The Uptake of Engineered Nanoparticles by Sludge Particulates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Engineered Nanoparticles and Sludge Particulates
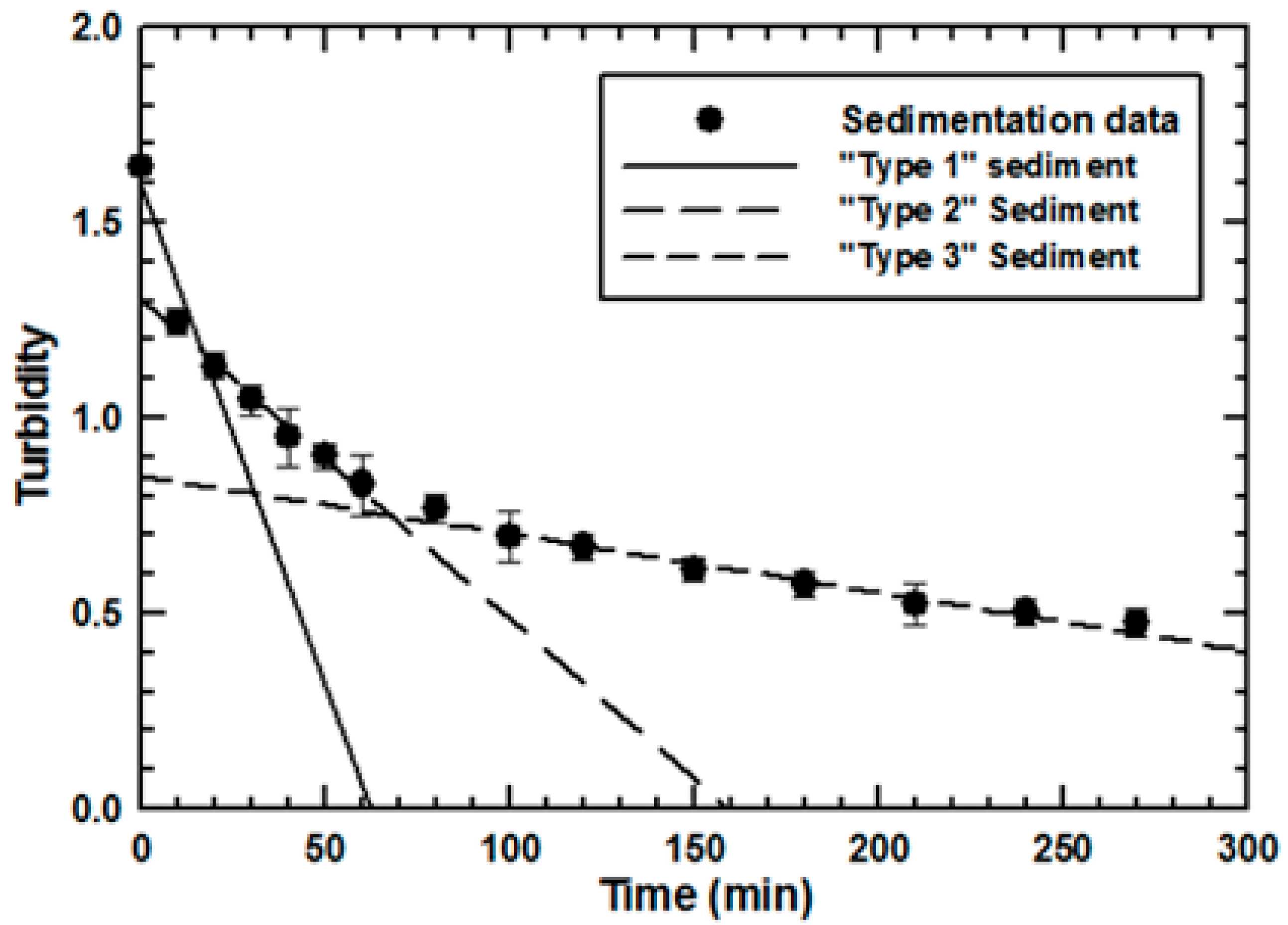
2.2. Differential Sedimentation
2.3. Determination of Free-Engineered Nanoparticle Concentration
2.4. ENP Sorption Experiment and Evaluation
3. Results and Discussion
3.1. Effect of Sludge Particulate Size on Engineered Nanoparticle Sorption
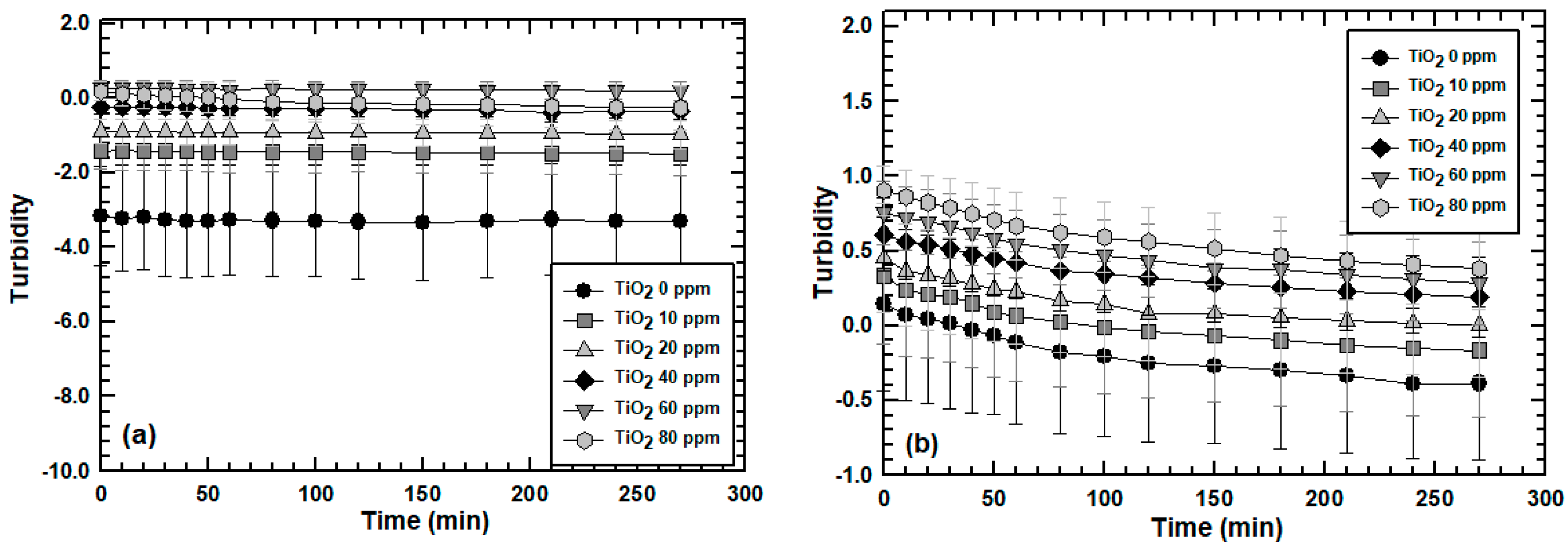
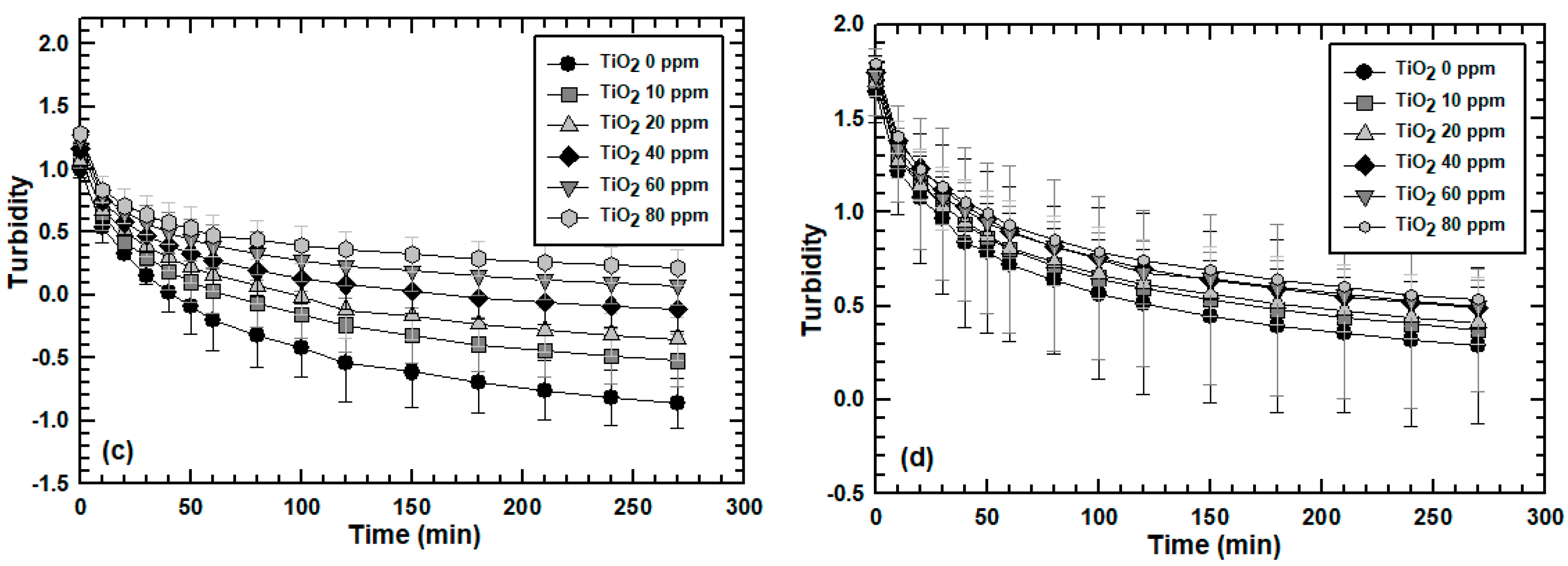
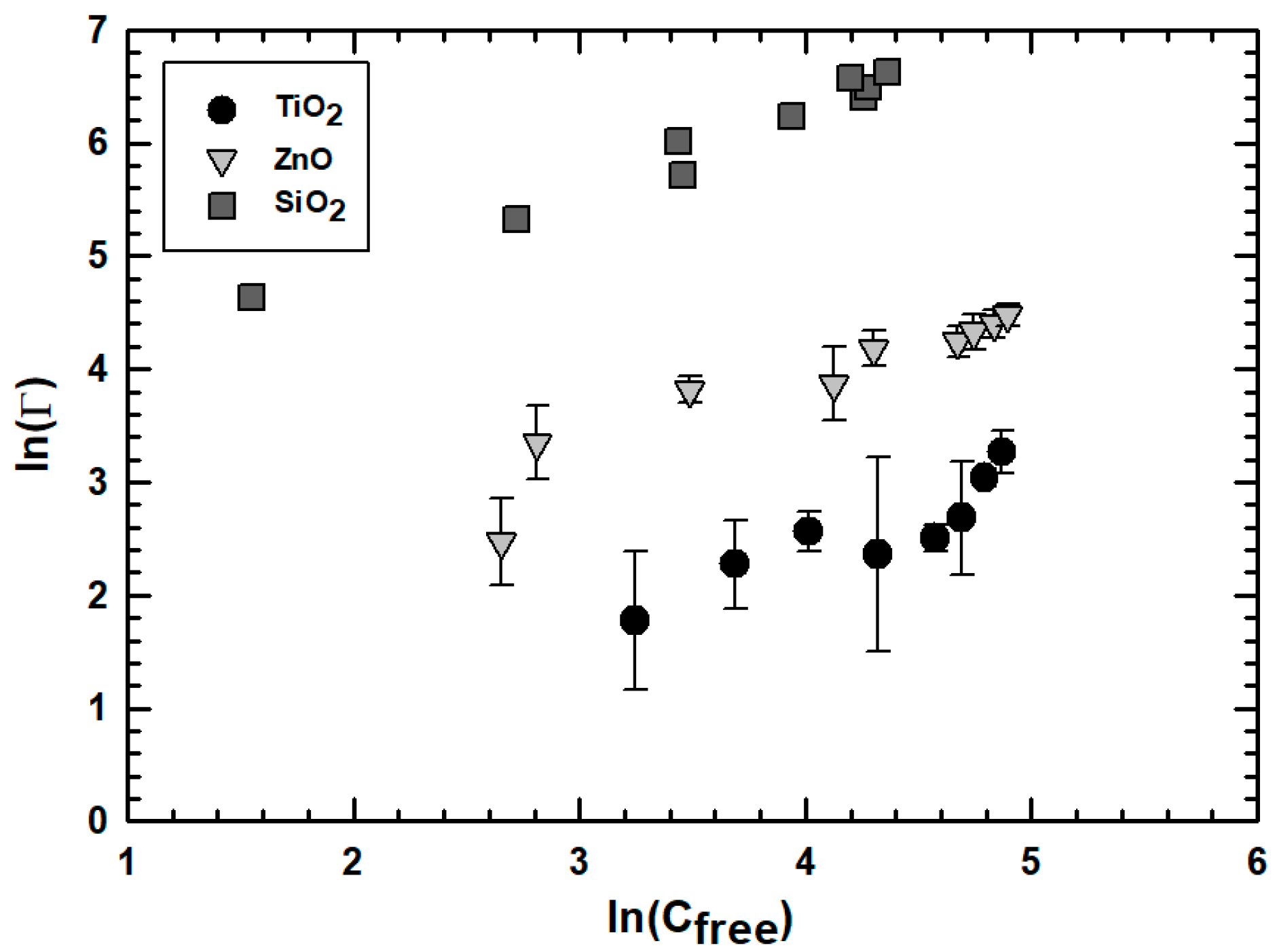
3.2. Effect of Engineered Nanoparticle Type: ZnO, TiO2, SiO2
3.3. Effect of MLSS Concentration on Engineered Nanoparticle Sorption
3.4. Effect of Ionic Strength on Engineered Nanoparticle Uptake
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, D.; Tian, X.; Wu, F.; Xing, B. Fate and Transport of Engineered Nanomaterials in the Environment. J. Environ. Qual. 2010, 39, 1896. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Gao, H.; Wu, J.; Yu, R. Effects of ZnO nanoparticles on flocculation and sedimentation of activated sludge in wastewater treatment process. Environ. Res. 2021, 192, 110256. [Google Scholar] [CrossRef]
- Zhou, L.; Zhuang, W.Q.; De Costa, Y.; Xia, S. Potential effects of suspended TiO2 nanoparticles on activated sludge floc properties in membrane bioreactors. Chemosphere 2019, 223, 148–156. [Google Scholar] [CrossRef]
