Water-Level Fluctuations and Ungulate Community Dynamics in Central Uganda
Abstract
:1. Introduction
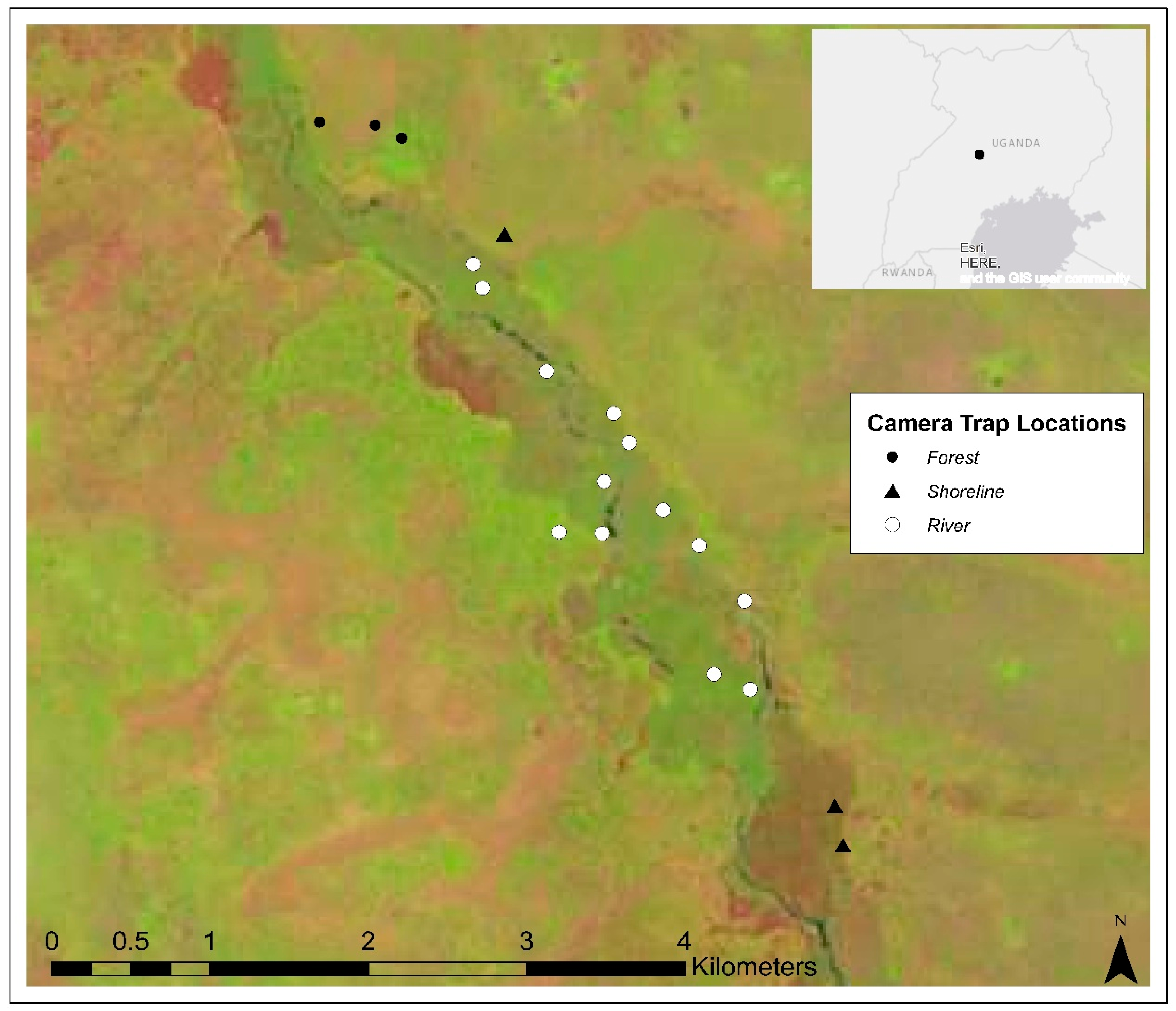
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, P.R.; Skea, J.; Slade, R.; van Diemen, R.; Haughey, E.; Malley, J.; Pathak, M.; Portugal Pereira, J. Technical Summary, 2019. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Erwin, K.L. Wetlands and Global Climate Change: The Role of Wetland Restoration in a Changing World. Wetl. Ecol. Manag. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- Engelbrecht, F.; Adegoke, J.; Bopape, M.-J.; Naidoo, M.; Garland, R.; Thatcher, M.; McGregor, J.; Katzfey, J.; Werner, M.; Ichoku, C.; et al. Projections of Rapidly Rising Surface Temperatures over Africa under Low Mitigation. Environ. Res. Lett. 2015, 10, 085004. [Google Scholar] [CrossRef] [Green Version]
- Maclean, I.M.D.; Boar, R.R.; Lugo, C. A Review of the Relative Merits of Conserving, Using, or Draining Papyrus Swamps. Environ. Manag. 2011, 47, 218–229. [Google Scholar] [CrossRef]
- Kayendeke, E.J.; Kansiime, F.; French, H.K.; Bamutaze, Y. Spatial and Temporal Variation of Papyrus Root Mat Thickness and Water Storage in a Tropical Wetland System. Sci. Total Environ. 2018, 642, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Pacini, N.; Hesslerová, P.; Pokorný, J.; Mwinami, T.; Morrison, E.H.J.; Cook, A.A.; Zhang, S.; Harper, D.M. Papyrus as an Ecohydrological Tool for Restoring Ecosystem Services in Afrotropical Wetlands. Ecohydrol. Hydrobiol. 2018, 18, 142–154. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine Flood Plains: Present State and Future Trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef] [Green Version]
