Abstract
Non-graphitic nitrogen-doped nano-scale diamonds were tested in the laboratory for their ability to activate peroxydisulfate for treating Microcystis-laden water. Flocculation was observed and up to 99.8% of the cyanobacterial cells were removed. The flocs’ composition showed that nano-scale diamonds with activated persulfate promoted the release of protein-like and humic-like substances during the treatment, which could have promoted agglomeration. Comprehensive analyses suggest that both radical and radical-free mechanisms were involved. Extracellular substances associated with the Microcystis cells were modified to function as active bioflocculants, leading to self-flocculation and sedimentation.
1. Introduction
Blooms of cyanobacteria like Microcystis sp. threaten water bodies worldwide. Bacterial cells which are not removed can survive and grow in filtration tanks and on membranes [], even in the pipes of a water distribution system []. They may release toxins, odorants and metabolites that degrade water quality and serve as precursors of disinfection by-products that are dangerous for human health [,]. Removing cyanobacteria during water treatment is therefore of great importance.
An ideal removal treatment should remove cyanobacteria without cell rupture and control the release of intracellular compounds. In this regard, chemical pre-oxidation and coagulation or flocculation are employed to destroy the multicellular structures such bacteria form to promote efficient removal via sedimentation [,]. However, an oxidant such as chlorine [,], ozone [] or permanganate [,] needs to be in contact with cyanobacterial cells for a long time to achieve this, and an extra dose is usually required due to the presence of organic matter and salts in the water [,]. A common problem with flocculation is that induced flocculation usually requires a high load, and the flocculant may then cause problems in the downstream filtration of drinking water processing. In addition, residuals such as AlCl3 and poly-aluminum chloride (PAC) also affect human health negatively [,].
Several persulfate oxidation processes using peroxymonosulfate (PMS) or peroxydisulfate (PDS) in conjunction with Fe2 + or ultraviolet light have been developed for the removal of cyanobacteria [,,,,,,]. In fact, persulfate is easily activated by UV irradiation [], heating [], ultrasound irradiation [] or transition metal ions [,]. This generates sulfate radicals with a higher oxidation potential than that of hydroxyl radicals []. Recently, l-cysteine (Cys), an organic ligand with reducing capability, was used to boost an Fe3+/PMS system []. However, the homogeneous Fe2+ activation of persulfate may have potential risks, such as metal leaching [], threatening the environment, drinking water safety and human health.
Carbon-based catalysts have been suggested for environmental applications due to their good catalytic performance, lower risks of secondary pollution and less negative effects on health [,]. The active surface sites on carbon-based catalysts can transfer electrons to PDS molecules [], producing sulfate radicals and hydroxyl radicals [,,]. In particular, catalysts doped with graphitic, pyridinic or pyrrolic nitrogen on the surface of activated carbon remarkably promote bonding between the nitrogen-doped catalyst and PDS, leading to a better catalytic performance than that of unmodified materials []. Lee’s group has shown [] that graphite in the form of nano-scale diamond crystals annealed at 1000 °C can, with PDS, oxidize pollutants via a non-radical mechanism. The good selectivity without interference makes the no-radical pathway effective for oxidation. An important question, however, is whether or not diamonds annealed at a lower temperature might be effective enough. Little has been published on using nano-scale diamonds with PDS for removing cyanobacteria. It should be pointed out that although the price of nano-scale diamond is relatively expensive at present, with the development of production technology, it is expected to become a popular catalyst with good performance.
Unlike conventional chemical flocculation, bioflocculation uses microorganisms or their metabolites to interact with algae and cyanobacteria to achieve effective flocculation []. For example, bacterial cellulose produced by Gluconacetobacter xylinus can effectively flocculate and collect microalgae. The flocculation efficiency of Scenedesmus obliquus and Chlamydomonas reinhardtii in 4 h is more than 90% []. A group led by Zhang has demonstrated a bacterial culture which could flocculate 90% of a suspension of cyanobacteria within 2 h []. Compared with traditional chemical flocculation, bioflocculation is safer, avoiding the environmental pollution caused by the residue of the chemical flocculant. However, bioflocculant removal of Microcystis aeruginosa may not be satisfactory. Cai has reported that Glyptotendipes tokunagai bioflocculated Microcystis wesenbergii more effectively than Microcystis aeruginosa [].
Under some conditions, some cyanobacterial self-flocculate. They aggregate and precipitate in a mass to escape harsh conditions []. M. aeruginosa’s self-flocculation may be caused by extracellular proteins and carbohydrates (EOM) excreted by the bacteria themselves. They interact to reduce the electrostatic repulsion on the surface of cyanobacterial cells, allowing the formation of flocs in which cyanobacterial cells are closely linked with each other [,]. Harnessing such safe, rapid and effective bioflocculation for removing cyanobacteria would provide a useful new technology for treating water with high loads of cyanobacteria.
Inspirited by this objective, these experiments first used non-graphitic nitrogen-doped nano-scale diamonds (N-NDs) as a catalyst to trigger persulfate pre-oxidation and then removed Microcystis aeruginosa from aqueous suspension via self-flocculation and sedimentation. The specific objectives of this work were (1) to evaluate the effectiveness of using N-NDs with PDS to remove Microcystis aeruginosa; (2) to optimize the N-ND and PDS dosages; (3) to elucidate the interaction between the N-ND and cyanobacteria’s extracellular polymers (EPSs) and its impacts on cyanobacterial self-flocculation; and (4) to reveal the possible radical and non-radical oxidation mechanisms involved in cyanobacteria removal using non-graphitic N-ND and PDS. The study’s overall aim was to develop a new self-flocculation technique for cyanobacteria removal (Scheme 1). The cyanobacteria’s extracellular organic matter was to be regulated to function as an active flocculating substance to form large flocs of cyanobacteria by neutralizing the charge on the cells’ surface. A treatment was developed which removed most Microcystis cells via flocculation and sedimentation with two hours.
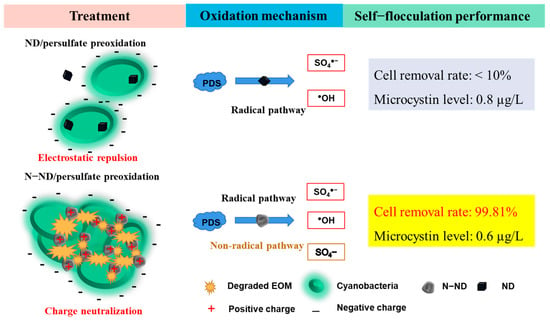
Scheme 1.
Peroxydisulfate peroxidation of cyanobacterial extracellular organic matter catalyzed by non-graphitic N-doped nano-scale diamonds and flocculation of Microcystis aeruginosa.
2. Materials and Methods
2.1. Nitrogen Doping of Nano-Scale Diamonds
The N-ND particles were synthesized using pyrolysis. A total of 500 mg of purchased nano-scale diamond powder (ND, XFJ16, Jiangsu XFNANO Materials Tech Co., Ltd., Nanjing, China) was heated to 600 °C at 5 °C/min under NH3 gas flowing at 160 mL/min. After 4 h, the oven was cooled to room temperature and the carrier gas flow was stopped, yielding N-ND.