- Yuan, L.; Li, Y.; Zeng, T.; Wang, D.; Liu, X.; Xu, Q.; Yang, Q.; Yang, F.; Chen, H. Revealing how the entering nano-titanium dioxide in wastewater worsened sludge dewaterability. Chem. Eng. J. 2021, 411, 128465. [Google Scholar] [CrossRef]
- Jiang, Y.; Shang, Y.; Zhang, W.; Zhang, X.; Li, J.; Shao, S. Assessing the effect of SiO2 and TiO2 nanoparticles on granule stability and microbial community shift in aerobic granular sludge process. Chemosphere 2022, 307, 135677. [Google Scholar] [CrossRef] [PubMed]
- Sibag, M.L.; Lee, S.M.; Lee, S.M.; Kim, H.J.; Cho, J. Retention of Silica Nanoparticles in a Lab-Scale Membrane Bioreactor: Implications for Process Performance and Membrane Fouling. Water 2016, 8, 277. [Google Scholar] [CrossRef] [Green Version]
- Cervantes-Avilés, P.; Ida, J.; Toda, T.; Cuevas-Rodríguez, G. Effects and fate of TiO2 nanoparticles in the anaerobic treatment of wastewater and waste sludge. J. Environ. Manag. 2018, 222, 227–233. [Google Scholar] [CrossRef]
- He, Q.; Yuan, Z.; Zhang, J.; Zhang, S.; Zhang, W.; Zou, Z.; Wang, H. Insight into the impact of ZnO nanoparticles on aerobic granular sludge under shock loading. Chemosphere 2017, 173, 411–416. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Eng, C.Y.; Stuckey, D.C.; Zhou, Y. Effects of ZnO nanoparticle exposure on wastewater treatment and soluble microbial products (SMPs) in an anoxic-aerobic membrane bioreactor. Chemosphere 2017, 171, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Auwerter, L.C.C.; Ouki, S.K.; Asaadi, M.; Shana, A. Effects of nanosized titanium dioxide (TiO2) and fullerene (C60) on wastewater microorganisms activity. J. Water Process Eng. 2017, 16, 35–40. