Assessing the Dual Use of Red and Yellow Algerian Pomegranate Husks: Natural Antiradical Agents and Low-Cost Biosorbents for Chromium (VI) Removal from Contaminated Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Biosorbents
2.3. Preparation of Stock Solution
2.4. Concentration Determination Method
2.5. Biosorbents Characterization
2.6. Quencher-Free Radical Scavenging Activity
2.7. Biosorption Studies
2.8. Isotherms Modeling
2.9. Kinetics Modeling
2.10. Statistical Analysis
3. Results
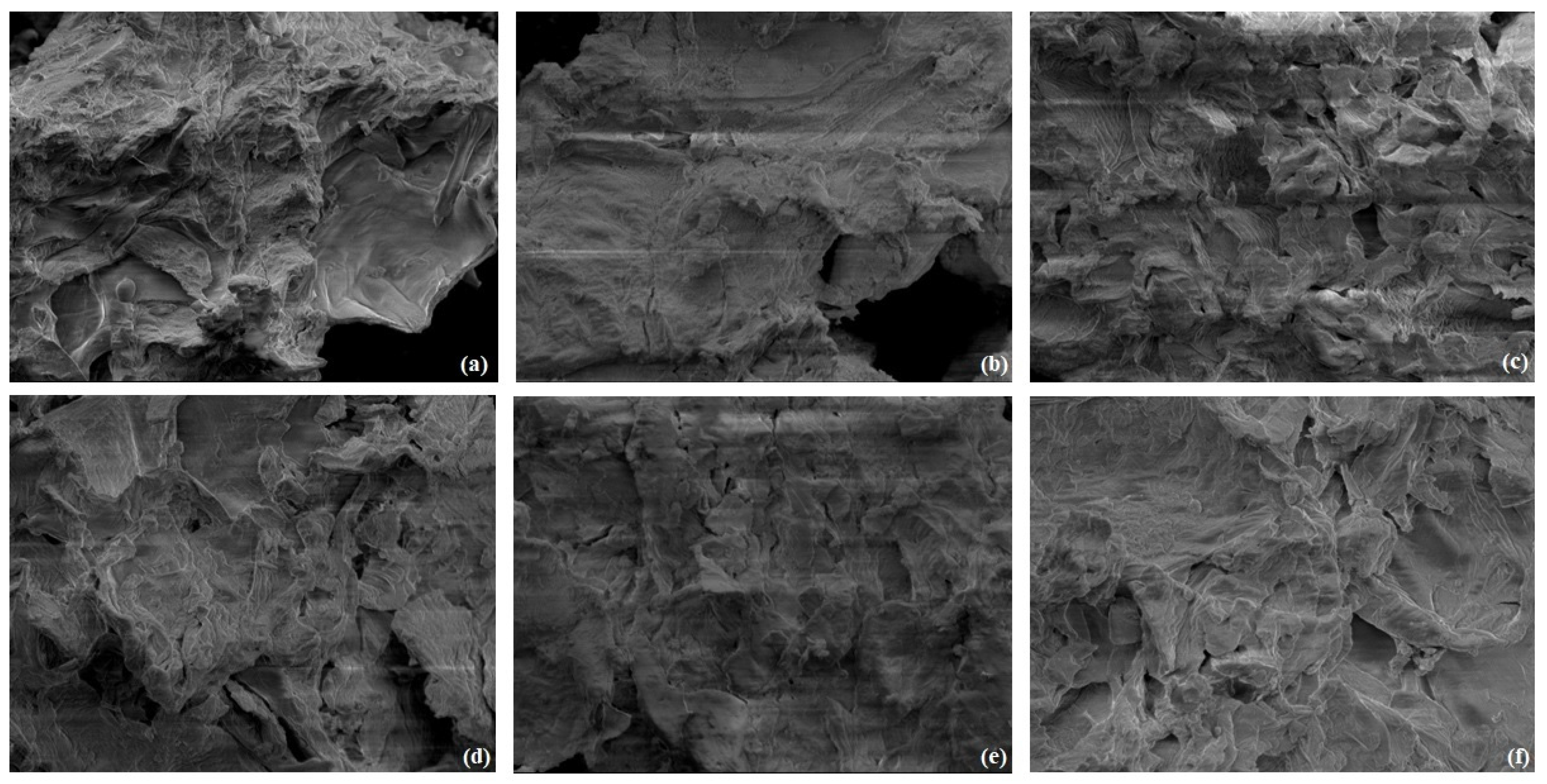
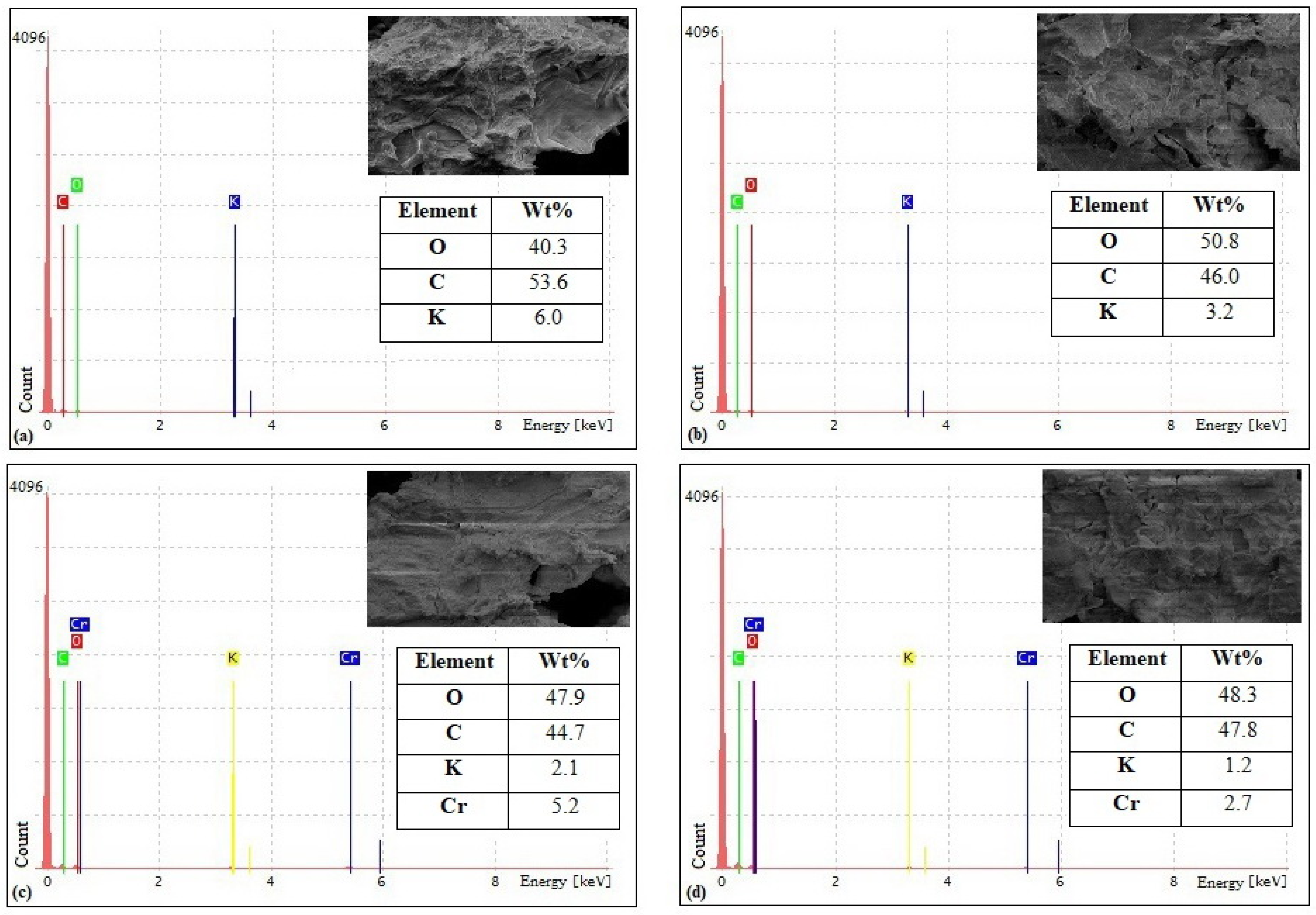
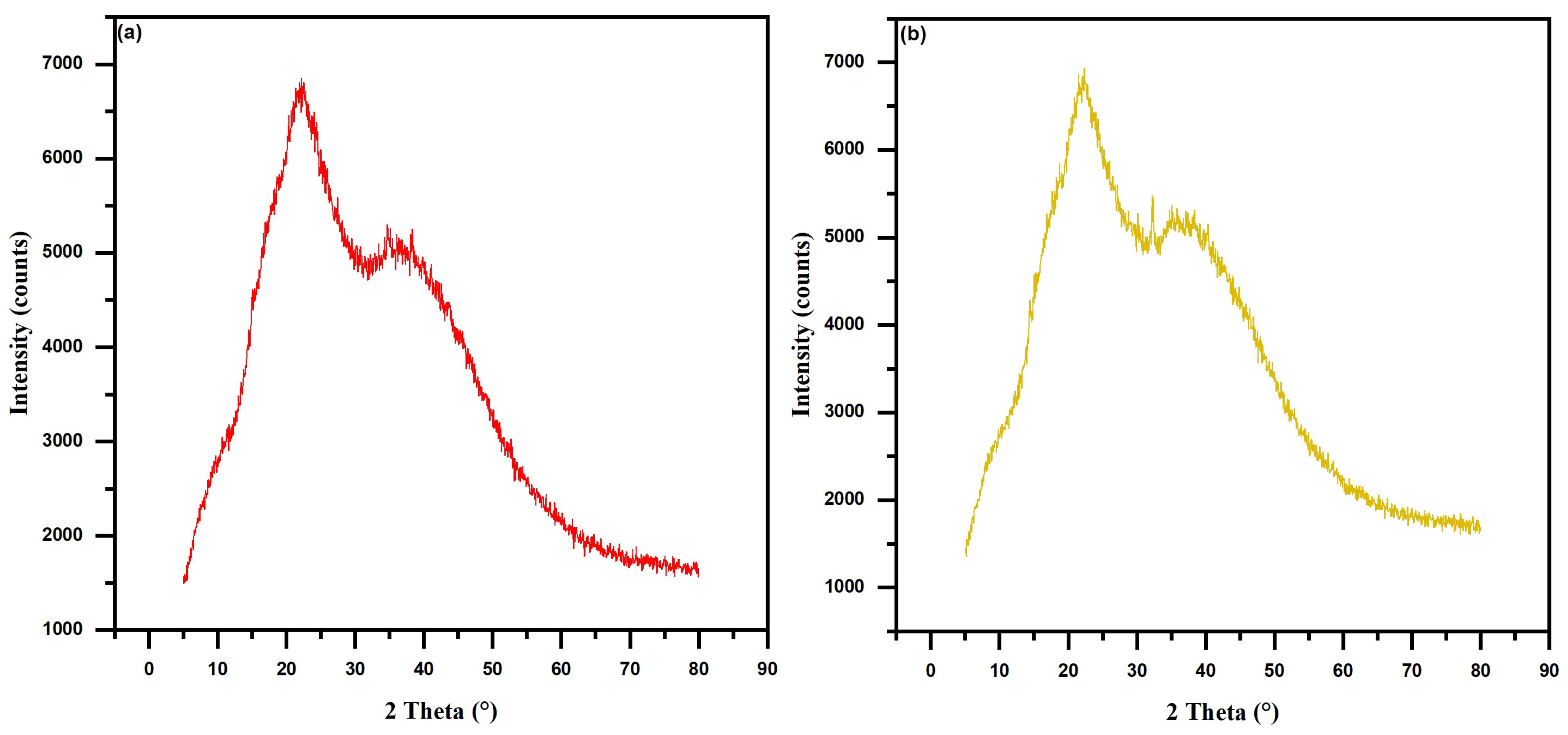
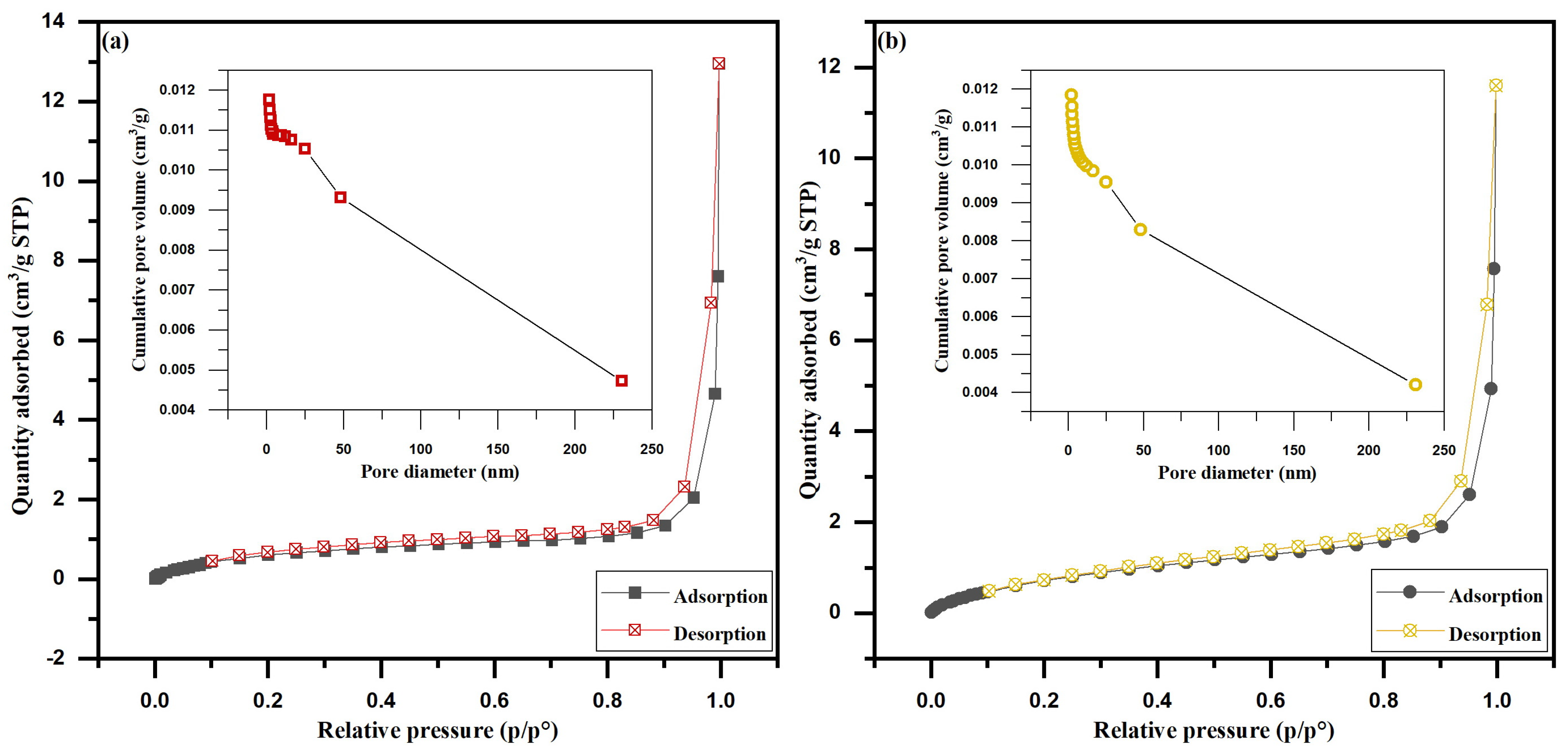
3.1. Biosorbents Characterization
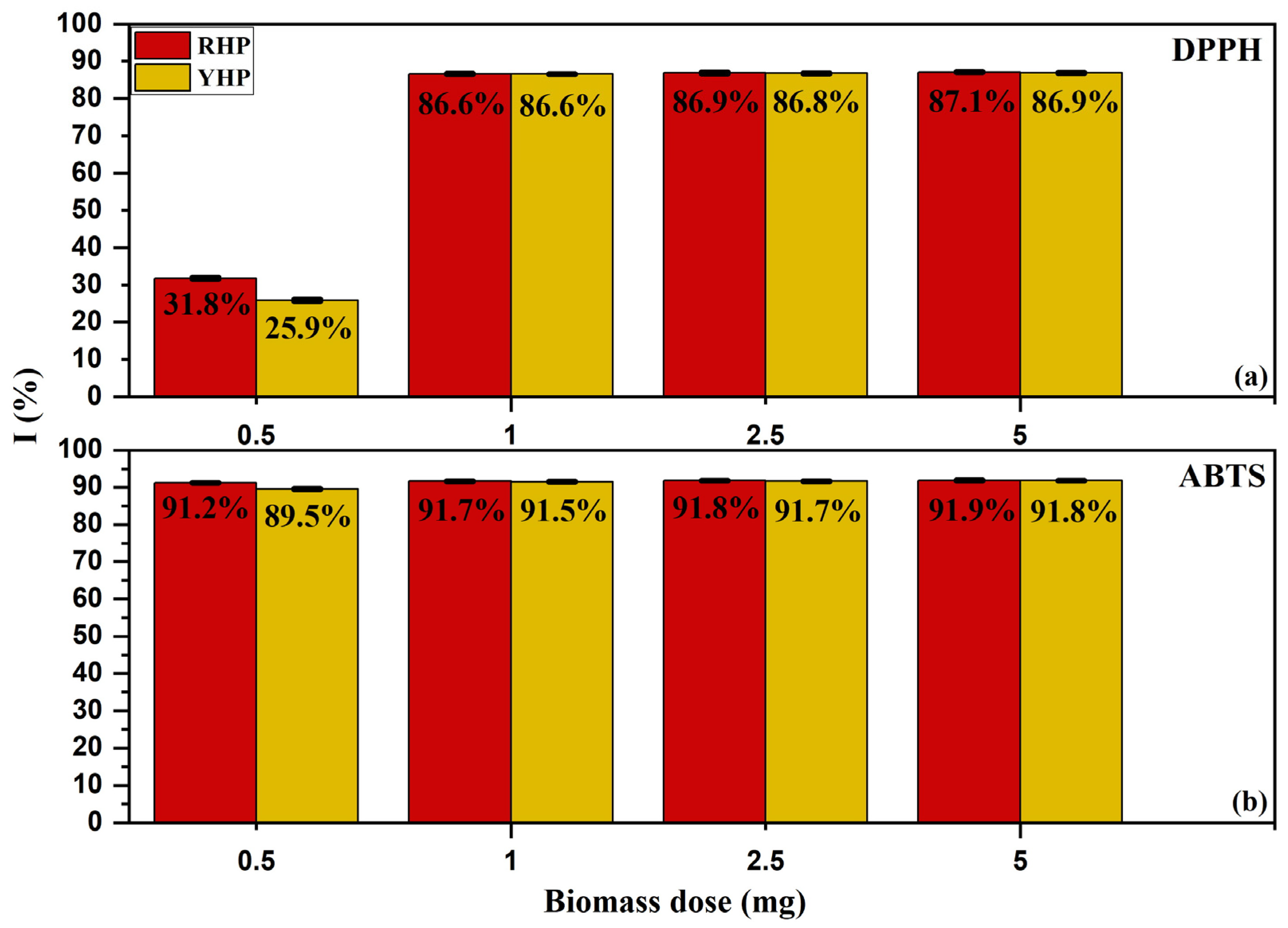
3.2. Quencher-Free Radical Scavenging Activity
3.3. Biosorption Studies
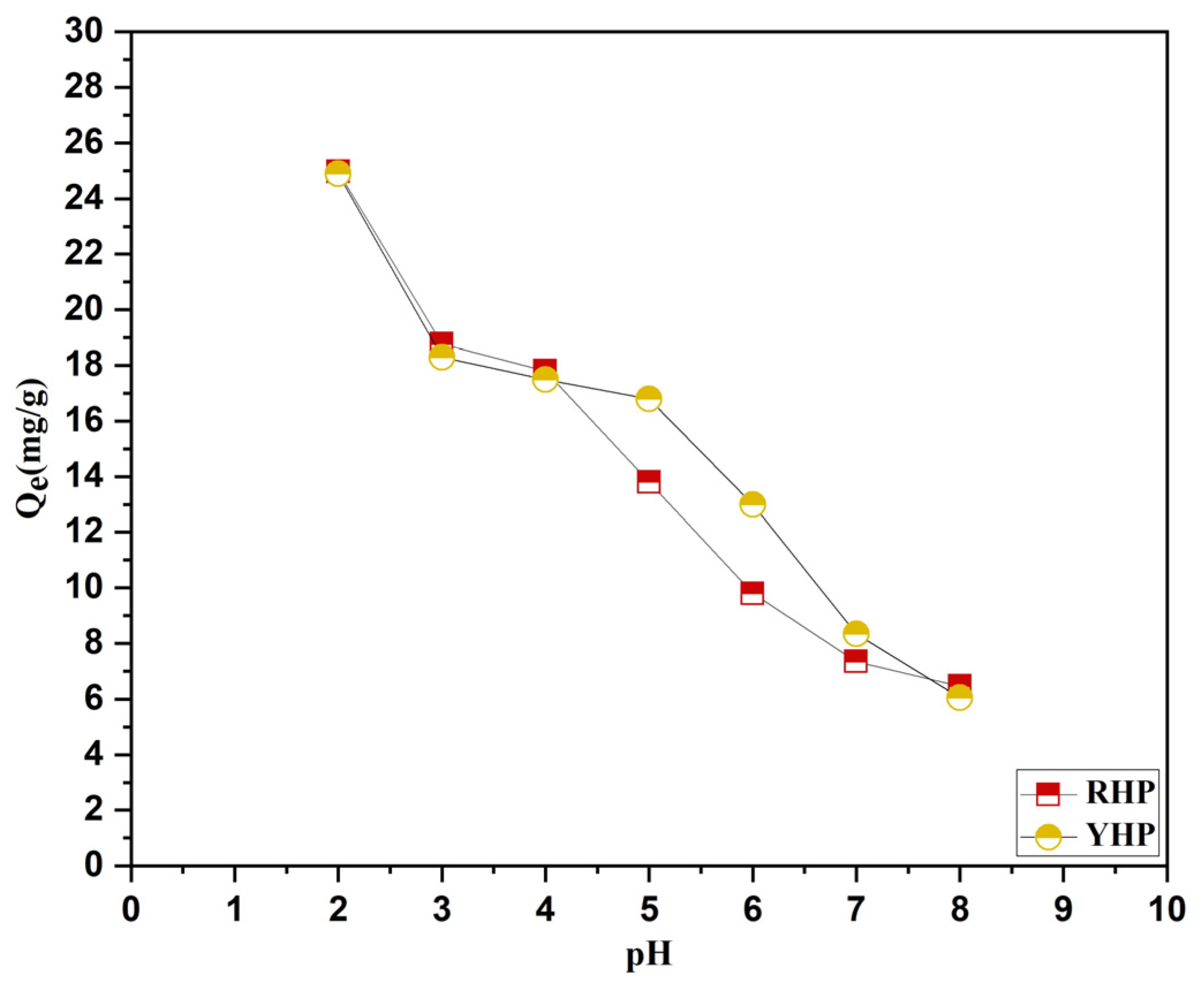
3.3.1. pH Effect
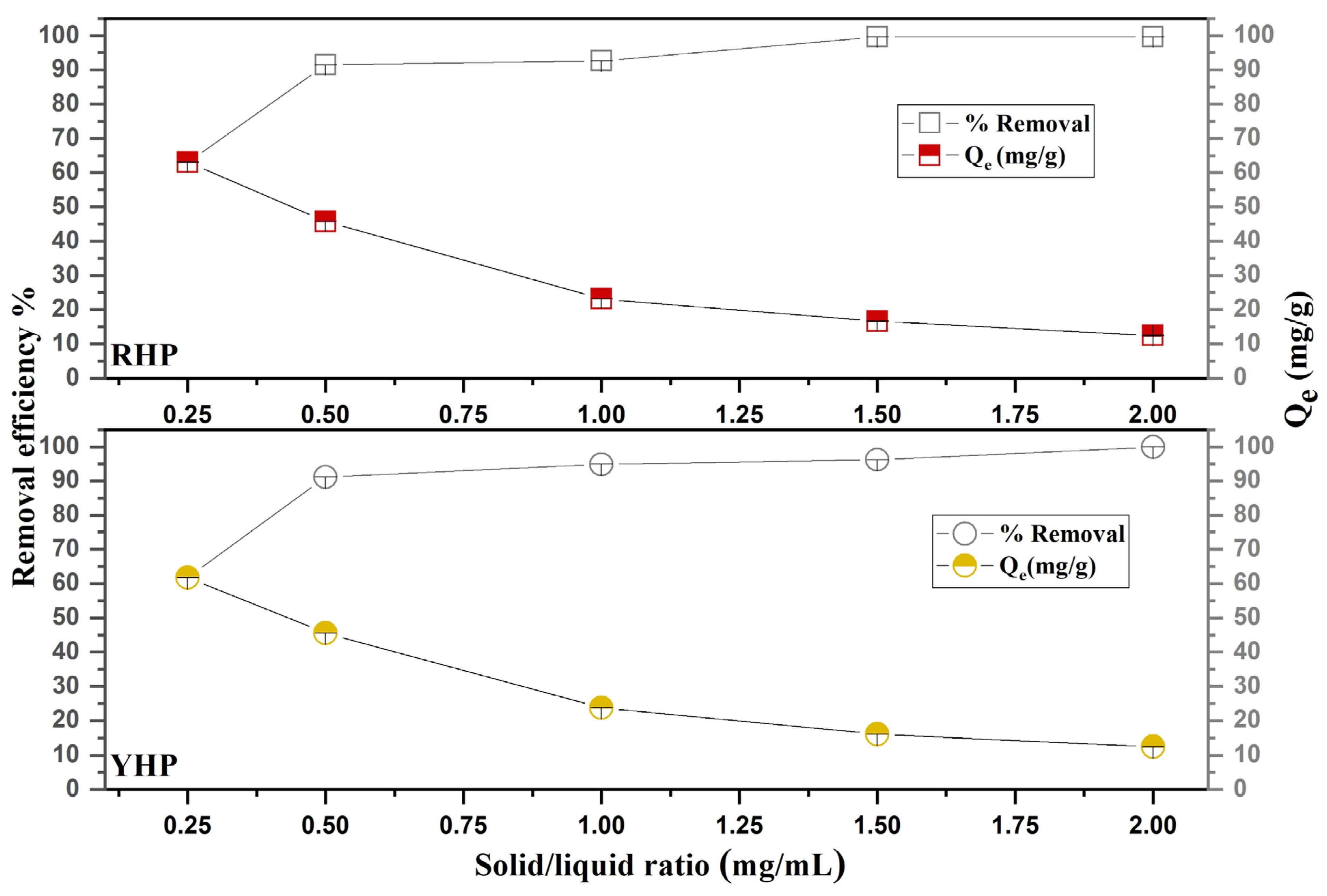
3.3.2. Biosorbent Dose and Solid/Liquid Ratio Effect
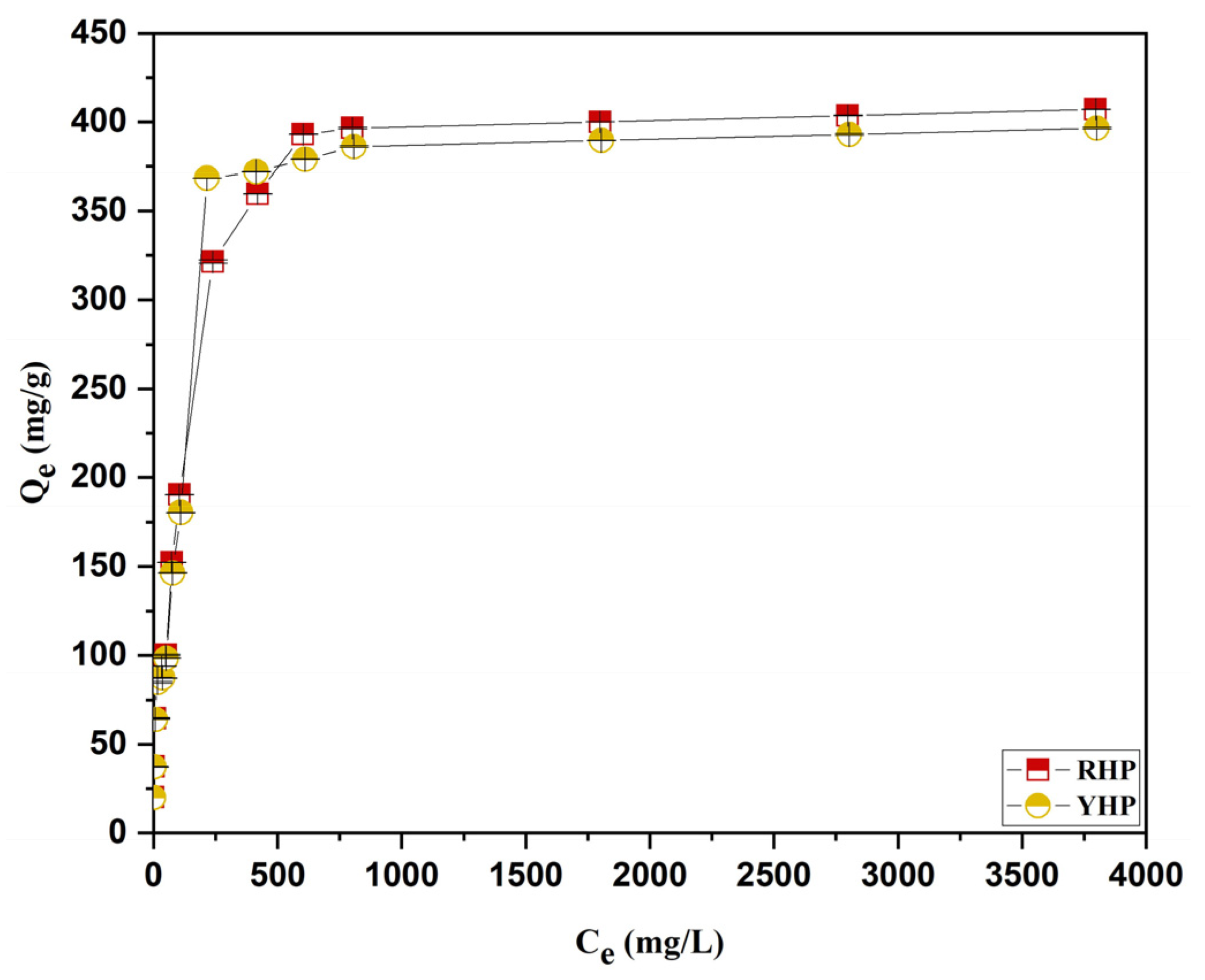
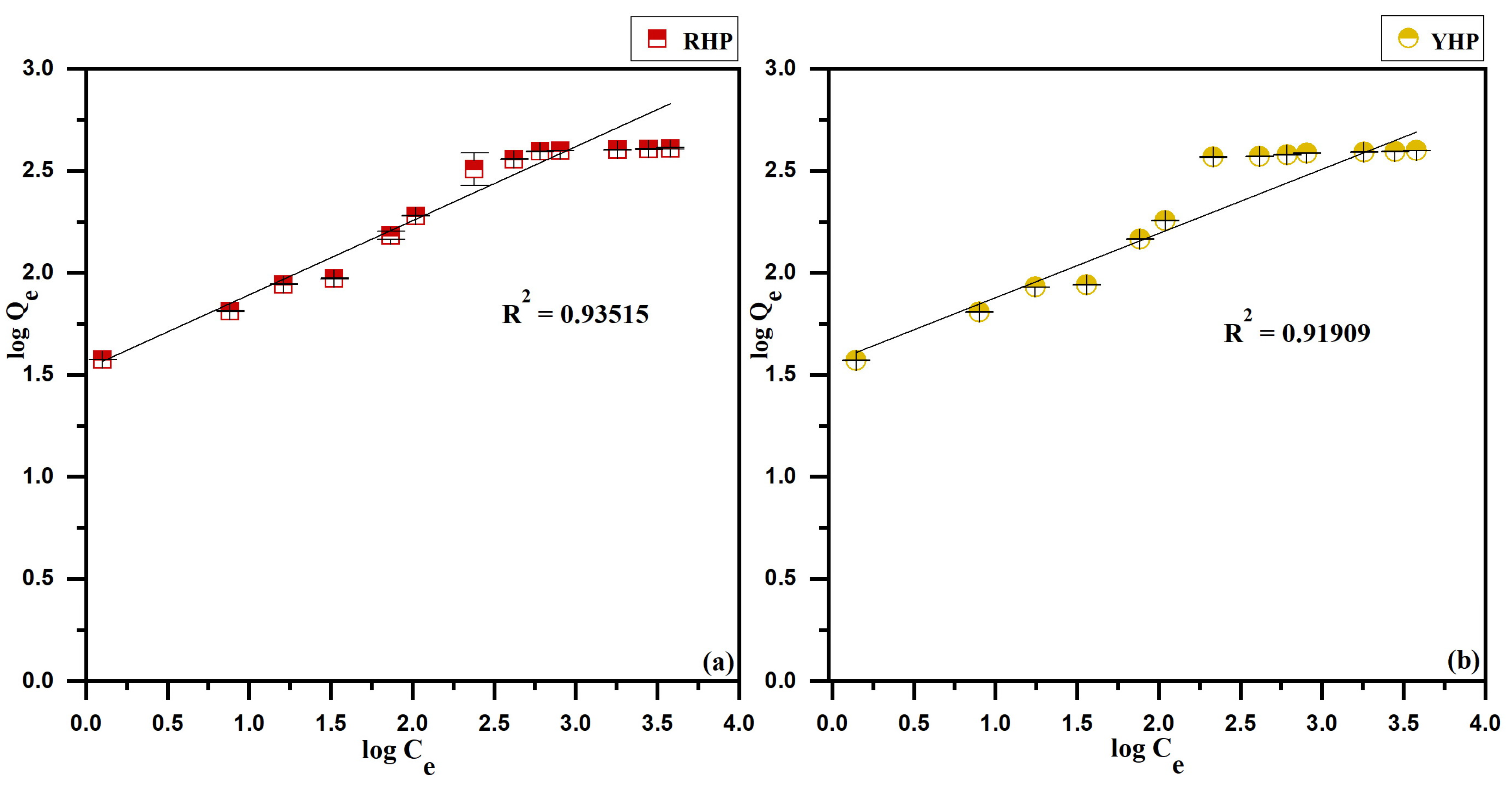
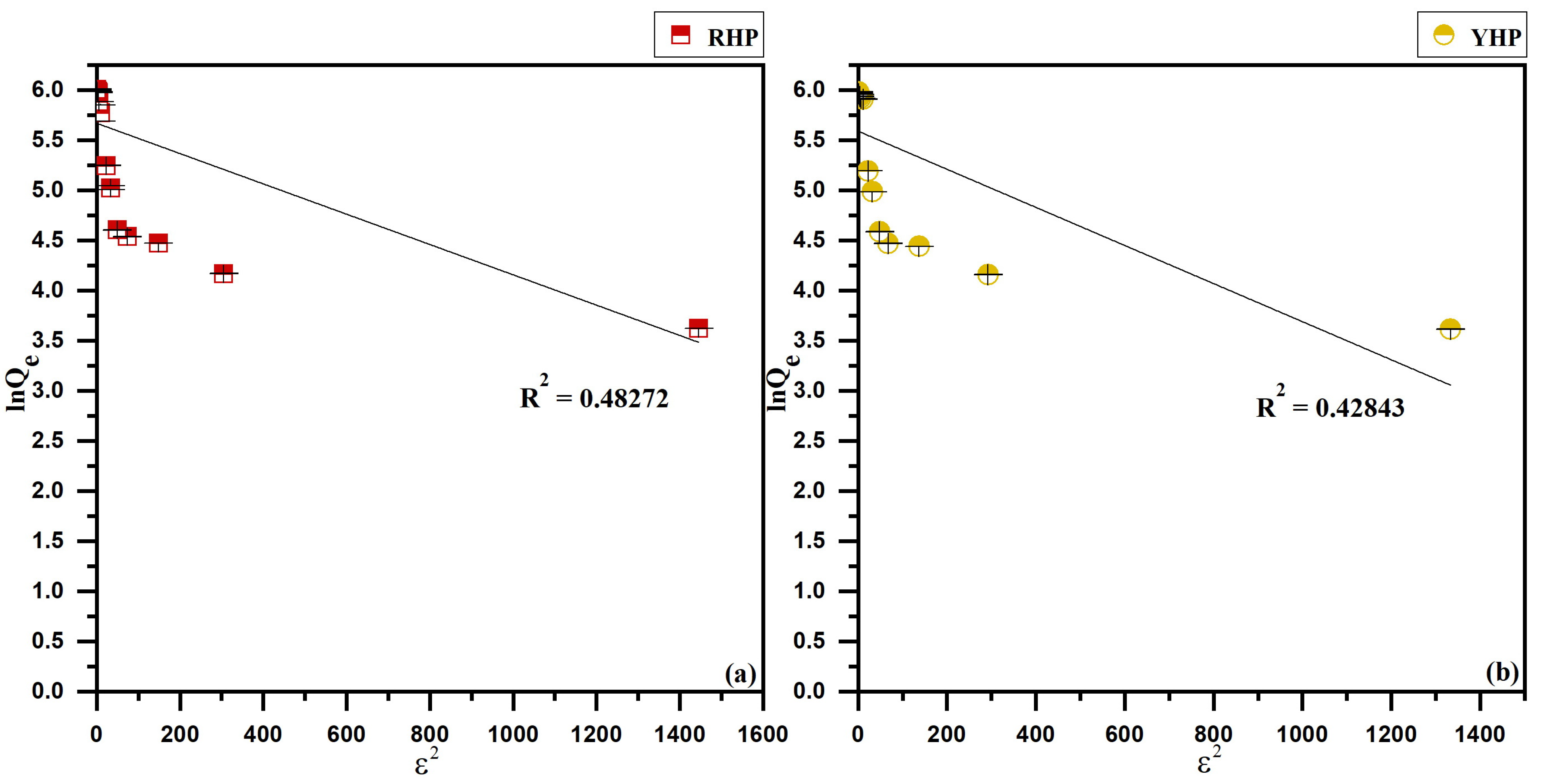
3.4. Isotherms Modeling
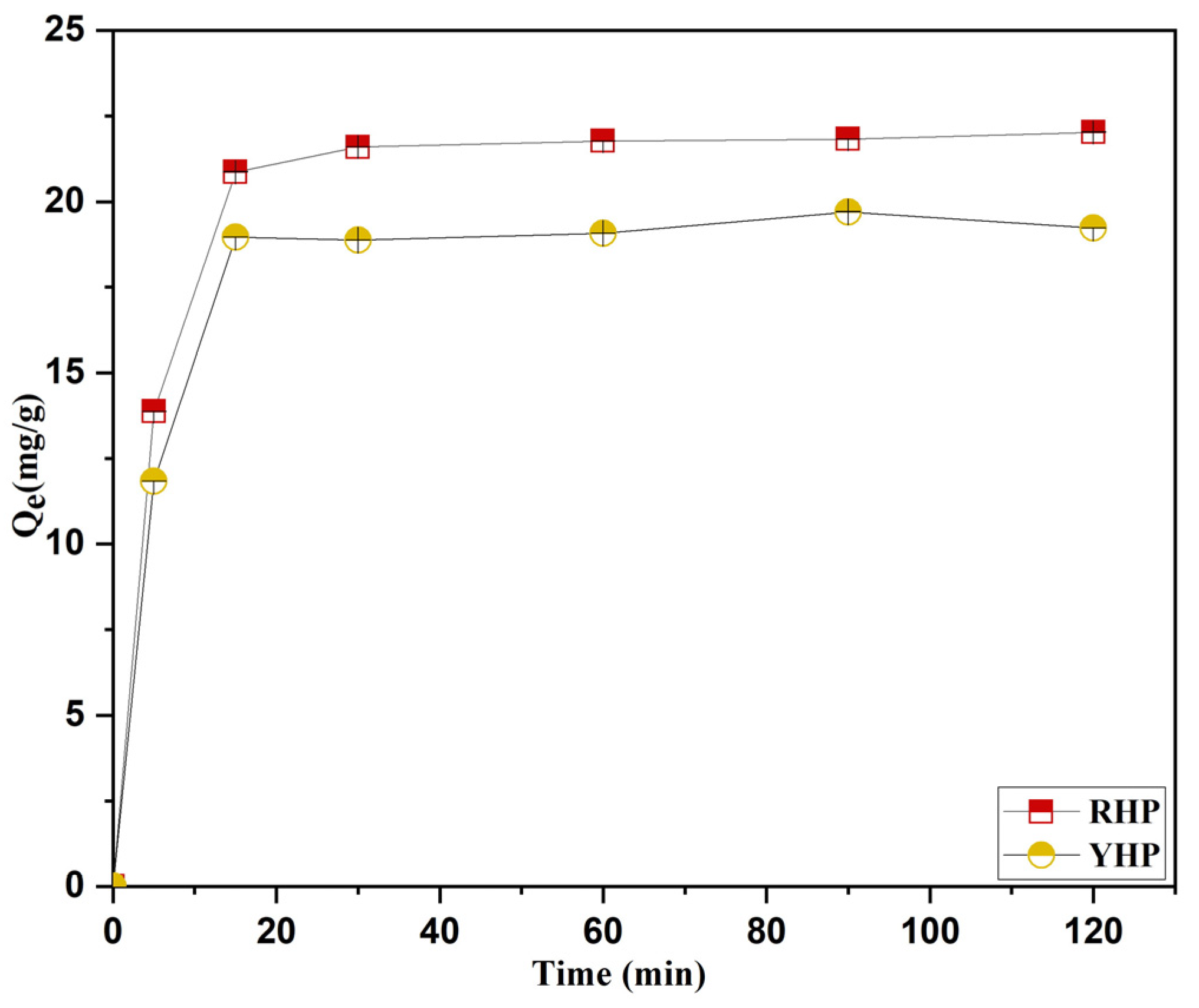
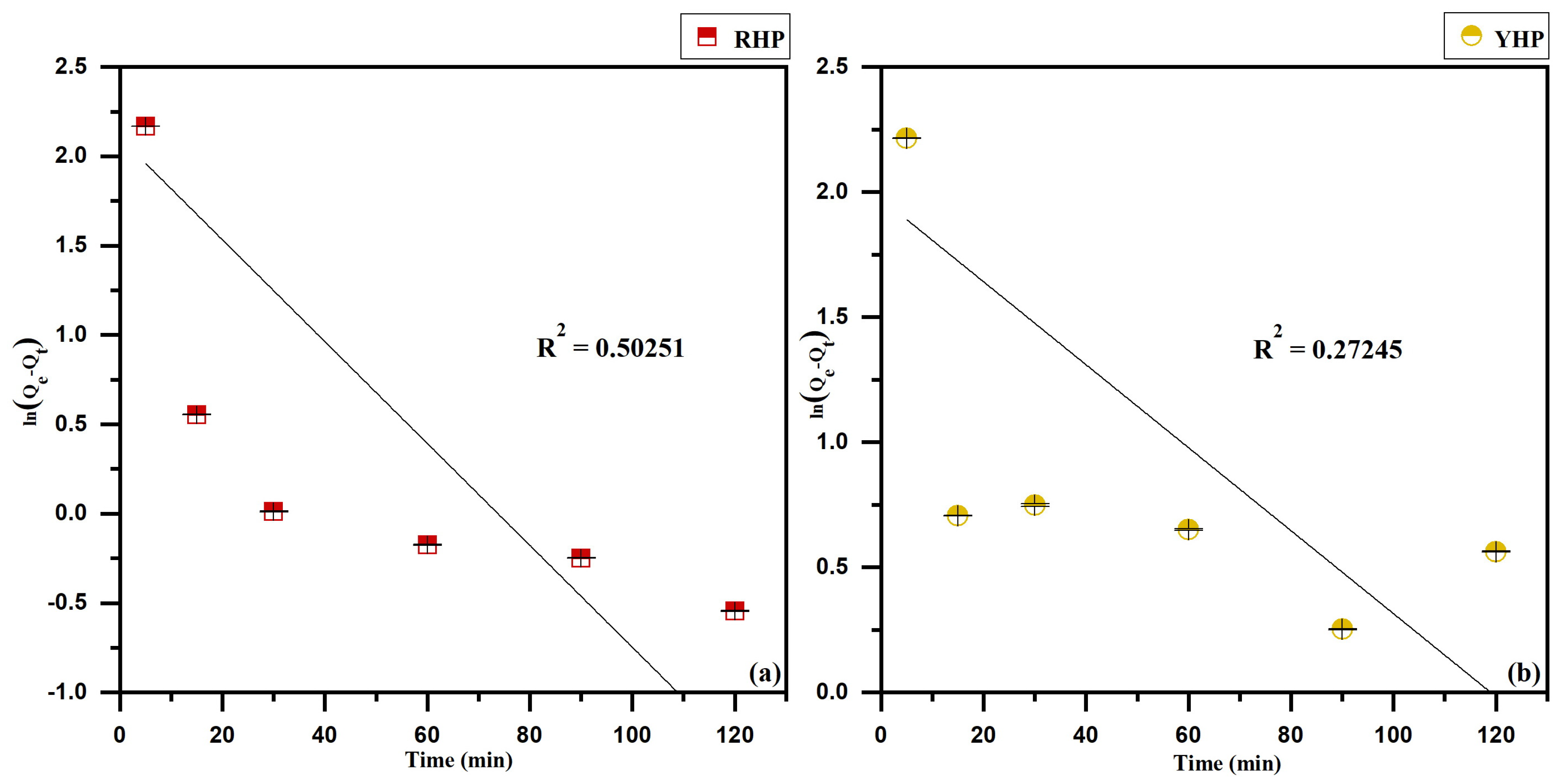
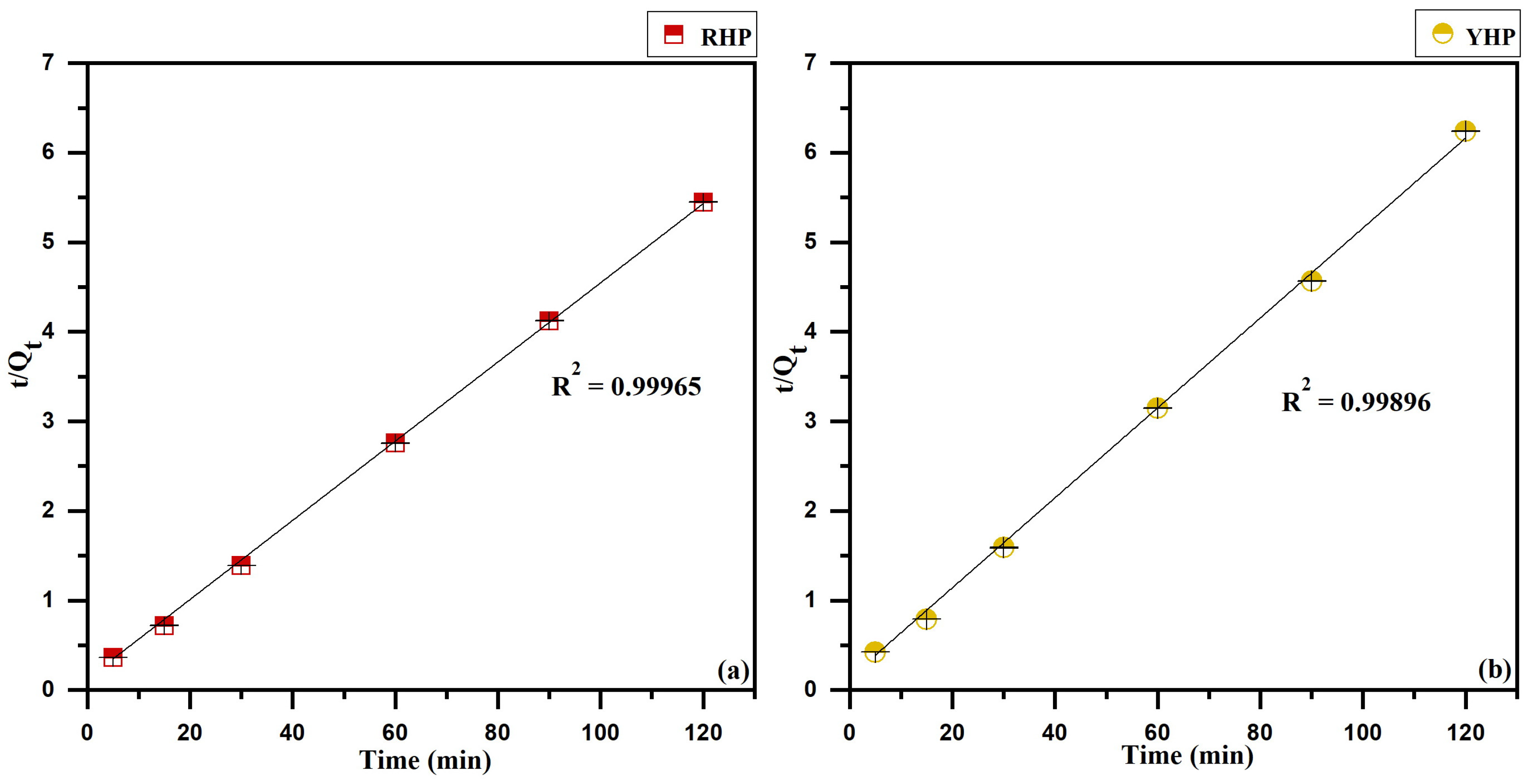
3.5. Kinetics Modeling
4. Discussion
4.1. Biosorbents Characterization
4.2. Quencher-Free Radical Scavenging Activity
4.3. Biosorption Optimization
4.3.1. pH Effect
4.3.2. Biosorbent Dose and Solid/Liquid Ratio Effect
4.4. Biosorption Isotherms
4.5. Biosorption Kinetics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Zhou, H.; Ouyang, T.; Guo, Y.; Peng, S.; He, C.; Zhu, Z. Assessment of Soil Heavy Metal Pollution and Its Ecological Risk for City Parks, Vicinity of a Landfill, and an Industrial Area within Guangzhou, South China. Appl. Sci. 2022, 12, 9345. [Google Scholar] [CrossRef]
- Pavesi, T.; Moreira, J.C. Mechanisms and Individuality in Chromium Toxicity in Humans. J. Appl. Toxicol. 2020, 40, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Gao, Y.; Xue, K.; Qi, Y.; Fan, Y.; Tian, X.; Wang, J.; Zhao, R.; Zhang, P.; Liu, Y.; et al. Toxicity Mechanisms and Re-mediation Strategies for Chromium Exposure in the Environment. Front. Environ. Sci. 2023, 11, 1131204. [Google Scholar] [CrossRef]
- Shammout, M.; Shatanawi, M.; Awwad, A. Fate and Management of Pollution of Hexavalent Chromium Cr(VI) and Heavy Metals in the Zarqa River Basin in Jordan. J. Ecol. Eng. 2022, 23, 108–115. [Google Scholar] [CrossRef]
- Georgaki, M.N.; Charalambous, M. Toxic Chromium in Water and the Effects on the Human Body: A Systematic Review. J. Water Health 2023, 21, 205–223. [Google Scholar] [CrossRef]
- Shin, D.Y.; Lee, S.M.; Jang, Y.; Lee, J.; Lee, C.M.; Cho, E.-M.; Seo, Y.R. Adverse Human Health Effects of Chromium by Exposure Route: A Comprehensive Review Based on Toxicogenomic Approach. Int. J. Mol. Sci. 2023, 24, 3410. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The Double Face of Metals: The Intriguing Case of Chromium. Appl. Sci. 2021, 11, 638. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N.; Verma, M.; Kamal, R.; Tiwari, R.; Sanjay Chivate, M.; Rai, S.N.; Kumar, A.; Singh, A.; Singh, M.P.; et al. Hexavalent-Chromium-Induced Oxidative Stress and the Protective Role of Antioxidants against Cellular Toxicity. Antioxidants 2022, 11, 2375. [Google Scholar] [CrossRef]
- Husain, N.; Mahmood, R. Hexavalent Chromium Induces Reactive Oxygen Species and Impairs the Antioxidant Power of Human Erythrocytes and Lymphocytes: Decreased Metal Reducing and Free Radical Quenching Ability of the Cells. Toxicol. Ind. Health 2017, 33, 623–635. [Google Scholar] [CrossRef]
