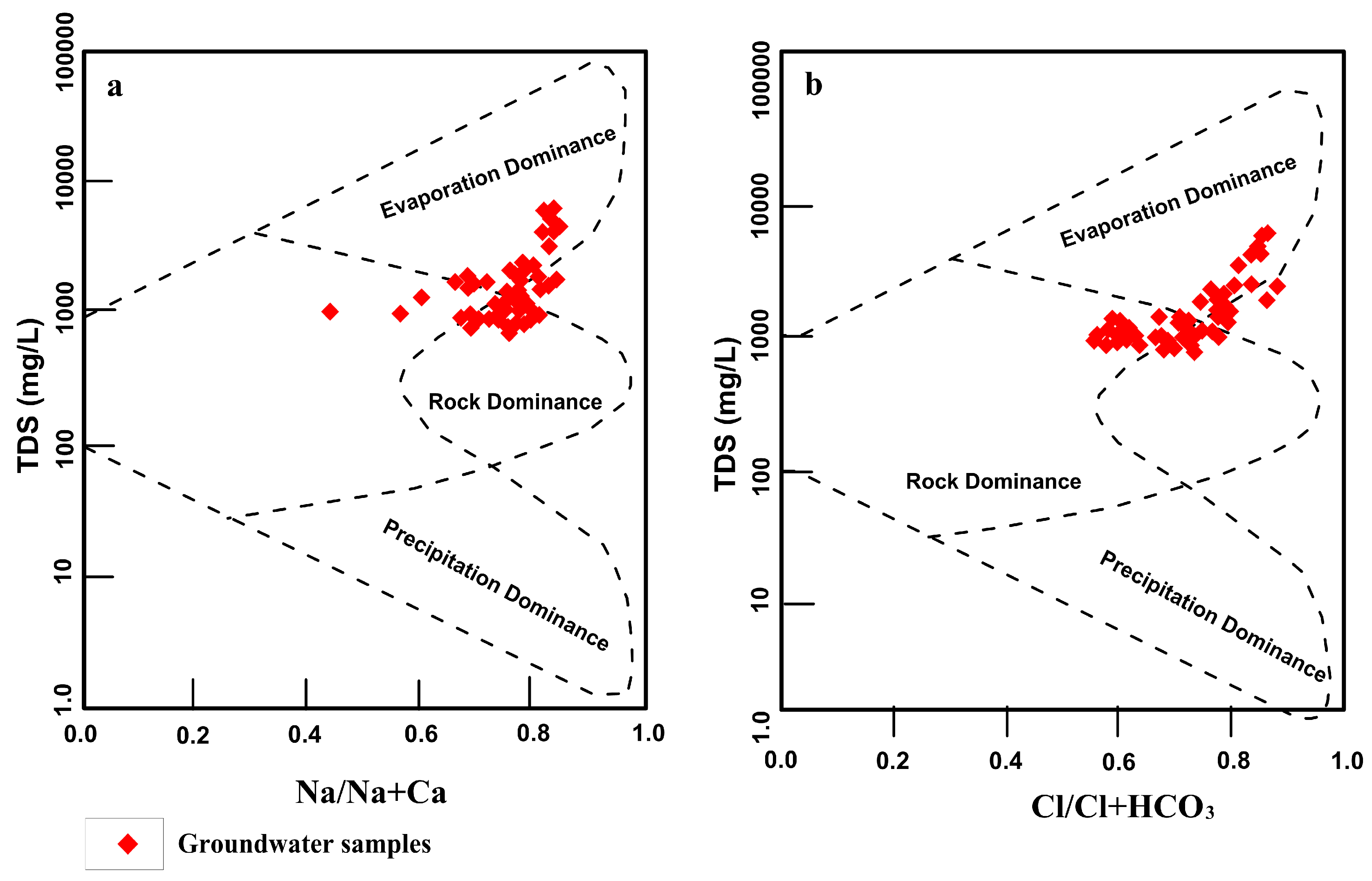
4.5. Geochemical Processes
4.6. Evaluation of Drinking and Household Groundwater Quality
4.7. Evaluation of Groundwater Quality for Irrigation Use
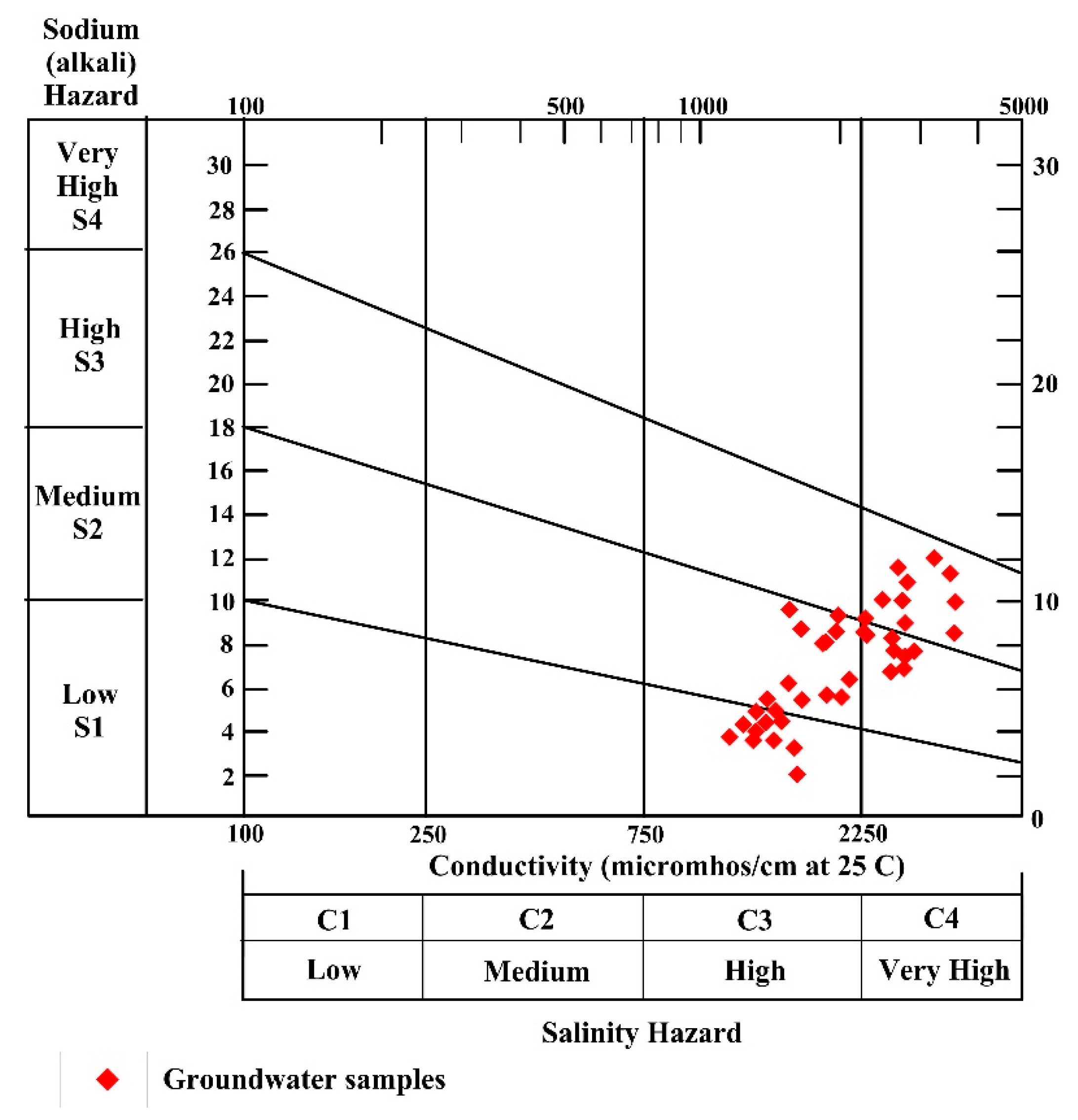
4.7.1. Sodium Absorption Ratio (SAR)
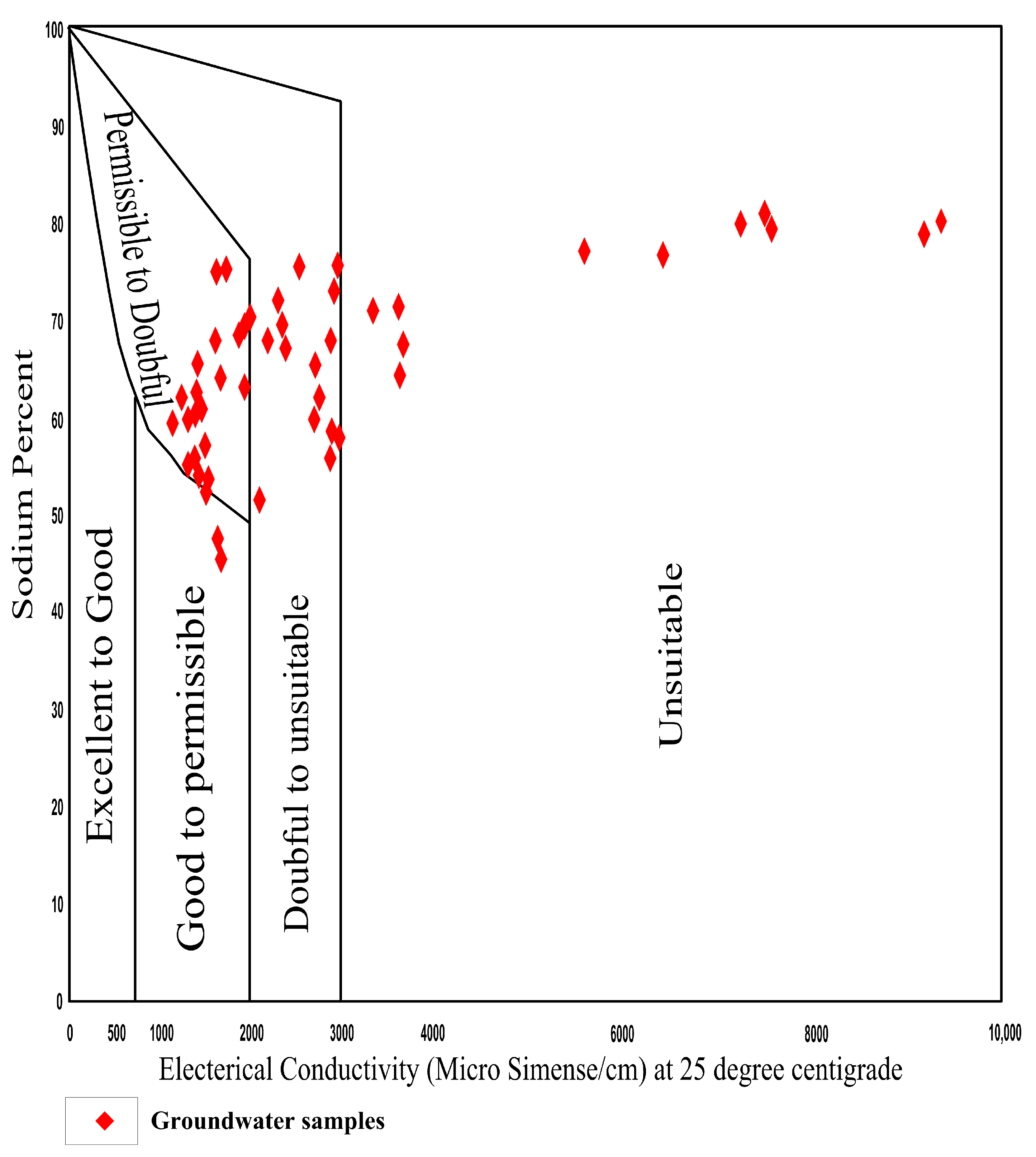
4.7.2. Sodium Percentage (Na%)
4.7.3. Residual Sodium Carbonate (RSC)
4.7.4. Kelley’s Ratio (KR)
4.7.5. Magnesium hazard (MH)
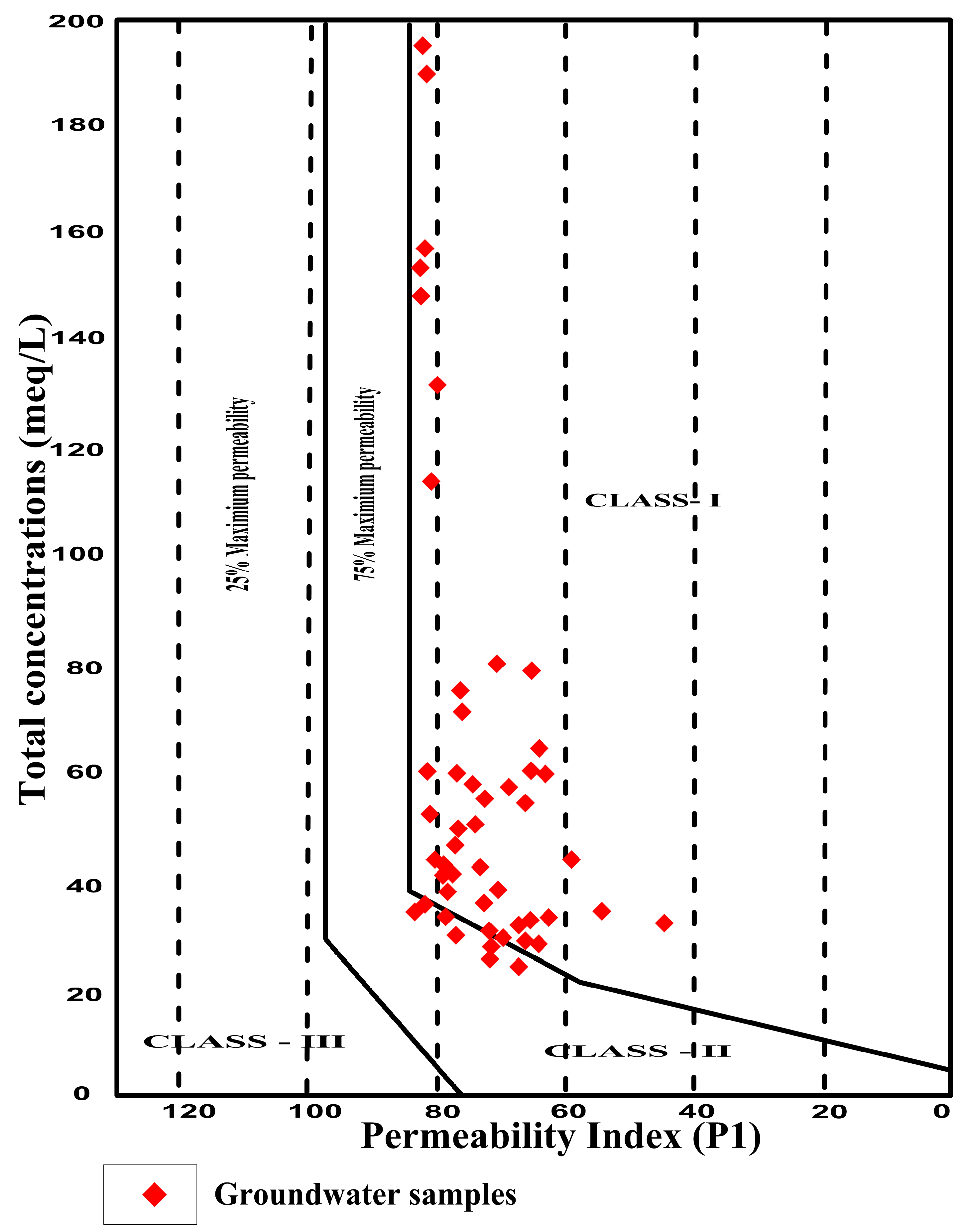
4.7.6. Permeability Index
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexakis, D.; Gotsis, D.; Giakoumakis, S. Assessment of drainage water quality in pre- and post-irrigation seasons for supplemental irrigation use. Environ. Monit. Assess. 2012, 184, 5051–5063. [Google Scholar] [CrossRef] [PubMed]
- Bekas, G.K.; Alexakis, D.E.; Gamvroula, D.E. Forecasting discharge rate and chloride content of karstic spring water by applying the Levenberg–Marquardt algorithm. Environ. Earth Sci. 2021, 80, 404. [Google Scholar] [CrossRef]
- Gamvroula, D.; Alexakis, D.; Stamatis, G. Diagnosis of groundwater quality and assessment of contamination sources in the Megara basin (Attica, Greece). Arab. J. Geosci. 2013, 6, 2367–2381. [Google Scholar] [CrossRef]
- Gamvroula, D.E.; Alexakis, D.E. Evaluating the Performance of Water Quality Indices: Applicationin Surface Waterof Lake Union, Washington State-USA. Hydrology 2022, 9, 116. [Google Scholar] [CrossRef]
- Tsitsis, C.; Alexakis, D.E.; Moustris, K.; Gamvroula, D.E. Combining Artificial Neural Network and Driver–Pressure–State–Impact–Response Approach for Evaluating a Mediterranean Lake. Water 2023, 15, 266. [Google Scholar] [CrossRef]
- Latha, P.S.; Rao, K.N.; Kumar, P.V. Hydrochemical evaluation of subsurface water in the aquaculture region of coastal India using multivariate statistics, GWQI and GIS. Int. J. Energy Water Resour. 2022, 1–21. [Google Scholar] [CrossRef]
- Sajjad, M.M.; Wang, J.; Abbas, H.; Ullah, I.; Khan, R.; Ali, F. Impact of Climate and Land-Use Changeon Ground water Resources, Study of Faisalabad District, Pakistan. Atmosphere 2022, 13, 1097. [Google Scholar] [CrossRef]