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Wang, Q.; Hou, N.; Li, C.; Cheng, X. Toxicity of TiO2 nanoparticle to denitrifying strain CFY1 and the impact on microbial community structures in activated sludge. Chemosphere 2016, 144, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, X.; Ma, B.; Wang, S.; Zheng, D.; She, Z.; Guo, L.; Zhao, Y.; Xu, Q.; Jin, C.; et al. Long-term impacts of titanium dioxide nanoparticles (TiO2 NPs) on performance and microbial community of activated sludge. Bioresour. Technol. 2017, 238, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Su, Y.; Chen, Y. Acute and Chronic Responses of Activated Sludge Viability and Performance to Silica Nanoparticles. Environ. Sci. Technol. 2012, 46, 7182–7188. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Avilés, P.; Brito, E.M.S.; Duran, R.; Martínez, A.B.; Cuevas-Rodríguez, G. Effect of ZnO nanoparticles in the oxygen uptake during aerobic wastewater treatment. J. Nanoparticle Res. 2016, 18, 173. [Google Scholar] [CrossRef]
- Puay, N.Q.; Qiu, G.; Ting, Y.P. Effect of Zinc oxide nanoparticles on biological wastewater treatment in a sequencing batch reactor. J. Clean. Prod. 2015, 88, 139–145. [Google Scholar] [CrossRef]