The catalyst was observed using a transmission electron microscope (Tecnai F30, FEI Company, Hillsborough, OR, USA) and a field emission scanning electron microscope (Gemini 3000, Carl Zeiss, Oberkohen, Batenwerburg, Germany). X-ray photoelectron spectroscopy (XPS) was also performed using an ESCALAB instrument with a monochromatic Al Kα X-ray source. FTIR spectra were obtained from a Brukerspectrometer (NICOLET-8700, Thermo Fisher Scientific, Waltham, MA, USA).
2.2. Flocculation of Microcystis Cells
Microcystis aeruginosa (FACHB-905) was cultivated with BG 11 blue-green medium and a 12 h light–12 h dark cycle. The room temperature was kept at 25 °C. The cyanobacterial cells were harvested in the stationary phase. The initial cell number used in the experiments was 2 × 106 cells/mL.
The flocculation tests were performed in 150 mL glass beakers. All tests were conducted in triplicate, and Microcystis suspensions without catalyst or persulfate were run as controls. N-NDs were applied as the catalyst as well as coagulant. In each test, 10 mL of a suspension of N-NDs was mixed into a suspension of Microcystis aeruginosa. A total of 10 mL of a PDS stock solution at a pre-specified concentration was then added to initiate the oxidation and sedimentation. After 5 min of mixing, the beaker was held at room temperature in an incubator for 120 min. During the experiments, water samples were collected from 2 cm below the surface of the suspension, initially and then after 10, 30, 60, 90 and 120 min. After collecting each sample, Na2S2O3 was added to quench the reaction.
For comparison, 10 mL of a suspension of undoped ND was mixed with the Microcystis aeruginosa suspension, and 10 mL of a 10 mg/L PDS stock solution was added to perform the pre-oxidation and sedimentation without nitrogen doping.
2.3. Water Quality Measurement
Microcystis cell concentrations 2 cm below the surface were measured using haemocytometry. After filtering, the supernatant of the Microcystis aeruginosa suspension was analyzed using a Microcystin 13119L ELISA kit (Beacon Analytical Systems) to measure the remaining unflocculated microcystins. The dissolved organic carbon (DOC) was measured using a Shimadzu TOC-V CPN DOC analyzer. The supernatant’s pH was also measured using a pH meter.
2.4. Floc Characterization
Excitation–emission matrices (EEMs) of the dissolved extracellular organic matter, the bound extracellular organic matter and the organic matter extracted from the BG11 medium were recorded using a Hitachi 5J1-0004 fluorescence spectrophotometer. EEMs were recorded with excitation wavelengths from 200 to 450 nm at 5 nm intervals and emission wavelengths from 280 to 550 nm at 2 nm intervals.
A Zeiss LSM 880 confocal microscope was used to observe the surfaces of Microcystis cells, the precipitated cells and catalyst particles. Unoxidized Microcystis cells were photographed under the microscope, as were cell-catalyst aggregates after pre-oxidation and Microcystis flocs. Aspect ratios (ARs) were calculated to indicate the width of the flocs []. The zeta potential of the flocs was also measured. After each treatment, Microcystis aeruginosa suspensions were harvested, washed twice and resuspended in MilliQ water to obtain a final concentration of about 5.0 × 106 cells. Zeta potential was measured using a Nanoplus Zeta/nano particle analyzer (Model: Nano Plus-3, Serial no. 405613, Meiningen, made in German. Microcystis flocs were freeze-dried and then analyzed using Fourier Transform infrared spectroscopy (NICOLET- 8700, Thermo Fisher Scientific, Waltham, MA, USA).
2.5. Oxidation Mechanism
Electron paramagnetic resonance spectroscopy (EPR) was used to investigate free radical generation with PDS using DMPO (3,4-dihydro-2,3-dimethyl-2H-pyrrole 1-oxide) as a spin trapper. A total of 10 mg/L of N-NDs or NDs was added to the test suspension, and the EPR samples were extracted after 10 min of reaction.
In scavenger quenching tests, the removal performance of Microcystis was tested by adding 148.24 mg/L tert-butyl alcohol (TBA) or 92.14 mg/L ethanol into the N-ND/PDS system (equal to 2 mM concentration in both cases). The initial PDS concentration was 10 mg/L (0.42 mM), and the N-ND concentration was 20 mg/L. TBA is a typical scavenger for hydroxyl radicals, while ethanol scavenges both sulfate radicals and hydroxyl radicals [,].
Chronoamperometry was employed to monitor the electron transfer among the EPS, N-NDs and PDS []. To do so, 5 mg of the catalyst was dispersed in 50 mL of ethanol solution with sonication. A total of 15 µL of the mixture was then dropped on a glass plate coated with indium tin oxide and dried at 60 °C overnight. The measurement involved a Pt wire, a Ag/AgCl/KCl electrode and a fabricated working electrode. In addition, 0.1 M PBS (pH = 7) was employed as an electrolyte. PDS and EPS extracted from Microcystis cells were added into the system for chronoamperometry using a Beijing Science Days CHI760E electrochemical work station. For comparison, the same measurement was also performed in the EPS/NDs/PDS system. To investigate the outcomes of the PDS oxidation, cyanobacterial sediments from the various treatments were analyzed using Fourier transform infrared spectroscopy.
2.6. Statistical Analysis
Each treatment was conducted with three replications. Statistical analyses were performed using Microsoft Excel 16.0 (Microsoft, Redmond, WA, USA), and the results were presented as mean ± SD (standard deviation).
3. Results and Discussion
3.1. Structure of the N-NDs
Transmission electron microscopy (TEM) was used to characterize the nano-scale diamond particles. After annealing, the morphology and crystal lattice of the nitrogen-modified diamonds remained similar to those of the untreated diamonds. In Figure 1, the modified N-NDs appear as irregular agglomerates of particles []. Compared with the control samples, the TEM results suggested that the relatively low-temperature annealing and nitrogen doping at 600 °C resulted in no obvious morphological changes []. In the results of the chemical element mapping, a large number of N and O atoms appear, uniformly adhering to the surface of the N-NDs.
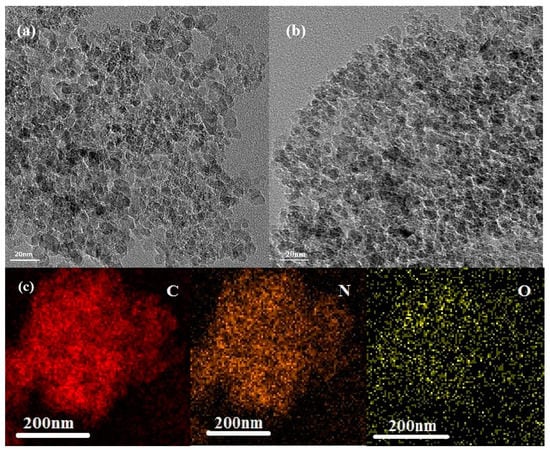
Figure 1.
Transmission electron micrographs of (a,b) N-NDs with the lattice plane spacing indicated; (c) element mapping showing the distribution of C, N and O.