- Alexakis, D.E. Linking DPSIR model and water quality indices to achieve sustainable development goals in groundwater resources. Hydrology 2021, 8, 90. [Google Scholar] [CrossRef]
- Stamatis, G.; Alexakis, D.; Gamvroula, D.; Migiros, G. Groundwater quality assessment in Oropos-Kalamos basin, Attica, Greece. Environ. Earth Sci. 2011, 64, 973–988. [Google Scholar] [CrossRef]
- Giakoumakis, S.; Alexakis, D.; Gotsis, D. An approximate method for estimating nutrient loads in drainage water from a coastal irrigated area. Earth Sci. Res. J. 2013, 17, 115–118. [Google Scholar]
- Alexakis, D.; Kiskira, K.; Gamvroula, D.; Emmanouil, C.; Psomopoulos, C. Evaluating toxic element contamination sources in groundwater bodies of two Mediterranean sites. Environ. Sci. Pollut. Res. 2021, 28, 34400–34409. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.K.; Maharana, C.; Sharma, D.; Singh, A.K.; Tripathi, J.K.; Singh, S.K. Evaluation of groundwater quality in the Chotanagpur plateau region of the Subarnarekha river basin, Jharkhand State, India. Sustain. Water Qual. Ecol. 2015, 6, 57–74. [Google Scholar] [CrossRef]
- WHO; UNICEF. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2020: Years into the SDGs; World Health Organisation and United Nations Children Fund 2021: Geneva, Switzerland, 2021. [Google Scholar]
- Nag, S.K.; Das, S. Quality assessment of groundwater with special emphasis on irrigation and domestic suitability in suri I & II Blocks, Birbhum District, West Bengal, India. Am. J. Water. Res. 2014, 2, 81–98. [Google Scholar]
- Honarbakhsh, A.; Tahmoures, M.; Tashayo, B.; Mousazadeh, M.; Ingram, B.; Ostovari, Y. GIS-based assessment of groundwater quality for drinking purposes in northern part of Fars province, Marvdasht. J. Water Supply Res. Technol. Aqua 2019, 68, 187–196. [Google Scholar] [CrossRef]
- Mukherjee, I.; Singh, U.K.; Chakma, S. Evaluation of groundwater quality for irrigation water supply using multi-criteria decision-making techniques and GIS in an agroeconomic tract of Lower Ganga basin, India. J. Environ. Manag. 2022, 309, 114691. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Kumar, S.; Malik, A.; Kuriqi, A. Artificial neural network optimized with a genetic algorithm for seasonal groundwater table depth prediction in Uttar Pradesh, India. Sustainability 2020, 12, 8932. [Google Scholar] [CrossRef]