- Lazareva, A.; Keller, A.A. Estimating Potential Life Cycle Releases of Engineered Nanomaterials from Wastewater Treatment Plants. ACS Sustain. Chem. Eng. 2014, 2, 1656–1665. [Google Scholar] [CrossRef]
- Gómez-Rivera, F.; Field, J.A.; Brown, D.; Sierra-Alvarez, R. Fate of cerium dioxide (CeO2) nanoparticles in municipal wastewater during activated sludge treatment. Bioresour. Technol. 2012, 108, 300–304. [Google Scholar] [CrossRef]
- Kiser, M.A.; Westerhoff, P.; Benn, T.; Wang, Y.; Pérez-Rivera, J.; Hristovski, K. Titanium Nanomaterial Removal and Release from Wastewater Treatment Plants. Environ. Sci. Technol. 2009, 43, 6757–6763. [Google Scholar] [CrossRef]
- Li, L.; Hartmann, G.; Döblinger, M.; Schuster, M. Quantification of Nanoscale Silver Particles Removal and Release from Municipal Wastewater Treatment Plants in Germany. Environ. Sci. Technol. 2013, 47, 7317–7323. [Google Scholar] [CrossRef]
- North East Biosolids and Residuals Association. A National Biosolids Regulation, Quality End Use and Disposal Survey; North East Biosolids and Residuals Association: Tamworth, NH, USA, 2007. [Google Scholar]
- Keller, A.A.; Lazareva, A. Predicted Releases of Engineered Nanomaterials: From Global to Regional to Local. Environ. Sci. Technol. Lett. 2014, 1, 65–70. [Google Scholar] [CrossRef] [Green Version]
- US EPA. Targeted National Sewage Sludge Survey—Overview Report; Environmental Protection; US EPA: Washington, DC, USA, 2009; Volume 88.