The content of nitrogen and oxygen in the NDs and N-NDs was further studied using XPS with the results shown in Figure 2. In nitrogen’s 1 s spectra, the N atoms are mainly in the form of pyridinic N at about 398.7 eV, lactam or amide (399.5 eV), pyrrolic N (400.4 eV) or pyridinic N-oxide in the 402 to 407 eV range [,]. In the 1 s spectra of oxygen, the peaks can be divided into O1 (530.2 eV), indicating quinones or pyrone, and O2 (533 eV), indicating carbonyl groups. The O2 peak supports the existence of pyridinic N-oxide []. The 1 s spectrum of carbon can be divided into two bands: C1 (284.4 ev) is assigned to C=C/C-C, and C2 (286.0 eV) is attributed to C-N bonds. The annealing under ammonia enhanced the nitrogen content from 1.67% (ND) to 3.08% (N-ND). Figure 2e shows that the concentration of oxygen decreased from 6.52% (ND) to 3.65% (N-ND). In Figure 2f, the pyridinic N (N1) and pyridinic N-oxide (N4) have increased markedly compared to pristine ND. In addition, the results showed that the N-NDs were not graphitized. Conventionally, graphitized NDs could effectively activate PDS [], but here nitrogen-doped NDs without obvious graphitization are shown to effectively activate PDS also, suggesting that non-graphitic N groups such as pyridinic N and N groups containing oxygen on an N-ND surface may also provide active sites for catalytic reactions []. Fourier transform infrared spectroscopy was also performed to analyze the catalysts. Compared with ND, the C=O bond peak of N-ND was weakened, while a C-N bond peak of N-ND appeared (Section 3.4).
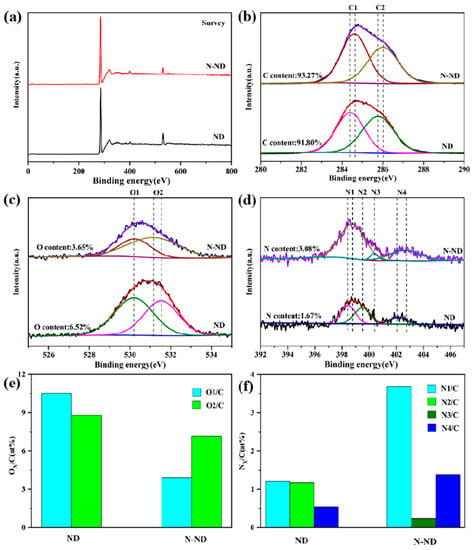
Figure 2.
XPS spectra (a) survey, (b) N 1 s, (c) O 1 s and (d) C 1 s. (e) The O/C atomic ratio and (f) N/C atomic ratio.
3.2. Microcystis Removal
Microcystis removal was quantified using measurements of cell number and DOC. The removal tests using PDS alone or the carbonaceous catalysts without PDS showed only very slight removal (Figure 3). The green Microcystis cells remained in suspension after 120 min, so those pre-treatments did not significantly promote Microcystis removal. Considering that the PDS dose was 10 mg/L, such a low concentration of oxidizing reagent may not cause any obvious reactions without a suitable catalyst. However, when 10 mg/L of PDS was added into a suspension containing the N-ND catalyst, the removal was obviously enhanced. After 120 min, 98.6% of the Microcystis cells had been precipitated. And the DOC content had been reduced from 4.5 to 1.2 mg/L. Visually, the water became clear since most of the cells had settled to the bottom of the beaker. N-ND clearly activates PDS, and modifying the diamonds by nitrogen doping at low temperature has achieved the purpose of Microcystis aeruginosa removal. In contrast, undoped NDs and PDS only removed about 20% of the cells, and the DOC remained at 3 mg/L after 120 min. The water was still green with suspended cells. Unmodified ND is not an effective catalyst for Microcystis removal. It should be noted that no traditional coagulant such as PAC was added. That could be an additional advantage of N-ND/PDS treatment.
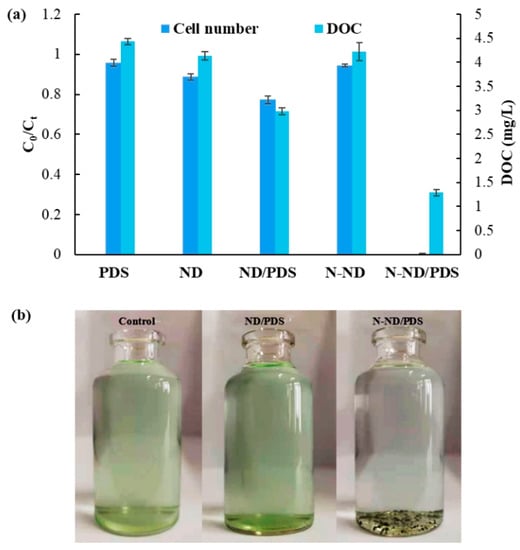
Figure 3.
Microcystis removal using different treatments; (a) the removal percentage and DOC results; (b) the suspensions after treatment. The concentration of N-ND was 20 mg/L. The concentration of PDS was 10 mg/L. The treatment time was 120 min. Error bars represent the standard deviations from triplicate experiments.
Combining 10 mg/L of PDS with nitrogen-modified ND provides a new, effective and relatively green technique for effectively removing Microcystis from water. The PDS concentration is relatively low, and no coagulant is used during sedimentation. No direct comparison of cell removal using N-ND/PDS with other pre-oxidation methods without the use of a coagulant has been published. The effectiveness of N-ND/PDS was therefore compared to that of existing PDS-based pre-oxidation and coagulation. UV/PS pre-oxidation (PDS concentration: 60 mg/L) and subsequent coagulation and sedimentation has been reported to remove 93.8% of Microcystis cells []. Using 23.81 mg/L of PDS with 27.08 mg/L of FeSO4 has been shown to achieve 90.4% removal of Microcystis cells []. So, the removal of cyanobacterial cells using other pre-oxidation and coagulation processes commonly reaches 95%.
Using ferrous ions as the catalyst for persulfate to enhance the coagulation of Microcystis-laden water has also been reported [,], but after pre-oxidation and coagulation with Fe2+ and PDS, 9.3% of Microcystis cells are reported to remain in the water []. With 20 mg/L of PMS and 4.5 mg/L Fe2+, that can be reduced to 5.7% []. Note that Fe2+ can serve not only as a catalyst for persulfate but also as a coagulant in Microcystis removal without adding a conventional aluminum flocculant []. But the system tested here showed more complete removal with only 0.2% remaining after 120 min. N-ND can apparently be used as a substitute for iron ions in catalyzing PDS and as a coagulant to promote sedimentation.
3.3. Dosage
In order to study the effect of different PDS concentrations on the removal of Microcystis in the N-ND/PDS system, PDS concentrations of 0, 5, 10, 20 and 50 mg/L were tested with an initial N-ND dose of 20 mg/L. Figure 4 shows the results. The removal dynamics are presented in Figure S1 of the Supplementary Information. Increasing the initial PDS dose generally enhanced the reduction in cell numbers, microcystin concentration and DOC. The residual cells were slightly fewer using 10 mg/L than with higher doses.