- ElBaba, M.; Kayastha, P.; Huysmans, M.; DeSmedt, F. Evaluation of the Groundwater Quality Using the Water Quality Index and Geostatistical Analysis in the Dieral-Balah Governorate, Gaza Strip, Palestine. Water 2020, 12, 262. [Google Scholar] [CrossRef] [Green Version]
- BenMoussa, A.; Chandoul, S.; Mzali, H.; BelHajSalem, S.; Elmejri, H.; Zouari, K.; Hafiane, A.; Mrabet, H. Hydrogeochemistry and evaluation of groundwater suitability for irrigation purpose in the Mornag region, northeastern Tunisia. Environ. Dev. Sustain. 2021, 23, 2698–2718. [Google Scholar] [CrossRef]
- Shadrin, D.; Nikitin, A.; Tregubova, P.; Terekhova, V.; Jana, R.; Matveev, S.; Pukalchik, M. An Automated Approach to Groundwater Quality Monitoring—Geospatial Mapping Based on Combined Application of Gaussian Process Regression and Bayesian Information Criterion. Water 2021, 13, 400. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Q.; Peng, W.; Liu, X. Source apportionment and natural background levels of major ions in shallow groundwater using multivariate statistical method: A case study in Huaibei Plain, China. J. Environ. Manag. 2022, 301, 113806. [Google Scholar] [CrossRef]
- Farid, H.U.; Ayub, H.U.; Khan, Z.M.; Ahmad, I.; Anjum, M.N.; Kanwar, R.M.A.; Mubeen, M.; Sakinder, P. Groundwater quality risk assessment using hydro-chemical and geospatial analysis. Environ. Dev. Sustain. 2022, 25, 8343–8365. [Google Scholar] [CrossRef]
- ElOsta, M.; Masoud, M.; Lqarawy, A.; Elsayed, S.; Gad, M. Groundwater Suitability for Drinking and Irrigation Using Water Quality Indices and Multivariate Modeling in Makkah Al-Mukarramah Province, Saudi Arabia. Water 2022, 14, 483. [Google Scholar] [CrossRef]
- El-Kholy, R.A.; Zaghlool, E.; Isawi, H.; Soliman, E.A.; Khalil, M.M.; El-Aassar, A.H.M.; Said, M.M. Groundwater quality assessment using water quality index and multivariate statistical analysis case study: East Matrouh, Northwestern coast, Egypt. Environ. Sci. Pollut. Res. 2022, 29, 65699–65722. [Google Scholar] [CrossRef]