- Ge, Y.; Schimel, J.P.; Holden, P.A. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ. Sci. Technol. 2011, 45, 1659–1664. [Google Scholar] [CrossRef]
- Tian, L.; Shen, J.; Sun, G.; Wang, B.; Ji, R.; Zhao, L. Foliar Application of SiO2 Nanoparticles Alters Soil Metabolite Profiles and Microbial Community Composition in the Pakchoi (Brassica chinensis L.) Rhizosphere Grown in Contaminated Mine Soil. Environ. Sci. Technol. 2022, 54, 13137–13146. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.S.; Filser, J. Impacts of metal-based engineered nanomaterials on soil communities. Environ. Sci. Nano 2016, 3, 506–533. [Google Scholar] [CrossRef] [Green Version]
- Simonin, M.; Richaume, A.; Guyonnet, J.P.; Dubost, A.; Martins, J.M.F.; Pommier, T. Titanium dioxide nanoparticles strongly impact soil microbial function by affecting archaeal nitrifiers. Sci. Rep. 2016, 6, 33643. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Huang, Y.C.; Grusak, M.A.; Huang, C.P.; Sherrier, D.J. Effects of nano-TiO2 on the agronomically-relevant Rhizobium–legume symbiosis. Sci. Total Environ. 2014, 466–467, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Fan, R.; Grusak, M.A.; Sherrier, J.D.; Huang, C.P. Effects of nano-ZnO on the agronomically relevant Rhizobium-legume symbiosis. Sci. Total Environ. 2014, 497–498, 78–90. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Borrás, M.C.; Sluyter, R.; Barker, P.J.; Konstantinov, K.; Bakand, S. Y2O3 decorated TiO2 nanoparticles: Enhanced UV attenuation and suppressed photocatalytic activity with promise for cosmetic and sunscreen applications. J. Photochem. Photobiol. B Biol. 2020, 207, 111883. [Google Scholar] [CrossRef]
- Cheng, X.; Kan, A.T.; Tomson, M.B. Study of C60 transport in porous media and the effect of sorbed C60 on naphthalene transport. J. Mater. Res. 2005, 20, 3244–3254. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.; Lee, S. TiO2 nanoparticle sorption to sand in the presence of natural organic matter. Environ. Earth Sci. 2015, 73, 5585–5591. [Google Scholar] [CrossRef]