- Georgaki, M.-N.; Charalambous, M.; Kazakis, N.; Talias, M.A.; Georgakis, C.; Papamitsou, T.; Mytiglaki, C. Chromium in Water and Carcinogenic Human Health Risk. Environments 2023, 10, 33. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Abbas, A. Enhanced Chromium (VI) Adsorption onto Waste Pomegranate-Peel-Derived Biochar for Wastewater Treatment: Performance and Mechanism. Toxics 2023, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency (USEPA). National Recommended Water Quality Criteria (4304T); Office of Water, Office of Science and Technology: Washington, DC, USA, 2009. [Google Scholar]
- Environmental Protection Agency (USEPA). Edition of the Drinking Water Standards and Health Advisories; Agency, Office of Water: Washington, DC, USA, 2012. [Google Scholar]
- Peng, X.; Liu, S.; Luo, Z.; Yu, X.; Liang, W. Selective Removal of Hexavalent Chromium by Novel Nitrogen and Sulfur Containing Cellulose Composite: Role of Counter Anions. Materials 2023, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Kabdaşlı, I.; Tünay, O. Hexavalent Chromium Removal from Water and Wastewaters by Electrochemical Processes: Review. Molecules 2023, 28, 2411. [Google Scholar] [CrossRef] [PubMed]
- Michael Smarte Anekwe, I.; Adedeji, J.; OkiemuteAkpasi, S.; Lewis Kiambi, S. Available Technologies for Wastewater Treatment. In Wastewater Treatment; Ince, M., Kaplan Ince, O., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-80355-846-2. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Antoniadou, M.; Arfanis, M.K.; Ibrahim, I.; Falaras, P. Bifunctional g-C3N4/WO3 Thin Films for Photocatalytic Water Purification. Water 2019, 11, 2439. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Arfanis, M.K.; Athanasekou, C.; Philippopoulos, A.I.; Mitsopoulou, C.A.; Romanos, G.E.; Falaras, P. Surfactant Effects on the Synthesis of Redox Bifunctional V2O5 Photocatalysts. Materials 2020, 13, 4665. [Google Scholar] [CrossRef]
- Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef]
- Benettayeb, A.; Seihoub, F.Z.; Pal, P.; Ghosh, S.; Usman, M.; Chia, C.H.; Usman, M.; Sillanpää, M. Chitosan Nanoparticles as Potential Nano-Sorbent for Removal of Toxic Environmental Pollutants. Nanomaterials 2023, 13, 447. [Google Scholar] [CrossRef]
- Ali, K.; Javaid, M.U.; Ali, Z.; Zaghum, M.J. Biomass-Derived Adsorbents for Dye and Heavy Metal Removal from Wastewater. Adsorp. Sci. Technol. 2021, 2021, 9357509. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Tran, T.V.; Kumar, P.S.; Din, A.T.M.; Jalil, A.A.; Vo, D.-V.N. Invasive Plants as Biosorbents for Environmental Remediation: A Review. Environ. Chem. Lett. 2022, 20, 1421–1451. [Google Scholar] [CrossRef]
- Karić, N.; Maia, A.S.; Teodorović, A.; Atanasova, N.; Langergraber, G.; Crini, G.; Ribeiro, A.R.L.; Đolić, M. Bio-Waste Valorisation: Agricultural Wastes as Biosorbents for Removal of (in)Organic Pollutants in Wastewater Treatment. Chem. Eng. J. Adv. 2022, 9, 100239. [Google Scholar] [CrossRef]
- Muhammad, S.; Abdul Khalil, H.P.S.; Abd Hamid, S.; Albadn, Y.M.; Suriani, A.B.; Kamaruzzaman, S.; Mohamed, A.; Allaq, A.A.; Yahya, E.B. Insights into Agricultural-Waste-Based Nano-Activated Carbon Fabrication and Modifications for Wastewater Treatment Application. Agriculture 2022, 12, 1737. [Google Scholar] [CrossRef]
- Lakshmana Naik, R.; Rupas Kumar, M.; Bala Narsaiah, T. Removal of Heavy Metals (Cu & Ni) from Wastewater Using Rice Husk and Orange Peel as Adsorbents. Mater. Today Proced. 2023, 72, 92–98. [Google Scholar] [CrossRef]