- Selvakumar, S.; Chandrasekar, N.; Srinivas, Y.; Selvam, S.; Kaliraj, S.; Magesh, N.S.; Venkatramanan, S. Hydrogeochemical processes controlling the groundwater salinity in the coastal aquifers of Southern Tamil Nadu, India. Mar. Pollut. Bull. 2022, 174, 113264. [Google Scholar] [CrossRef] [PubMed]
- Ismail, E.; El-Sayed, E.; Sakr, S.; Youssef, E. Characteristic of groundwater potentialities in West Nile Valley South, Minia Governorate, Egypt. Arab. J. Geosci. 2017, 10, 521. [Google Scholar] [CrossRef]
- Ismail, E.; Abdelhalim, A.; AbouHeleika, M. Hydrochemical characteristics and quality assessment of groundwater aquifers northwest of Assiut district, Egypt. J. Afr. Earth Sci. 2021, 181, 104260. [Google Scholar] [CrossRef]
- Ismail, E.; El-Rawy, M. Assessment of groundwater quality in West Sohag, Egypt. Desal. Wat. Treat. 2018, 123, 101–108. [Google Scholar] [CrossRef]
- Abd-Elaty, I.; Pugliese, L.; Zelenakova, M.; Mesaros, P.; Shinawi, A.E. Simulation-based solutions reducing soil and groundwater contamination from fertilizers in arid and semi-arid regions: Case study the eastern Nile delta, Egypt. Int. J. Environ. Res. Publ. Health 2020, 17, 9373. [Google Scholar] [CrossRef]
- Snousy, M.G.; Ismail, E.; Zaki, R. Trace element occurrence and distribution problems in the irrigation water at El-Minia district, north upper Egypt. Arab. J. Geosci. 2019, 12, 582. [Google Scholar] [CrossRef]
- Geriesh, M.H.; Mansour, B.M.H.; Farouk, H. Assessment of drinking water quality along Port Said Canal treatment plants, Suez Canal corridor, Egypt. Arab. J. Geosci. 2019, 12, 738. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Sefelnasr, A.; Ismail, E. Numerical modeling technique for groundwater management in Samalutcity, Minia Governorate, Egypt. Arab. J. Geosci. 2019, 12, 124. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Sefelnasr, A.; Ismail, E. Response of the interaction between surface water and groundwater to climate change and proposed megastructure. J. Afr. Earth Sci. 2020, 162, 103723. [Google Scholar] [CrossRef]
- Abdalazem, A.H.; Gamee, M.A.; Hamdan, A.; Awad, A.A.M.; Mohamed, A.G. Groundwater quality assessment for irrigation in West Edfuregion, Aswan, Egypt. Assiut J. Agric. Sci. 2020, 51, 125–149. [Google Scholar]