- Klitzke, S.; Metreveli, G.; Peters, A.; Schaumann, G.E.; Lang, F. The fate of silver nanoparticles in soil solution—Sorption of solutes and aggregation. Sci. Total Environ. 2015, 535, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brant, J.; Lecoanet, H.; Wiesner, M.R. Aggregation and Deposition Characteristics of Fullerene Nanoparticles in Aqueous Systems. J. Nanoparticle Res. 2005, 7, 545–553. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Pennell, K.D.; Abriola, L.M. Investigation of the Transport and Deposition of Fullerene (C60) Nanoparticles in Quartz Sands under Varying Flow Conditions. Environ. Sci. Technol. 2008, 42, 7174–7180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Pennell, K.D. Influence of electrolyte species and concentration on the aggregation and transport of fullerene nanoparticles in quartz sands. Environ. Toxicol. Chem. 2008, 27, 1860. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Elimelech, M. Aggregation and Deposition Kinetics of Fullerene (C60) Nanoparticles. Langmuir 2006, 22, 10994–11001. [Google Scholar] [CrossRef]
- Jiang, X.; Tong, M.; Li, H.; Yang, K. Deposition kinetics of zinc oxide nanoparticles on natural organic matter coated silica surfaces. J. Colloid Interface Sci. 2010, 350, 427–434. [Google Scholar] [CrossRef]
- Nicolosi, V.; Vrbanic, D.; Mrzel, A.; McCauley, J.; O’Flaherty, S.; McGuinness, C.; Compagnini, G.; Mihailovic, D.; Blau, W.J.; Coleman, J.N. Solubility of Mo6S4.5I4.5 nanowires in common solvents: A sedimentation study. J. Phys. Chem. B 2005, 109, 7124–7133. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; McGinley, P.M.; Katz, L.E. Sorption phenomena in subsurface systems: Concepts, models and effects on contaminant fate and transport. Water Res. 1991, 25, 499–528. [Google Scholar] [CrossRef] [Green Version]
- Petosa, A.R.; Brennan, S.J.; Rajput, F.; Tufenkji, N. Transport of two metal oxide nanoparticles in saturated granular porous media: Role of water chemistry and particle coating. Water Res. 2012, 46, 1273–1285. [Google Scholar] [CrossRef] [Green Version]