- Ferrati, R.; Canziani, G.A.; Ruiz Moreno, D. Esteros Del Ibera: Hydrometeorological and Hydrological Characterization. Ecol. Modell. 2005, 186, 3–15. [Google Scholar] [CrossRef]
- Antle, J.; Apps, M.; Beamish, R.; Chapin, T.; Cramer, W.; Frangi, J.; Laine, J.; Lin, E.; Magnuson, J.; Noble, I.; et al. Ecosystems and Their Goods and Services. In Climate Change 2001: Impacts, Adaptation, and Vulnerability; McCarthy, J.J., Canziani, O.F., Leary, N.A., Dokken, D.J., White, K.S., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 235–342. ISBN 0521015006. [Google Scholar]
- Silvius, M.J.; Oneka, M.; Verhagen, A. Wetlands: Lifeline for People at the Edge. Phys. Chem. Earth Part B Hydrol. Ocean. Atmos. 2000, 25, 645–652. [Google Scholar] [CrossRef]
- Sakané, N.; Alvarez, M.; Becker, M.; Böhme, B.; Handa, C.; Kamiri, H.W.; Langensiepen, M.; Menz, G.; Misana, S.; Mogha, N.G.; et al. Classification, Characterisation, and Use of Small Wetlands in East Africa. Wetlands 2011, 31, 1103–1116. [Google Scholar] [CrossRef]
- Namaalwa, S.; Van Dam, A.A.; Funk, A.; Ajie, G.S.; Kaggwa, R.C. A Characterization of the Drivers, Pressures, Ecosystem Functions and Services of Namatala Wetland, Uganda. Environ. Sci. Policy 2013, 34, 44–57. [Google Scholar] [CrossRef]
- Uganda Bureau of Statistics. National Mid Year Population Projections by Single Age (2015–2050). Available online: https://www.ubos.org/wp-content/uploads/statistics/Single_year_mid_year_projections.xlsx (accessed on 25 February 2019).