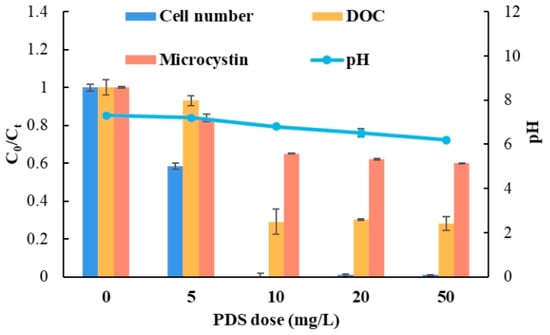
Figure 4.
The effect of PDS dosage on Microcystis removal. The concentration of N-ND was 20 mg/L. The treatment time was 120 min. The error bars represent the standard deviations of triplicate experiments.
The pH of the treated water decreased slightly with the PDS dosage into weakly acidic territory. When the PDS dose was 50 mg/L, the pH was 6.2. The decrease in pH is due to the production of sulfate radical and its oxidation of water molecules []. It is well known that PAC coagulation performs best under neutral and weakly alkaline conditions, but the N-ND/PDS system is effective in acidic water. The mechanism of N-ND/PDS flocculation should be further explored. Most water treatment includes chlorine disinfection, which is more effective under acidic conditions. In fact, above pH 8, the effectiveness of chlorine disinfection decreases significantly. Therefore, a slightly lower pH environment after N-ND/PDS treatment should promote subsequent disinfection.
The optimum 10 mg/L PDS dose demonstrated here is much lower than the 250 mg/L of sulfate prescribed in China’s surface water purification standard.
The effect of different N-ND concentrations on the removal was studied by adding 0, 5, 10, 20 and 50 mg/L of N-NDs into Microcystis suspensions. Figure 5 shows the results. The removal dynamics are presented in Figure S2. The initial PDS dose was 10 mg/L in all cases. When the N-ND dose was 5 mg/L, 60% of the Microcystis cells remained suspended after 120 min. At 10 mg/L, 32% remained, but with 20 mg/L, only 0.2% remained. Using 50 mg/L of the catalyst produced no further improvement. The DOC and microcystin concentration also decreased, as might be expected. Therefore, 20 mg/L was concluded to be the optimum N-ND dose.
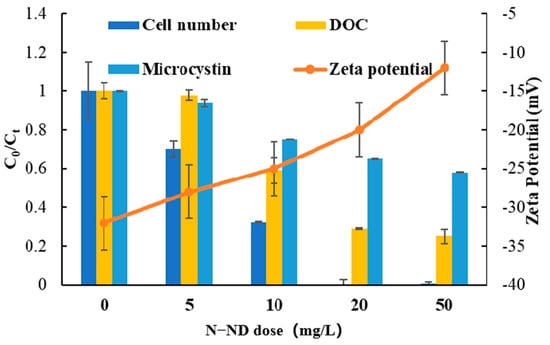
Figure 5.
The effect of N-ND dosage on Microcystis removal. The concentration of PDS was 10 mg/L. The treatment time was 120 min. The error bars represent the standard deviations of triplicate experiments.
The suspension’s zeta potential was also monitored. Initially, it was about −30.7 mV, indicating a strong electrostatic repulsion among the cells, forming a stable suspension. With the addition of N-ND and PDS, the zeta potential increased from −30.7 to −3.8 mV after 120 min. That increase tended to stabilize cell agglomerates, which resulted in the decrease in cell numbers, DOC concentration and microcystin level.
According to the XDLVO theory, microalgae flocculation is caused by the elimination or reduction of the electrostatic repulsion (zeta potential) between algal cells. The water and the formation of Lewis base pairs then allow cells to come together to form flocs. In industrial flocculation, an inorganic flocculant is added to neutralize the electronegativity on the surface of algae cells and thus reduce or eliminate their electrostatic repulsion. However, in this work, N-ND alone did not show an obvious promotion of flocculation. This suggests that N-ND and PDS must work together to condition Microcystis cells’ EPSs, leading to self-flocculation. At present, little has been published about self-flocculation induced by EPSs. Such a study promises to provide very valuable information for the understanding of the regulation of Microcystis aeruginosa’s self-flocculation.
3.4. Extracellular Organic Matter from Microcystis aeruginosa
To further explore the flocculation mechanisms, the settled Microcystis flocs without dehydration were observed using confocal microscopy (Figure 6). After either N-ND or N-ND/PDS treatment, most of the cells precipitated were intact. The images show a few NDs attached to a cell surface to form small clusters. In contrast, most Microcystis cells appear “coated” with N-NDs to form relatively larger agglomerates, which may result in more efficient Microcystis settling. N-ND with PDS apparently neutralizes the electrostatic repulsion between cyanobacterial cells, which then aggregate via patching and bridging, and gravity alone can settle the aggregates out.
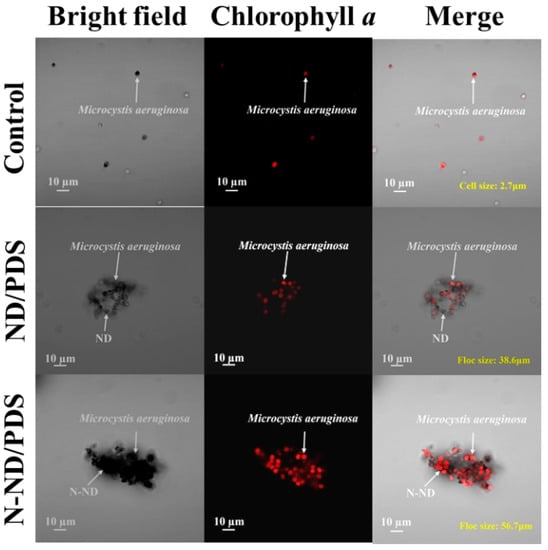
Figure 6.
Confocal micrographs of Microcystis floc after ND/PDS and N-ND/PDS treatment. The concentration of N-ND and ND was 20 mg/L in both cases. The dosage of persulfate was 10 mg/L. The initial cell concentration was 2.0 × 106/mL.
The extracellular organic matter (EOM) associated with the cells was analyzed using its 3D EEM fluorescence spectra. EOM can be either dissolved (dEOM) or bound (bEOM). The EEMs are shown in Figure 7. The dEOM of the control sample shows two peaks marked A (Ex/Em = 280/320) and B (Ex/Em = 220/300) [,]. The treatment with PDS, NDs or N-NDs alone did not release protein-like substances (Figure S3). ND with PDS (Figure 7b) enhanced the release of protein-like substances, as did treatment with N-ND and PDS (Figure 7c). But it is interesting that the fluorescence intensity of protein-like substances was lower after N-ND/PDS treatment. Tryptophan-like proteins could be the main components of dEOM, and they are reported to be easily removed by binding with functional groups of flocculants []. Alternatively, they could also be degraded via persulfate oxidation []. Whatever the explanation, these data show that dissolved protein-like substances from Microcystis cells were reduced in N-ND treatment with PDS.
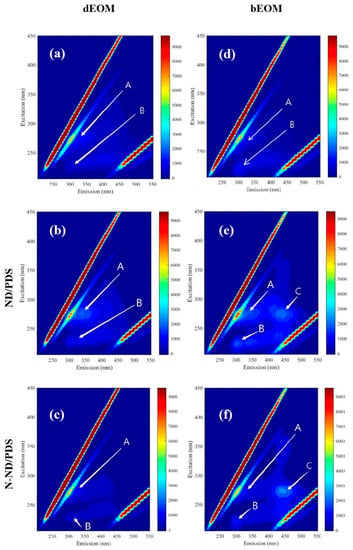
Figure 7.