- Sharaky, A.M.; Abdoun, S.H. Assessment of groundwater quality in bahariyaoasis, westerndesert, Egypt. Environ. Earth Sci. 2020, 79, 145. [Google Scholar] [CrossRef]
- Snousy, M.G.; Wu, J.; Su, F.; Abdelhalim, A.; Ismail, E. Groundwater Quality and Its Regulating Geochemical Processes in Assiut Province, Egypt. Expo.Health 2022, 14, 305–323. [Google Scholar] [CrossRef]
- Omara, S.; El-Tahlawi, M.R.; Mansour, H.H. The geology of the environs of Assiut. Bull. Soc. Geograph. Egypt 1970, 8, 43. [Google Scholar]
- Osman, H.Z. Geological Studies on the Northwest of Assiut, Egypt. Ph.D. Thesis, Assiut University, Asyut, Egypt, 1980. [Google Scholar]
- CONOCO. Geological Maps of Egypt Scale 1: 500,000; Sheet Nos. NG36NW Cairo; The Egyptian General Petroleum Corporation: Cairo, Egypt, 1987. [Google Scholar]
- Said, R. The Geology of Egypt; Balkema Publ.: Rotterdam, The Netherlands, 1990; p. 734. [Google Scholar]
- AbouHeleika, M.M.; Niesner, E. Configuration of the limestone aquifers in the central part of Egypt using electrical measurements. Hydrogeology 2009, 17, 433–446. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Howard, G.; Howden, N.J.K.; Ahmed, M.S.; Ismail, E. Carcinogenic and non-carcinogenic health risk assessment of heavy metals contamination in groundwater in the west of Miniaarea, Egypt. Hum. Ecol. Risk Assess. Int. J. 2022, 29, 571–596. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Waste water, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012; p. 1496. [Google Scholar]
- APHA. Standard Methods of Analysis of Water, Wastewater, 14th ed.; American Public Health Association: Washington, DC, USA, 2005; p. 1457. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; Licence: CCBY-NC-SA3.0IGO. [Google Scholar]
- Tiwari, K.; Goyal, R.; Sarkar, A. GIS-based spatial distribution of groundwater quality and regional suitability evaluation for drinking water. Environ. Proc. 2017, 4, 645–662. [Google Scholar] [CrossRef]
- Duarte, L.B.C.B.; Gonçalves, J.A.A.P.; Teodoro, A.C. Open-Source GIS Tools: Two Environmental Applications. Front. Inf. Syst. GIS—Overv. Appl. 2018, 1, 102. [Google Scholar] [CrossRef]