- Barakagira, A.; de Wit, A.H. Community Livelihood Activities as Key Determinants for Community Based Conservation of Wetlands in Uganda. Environ. Socio-Econ. Stud. 2017, 5, 11–24. [Google Scholar] [CrossRef]
- Mwanjalolo, M.; Barasa, B.; Mukwaya, P.; Wanyama, J.; Kutegeka, S.; Nakyeyune, C.; Nakileza, B.; Diisi, J.; Ssenyonjo, E.; Nakangu, B. Assessing the Extent of Historical, Current, and Future Land Use Systems in Uganda. Land 2018, 7, 132. [Google Scholar] [CrossRef] [Green Version]
- Gosling, A.; Shackleton, C.M.; Gambiza, J. Community-Based Natural Resource Use and Management of Bigodi Wetland Sanctuary, Uganda, for Livelihood Benefits. Wetl. Ecol. Manag. 2017, 25, 717–730. [Google Scholar] [CrossRef]
- Junk, W.J.; Brown, M.; Campbell, I.C.; Finlayson, M.; Gopal, B.; Ramberg, L.; Warner, B.G. The Comparative Biodiversity of Seven Globally Important Wetlands: A Synthesis. Aquat. Sci. 2006, 68, 400–414. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource Partitioning in Ecological Communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef]
- Redfern, J.V.; Grant, R.; Biggs, H.; Getz, W.M. Surface-Water Constraints on Herbivore Foraging in the Kruger National Park, South Africa. Ecology 2003, 84, 2092–2107. [Google Scholar] [CrossRef]
- Spinage, C.A. Kobus ellipsiprymnus waterbuck. In Mammals of Africa Volume VI: Pigs, Hippopotamuses, Chevrotain, Giraffes, Deer and Bovids; Kingdon, J., Hoffmann, M., Eds.; Bloomsbury: London, UK, 2013; pp. 461–468. [Google Scholar]
- Lewison, R.L.; Pluháček, J. Hippopotamus amphibius. The IUCN Red List of Threatened Species. 2017. Available online: https://doi.org/10.2305/IUCN.UK.2017-2.RLTS.T10103A18567364.en (accessed on 25 June 2023).
- May, J.; Lindholm, R. Tragelaphus spekii Sitatunga. In Mammals of Africa Volume VI: Pigs, Hippopotamuses, Chevrotain, Giraffes, Deer and Bovids; Kingdon, J., Hoffmann, M., Eds.; Bloomsbury Publishing: London, UK, 2013; pp. 172–178. [Google Scholar]
- Ogutu, J.O.; Kuloba, B.; Piepho, H.P.; Kanga, E. Wildlife Population Dynamics in Human-Dominated Landscapes under Community-Based Conservation: The Example of Nakuru Wildlife Conservancy, Kenya. PLoS ONE 2017, 12, e0169730. [Google Scholar] [CrossRef] [Green Version]
- Ogutu, J.O.; Owen-Smith, N.; Piepho, H.-P.; Kuloba, B.; Edebe, J. Dynamics of Ungulates in Relation to Climatic and Land Use Changes in an Insularized African Savanna Ecosystem. Biodivers. Conserv. 2012, 21, 1033–1053. [Google Scholar] [CrossRef]
- Dunham, K.M. The Effect of Drought on the Large Mammal Populations of Zambezi Riverine Woodlands. J. Zool. 1994, 234, 489–526. [Google Scholar] [CrossRef]
- Jenkins, R.K.B.; Corti, G.R.; Fanning, E.; Roettcher, K. Management Implications of Antelope Habitat Use in the Kilombero Valley, Tanzania. Oryx 2002, 36, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, A.R.E. Does Interspecific Competition or Predation Shape the African Ungulate Community? J. Anim. Ecol. 1985, 54, 899. [Google Scholar] [CrossRef]
- Ndawula, J.; Tweheyo, M.; Tumusiime, D.M.; Eilu, G. Understanding Sitatunga (Tragelaphus spekii) Habitats through Diet Analysis in Rushebeya-Kanyabaha Wetland, Uganda. Afr. J. Ecol. 2011, 49, 481–489. [Google Scholar] [CrossRef]
- Gordon, I.J.; Illius, A.W. Resource Partitioning by Ungulates on the Isle of Rhum. Oecologia 1989, 79, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Bennitt, E.; Hubel, T.Y.; Bartlam-Brooks, H.L.A.; Wilson, A.M. Possible Causes of Divergent Population Trends in Sympatric African Herbivores. PLoS ONE 2019, 14, e0213720. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Muwanguzi, A.J.B.; Eden, U.; Sauer, T.; Bwanga, G.; Kateregga, G.; Aribo, L.; Ojara, M.; Mugerwa, W.K.; Schiff, S.J. Changes in Ugandan Climate Rainfall at the Village and Forest Level. Sci. Rep. 2018, 8, 3551. [Google Scholar] [CrossRef] [Green Version]
- Gideon, O.J.; Bernard, B. Effects of Human Wetland Encroachment on the Degradation of Lubigi Wetland System, Kampala City Uganda. Environ. Ecol. Res. 2018, 6, 562–570. [Google Scholar] [CrossRef]
- Jacob, A.L.; Bonnell, T.R.; Dowhaniuk, N.; Hartter, J. Topographic and Spectral Data Resolve Land Cover Misclassification to Distinguish and Monitor Wetlands in Western Uganda. ISPRS J. Photogramm. Remote Sens. 2014, 94, 114–126. [Google Scholar] [CrossRef]
- Oliver, W.L.R. Pigs, Peccaries and Hippos: Status Survey and Conservation Action Plan; IUCN/SSC Pigs and Peccaries Specialist Group, Hippo Specialist Group: Gland, Switzerland, 1993. [Google Scholar]
- East, R. African Antelope Database 1998; IUCN/SSC Antelope Specialist Group: Gland, Switzerland, 1998; ISBN 2831704774. [Google Scholar]
- IUCN SSC Antelope Specialist Group. Kobus ellipsiprymnus. The IUCN Red List of Threatened Species. 2016. Available online: https://doi.org/10.2305/IUCN.UK.2016-2.RLTS.T11035A50189324.en (accessed on 25 June 2023).