- Guevara-Bernal, D.F.; Cáceres Ortíz, M.Y.C.; Gutiérrez Cifuentes, J.A.; Bastos-Arrieta, J.; Palet, C.; Candela, A.M. Coffee Husk and Lignin Revalorization: Modification with Ag Nanoparticles for Heavy Metals Removal and Antifungal Assays. Water 2022, 14, 1796. [Google Scholar] [CrossRef]
- Gryko, K.; Kalinowska, M.; Swiderski, G. The Use of Apple Pomace in Removing Heavy Metals from Water and Sewage. Environ. Sci. Proc. 2021, 9, 24. [Google Scholar] [CrossRef]
- Khan, M.N.; Ullah, H.; Naeem, S.; Uddin, J.; Hamid, Y.; Ahmad, W.; Ding, J. Remediation of Emerging Heavy Metals from Water Using Natural Adsorbent: Adsorption Performance and Mechanistic Insights. Sustainability 2021, 13, 8817. [Google Scholar] [CrossRef]
- Jeyaseelan, C.; Gupta, A. Green Tea Leaves as a Natural Adsorbent for the Removal of Cr(VI) from Aqueous Solutions. Air Soil Water Res. 2016, 9, 13–19. [Google Scholar] [CrossRef]
- Ain, H.B.U.; Tufail, T.; Bashir, S.; Ijaz, N.; Hussain, M.; Ikram, A.; Farooq, M.A.; Saewan, S.A. Nutritional Importance and Industrial Uses of Pomegranate Peel: A Critical Review. Food Sci. Nutr. 2023, 11, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, M.R.V.; Martins, V.C.A.; Plepis, A.M.G.; Bogusz, S. Utilization of Pomegranate Peel Waste: Natural Deep Eutectic Solvents as a Green Strategy to Recover Valuable Phenolic Compounds. J. Clean. Prod. 2021, 327, 129471. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Gao, W.; Zhang, L.; Li, J.; Li, J.; Zang, J. Pomegranate Peel as a Source of Bioactive Compounds: A Mini Review on Their Physiological Functions. Front. Nutr. 2022, 9, 887113. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Hlima, H.B.; Mtibaa, A.C.; Fourati, M.; Sellem, I.; Elhadef, K.; Ennouri, K.; Mellouli, L. Pomegranate Peel as Phenolic Compounds Source: Advanced Analytical Strategies and Practical Use in Meat Products. Meat Sci. 2019, 158, 107914. [Google Scholar] [CrossRef] [PubMed]
- El-Hadary, A.E.; Taha, M. Pomegranate Peel Methanolic-extract Improves the Shelf-life of Edible-oils under Accelerated Oxidation Conditions. Food Sci.Nutr. 2020, 8, 1798–1811. [Google Scholar] [CrossRef] [PubMed]
- Abed, L.; Belattar, N. Polyphenols Content, Chelating Propertiesand Adsorption Isotherms and Kinetics of Redand Yellow Pomegranate Peels (Punica Granatum L.) Towards Lead (II). Pol. J. Environ. Stud. 2022, 31, 5765–5779. [Google Scholar] [CrossRef]
- Lace, A.; Ryan, D.; Bowkett, M.; Cleary, J. Chromium Monitoring in Water by Colorimetry Using Optimised 1,5-Diphenylcarbazide Method. Int. J. Environ. Res. Public Health 2019, 16, 1803. [Google Scholar] [CrossRef]
- Ferslew, K.E.; Nicolaides, A.N.; Robert, T.A. Determination of Chromate Adulteration of Human Urine by Automated Colorimetric and Capillary Ion Electrophoretic Analyses. J. Anal. Toxicol. 2003, 27, 36–39. [Google Scholar] [CrossRef][Green Version]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct Measurement of the Total Antioxidant Capacity of Foods: The ‘QUENCHER’ Approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Condezo-Hoyos, L.; Abderrahim, F.; Arriba, S.M.; Carmen González, M. A Novel, Micro, Rapid and Direct Assay to Assess Total Antioxidant Capacity of Solid Foods. Talanta 2015, 138, 108–116. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Struktur der Kolloidteilchen und über den Aufbau von Solen und Gelen. Berichte Dtsch. Chem. Ges. A/B 1928, 61, 2219–2233. [Google Scholar] [CrossRef]