Fluorescence EEM spectra of dEOM after treatment (a) control, (b) ND/PDS treatment and (c) N-ND/PDS treatment and bEOM (d) control, (e) ND/PDS treatment and (f) N-ND/PDS treatment. The concentration of N-ND was 20 mg/L, and the dosage of PDS was 10 mg/L. The initial concentration of cell number was 2.0 × 106 cells/mL. The pre-oxidation and sedimentation times were both 120 min.
The bEOM in Figure 7 shows three peaks (Ex/Em = 270/440), indicating the existence of protein-like substances and humic-like substances on the surface of the cells []. Treatment with PDS, NDs or N-NDs alone did not release protein-like or humic-like substances (Figure S3). But treatment with ND and PDS increased protein-like substances surrounding the Microcystis cells (Figure 7f), suggesting that PDS pre-treatment can expose proteins at the cell surface. When N-NDs are used with PDS, the fluorescence intensity of peaks A and B is also higher than that of the raw samples, and the fluorescence spectra show a marked peak C, which corresponds to humic-like substances []. Generally, the data show that using N-NDs with PDS can promote the release of the protein-like and humic-like substances from bEOM during the treatment, which could be why the cells aggregate.
The infrared spectra of the coagulated Microcystis aeruginosa are shown in Figure 8. With the catalysts, the main characteristic absorption bands at 3450 cm−1 represent O-H, at 2913 C-H, at 1717 C=O and at 1588 C=C [,]. Compared with the unmodified sample, the C=O bond peak (1717 cm−1) in the modified material is weakened, which is consistent with the XPS results. It should be noted that there is a new characteristic absorption band at 1458 cm−1 in the N-ND modified material. This corresponds to the stretching vibration of C-N bonds []. Misra and his colleagues have previously proposed that the C-N stretching vibration is related to the nitrogen atom inserted in the SP2 carbon network, which further indicates that nitrogen is successfully doped into the diamond []. This improves the catalytic performance because N-ND’s defects and low oxygen content facilitate electron transfer.
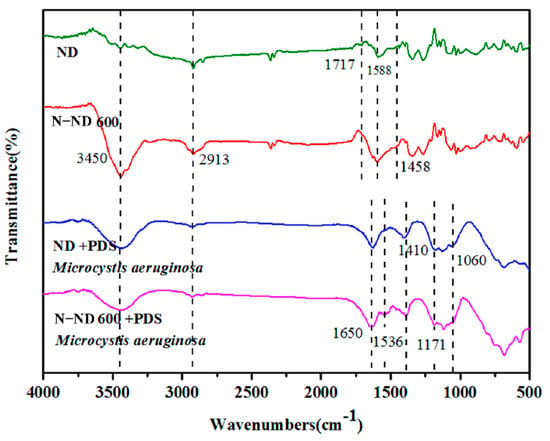
Figure 8.
Fourier transform infrared spectra showing the changes in spectral features in ND, N-ND, ND/PDS and N-ND/PDS treatment. The concentration of catalyst was 20 mg/L, and the dosage of persulfate was 10 mg/L. The initial concentration of cells was 2.0 × 106/mL.
For the oxidant/catalyst/Microcystis aeruginosa integrated system, the spectra of ND/PDS/Microcystis aeruginosa show obvious characteristic EPS peaks. The bands at 1650 cm−1 and 1536 cm−1 represent amides and the band at 1410 cm−1 (stretching vibration of CH2) corresponds to polysaccharides [,]. In comparable experiments using N-ND, the band at 1536 cm−1 was significantly enhanced, indicating that one of the amides in the polymer is released. At the same time, the absorption peaks at about 1171 cm− 1 and 1060 cm−1 are also larger. They represent the symmetric and asymmetric vibration of sulfonic acid groups (S=O=S), indicating that PDS has been absorbed on the catalyst [].
The zeta potentials before and after flocculation confirm electro-neutralization as the main flocculation mechanism. The fluorescence spectroscopy further reveals that the release of the protein-like and humic-like substances could be the reason the cells aggregate. The Fourier transform infrared spectrographs show that polymer is released which affixes itself to the N-ND particles. Together, this suggests that protein-like and humic-like substances and biopolymer are the key to good flocculation. Further investigation of the interactions of these compounds may lead to a better understanding of self-flocculation.
3.5. Oxidation Mechanisms
It should be noted that neither PDS nor N-ND alone triggered the self-flocculation of these Microcystis aeruginosa cells (Figure 3). The oxidation clearly plays a vital role. The removal mechanism was explored in experiments using oxidant scavengers (Figure 9a). No obvious removal was observed after adding TBA or ethanol. The inhibition of Microcystis removal was weak with TBA. Only a few Microcystis cells were removed by •OH from the N-ND/PDS system. The contribution of SO4•− was confirmed by a partial inhibitory effect of ethanol on Microcystis removal. The EPR results reported in Figure 9b confirm the generation of SO4•− and •OH from the DMPO−•OH (1:2:2:1) and DMPO−•SO4− (1:1:1:1:1:1) signals []. Although the dosage of scavengers (2 mM) was several times higher than that of persulfate (~0.042 mM), the addition of a scavenger only partly inhibited Microcystis removal.
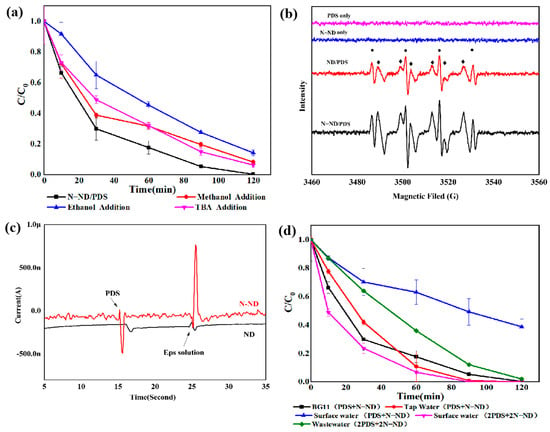
Figure 9.
(a) Scavenger quenching of Microcystis removal by N-ND with PDS; (b) the spectra with DMPO spin-trapping; (c) chronoamperometry with ND and N-ND electrodes after adding PDS and extracting EPS; (d) Microcystis removal from different waters. The concentration of N-ND was 20 mg/L, and the dosage of persulfate was 10 mg/L. The initial concentration of cells was 2.0 × 106/mL.