- Zolekar, R.B.; Bhagat, V.S. Multi-criteria land suitability analysis for agriculture in hilly zone: Remote sensing and GIS approach. Comput. Electron. Agric. 2018, 118, 300–321. [Google Scholar] [CrossRef]
- El-Rawy, M.; Ismail, E.; Abdalla, O. Assessment of groundwater quality using GIS, hydrogeochemistry, and factor statistical analysis in Qena Governorate, Egypt. Desalination Water Treat. 2019, 162, 14–29. [Google Scholar] [CrossRef]
- Raju, N.J.; Ram, P.; Dey, S. Groundwater quality in the lower Varuna river basin, Varanasi district, Uttar Pradesh. J. Geol. Soc. India 2009, 73, 178–192. [Google Scholar] [CrossRef]
- Piper, A.M. A Graphical Interpretation of Water Analysis. Eos. Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Hem, J.D. Study and interpretation of the chemical characteristics of natural water. USGS Water-Supply Pap. 1985, 2254, 19–32. [Google Scholar] [CrossRef]
- Kumar, P.S.; Balamurugan, P. Suitability of groundwater for irrigation purpose in Omalur Taluk, Salem, Tamil Nadu, India. Indian J. Ecol. 2019, 46, 1–6. [Google Scholar]
- Tijani, M.N. Hydrogeochemical assessment of groundwater in Moro area, Kwara State, Nigeria. Environ. Geol. 1994, 24, 194–202. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils, Agriculture Handbook; US Department of Agriculture: Washington, DC, USA, 1954; p. 160. [Google Scholar] [CrossRef]
- United States Salinity Laboratory (USSL). Diagnosis and Improvement of Saline and Alkaline Soils; Handbook No. 60; US Department of Agriculture: Washington, DC, USA, 1954; p. 160. [CrossRef]
- Amiri, V.; Sohrabi, N.; Dadgar, M.A. Evaluation of groundwater chemistry and its suitability for drinking and agricultural uses in the Lenjanat plain, central Iran. Environ. Earth Sci. 2015, 74, 6163–6176. [Google Scholar] [CrossRef]
- Wilcox, L.V. Classification and Use of Irrigation Waters; Circular 969; US Department of Agriculture: Washington, DC, USA, 1955; p. 19. [Google Scholar]
- Kelley, W.P. Permissible composition and concentration of irrigation waters. Am. Soc. Civ. Eng. 1963, 66, 607–613. [Google Scholar] [CrossRef]
- Szabolcs, I.; Darab, C. The influence of irrigation water of high sodium carbonate content of soils. In Proceedings of the 8th International Congress of ISSS, Bucharest, Romania, 8 August 1964; pp. 802–812. [Google Scholar]