- Dubinin, M.M. The Potential Theory of Adsorption of Gases and Vapors for Adsorbents with Energetically Nonuniform Surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and Solid Diffusion Models for Fixed-Bed Adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Abin-Bazaine, A.; Campos Trujillo, A.; Olmos-Marquez, M. Adsorption Isotherms: Enlightenment of the Phenomenon of Adsorption. In Wastewater Treatment; Ince, M., Kaplan Ince, O., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-80355-846-2. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption GelösterStoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Low, M.J.D. Kinetics of Chemisorption of Gases on Solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Cordova Estrada, A.K.; Cordova Lozano, F.; Lara Díaz, R.A. Thermodynamics and Kinetic Studies for the Adsorption Process of Methyl Orange by Magnetic Activated Carbons. Air Soil Water Res. 2021, 14, 11786221211013336. [Google Scholar] [CrossRef]
- Larkin, P.J. General Outline for IR and Raman Spectral Interpretation. In Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–151. ISBN 978-0-12-804162-8. [Google Scholar] [CrossRef]
- Mekhamer, W.; Al-Tamimi, S. Removal of Ciprofloxacin from Simulated Wastewater by Pomegranate Peels. Environ. Sci.Pollut. Res. 2019, 26, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Saadi, W.; Rodríguez-Sánchez, S.; Ruiz, B.; Najar-Souissi, S.; Ouederni, A.; Fuente, E. From Pomegranate Peels Waste to One-Step Alkaline Carbonate Activated Carbons. Prospect as Sustainable Adsorbent for the Renewable Energy Production. J. Environ. Chem. Eng. 2022, 10, 107010. [Google Scholar] [CrossRef]
- M Khairy, G.; Hesham, A.M.; Jahin, H.E.S.; El-Korashy, S.A.; Mahmoud Awad, Y. Green Synthesis of a Novel Eco-Friendly Hydrochar from Pomegranate Peels Loaded with Iron Nanoparticles for the Removal of Copper Ions and Methylene Blue from Aqueous Solutions. J. Mol. Liq. 2022, 368, 120722. [Google Scholar] [CrossRef]
- Jawad, A.H.; Sauodi, M.; Mastuli, M.S.; Aouda, M.; Radzun, K. Pomegranate peels collected from fresh juice shop as a renewable precursor for high surface area activated carbon with potential application for methylene blue adsorption. Desalin. Water Treat. 2018, 124, 287–296. [Google Scholar] [CrossRef]
- Abbach, W.; Laghlimi, C.; Isaad, J. Simultaneous adsorption of cationic and anionic dyes by raw pomegranate peel: Modelling of equilibrium, kinetical and thermodynamical studies. Mor. J. Chem. 2023, 11, 832–853. [Google Scholar] [CrossRef]
- Ahmed, E.; Zeitoun, A.; Hamad, G.; Zeitoun, M.A.M.; Taha, A.; Korma, S.A.; Esatbeyoglu, T. Lignocellulosic Biomasses from Agricultural Wastes Improved the Quality and Physicochemical Properties of Frying Oils. Foods 2022, 11, 3149. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Jawad, A.H.; Waheeb, A.S.; Abd Rashid, R.; Nawawi, W.I.; Yousif, E. Equilibrium isotherms, kinetics, and thermodynamics studies of methylene blue adsorption on pomegranate (Punica granatum) peels as a natural low-cost biosorbent. Desalin. Water Treat. 2018, 105, 322–331. [Google Scholar] [CrossRef]
- Akkari, I.; Graba, Z.; Bezzi, N.; Merzeg, F.A.; Bait, N.; Ferhati, A. Raw pomegranate peel as promise efficient biosorbent for the removal of Basic Red 46 dye: Equilibrium, kinetic, and thermodynamic studies. Biomass Conv. Bioref. 2023, 13, 8047–8060. [Google Scholar] [CrossRef]
- Salam, F.A.; Narayanan, A. Biosorption—A Case Study of Hexavalent Chromium Removal with Raw Pomegranate Peel. Desalin. Water Treat. 2019, 156, 278–291. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.; Macena, M.; Esteves, B.; Santos-Vieira, I. Lignocellulosic Materials Used as Biosorbents for the Capture of Nickel (II) in Aqueous Solution. Appl. Sci. 2022, 12, 933. [Google Scholar] [CrossRef]
