A Study on Nitrogen and Phosphorus Budgets in a Polyculture System of Oreochromis niloticus, Aristichthys nobilis, and Cherax quadricarinatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Ponds and Materials
2.2. Aquaculture Management
2.3. Response Variables and Analytical Methods
2.3.1. Determination of Dissolved Oxygen, pH Value, and Temperature of Pond Water
2.3.2. Measurement of Nutrients of Pond Water Samples and Interstitial Water
2.3.3. Collection and Measurement of Breeding Organisms, Sediment, and Feed
2.3.4. Determination of the Growth Parameters of Breeding Organisms
2.3.5. Determination of Other Indicators
2.3.6. Calculation Formula
2.4. Statistical Analysis of Data
3. Results
3.1. Environmental Parameters
3.2. Harvesting Conditions
3.3. Dry Matter and Nitrogen and Phosphorus Contents of Feed and Breeding Organisms
3.4. Contents of Dry Matter, N, and P, and Densities in the Sediment of Different Enclosures before and after the Experiment
3.5. N and P Inputs
3.6. N and P Outputs
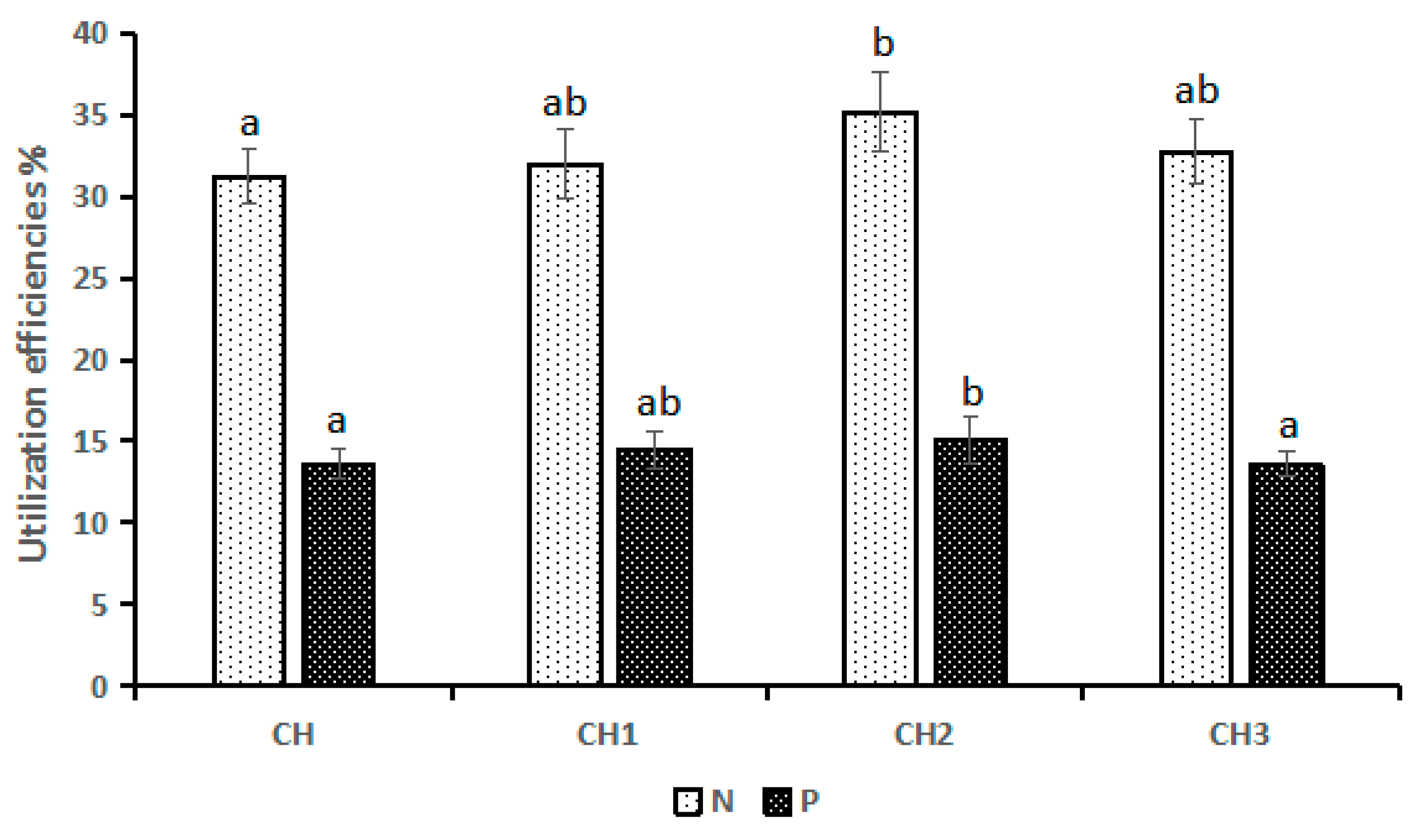
3.7. N and P Utilization Efficiencies
3.8. Pollution Production Coefficients of the Ponds (PPCP) and Sediment Deposition (PPCSD)
4. Discussion
4.1. N and P Inputs and Outputs
4.2. N and P Accumulation
4.3. Suitable Breeding Ratio and N and P Utilization Efficiencies
4.4. Pollution Production Coefficient and Environmental Impact of Different O. niloticus and Breeding Modes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherif, A.H.; Prince, A.; Seida, A.A.; Sharaf, M.S.; Eldessouki, E.A.; Harfoush, M.A. Moringa oleifera mitigates oxytetracycline stress in Oreochromis niloticus. Aquac. Res. 2022, 53, 1790–1799. [Google Scholar] [CrossRef]
- Doan, H.V.; Hoseinifar, S.H.; Naraballobh, W.; Jaturasitha, S.; Tongsiri, S.; Chitmanat, C.; Ring, E. Dietary inclusion of orange peels derived pectin and Lactobacillus plantarum for Nile tilapia (Oreochromis niloticus) cultured under indoor biofloc systems. Aquaculture 2019, 508, 98–105. [Google Scholar] [CrossRef]
- Rahman, M.M.; Nagelkerke, L.A.J.; Verdegem, M.C.J.; Abdul Wahab, M.; Verreth, J.A.J. Relationships among water quality, food resources, fish diet and fish growth in polyculture ponds: A multivariate approach. Aquaculture 2008, 275, 108–115. [Google Scholar] [CrossRef]
- Storer, T. Ethology and Production of Freshwater Crayfish in Aquatic Polysystems in Western Australia. Ph.D. Thesis, Curtin University, Singapore, 2005. [Google Scholar]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.-G. Ecological engineering in aquaculture Potential for integrated multitrophic aquaculture (IMTA) in marine offshore systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Liu, Y.; Ai, G.M.; Miao, L.L.; Zheng, H.Y.; Liu, Z.P. The characteristics of a novel heterotrophic nitrification-aerobic denitrification bacterium, bacillus methylotrophicus strain L7. Bioresour. Technol. 2012, 108, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.F.; Wen, Y.H.; Ge, X.P.; Sun, R.J. Current situation and development trend of bighead fish culture in the Pearl River Delta region. Sci. Fish Farming 2016, 10, 82–84. [Google Scholar]