- Cumming, D.H.M. Phacochoerus africanus Common Warthog. In Mammals of Africa Volume VI: Pigs, Hippopotamuses, Chevrotain, Giraffes, Deer and Bovids; Kingdon, J., Hoffmann, M., Eds.; Bloomsbury: London, UK, 2013; pp. 54–60. [Google Scholar]
- Plumptre, A.J.; Wronski, T. Tragelaphus scriptus Bushbuck. In Mammals of Africa Volume VI: Pigs, Hippopotamuses, Chevrotain, Giraffes, Deer and Bovids; Kingdon, J., Hoffmann, M., Eds.; Bloomsbury: London, UK, 2013; pp. 163–172. [Google Scholar]
- Games, I. Observations on the Sitatunga Tragelaphus spekei selousi in the Okavango Delta of Botswana. Biol. Conserv. 1983, 27, 157–170. [Google Scholar] [CrossRef]
- Klingel, H. Hippopotamus amphibius Common Hippopotamus. In Mammals of Africa Volume VI: Pigs, Hippopotamuses, Chevrotain, Giraffes, Deer and Bovids; Kingdon, J., Hoffmann, M., Eds.; Bloomsbury: London, UK, 2013; pp. 68–78. [Google Scholar]
- Amin, R.; Bowkett, A.E.; Wacher, T. The Use of Camera-Traps to Monitor Forest Antelope Species. In Antelope Conservation: From Diagnosis to Action; Bro-Jørgensen, J., Mallon, D.P., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 190–216. ISBN 9781118409572. [Google Scholar]
- Central Intelligence Agency. Africa: Uganda—The World Factbook—Central Intelligence Agency. Available online: https://www.cia.gov/library/publications/the-world-factbook/geos/ug.html (accessed on 21 February 2019).
- Climate-Data.org. Nakaseke Climate: Average Temperature, Weather by Month, Nakaseke Weather Averages. Available online: https://en.climate-data.org/africa/uganda/central-region/nakaseke-925183/ (accessed on 26 February 2019).
- River Mayanja Washes Away Bridge. New Vision, 6 May 2016. Available online: https://www.newvision.co.ug/new_vision/news/1424016/river-mayanja-washes-away-bridge(accessed on 4 August 2022).
- Masindi, Uganda Travel Weather Averages (Weatherbase). Available online: http://www.weatherbase.com/weather/weather.php3?s=605888&cityname=Masindi-Uganda (accessed on 12 February 2020).
- Masindi, Masindi, Uganda Historical Weather Almanac. Available online: https://www.worldweatheronline.com/masindi-weather-history/masindi/ug.aspx (accessed on 12 February 2020).