- Esmaeili Bidhendi, M.; Poursorkh, Z.; Sereshti, H.; Rashidi Nodeh, H.; Rezania, S.; Afzal Kamboh, M. Nano-Size Biomass Derived from Pomegranate Peel for Enhanced Removal of Cefixime Antibiotic from Aqueous Media: Kinetic, Equilibrium and Thermodynamic Study. Int. J. Environ. Res. Public Health 2020, 17, 4223. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-Antioxidant Activity Relationship of Methoxy, Phenolic Hydroxyl, and Carboxylic Acid Groups of Phenolic Acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Cruz-Valenzuela, M.R.; Ayala-Soto, R.E.; Ayala-Zavala, J.F.; Espinoza-Silva, B.A.; González-Aguilar, G.A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Nazzaro, F.; Fratianni, F.; Tapia-Rodríguez, M.R.; et al. Pomegranate (Punica granatum L.) Peel Extracts as Antimicrobial and Antioxidant Additives Used in Alfalfa Sprouts. Foods 2022, 11, 2588. [Google Scholar] [CrossRef]
- Gullón, P.; Astray, G.; Gullón, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate Peel as Suitable Source of High-Added Value Bioactives: Tailored Functionalized Meat Products. Molecules 2020, 25, 2859. [Google Scholar] [CrossRef]
- Wakeel, A.; Xu, M.; Gan, Y. Chromium-Induced Reactive Oxygen Species Accumulation by Altering the Enzymatic Antioxidant System and Associated Cytotoxic, Genotoxic, Ultrastructural, and Photosynthetic Changes in Plants. Int. J. Mol. Sci. 2020, 21, 728. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.P.; Macena, M.; Esteves, B.; Guiné, R.P.F. Ideal PH for the Adsorption of Metal Ions Cr6+, Ni2+, Pb2+ in Aqueous Solution with Different Adsorbent Materials. Open Agric. 2021, 6, 115–123. [Google Scholar] [CrossRef]
- Islam, M.M.; Mohana, A.A.; Rahman, M.A.; Rahman, M.; Naidu, R.; Rahman, M.M. A Comprehensive Review of the Current Progress of Chromium Removal Methods from Aqueous Solution. Toxics 2023, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, A.; Lu, J.; Niu, X.; Jiang, M.; Ma, Y.; Liu, X.; Li, M. Adsorption Mechanism of Hexavalent Chromium on Biochar: Kinetic, Thermodynamic, and Characterization Studies. ACS Omega 2020, 5, 27323–27331. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Akpan, B.M.; Akpomie, K.G. Sequestered Capture and Desorption of Hexavalent Chromium from Solution and Textile Wastewater onto Low Cost Heinsiacrinita Seed Coat Biomass. Appl. Water Sci. 2020, 10, 32. [Google Scholar] [CrossRef]
- Puszkarewicz, A.; Kaleta, J. Chromium (VI) Adsorption on Modified Activated Carbons. Appl. Sci. 2019, 9, 3549. [Google Scholar] [CrossRef]
- Badessa, T.S.; Wakuma, E.; Yimer, A.M. Bio-Sorption for Effective Removal of Chromium(VI) from Wastewater Using Moringa stenopetala Seed Powder (MSSP) and Banana Peel Powder (BPP). BMC Chem. 2020, 14, 71. [Google Scholar] [CrossRef]
- Biswas, S.; Bal, M.; Behera, S.; Sen, T.; Meikap, B. Process Optimization Study of Zn2+ Adsorption on Biochar-Alginate Com-posite Adsorbent by Response Surface Methodology (RSM). Water 2019, 11, 325. [Google Scholar] [CrossRef]
- Elkhaleefa, A.; Ali, I.H.; Brima, E.I.; Shigidi, I.; Elhag, A.B.; Karama, B. Evaluation of the Adsorption Efficiency on the Removal of Lead(II) Ions from Aqueous Solutions Using Azadirachta indica Leaves as an Adsorbent. Processes 2021, 9, 559. [Google Scholar] [CrossRef]
- Akhtar, A.; Hanif, M.A.; Rashid, U.; Bhatti, I.A.; Alharthi, F.A.; Kazerooni, E.A. Advanced Treatment of Direct Dye Wastewater Using Novel Composites Produced from Hoshanar and Sunny Grey Waste. Separations 2022, 9, 425. [Google Scholar] [CrossRef]