- Zhang, Z.; Shi, Y.; Zhang, J. Experimental observation on the efects of bighead carp (Hypophthalmichthys nobilis) on the plankton and water quality in ponds. Environ. Sci. Pollut. Res. 2002, 29, 19923–19925. [Google Scholar]
- Liu, B.H.; Fang, Z.S.; Qiao, B.H.; Qiu, J.J. Economic benefit analysis of Nile Tilapia Polyculture model. Ocean Fish 2020, 9, 182–183. [Google Scholar]
- Sha, T.A.; Dar, C.J.Y. Feral populations of the Australian Red-Claw crayfifish (Cherax quadricarinatus von Martens) in water supply catchments of Singapore. Biol. Invasions 2007, 9, 943–946. [Google Scholar]
- Mai, W.; Wang, C.; Zhong, X.Q. Culture experiment of Australian freshwater crayfish mixed with conventional freshwater fish. Sci. Fish Farming 2019, 35, 80–81. [Google Scholar]
- Wang, M.D.; Meng, L.X. Research Progress on Breeding Cherax quadricarinatus. Aquaculture 2021, 6, 35–38. [Google Scholar]
- Coronado-Castro, E.; Silva-Ledezma, L.; Gasca-Leyva, E. Economic analysis of polyculture tilapia and Australian redclaw crayfish. In Proceedings of the IIFET 2010, Montpellier, France, 13–16 July 2010; pp. 1–10. [Google Scholar]
- Karplus, I.; Barki, A.; Cohen, S.; Hulata, G. Culture of The Australian Red-Claw Crayfish (Cherax quadricarinatus) in Israel Crayfish Incorporation into Intensive Tilapia Production Units. Isr. J. Aquac.-Bamidgeh 2001, 53, 23–33. [Google Scholar] [CrossRef]
- Brom, R.E.; Alon, N.C. Polyculture of Nile tilapia (Oreochromis niloticus) and Australian red claw red claw (Cherax quadricarinatus) in earthen ponds. Aquaculture 1994, 1, 47–54. [Google Scholar]
- Karplus, I.; Barki, A.; Cohen, S.; Hulata, G. Culture of the Australian red-claw crayfish (Cherax quadricarinatus) in Israel. I. Polyculture with fish in earthen ponds. Isr. J. Aquac. Bamidgeh. 1995, 47, 6–16. [Google Scholar]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Sahu, B.C.; Adhikari, S.; Dey, L. Carbon, nitrogen and phosphorus budget in shrimp (Penaeus monodon) culture ponds in eastern India. Aquac. Int. 2013, 21, 453–466. [Google Scholar] [CrossRef]
- Boyd, C.E. Chemical budgets for channel catfish ponds. Trans. Am. Fish. Soc. 1985, 114, 291–298. [Google Scholar] [CrossRef]
- Krom, M.D.; Neori, A. A total nutrient budget for an experimental intensive fish-pond with circularly moving seawater. Aquaculture 1989, 83, 345–358. [Google Scholar] [CrossRef]
- Dong, J.; Tian, X.L.; Dong, S.L.; Zhang, K.; Feng, J.; He, R.P. Study on nitrogen and phosphorus budget on different polyculture system of Litopenaeus vannamei and Portunus trituberculatus. J. Ocean Univ. China 2013, 43, 16–24. [Google Scholar]
- Sahu, B.C.; Adhikari, S.; Mahapatra, A.S.; Dey, L. Carbon, nitrogen, and phosphorus budget in scampi (Macrobrachium rosenbergii) culture ponds. Environ. Monit. Assess. 2013, 185, 10157–10166. [Google Scholar] [CrossRef]
- Zhang, K.; Tian, X.L.; Dong, S.L.; Feng, J.; He, R.P. An experimental study on the budget of organic carbon in polyculture systems of swimming crab with white shrimp and short necked clam. Aquaculture 2016, 451, 58–64. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, J.; Yu, D.G.; Wang, G.J.; Yu, E.R.; Li, Z.F.; Gong, W.B.; Wang, C.C.; Xia, Y. A comparative study on the budget of nitrogen and phosphorus in polyculture systems of snakehead with bighead carp. Aquaculture 2018, 483, 69–75. [Google Scholar] [CrossRef]