- Bbosa, T. Construction/Reconstruction of Strategic Bridges in Eastern and Central Regions (3 Lots): Lot 1: Kaabale Bridge on Kyankwanzi-Ngoma Road; Makarere University: Kampala, Uganda, 2019. [Google Scholar]
- Sollmann, R.; Mohamed, A.; Samejima, H.; Wilting, A. Risky Business or Simple Solution—Relative Abundance Indices from Camera-Trapping. Biol. Conserv. 2013, 159, 405–412. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.5.1; Windows; R foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Nakagawa, S. A Farewell to Bonferroni: The Problems of Low Statistical Power and Publication Bias. Behav. Ecol. 2004, 15, 1044–1045. [Google Scholar] [CrossRef] [Green Version]
- Moran, M.D. Arguments for Rejecting the Sequential Bonferroni in Ecological Studies. Oikos 2003, 100, 403–405. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Science, 2nd ed; Routledge: London, UK, 1988; ISBN 0805802835. [Google Scholar]
- Warbington, C.; Boyce, M. Species Detections from Camera Traps Along the Mayanja River of Uganda, 2015–2017; UAlberta Research Data Collection: Edmonton, AB, Canada, 2020. [Google Scholar] [CrossRef]
- Cooke, R.S.C.; Woodfine, T.; Petretto, M.; Ezard, T.H.G. Resource Partitioning between Ungulate Populations in Arid Environments. Ecol. Evol. 2016, 6, 6354–6365. [Google Scholar] [CrossRef] [Green Version]
- Starin, E.D. Notes on Sitatunga in The Gambia. Afr. J. Ecol. 2000, 38, 339–342. [Google Scholar] [CrossRef]
- Seydack, A.H.W. Potamochoerus larvatus Bushpig. In Mammals of Africa Volume VI: Pigs, hippopotamuses, Chevrotain, Giraffes, Deer, and Bovids; Kingdon, J., Hoffmann, M., Eds.; Bloomsbury: London, UK, 2013; pp. 32–36. [Google Scholar]
- Williams, J.J.; Newbold, T. Local Climatic Changes Affect Biodiversity Responses to Land Use: A Review. Divers. Distrib. 2020, 26, 76–92. [Google Scholar] [CrossRef]
- Khaliq, I.; Hof, C.; Prinzinger, R.; Böhning-Gaese, K.; Pfenninger, M. Global Variation in Thermal Tolerances and Vulnerability of Endotherms to Climate Change. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141097. [Google Scholar] [CrossRef] [PubMed]
- Voeten, M.M.; Prins, H.H.T. Resource Partitioning between Sympatric Wild and Domestic Herbivores in the Tarangire Region of Tanzania. Oecologia 1999, 120, 287–294. [Google Scholar] [CrossRef]
- Ogutu, J.O.; Piepho, H.-P.; Said, M.Y.; Kifugo, S.C. Herbivore Dynamics and Range Contraction in Kajiado County Kenya: Climate and Land Use Changes, Population Pressures, Governance, Policy and Human-Wildlife Conflicts. Open Ecol. J. 2014, 7, 9–31. [Google Scholar] [CrossRef] [Green Version]
- Ehlers Smith, Y.C.; Ehlers Smith, D.A.; Ramesh, T.; Downs, C.T. Novel Predators and Anthropogenic Disturbance Influence Spatio-Temporal Distribution of Forest Antelope Species. Behav. Process. 2019, 159, 9–22. [Google Scholar] [CrossRef]
- Averbeck, C.; Plath, M.; Wronski, T.; Apio, A. Effect of Human Nuisance on the Social Organisation of Large Mammals: Group Sizes and Compositions of Seven Ungulate Species in Lake Mburo National Park and the Adjacent Ankole Ranching Scheme. Wildl. Biol. 2012, 18, 180–193. [Google Scholar] [CrossRef] [Green Version]