- Al-Najar, J.A.; Al-Humairi, S.T.; Lutfee, T.; Balakrishnan, D.; Veza, I.; Soudagar, M.E.M.; Fattah, I.M.R. Cost-Effective Natural Adsorbents for Remediation of Oil-Contaminated Water. Water 2023, 15, 1186. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, Y.; Zhu, Z. Biosorption Characteristic and Cytoprotective Effect of Pb2+, Cu2+ and Cd2+ by a Novel Polysaccharide from Zingiber strioatum. Molecules 2022, 27, 8036. [Google Scholar] [CrossRef]
- Mustapha, S.; Shuaib, D.T.; Ndamitso, M.M.; Etsuyankpa, M.B.; Sumaila, A.; Mohammed, U.M.; Nasirudeen, M.B. Adsorption Isotherm, Kinetic and Thermodynamic Studies for the Removal of Pb(II), Cd(II), Zn(II) and Cu(II) Ions from Aqueous Solutions Using Albizia lebbeck Pods. Appl. Water Sci. 2019, 9, 142. [Google Scholar] [CrossRef]
- Mnasri-Ghnimi, S.; Frini-Srasra, N. Removal of Heavy Metals from Aqueous Solutions by Adsorption Using Single and Mixed Pillared Clays. Appl. Clay Sci. 2019, 179, 105151. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Rafiaee, S.; Samani, M.R.; Toghraie, D. Removal of Hexavalent Chromium from Aqueous Media Using Pomegranate Peels Modified by Polymeric Coatings: Effects of Various Composite Synthesis Parameters. Synth. Met. 2020, 265, 116416. [Google Scholar] [CrossRef]
- Giri, R.; Kumari, N.; Behera, M.; Sharma, A.; Kumar, S.; Kumar, N.; Singh, R. Adsorption of Hexavalent Chromium from Aqueous Solution Using Pomegranate Peel as Low-Cost Biosorbent. J. Environ. Sustain. 2021, 4, 401–417. [Google Scholar] [CrossRef]
- Boutaleb, Y.; Zerdoum, R.; Bensid, N.; Abumousa, R.A.; Hattab, Z.; Bououdina, M. Adsorption of Cr(VI) by Mesoporous Pomegranate Peel Biowaste from Synthetic Wastewater under Dynamic Mode. Water 2022, 14, 3885. [Google Scholar] [CrossRef]
- Khalil, U.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Alyemeni, M.N.; Wijaya, L. Adsorption-Reduction Performance of Tea Waste and Rice Husk Biochars for Cr(VI) Elimination from Wastewater. J. Saudi Chem. Soc. 2020, 24, 799–810. [Google Scholar] [CrossRef]
- Nithya, K.; Sathish, A.; Kumar, P.S. Packed Bed Column Optimization and Modeling Studies for Removal of Chromium Ions Using Chemically Modified Lantana camara Adsorbent. J. Water Process. Eng. 2020, 33, 101069. [Google Scholar] [CrossRef]
- Enniya, I.; Rghioui, L.; Jourani, A. Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain. Chem. Pharm. 2018, 7, 9–16. [Google Scholar] [CrossRef]
- Yarkandi, N.H. Kinetic and Isotherm of Toxic Hexavelant Chromium Adsorption onto Natural Adsorbent. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1–15. [Google Scholar]
- Bayuo, J.; Pelig-Ba, K.B.; Abukari, M.A. Adsorptive Removal of Chromium(VI) from Aqueous Solution unto Groundnut Shell. Appl. Water Sci. 2019, 9, 107. [Google Scholar] [CrossRef]
- Mitra, T.; Bar, N.; Das, S.K. Rice Husk: Green Adsorbent for Pb(II) and Cr(VI) Removal from Aqueous Solution–Column Study and GA–NN Modeling. SN Appl. Sci. 2019, 1, 486. [Google Scholar] [CrossRef]
- El-Baz, A.A.; Hendy, I.; Dohdoh, A.M.; Srour, M.I. Adsorption of High Chromium Concentrations from Industrial Wastewater Using Different Agricultural Residuals. J. Environ. Treat. Tech. 2020, 9, 122–138. [Google Scholar] [CrossRef]
- Garg, R.; Garg, R.; Sillanpää, M.; Alimuddin; Khan, M.A.; Mubarak, N.M.; Tan, Y.H. Rapid Adsorptive Removal of Chromium from Wastewater Using Walnut-Derived Biosorbents. Sci. Rep. 2023, 13, 6859. [Google Scholar] [CrossRef] [PubMed]
- Pertile, E.; Dvorský, T.; Václavík, V.; Heviánková, S. Use of Different Types of Biosorbents to Remove Cr(VI) from Aqueous Solution. Life 2021, 11, 240. [Google Scholar] [CrossRef]