- Paliwal, K.V. Irrigation with Saline Water, Monogram No.2 (New Series); IARI: NewDelhi, India, 1972; p. 198. [Google Scholar]
- Doneen, I.D. Noteson Water Quality in Agriculture; Published as a Water Science and Engineering. Paper 4001; Department of Water Sciences and Engineering, University of California: Berkeley, CA, USA, 1964. [Google Scholar]
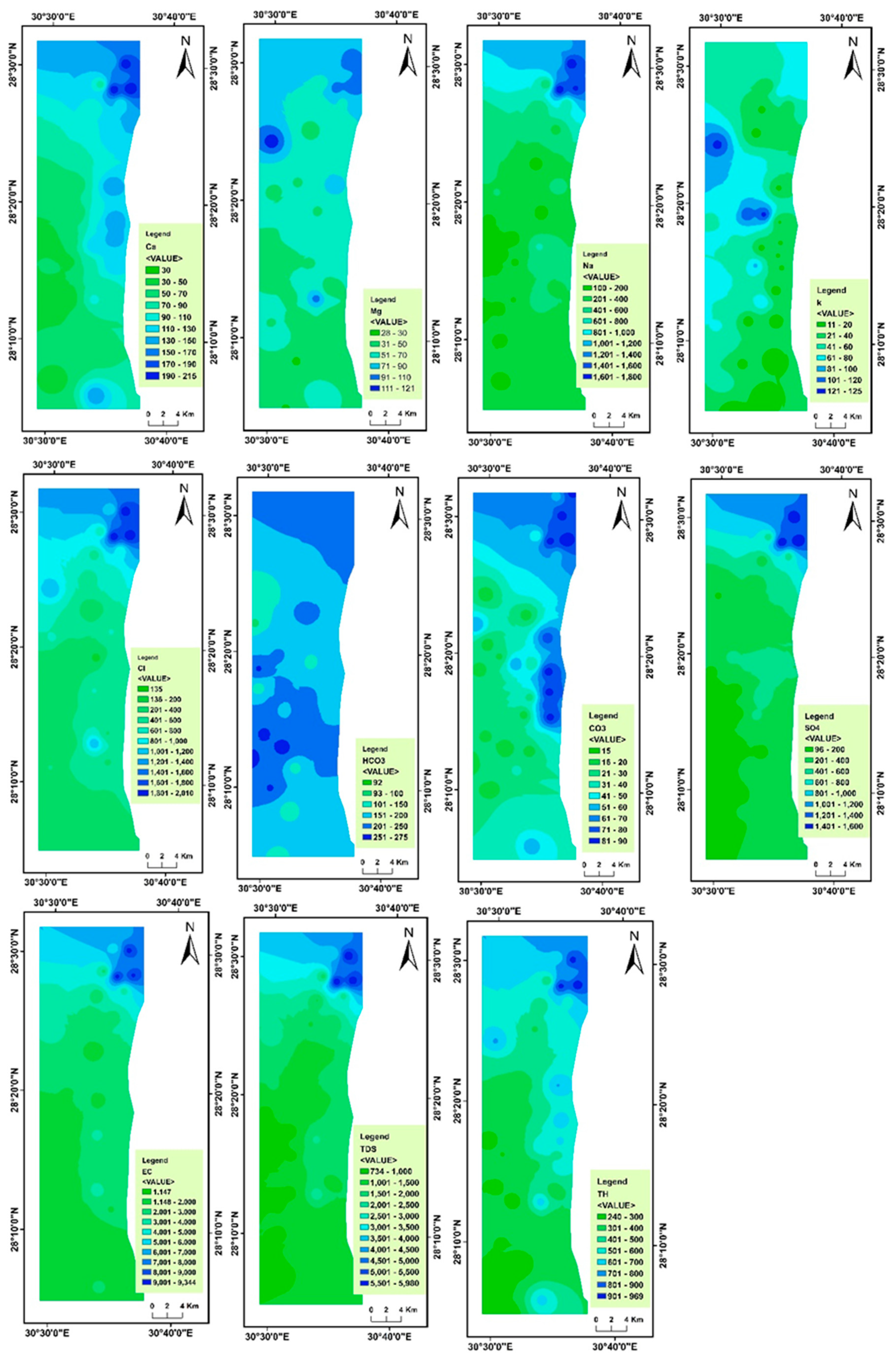

| Min | Max | Mean | Std. Deviation | Variance | Skewness | Kurtosis | WHO Maximum Permissible Limit | No. of Samples Exceeding WHO Limit | Percentage (%) of Samples Exceeding WHO Limit | |
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | 100 | 1800 | 500.24 | 449.046 | 201,642.314 | 1.825 | 2.356 | 200 | 36 | 73 |
| Ca2+ | 30 | 215 | 91.84 | 50.922 | 2593.056 | 0.849 | −0.214 | 200 | 3 | 6 |
| Mg2+ | 28 | 121 | 59.80 | 21.396 | 457.791 | 1.058 | 0.443 | 100 | 2 | 4 |
| K+ | 11 | 125 | 51.47 | 30.899 | 954.754 | 0.430 | −0.255 | 12 | 46 | 94 |
| CO32− | 15 | 90 | 43.12 | 25.009 | 625.443 | 0.578 | −1.077 | - | - | - |
| HCO3− | 92 | 275 | 199.14 | 45.084 | 2032.583 | −0.458 | −0.304 | 100 | 48 | 98 |
| Cl− | 135 | 2010 | 650.76 | 511.884 | 262,025.272 | 1.554 | 1.408 | 250 | 43 | 88 |
| SO42− | 96 | 1600 | 453.43 | 424.131 | 179,886.792 | 1.759 | 1.924 | 250 | 30 | 61 |
| EC | 1147 | 9344 | 3044.18 | 2263.390 | 5,122,932.486 | 1.787 | 2.149 | 1400 | 42 | 86 |
| TDS | 734 | 5980 | 1948.29 | 1448.579 | 2,098,380.708 | 1.787 | 2.149 | 1000 | 37 | 78 |
| TH | 240 | 969 | 467 | 199.65 | 39,863.27 | 0.915 | −0.138 | 500 | 18 | 37 |
| Variable | PC1 | PC2 | PC3 | Communalities |
|---|---|---|---|---|
| Na+ | 0.969 | 0.011 | 0.035 | 0.941 |
| Ca2+ | 0.904 | −0.297 | 0.005 | 0.905 |
| Mg2+ | 0.845 | 0.295 | −0.141 | 0.821 |
| K+ | 0.212 | 0.889 | −0.303 | 0.927 |
| CO32− | 0.741 | −0.499 | −0.152 | 0.821 |
| HCO3− | 0.294 | 0.261 | 0.905 | 0.974 |
| Cl− | 0966 | 0.083 | −0.062 | 0.944 |
| SO42− | 0.950 | 0.006 | 0.082 | 0.909 |
| EC | 0.986 | 0.043 | 0.016 | 0.974 |
| TDS | 0.986 | 0.043 | 0.016 | 0.974 |
| TH | 0.949 | −0.059 | −0.061 | 0.907 |
| % of Variance | 71.174 | 11.795 | 8.820 | |