A no-radical mechanism involving electron transfer has been reported to be involved in persulfate’s activation by graphitized nano-scale diamonds [,] in which organics complex with modified nano-scale diamonds (as mediators) and transfer electrons to PDS (an electron acceptor). This degrades the organic substances (the donor) []. Chronoamperometry was applied to identify whether such a no-radical mechanism is involved in the N-ND/PDS system. Figure 9c shows that with the N-ND electrode, there was a negative current peak when PDS was added. Upon the addition of the EPS extracted from Microcystis cells, an obvious positive current peak formed. This confirms electron movement from the N-ND electrode to the PDS, activating it. The oxidation of EPS involved electron transfer from the complex of EPS with N-NDs to the PDS. The N-ND plays a vital role there as an electronic mediator. By comparison, using the ND electrode, a much smaller negative current peak was observed when PS was added, while with the addition of the extracted EPS, a smaller positive current peak appeared []. The chronoamperometric data indicate that there is indeed charge transfer from both N-ND and ND electrodes to PDS and that N-ND is the better electron mediator. These findings illuminate the important roles of the nitrogen functional groups on the surface of N-ND in N-ND/PDS catalysis.
These experiments were extended to systems based on natural surface water (SW), tap water and secondary wastewater (WW). Figure 9d shows that N-ND/PDS treatment removed most Microcystis aeruginosa cells from a solution of BG11 and from tap water, but it was less effective in SW and WW. The relatively poor performance of oxidation and coagulation in SW and WW is a result of the organic matter present. This content was determined using the potassium dichromate method. The COD content of the SW was about 10 mg/L, and that of the WW was about 20 mg/L. However, when the PDS and N-ND dosages were doubled, the cyanobacterial cells were completely removed within 2 h. This group of experiments confirmed that the N-ND/PDS technique can resist interference to some extent. In engineering practice, the variables would have to be adjusted according to the actual water quality. In fact, in organic matter degradation, electrons are transferred from the organic matter to PDS through the graphitized ND, which will cause organic molecules to lose electrons and decompose [,].
Turning to bactericidal effects, electron transfer from the cell membrane of a bacterium to the catalytic mediator may also lead to a marked destruction of extracellular polymers [,]. In this study, instead of degrading or destroying the EPS, N-NDs without graphitization functioned as an electron mediator and a PDS catalyst to condition the extracellular substances from Microcystis cells. This facilitated floc formation and efficient removal via sedimentation.
These results show that Microcystis removal can be significantly enhanced using N-NDs and PDS. The possible mechanisms involve electron transfer via the diamond particles with functionalized nitrogen groups. The following points summarize the results of this work:
(a) Oxidation at the cell surface. Activated N-ND/PDS* complexes can provide SO4•− and/or •OH for direct oxidation via electron transfer. This is mediated by the non-graphitic nitrogen of the N-NDs. The introduction of pyridinic N and pyridinic N-oxide improves the catalytic performance of PDS.
(b) Controlling on the release of bEOM. Protein-like and humic-like substances and biopolymer in cyanobacteria’s bEOM are released. The electrostatic repulsion between cyanobacterial cells is reduced. With the addition of N-ND, cells aggregate via patching and bridging.
(c) Flocculation and sedimentation. In the subsequent flocculation and sedimentation, cells agglomerate to form large flocs which gravity alone can precipitate.
It should be pointed out that at these low PDS dosages, the pre-oxidation was moderate and did not cause cell lysis. Microcystin and COD did not increase significantly after the treatment.
PDS activation via mediated electron transfer without involving radicals has some benefits, such as rooting out carcinogenic halogen-containing moieties, good selectivity without interference from the water matrix and reduced PDS consumption [,]. Only a few carbonaceous materials that can trigger PDS via non-radical activation are known today. Among them are doped CNTs and graphitized NDs []. In this study, nitrogen modification at 600 °C produced N-NDs without graphitization that could do the job. They should be more economical than the other reported carbonaceous catalysts and have better biocompatibility. This system may provide a useful treatment option for the removal of Microcystis from raw water.
Little has been published about self-flocculation induced by extracellular EPS from Microcystis aeruginosa cells. This study has shown the potential, but the roles of protein, humic substances and polymers in the flocculation remain unclear. The data suggest that the surface of Microcystis cells may supply these substances and that their levels could be regulated by N-ND/PDS oxidation. The interaction of protein, humic substance, and polymer should be further investigated. Their influences on Microcystis aeruginosa flocculation will provide helpful insights for developing industrial-scale Microcystis aeruginosa removal processes.
4. Conclusions
This work has shown that moderate N-ND/PDS pre-oxidation will trigger the self-flocculation and sedimentation of Microcystis removal with relatively low PDS consumption and an environment-friendly catalyst. N-ND with non-graphitic nitrogen was obtained through low-temperature annealing. With optimized conditions, 99.8% of suspended cells were removed. Positively charged N-ND nanoparticles “coated” the Microcystis cells and transferred electrons from the cells to PDS, leading to the regulation of bEOM. The electron transfer and radicals may cooperate to change the composition of cyanobacterial EPS. Extracellular organic matter thus regulated can be induced to form large flocs as charges on the cell surface are neutralized. Due to the low dosage of PDS and the good biocompatibility of the catalyst, the integrity of the Microcystis cells is not markedly damaged, while the release of microcystins is controllable. That helps to ensure safe water treatment. This work shows that using biocompatible N-NDs as a PDS catalyst may effectively remove Microcystis cells through flocculation and sedimentation triggered by moderate pre-oxidation. In laboratory-scale experiments, catalyst-mediated electron transfer played an important role.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15152768/s1, Figure S1. Effect of PDS doses on Microcystis removal kinetics. The concentration of N-ND was 20 mg/L. The treatment time was 120 min. Error bars represent the standard deviations (n = 3). Figure S2. Effect of N-ND doses on Microcystis removal kinetics. The concentration of PDS was 10 mg/L. The treatment time was 120 min. Error bars represent the standard deviations (n = 3). Figure S3. Fluorescence EEM spectra of dEOM (a) PDS treatment, (b) ND treatment, (c) N-ND treatment, and bEOM (d) PDS, (e) ND treatment, (f) N-ND treatment. The concentration of N-ND was 20 mg/L, and the dosage of PDS was 10 mg/L. The initial concentration of cell number was 2.0 × 106 cells/mL. The pre-oxidation and sedimentation time were both 120 min.
Author Contributions
X.W. designed the experiments; W.L., Y.W. and J.L. performed the experiments; X.W., Y.W. and W.L. analyzed the data; X.W. contributed reagents, materials, and access to analytical equipment; X.W., C.L. and Y.P. wrote the paper. All authors have read and agreed to the published version of this manuscript.
Funding
This work was supported by China’s National Natural Science Foundation (grant 52100014).
Data Availability Statement
Data are available by requirement.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships which could appear to have influenced the work reported in this paper.