- Babu, J.D.; Sumalatha, B.; Narayana, V.A.; Pulipati, K.; Rao, R.A. Simultaneous Biosorption of Chromium (III) and Chromium (VI): Application of Multiple Response Optimizations. J. Agric. Sci. Technol. 2019, 22, 556230. [Google Scholar]
- Castañeda-Figueredo, J.S.; Torralba-Dotor, A.I.; Pérez-Rodríguez, C.C.; Moreno-Bedoya, A.M.; Mosquera-Vivas, C.S. Removal of Lead and Chromium from Solution by Organic Peels: Effect of Particle Size and Bio-Adsorbent. Heliyon 2022, 8, e10275. [Google Scholar] [CrossRef] [PubMed]
- Putz, A.-M.; Ciopec, M.; Negrea, A.; Grad, O.; Ianăşi, C.; Ivankov, O.I.; Milanović, M.; Stijepović, I.; Almásy, L. Comparison of Structure and Adsorption Properties of Mesoporous Silica Functionalized with Aminopropyl Groups by the Co-Condensation and the Post Grafting Methods. Materials 2021, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Allwin Mabes Raj, A.F.P.; Bauman, M.; Lakić, M.; Dimitrušev, N.; Lobnik, A.; Košak, A. Removal of Pb2+, CrT, and Hg2+ Ions from Aqueous Solutions Using Amino-Functionalized Magnetic Nanoparticles. Int. J. Mol. Sci. 2022, 23, 16186. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, D.; Wu, C.; Xie, R. Sulfidized Nanoscale Zerovalent Iron Supported by Oyster Powder for Efficient Removal of Cr (VI): Characterization, Performance, and Mechanisms. Materials 2022, 15, 3898. [Google Scholar] [CrossRef]
- De Borja Ojembarrena, F.; Sammaraie, H.; Campano, C.; Blanco, A.; Merayo, N.; Negro, C. Hexavalent Chromium Removal from Industrial Wastewater by Adsorption and Reduction onto Cationic Cellulose Nanocrystals. Nanomaterials 2022, 12, 4172. [Google Scholar] [CrossRef]
- Ali, A.; Alharthi, S.; Al-Shaalan, N.H.; Naz, A.; Fan, H.-J.S. Efficient Removal of Hexavalent Chromium (Cr(VI)) from Wastewater Using Amide-Modified Biochar. Molecules 2023, 28, 5146. [Google Scholar] [CrossRef]
- Angaru, G.K.R.; Lingamdinne, L.P.; Koduru, J.R.; Chang, Y.-Y. N-Cetyltrimethylammonium Bromide-Modified Zeolite Na-A from Waste Fly Ash for Hexavalent Chromium Removal from Industrial Effluent. J. Compos. Sci. 2022, 6, 256. [Google Scholar] [CrossRef]
- Li, J.; Dong, X.; Liu, X.; Xu, X.; Duan, W.; Park, J.; Gao, L.; Lu, Y. Comparative Study on the Adsorption Characteristics of Heavy Metal Ions by Activated Carbon and Selected Natural Adsorbents. Sustainability 2022, 14, 15579. [Google Scholar] [CrossRef]
- Tee, G.T.; Gok, X.Y.; Yong, W.F. Adsorption of Pollutants in Wastewater via Biosorbents, Nanoparticles and Magnetic Bio-sorbents: A Review. Environ. Res. 2022, 212, 113248. [Google Scholar] [CrossRef]
- Fernández Pérez, B.; Ayala Espina, J.; Fernández González, M.D.L.Á. Adsorption of Heavy Metals Ions from Mining Metallurgical Tailings Leachate Using a Shell-Based Adsorbent: Characterization, Kinetics and Isotherm Studies. Materials 2022, 15, 5315. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.A.K.; Rehman, F. Adsorption of Heavy Metal Ions on Pomegranate (Punica granatum) Peel: Removal and Recovery of Cr(VI) Ions from a Multi-Metal Ion System. Adsorp. Sci. Technol. 2010, 28, 195–211. [Google Scholar] [CrossRef]



| Parameter | RHP | YHP |
|---|---|---|
| SBET (m2/g) | 2.6844 | 3.3244 |
| Pore volume (cm3/g) | 0.003114 | 0.003987 |
| Average pore diameter (nm) | 21.1781 | 14.5149 |
| Material | pHpzc | Acidic Surface Functional Groups (mmol/g) | Polyphenolic Content (mg Gallic Acid Equivalent/g of Dry Weight) |
|---|---|---|---|
| RHP | 5.11 | 18.5 | 102 ± 0.9 |
| YHP | 4.55 | 16 | 85.9 ± 1.3 |
| Biosorbent | Langmuir | Freundlich | D-R | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qm Experimental (mg/g) | Qmax Theoretical (mg/g) | KL (L/mg) | RL | R2 | KF (mg/g(L/mg)1/nf) | 1/n | R2 | Qmax Theoretical (mg/g) | KDR (mol2/kJ2) | R2 | |