- Tipping, E.; Higgins, D.C. The effect of adsorbed humic substances on the colloid stability of haematite particles. Colloids Surf. 1982, 5, 85–92. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Ji, Z.; Jiang, X.; Dunphy, D.R.; Brinker, J.; Keller, A.A. Influence of Material Properties on TiO2 Nanoparticle Agglomeration. PLoS ONE 2013, 8, e81239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingos, R.F.; Tufenkji, N.; Wilkinson, K.J. Aggregation of Titanium Dioxide Nanoparticles: Role of a Fulvic Acid. Environ. Sci. Technol. 2009, 43, 1282–1286. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Villa, F.; Checca-Huaman, N.R.; Ramos-Guivar, J.A. Ecotoxicological Properties of Titanium Dioxide Nanomorphologies in Daphnia magna. Nanomaterials 2023, 13, 927. [Google Scholar] [CrossRef]
- Lindahl, M.; Faris, A.; Wadström, T.; Hjertén, S. A new test based on “salting out” to measure relative surface hydrophobicity of bacterial cells. Biochim. Biophys. Acta 1981, 677, 471–476. [Google Scholar] [CrossRef]
- Forster, C.F. Bound water in sewage sludges and its relationship to sludge surfaces and sludge viscosities. J. Chem. Technol. Biotechnol. Biotechnol. 1983, 33, 76–84. [Google Scholar] [CrossRef]
- Romanello, M.B.; Fidalgo de Cortalezzi, M.M. An experimental study on the aggregation of TiO2 nanoparticles under environmentally relevant conditions. Water Res. 2013, 47, 3887–3898. [Google Scholar] [CrossRef]
- Sanin, D.F. Effect of solution physical chemistry on the rheological properties of activated sludge. Water SA 2002, 28, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-H.; Ganczarczyk, J.J. Structure of activated sludge floes. Biotechnol. Bioeng. 1990, 35, 57–65. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.Y. Investigation of the effect of nanoparticle exposure on the flocculability of activated sludge using particle image volecimetry in combination with the extended DLVO analysis. Colloids Surf. B Biointerfaces 2016, 143, 382–389. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Hankins, N.P.; Hilal, N. A comparative study of the flocculation behaviour and final properties of synthetic and activated sludge in wastewater treatment. Desalination 2007, 204, 277–295. [Google Scholar] [CrossRef]