References
- Song, Q.; Niu, X.; Zhang, D.; Song, X.; Li, Y.; Ma, J.; Lai, S.; Yang, Z.; Zhou, S. The behaviors of Microcystis aeruginosa and microcystins during the Fe(2+)/persulfate (PS) preoxidation-coagulation and flocs storage period. Environ. Res. 2020, 186, 109549. [Google Scholar] [CrossRef]
- Skjevrak, I.; Lund, V.; Ormerod, K.; Herikstad, H. Volatile organic compounds in natural biofilm in polyethylene pipes supplied with lake water and treated water from the distribution network. Water Res. 2005, 39, 4133–4141. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wu, C.; Yu, H.; Gao, S.; Li, G.; Cui, F.; Qu, F. Applying ultraviolet/persulfate (UV/PS) pre-oxidation for controlling ultrafiltration membrane fouling by natural organic matter (NOM) in surface water. Water Res. 2018, 132, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Choi, S.Y.; Ahn, S.K.; Kweon, J.H. Disinfection by-product formation potential of algogenic organic matter from Microcystis aeruginosa: Effects of growth phases and powdered activated carbon adsorption. J. Hazard Mater. 2021, 408, 124864. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.; Parsons, S.A.; Jefferson, B. The impact of algal properties and pre-oxidation on solid-liquid separation of algae. Water Res. 2008, 42, 1827–1845. [Google Scholar] [CrossRef]
- Bai, Y.; Hu, W.; Jian, Z.; Qi, W.; Chang, Y.; Huo, Y.; Liao, K.; Qu, J. Combining KMnO4 pre-oxidation and bioaugmented sand filtration to simultaneously treat cyanobacterial bloom lake water and released Mn(II). Sep. Purif. Technol. 2019, 228, 115765. [Google Scholar] [CrossRef]
- Fan, J.; Rao, L.; Chiu, Y.T.; Lin, T.F. Impact of chlorine on the cell integrity and toxin release and degradation of colonial Microcystis. Water Res. 2016, 102, 394–404. [Google Scholar] [CrossRef]
- Li, L.; Zhu, C.; Xie, C.; Shao, C.; Yu, S.; Zhao, L.; Gao, N. Kinetics and mechanism of Pseudoanabaena cell inactivation, 2-MIB release and degradation under exposure of ozone, chlorine and permanganate. Water Res. 2018, 147, 422–428. [Google Scholar] [CrossRef]
- Tang, A.; Shi, X.; Bi, R.; Liao, X.; Zou, J.; Sun, W.; Yuan, B. Effects of pre-ozonation on the cell characteristics and N-nitrosodimethylamine formation at three growth phases of Microcystis aeruginosa. Env. Sci. Pollut. Res. Int. 2020, 27, 873–881. [Google Scholar] [CrossRef]
- Li, L.; Shao, C.; Lin, T.F.; Shen, J.; Yu, S.; Shang, R.; Yin, D.; Zhang, K.; Gao, N. Kinetics of cell inactivation, toxin release, and degradation during permanganation of Microcystis aeruginosa. Env. Sci. Technol. 2014, 48, 2885–2892. [Google Scholar] [CrossRef]
- Ma, B.; Qi, J.; Wang, X.; Ma, M.; Miao, S.; Li, W.; Liu, R.; Liu, H.; Qu, J. Moderate KMnO4-Fe(II) pre-oxidation for alleviating ultrafiltration membrane fouling by algae during drinking water treatment. Water Res. 2018, 142, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Lan, H.; Liu, R.; Liu, H.; Qu, J. Efficient Microcystis aeruginosa removal by moderate photocatalysis-enhanced coagulation with magnetic Zn-doped Fe3O4 particles. Water Res. 2020, 171, 115448. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cheng, J.; Zou, F.; Zhang, C.; Wang, M.; Li, Y.; Gu, J.; Yan, M. Effects of pre-oxidation on residual dissolved aluminum in coagulated water: A pilot-scale study. Water Res. 2021, 190, 116682. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, E.; Elsharkawy, E.; Alhumaydhi, F.A.; Salama, M. The protective effect of Indian Catechu methanolic extract against aluminum chloride-induced neurotoxicity, A rodent model of Alzheimer’s disease. Heliyon 2021, 7, e06269. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Y.; Yue, S.; Ding, J.; Xie, P.; Wang, Z. Simultaneous Removal of Microcystis aeruginosa and 2,4,6-Trichlorophenol by UV/Persulfate Process. Front. Chem. 2020, 8, 591641. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, J.; Zhao, Z.; Peng, W.; Cui, F.; Liang, Z. Microcystis aeruginosa-laden water treatment using peroxymonosulfate enhanced Fe(II) coagulation: Performance and the role of in situ formed Fe3O4. Chem. Eng. J. 2020, 382. [Google Scholar] [CrossRef]
- Wan, Y.; Xie, P.; Wang, Z.; Ding, J.; Wang, J.; Wang, S.; Wiesner, M.R. Comparative study on the pretreatment of algae-laden water by UV/persulfate, UV/chlorine, and UV/H2O2: Variation of characteristics and alleviation of ultrafiltration membrane fouling. Water Res. 2019, 158, 213–226. [Google Scholar] [CrossRef]
- Gu, N.; Wu, Y.; Gao, J.; Meng, X.; Zhao, P.; Qin, H.; Wang, K. Microcystis aeruginosa removal by in situ chemical oxidation using persulfate activated by Fe2+ ions. Ecol. Eng. 2017, 99, 290–297. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, P.; Wang, Z.; Shang, R.; Wang, S. UV/persulfate preoxidation to improve coagulation efficiency of Microcystis aeruginosa. J. Hazard Mater. 2017, 322, 508–515. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Xie, P.; Shang, R.; Ma, J. Removal of Microcystis aeruginosa by UV-activated persulfate: Performance and characteristics. Chem. Eng. J. 2016, 300, 245–253. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, Y.; Zhang, L.; Lu, D.; Lu, L.; Yang, X.; Cai, T. Degradation of Atrazine, Simazine and Ametryn in an arable soil using thermal-activated persulfate oxidation process: Optimization, kinetics, and degradation pathway. J. Hazard Mater. 2020, 400, 123201. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; El-Taliawy, H.; Duran, A.; Caro, G.; Bester, K. Sono-activated persulfate oxidation of diclofenac: Degradation, kinetics, pathway and contribution of the different radicals involved. J. Hazard Mater. 2018, 357, 457–465. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Env. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Qi, C.; Wen, Y.; Zhao, Y.; Dai, Y.; Li, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of organic contaminants by Fe(III)/peroxymonosulfate process with l-cysteine. Chin. Chem. Lett. 2022, 33, 2125–2128. [Google Scholar] [CrossRef]
- Xiao, P.-f.; An, L.; Wu, D.-d. The use of carbon materials in persulfate-based advanced oxidation processes: A review. New Carbon Mater. 2020, 35, 667–683. [Google Scholar] [CrossRef]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef]