| RHP | 407.01 | 413.22 | 0.0160 | (0.015 – 0.861) | 0.99889 | 4.4067 | 0.3629 | 0.93515 | 235.28 | 0.0015 | 0.48272 |
| YHP | 396.49 | 403.22 | 0.0165 | (0.014 – 0.858) | 0.9985 | 4.3398 | 0.3659 | 0.91909 | 232.52 | 0.0016 | 0.42843 |
| Biosorbent | Pseudo-First Order | Pseudo-Second Order | Elovich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe Experimental (mg/g) | Qe Calculated (mg/g) | K1 (min−1) | R2 | Qe Experimental (mg/g) | Qe Calculated (mg/g) | K2 (g/mg/min) | R2 | α (mg/g/min) | β (g/mg) | R2 | |
| RHP | 22.60 | 3.31 | 0.01697 | 0.50251 | 22.60 | 22.36 | 0.02464 | 0.99965 | 573.38 | 0.44580 | 0.6443 |
| YHP | 20.99 | 3.98 | 0.00987 | 0.27245 | 20.99 | 19.71 | 0.02948 | 0.99896 | 396.01 | 0.48942 | 0.58122 |
| Biosorbents | Qmax (mg/g) | S/L Ratio | Range of Initial Concentrations (mg/L) | pH | Temperature (°C) | References |
|---|---|---|---|---|---|---|
| Pomegranate husk | 403.22–413.22 | 0.5 mg/mL | 10–4000 | 2 | 25 | Current study |
| Tea waste biochar | 38.62 | 0.6 mg/mL | 10–250 | 5.2 | 20 ± 2 | [89] |
| Modified Lantana camara | 362.8 | Fixed bed column experiment | 100–300 | 1.5 | Not mentioned | [90] |
| (bed height 4 cm) | ||||||
| Apple peel | 36.01 | 1 mg/mL | 10–50 | 2 | 28 | [91] |
| Egg shell | 299.4 | 103 mg/150 mL | 1–100 | 3 | 25 | [92] |
| Groundnut shell | 3.792 | 2 mg/mL | 15–100 | 8 | 41.5 | [93] |
| Rice husk | 11.39 | Fixed bed column experiment | 10–30 | 5 | 25 ± 2 | [94] |
| (bed height 50 cm) | ||||||
| Banana waste | 105.84 | 25 mg/mL | 400–1000 | 3 | 30 | [95] |
| Walnut shell | 64.82 | 1 mg/mL | 20–120 | 2 | 25 | [96] |
| Peach stones | 25.5 | 20 mg/mL | 100–1000 | 1.1 | 25 ± 2 | [97] |
| Gelidilla acerosa (Micro algea) | 270.27 | 0.5 mg/mL | 20–100 | 2.81 | Ambient temperature | [98] |
| Passion fruit | 3.3 | 10 mg/mL | 5–500 | 6 | 22 | [99] |
| Aminopropyl functionalized mesoporous silica by co-condensation | 93.6 | 4 mg/mL | 25–1000 | 3.5 | 25 | [100] |
| Amino functionalized magnetic nanoparticles | 90.4 | 2.25 mg/mL | 200 | 7 | Room temperature | [101] |
| Sulfidized nanoscale zerovalent iron supported by oyster shell | 164.7 | 0.1 mg/mL | 0–10 | 3.5 | 25 | [102] |
| Cationic cellulose nanocrystals | 44 | 0.1 mg/mL | 0.1–70 | 3 | Room temperature | [103] |
| Amide-modified biochar | 229.88 | 2 mg/mL | 100–500 | 2 | 25 | [104] |
| N-cetyltrimethylammonium bromide (CTAB)-modified fly ash-based zeolite Na-A | 108.76 | 0.5 mg/mL | 10–300 | 3 | 25 | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abed, L.; Belattar, N. Assessing the Dual Use of Red and Yellow Algerian Pomegranate Husks: Natural Antiradical Agents and Low-Cost Biosorbents for Chromium (VI) Removal from Contaminated Waters. Water 2023, 15, 2869. https://doi.org/10.3390/w15162869
Abed L, Belattar N. Assessing the Dual Use of Red and Yellow Algerian Pomegranate Husks: Natural Antiradical Agents and Low-Cost Biosorbents for Chromium (VI) Removal from Contaminated Waters. Water. 2023; 15(16):2869. https://doi.org/10.3390/w15162869
Chicago/Turabian StyleAbed, Lina, and Noureddine Belattar. 2023. "Assessing the Dual Use of Red and Yellow Algerian Pomegranate Husks: Natural Antiradical Agents and Low-Cost Biosorbents for Chromium (VI) Removal from Contaminated Waters" Water 15, no. 16: 2869. https://doi.org/10.3390/w15162869
APA StyleAbed, L., & Belattar, N. (2023). Assessing the Dual Use of Red and Yellow Algerian Pomegranate Husks: Natural Antiradical Agents and Low-Cost Biosorbents for Chromium (VI) Removal from Contaminated Waters. Water, 15(16), 2869. https://doi.org/10.3390/w15162869