| Cumulative% | 71.174 | 82.969 | 91.789 |
| Water Type | TDS (mg/L) | No. of Samples | Percentage (%) |
|---|---|---|---|
| Fresh water | <1000 | 11 | 22 |
| Slightly saline | 1000–3000 | 30 | 62 |
| Moderately saline | 3000–10,000 | 8 | 16 |
| Very saline | 10,000–35,000 | 0 | 0 |
| Brine | >35,000 | 0 | 0 |
| Total Hardness (mg/L) | Water Class | No. of Samples | Percentage (%) |
|---|---|---|---|
| <70 | Soft | 0 | 0 |
| 70–150 | Moderate hard | 0 | 0 |
| 150–300 | Hard | 0 | 0 |
| >300 | Very hard | 49 | 100 |
| SAR | Alkalinity Hazard | Groundwater Class | No. of Samples | Percentage (%) |
|---|---|---|---|---|
| <10 | S1 | Excellent | 38 | 88 |
| 10–18 | S2 | Good | 6 | 12 |
| 18–26 | S3 | Doubtful | 5 | 10 |
| >26 | S4 | Unsuitable | - |
| Na% | Groundwater Class | No. of Samples | Percentage (%) |
|---|---|---|---|
| <20 | Excellent | 0 | 0 |
| 20–40 | Good | 0 | 0 |
| 40–60 | Permissible | 13 | 27 |
| 60–80 | Doubtful | 33 | 67 |
| >80 | Unsuitable | 3 | 6 |
| PI (%) | Classification | Water Quality | No. of Samples | Percentage (%) |
|---|---|---|---|---|
| >75 | Class I | Good | 23 | 47 |
| 25–75 | Class II | Moderate | 26 | 53 |
| <25 | Class III | Poor | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, E.; Snousy, M.G.; Alexakis, D.E.; Gamvroula, D.E.; Howard, G.; El Sayed, E.; Ahmed, M.S.; Ali, A.; Abdelhalim, A. Multivariate Statistical Analysis and Geospatial Mapping for Assessing Groundwater Quality in West El Minia District, Egypt. Water 2023, 15, 2909. https://doi.org/10.3390/w15162909
Ismail E, Snousy MG, Alexakis DE, Gamvroula DE, Howard G, El Sayed E, Ahmed MS, Ali A, Abdelhalim A. Multivariate Statistical Analysis and Geospatial Mapping for Assessing Groundwater Quality in West El Minia District, Egypt. Water. 2023; 15(16):2909. https://doi.org/10.3390/w15162909
Chicago/Turabian StyleIsmail, Esam, Moustafa Gamal Snousy, Dimitrios E. Alexakis, Dimitra E. Gamvroula, Guy Howard, Esam El Sayed, Mohamed S. Ahmed, Ahmed Ali, and Ahmed Abdelhalim. 2023. "Multivariate Statistical Analysis and Geospatial Mapping for Assessing Groundwater Quality in West El Minia District, Egypt" Water 15, no. 16: 2909. https://doi.org/10.3390/w15162909