- Qu, H.; Chen, L.; Yang, F.; Zhu, J.; Qi, C.; Peng, G. Synthesis of an Environmentally Friendly Modified Mulberry Branch-Derived Biochar Composite: High Degradation Efficiency of BPA and Mitigation of Toxicity in Silkworm Larvae. Int. J. Mol. Sci. 2023, 24, 3609. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.; Chen, D.; Ren, W.; Lin, H.; Zhang, H. Wood-based biochar as an excellent activator of peroxydisulfate for Acid Orange 7 decolorization. Chemosphere 2019, 231, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kwan, C.; Suvorova, A.; Ang, H.M.; Tadé, M.O.; Wang, S. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals. Appl. Catal. B Environ. 2014, 154–155, 134–141. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Wu, Q.; Luo, H.; Zhao, Q.; Si, Q.; Sseguya, F.; Ren, N. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism. Water Res. 2019, 160, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.I.; Weon, S.; Choi, W.; Hwang, Y.S.; Seo, J.; Lee, C.; Kim, J.H. Activation of Persulfates by Graphitized Nanodiamonds for Removal of Organic Compounds. Environ. Sci. Technol. 2016, 50, 10134–10142. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, L.; Jiang, X.; Zhang, K.; Zheng, T.; Wang, H. First evidence of bioflocculant from Shinella albus with flocculation activity on harvesting of Chlorella vulgaris biomass. Bioresour. Technol. 2016, 218, 807–815. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, Q.; Zhang, Z.; Mei, Y.; Wang, H. Effective in situ harvest of microalgae with bacterial cellulose produced by Gluconacetobacter xylinus. Algal Res. 2018, 35, 349–354. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, Q.; Zhang, F.; Shao, X.; Fan, Y.; Zhu, X.; Li, Y.; Yao, L.; Tian, Y.; Zheng, T.; et al. Flocculating properties and potential of Halobacillus sp. strain H9 for the mitigation of Microcystis aeruginosa blooms. Chemosphere 2019, 218, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wu, H.; Hong, P.; Donde, O.O.; Wang, C.; Fang, T.; Xiao, B.; Wu, X. Bioflocculation effect of Glyptotendipes tokunagai on different Microcystis species: Interactions between secreted silk and extracellular polymeric substances. Chemosphere 2021, 277, 130321. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.V.Y.; Ielasi, F.S.; Nookaew, I.; Stals, I.; Alonso-Sarduy, L.; Daenen, L.; Mulders, S.E.V.; Stassen, C.; van Eijsden, R.G.E.; Siewers, V.; et al. Molecular Mechanism of Flocculation Self-Recognition in Yeast and Its Role in Mating and Survival. MBio 2015, 6, e00427-15. [Google Scholar] [CrossRef]
- Rao, N.R.H.; Granville, A.M.; Wich, P.R.; Henderson, R.K. Detailed algal extracellular carbohydrate-protein characterisation lends insight into algal solid-liquid separation process outcomes. Water Res. 2020, 178, 115833. [Google Scholar] [CrossRef]
- Rao, N.R.H.; Granville, A.M.; Henderson, R.K. Understanding variability in algal solid-liquid separation process outcomes by manipulating extracellular protein-carbohydrate interactions. Water Res. 2021, 190, 116747. [Google Scholar] [CrossRef]
- Lin, J.L.; Hua, L.C.; Wu, Y.; Huang, C. Pretreatment of algae-laden and manganese-containing waters by oxidation-assisted coagulation: Effects of oxidation on algal cell viability and manganese precipitation. Water Res. 2016, 89, 261–269. [Google Scholar] [CrossRef]
- Sun, H.; Peng, X.; Zhang, S.; Liu, S.; Xiong, Y.; Tian, S.; Fang, J. Activation of peroxymonosulfate by nitrogen-functionalized sludge carbon for efficient degradation of organic pollutants in water. Bioresour. Technol. 2017, 241, 244–251. [Google Scholar] [CrossRef]
- Xia, D.; Li, Y.; Huang, G.; Yin, R.; An, T.; Li, G.; Zhao, H.; Lu, A.; Wong, P.K. Activation of persulfates by natural magnetic pyrrhotite for water disinfection: Efficiency, mechanisms, and stability. Water Res. 2017, 112, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Su, D. Fabrication of nitrogen-modified annealed nanodiamond with improved catalytic activity. ACS Nano 2014, 8, 7823–7833. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.-T.; Moon, G.-H.; Lee, H.; Jeon, T.H.; Lee, C.; Choi, W.; Lee, J. Oxidation of organic pollutants by peroxymonosulfate activated with low-temperature-modified nanodiamonds: Understanding the reaction kinetics and mechanism. Appl. Catal. B Environ. 2018, 237, 432–441. [Google Scholar] [CrossRef]
- Wu, K.-H.; Liu, Y.; Luo, J.; Wang, B.; Xu, J.; Pham-Huu, C.; Su, D. The Coulombic Nature of Active Nitrogen Sites in N-Doped Nanodiamond Revealed in Situ by Ionic Surfactants. ACS Catal. 2017, 7, 3295–3300. [Google Scholar] [CrossRef]
- Liu, B.; Qu, F.; Chen, W.; Liang, H.; Wang, T.; Cheng, X.; Yu, H.; Li, G.; Van der Bruggen, B. Microcystis aeruginosa-laden water treatment using enhanced coagulation by persulfate/Fe(II), ozone and permanganate: Comparison of the simultaneous and successive oxidant dosing strategy. Water Res. 2017, 125, 72–80. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Qin, Y.; Li, H. Removal of Microcystis aeruginosa and control of algal organic matter by Fe(II)/peroxymonosulfate pre-oxidation enhanced coagulation. Chem. Eng. J. 2021, 403, 126381. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, H.; Gao, B.; Zhao, C.; Liu, B.; Chen, W.; Guo, J. Interactions of specific extracellular organic matter and polyaluminum chloride and their roles in the algae-polluted water treatment. J. Hazard Mater. 2017, 332, 1–9. [Google Scholar] [CrossRef]
- Qu, F.; Liang, H.; He, J.; Ma, J.; Wang, Z.; Yu, H.; Li, G. Characterization of dissolved extracellular organic matter (dEOM) and bound extracellular organic matter (bEOM) of Microcystis aeruginosa and their impacts on UF membrane fouling. Water Res. 2012, 46, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, Y.; Dai, X.; Dong, B. The influence of organic-binding metals on the biogas conversion of sewage sludge. Water Res. 2017, 126, 329–341. [Google Scholar] [CrossRef]
- Tan, X.; Duan, Z.; Duan, P.; Parajuli, K.; Newman, J.; Shu, X.; Zhang, D.; Gao, L.; Li, M. Flocculation of Microcystis. unicells induced by pH regulation: Mechanism and potential application. Chemosphere 2021, 263, 127708. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-D.; Yang, Q.; Niu, C.H.; Badea, I. Protein-modified nanodiamond particles for Layer-by-Layer assembly. Diam. Relat. Mater. 2011, 20, 1193–1198. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, Y.; Ge, G. Nitrogen-doped nanotubes-decorated activated carbon-based hybrid nanoarchitecture as a superior catalyst for direct dehydrogenation. Catal. Sci. Technol. 2015, 5, 1548–1557. [Google Scholar] [CrossRef]
- Xu, H.; Yu, G.; Jiang, H. Investigation on extracellular polymeric substances from mucilaginous cyanobacterial blooms in eutrophic freshwater lakes. Chemosphere 2013, 93, 75–81. [Google Scholar] [CrossRef]
- Wang, G.; Tang, K.; Meng, Z.; Liu, P.; Mo, S.; Mehrjou, B.; Wang, H.; Liu, X.; Wu, Z.; Chu, P.K. A Quantitative Bacteria Monitoring and Killing Platform Based on Electron Transfer from Bacteria to a Semiconductor. Adv. Mater. 2020, 32, e2003616. [Google Scholar] [CrossRef]
- Shi, T.; Hou, X.; Guo, S.; Zhang, L.; Wei, C.; Peng, T.; Hu, X. Nanohole-boosted electron transport between nanomaterials and bacteria as a concept for nano-bio interactions. Nat. Commun. 2021, 12, 493. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).