- Gaynor, K.M.; Hojnowski, C.E.; Carter, N.H.; Brashares, J.S. The Influence of Human Disturbance on Wildlife Nocturnality. Science 2018, 360, 1232–1235. [Google Scholar] [CrossRef] [Green Version]
- Desbiez, A.L.J.; Santos, S.A.; Alvarez, J.M.; Tomas, W.M. Forage Use in Domestic Cattle (Bos indicus), Capybara (Hydrochoerus hydrochaeris) and Pampas Deer (Ozotoceros bezoarticus) in a Seasonal Neotropical Wetland. Mamm. Biol. 2011, 76, 351–357. [Google Scholar] [CrossRef]
- Warbington, C.H. Sitatunga Population Ecology and Habitat Use in Central Uganda. Doctoral Dissertation, University of Alberta, Edmonton, AB, Canada, 2020. [Google Scholar]
- Chui, J.; Warbington, C.; Boyce, M. Temporal Niche Differentiation among Large Herbivores in the Mayanja River, Uganda; UAlberta Research Data Collection: Edmonton, AB, Canada, 2020. [Google Scholar] [CrossRef]
- Turyahabwe, N.; Tumusiime, D.M.; Kakuru, W.; Barasa, B. Wetland Use/Cover Changes and Local Perceptions in Uganda. Sustain. Agric. Res. 2013, 2, 95. [Google Scholar] [CrossRef] [Green Version]
- Wasswa, H.; Kakembo, V.; Mugagga, F. A Spatial and Temporal Assessment of Wetland Loss to Development Projects: The Case of the Kampala–Mukono Corridor Wetlands in Uganda. Int. J. Environ. Stud. 2019, 76, 195–212. [Google Scholar] [CrossRef]






| Species | Scientific Name | Food Preferences | Habitat Preferences | Temporal Activity Pattern | References |
|---|---|---|---|---|---|
| Bushbuck | Tragelaphus scriptus Pallas, 1766 | Primarily Browser | Generalist, some cover | Crepuscular | [38] |
| Bushpig | Potamochoerus larvatus F. Cuvier, 1822 | Omnivorous | Dense vegetation, forests, thickets | Primarily nocturnal | [39] |
| Domestic Cattle | Bos taurus Linnaeus, 1758 | Grazing | NA | NA | |
| Hippopotamus | Hippopotamus amphibius Linnaeus, 1758 | Grazing | Water, grassland/bushland | Nocturnal | [21,40] |
| Sitatunga | Tragelaphus spekii Speke, 1863 | Selective mixed feeder | Dense wetlands | Diurnal | [22] |
| Warthog | Phacochoerus africanus Gmelin, 1788 | Grazing, rooting | Generalist, open | Generally diurnal | [37] |
| Waterbuck | Kobus ellipsiprymnus Ogilby, 1833 | Grazer-browser | Generalist, near permanent water | Diurnal | [20,36] |
| Species | Zone | 2015 Camera Days | 2015 Encounters | 2015 Proportion | 2016 Camera Days | 2016 Encounters | 2016 Proportion | 2017 Camera Days | 2017 Encounters | 2017 Proportion |
|---|---|---|---|---|---|---|---|---|---|---|
| Bushbuck | Forest | 318 | 46 ‡ | 0.145 | 286 | 105 ‡ | 0.367 | 168 | 116 ‡ | 0.690 |
| Bushpig | Forest | 318 | 24 ‡ | 0.075 | 286 | 22 ‡ | 0.077 | 168 | 53 ‡ | 0.315 |
| Cattle | Forest | 318 | 9 ‡ | 0.028 | 286 | 10 ‡ | 0.035 | 168 | 20 ‡ | 0.119 |
| Hippopotamus | Forest | 318 | 10 ‡ | 0.031 | 286 | 26 ‡ | 0.091 | 168 | 22 ‡ | 0.131 |
| Sitatunga | Forest | 318 | 33 ‡ | 0.104 | 286 | 4 ‡ | 0.014 | 168 | 0 ‡ | 0 |
| Warthog | Forest | 318 | 17 ‡ | 0.053 | 286 | 3 ‡ | 0.010 | 168 | 14 ‡ | 0.083 |