- Örmeci, B.; Vesilind, P.A. Development of an improved synthetic sludge: A possible surrogate for studying activated sludge dewatering characteristics. Water Res. 2000, 34, 1069–1078. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Huang, B.-C.; Zhou, T.; Liu, Y.-C.; Shi, H.-C. Aggregation behavior of engineered nanoparticles and their impact on activated sludge in wastewater treatment. Chemosphere 2015, 119, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Tseng, Y.H.; Huang, C.-P. Interactions between nano-TiO2 particles and algal cells at moderate particle concentration. Front. Chem. Sci. Eng. 2015, 9, 242–257. [Google Scholar] [CrossRef]
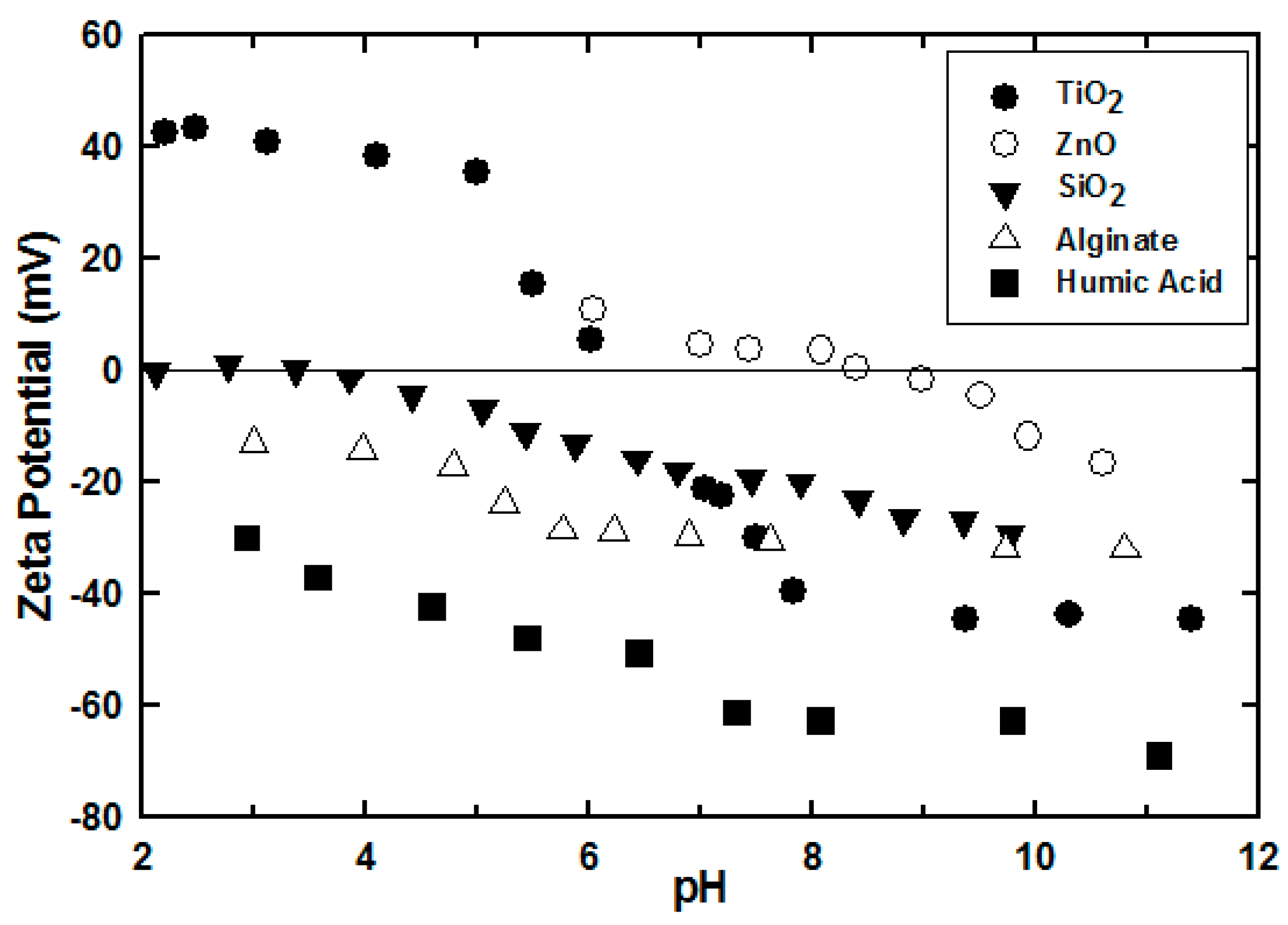

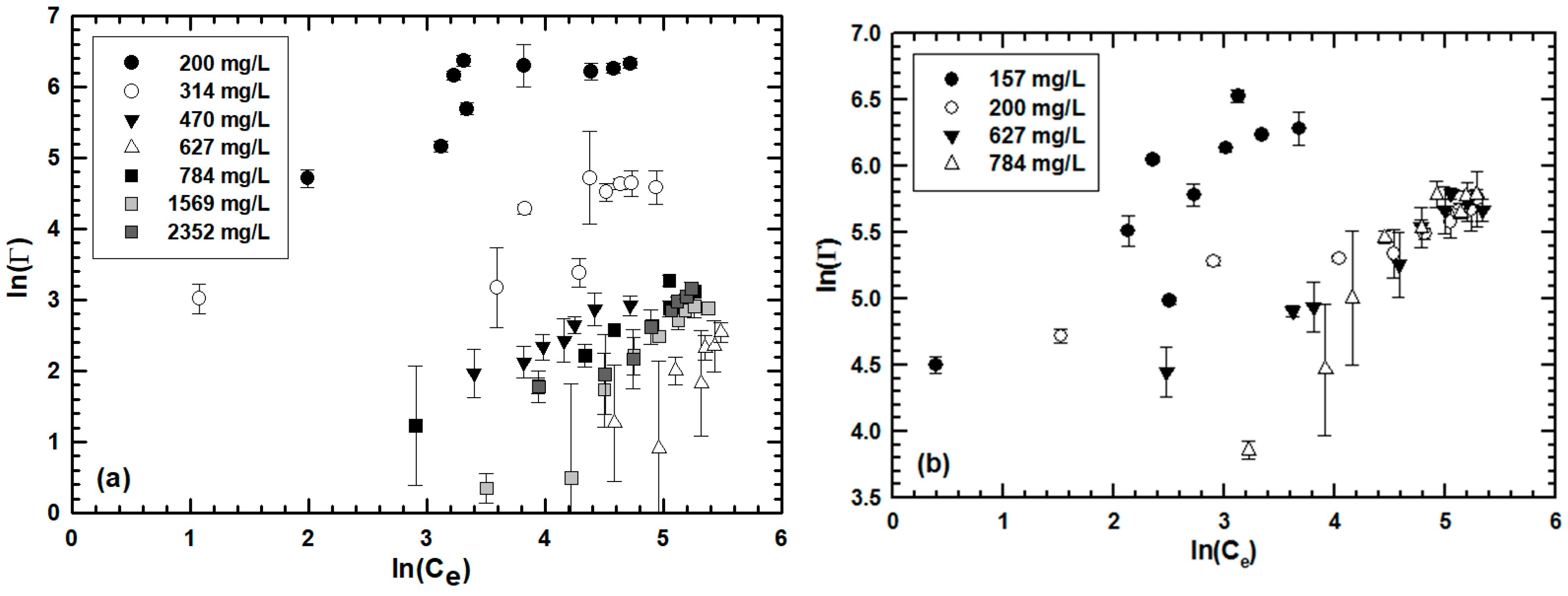
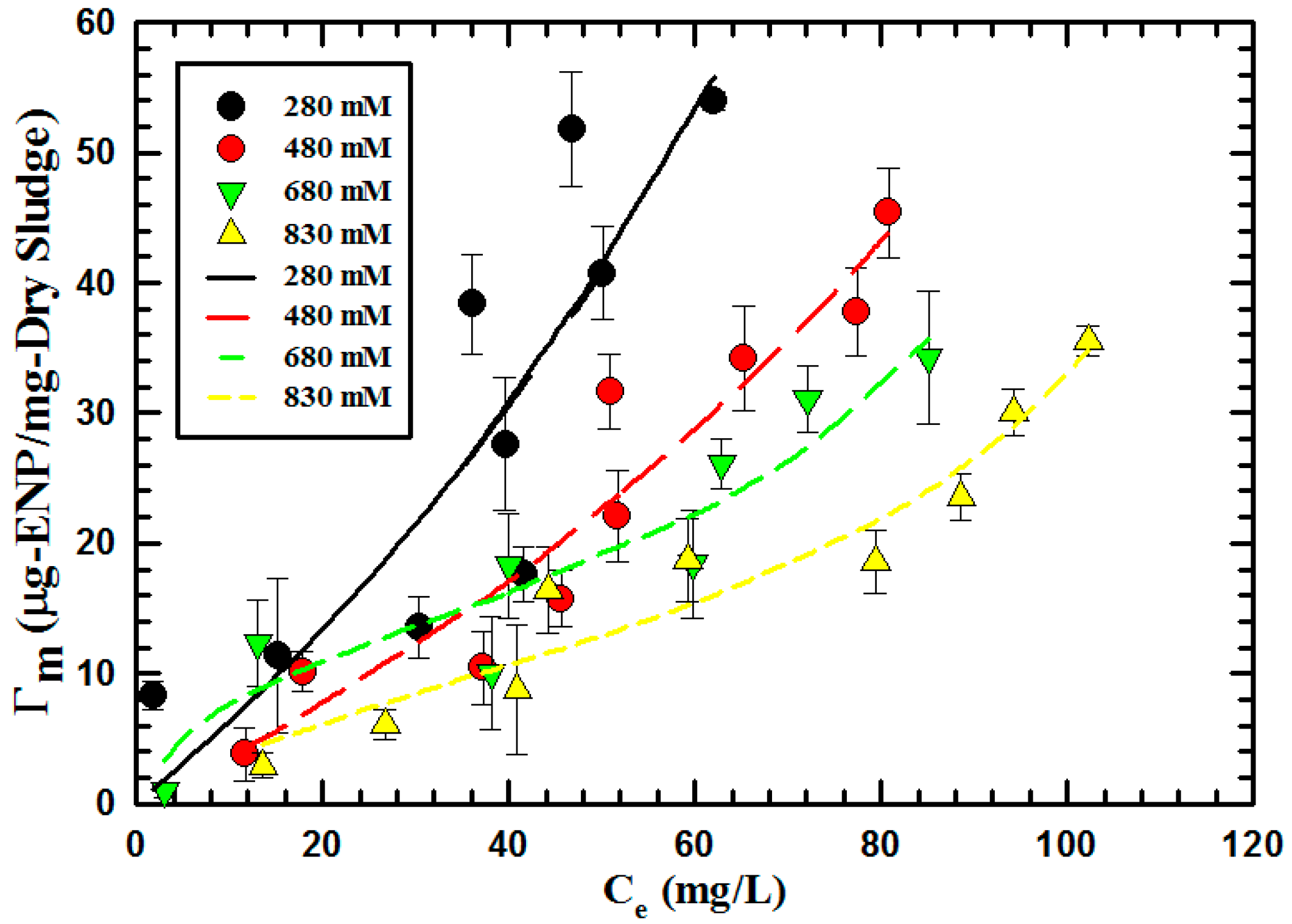
| NaCl (mM) | 280 | 480 | 680 | 830 | |
|---|---|---|---|---|---|
| BET | Γm (10−10 mol-ENP/mg-Dry sludge) | 210.03 | 121.12 | 60.59 | 9.48 |
| K1 (1010 M−1) | 0.0028 | 0.0073 | 0.016 | 0.045 | |
| K2 (1010 M−1) | 0.0051 | 0.0053 | 0.0074 | 0.0083 | |
| R2 | 72.51 | 96.78 | 99.51 | 93.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Johnston, M.; Wang, G.-S.; Huang, C.-P. The Uptake of Engineered Nanoparticles by Sludge Particulates. Water 2023, 15, 2872. https://doi.org/10.3390/w15162872
Choi S, Johnston M, Wang G-S, Huang C-P. The Uptake of Engineered Nanoparticles by Sludge Particulates. Water. 2023; 15(16):2872. https://doi.org/10.3390/w15162872
Chicago/Turabian StyleChoi, Soohoon, Murray Johnston, Gen-Shuh Wang, and Chin-Pao Huang. 2023. "The Uptake of Engineered Nanoparticles by Sludge Particulates" Water 15, no. 16: 2872. https://doi.org/10.3390/w15162872
APA StyleChoi, S., Johnston, M., Wang, G.-S., & Huang, C.-P. (2023). The Uptake of Engineered Nanoparticles by Sludge Particulates. Water, 15(16), 2872. https://doi.org/10.3390/w15162872