| Waterbuck | Forest | 318 | 32 ‡ | 0.101 | 286 | 40 ‡ | 0.140 | 168 | 59 ‡ | 0.351 |
| Sitatunga | Shoreline | 98 | 15 ‡ | 0.153 | 508 | 25 ‡ | 0.049 | † | † | † |
| Waterbuck | River | 1357 | 0 | 0 | 587 | 6 | 0.010 | 1012 | 0 | 0 |
| Bushbuck | River | 1357 | 0 | 0 | 587 | 0 | 0 | 1012 | 1 | 0.001 |
| Bushpig | River | 1357 | 0 | 0 | 587 | 0 | 0 | 1012 | 2 | 0.002 |
| Hippopotamus | River | 1357 | 0 | 0 | 587 | 0 | 0 | 1012 | 17 | 0.017 |
| Sitatunga | River | 1357 | 157 ‡ | 0.116 | 587 | 54 ‡ | 0.092 | 1012 | 127 ‡ | 0.125 |
| Species | Zone | Comparison | χ2 | p-Value | Comparison | χ2 | p-Value | Comparison | χ2 | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Bushbuck | Forest | 15, 16 | 38.572 | 5.23 × 10−10 *† | 16, 17 | 43.006 | 5.46 × 10−11 *† | 15, 17 | 14493 | 2.2 × 10−16 *† |
| Bushpig | Forest | 15, 16 | 6.35 × 10−30 | 1 | 16, 17 | 41.959 | 9.32 × 10−11 *† | 15, 17 | 45.707 | 1.37 × 10−11 *† |
| Cattle | Forest | 15, 16 | 0.055218 | 0.8142 | 16, 17 | 10.8 | 0.001015 *† | 15, 17 | 14.556 | 0.000136 *† |
| Hippopotamus | Forest | 15, 16 | 8.4675 | 0.003616 *† | 16, 17 | 1.3963 | 0.2373 | 15, 17 | 16.115 | 5.96 × 10−5 *† |
| Sitatunga | Forest | 15, 16 | 19.577 | 9.66 × 10−6 *‡ | 16, 17 | 1.0395 | 0.0379 *‡ | 15, 17 | 17.1 | 3.55 × 10−5 *‡ |
| Warthog | Forest | 15, 16 | 7.3935 | 0.006546 *‡ | 16, 17 | 13.625 | 0.000223 *† | 15, 17 | 1.1807 | 0.2772 |
| Waterbuck | Forest | 15, 16 | 1.8494 | 0.1739 | 16, 17 | 26.494 | 2.64 × 10−7 *† | 15, 17 | 43.717 | 3.8 × 10−11 *† |
| Sitatunga | Shoreline | 15, 16 | 12.736 | 0.000359 *‡ | -- | -- | -- | -- | -- | -- |
| Sitatunga | River | 15, 16 | 2.1406 | 0.1434 | 16, 17 | 3.8266 | 0.05045 | 15, 17 | 0.43864 | 0.5078 |
| Species | Zone | Comparison | Cohen’s h | Comparison | Cohen’s h | Comparison | Cohen’s h |
|---|---|---|---|---|---|---|---|
| Bushbuck | Forest | 15, 16 | 0.50 | 16, 17 | 0.66 | 15, 17 | 1.15 |
| Bushpig | Forest | -- | -- | 16, 17 | 0.63 | 15, 17 | 0.64 |
| Cattle | Forest | -- | -- | 16, 17 | 0.33 | 15, 17 | 0.37 |
| Hippopotamus | Forest | 15, 16 | 0.26 | -- | -- | 15, 17 | 0.38 |
| Sitatunga | Forest | 15, 16 | 0.42 | 16, 17 | 0.24 | 15, 17 | 0.66 |
| Warthog | Forest | 15, 16 | 0.26 | 16, 17 | 0.38 | -- | -- |
| Waterbuck | Forest | -- | -- | 16, 17 | 0.50 | 15, 17 | 0.62 |
| Sitatunga | Shoreline | 15, 16 | 0.36 | -- | -- | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warbington, C.H.; Boyce, M.S. Water-Level Fluctuations and Ungulate Community Dynamics in Central Uganda. Water 2023, 15, 2765. https://doi.org/10.3390/w15152765
Warbington CH, Boyce MS. Water-Level Fluctuations and Ungulate Community Dynamics in Central Uganda. Water. 2023; 15(15):2765. https://doi.org/10.3390/w15152765
Chicago/Turabian StyleWarbington, Camille H., and Mark S. Boyce. 2023. "Water-Level Fluctuations and Ungulate Community Dynamics in Central Uganda" Water 15, no. 15: 2765. https://doi.org/10.3390/w15152765
APA StyleWarbington, C. H., & Boyce, M. S. (2023). Water-Level Fluctuations and Ungulate Community Dynamics in Central Uganda. Water, 15(15), 2765. https://doi.org/10.3390/w15152765