- Yi, Y. Hybrid catfish (Clarias macrocephalus × C-gariepinus) and Nile tilapia (Oreochromis niloticus) culture in an integrated pen-cum-pond system: Growth performance and nutrient budgets. Aquaculture 2003, 217, 395–408. [Google Scholar] [CrossRef]
- Zhou, L. Study on nitrogen and phosphorus budget of intensive tilapia ponds in two culture models. Ph.D. Thesis, Guangdong Ocean University, Zhanjiang, China, 2010. [Google Scholar]
- Li, J.W.; Dong, S.L.; Gao, Q.F.; Zhu, C.B. Nitrogen and phosphorus budget of a polyculture system of sea cucumber (Apostichopus japonicus), jellyfish (Rhopilema esculenta) and shrimp (Fenneropenaeus chinensis). J. Ocean Univ. China 2014, 13, 503–508. [Google Scholar] [CrossRef]
- Zhong, Q.F. The Nitrogen and Phosphorus Budgets in Polyculture Ponds of Tilapia as The Main Species. J. Guangdong Ocean. Univ. 2019, 39, 48–53. [Google Scholar]
- Cao, B.; Abakari, G.; Luo, G.; Tan, H.; Xia, W. Comparative analysis of nitrogen and phosphorus budgets in a bioflocs aquaculture system and recirculation aquaculture system during over-wintering of tilapia (GIFT, Oreochromis niloticus). Aquac. Eng. 2020, 89, 102026. [Google Scholar] [CrossRef]
- Li, D.S. A Device of Land-Based Experimental Enclosure Used in Ponds. J. Ocean. Univ. Qingdao 1998, 28, 199–204. [Google Scholar]
- Laskov, C.; Herzog, C.; Lewandowski, J.; Hupfer, M. Miniaturized photometrical methods for the rapid analysis of phosphate, ammonium, ferrous iron and sulfate in pore water of freshwater sediments. Limnol. Oceanogr. Methods 2007, 5, 63–71. [Google Scholar] [CrossRef]
- Tu, X.H.; Xiao, B.D.; Xiong, J.; Chen, X. A simple miniaturised photometrical method for rapid determination of nitrate and nitrite in freshwater. Talanta 2010, 82, 976–983. [Google Scholar] [CrossRef]
- Hu, C.Y.; Wang, Z.F.; Lü, H.Y. A method of determination of total phosphate in the seawater and marine sediment. Mar. Environ. Sci. 1999, 18, 48–52. [Google Scholar]
- GB/T 6437-2002; Determination of Phosphorus in Feed-Spectphotometry. National Standardization Management Council of China: Beijing, China, 2002.
- Xue, L.Z. Transformation and removal effects of nitrogen and phosphorus in the ecological farming model of fish-vegetables-mushroom. J. Sub. Res. Environ. 2014, 9, 15–25. [Google Scholar]
- Meng, S.L.; Hu, G.D.; Qu, J.H.; Wu, W.F.; Fan, L.M.; Chen, J.Z. Preliminary Study on the Pollutants Producing Coefficient of Sediment in Tilapia Broodstock Rearing Pond with Monoculture Mode. J. Agric. Environ. Sci. 2010, 29, 1795–1800. [Google Scholar]
- Jackson, C.; Preston, N.; Thompson, P.J.; Burford, M. Nitrogen budget and effluent nitrogen components at an intensive shrimp farm. Aquaculture 2003, 218, 397–411. [Google Scholar] [CrossRef]
- Casillas-Hernnandez, R.; Ontiveros, C.A.S.; Estrada, M.A.C.; Ibarra-Gámez, J.C.; Lares-Villa, F. Water quality and nutrient budget in experimental closed tilapia Oreochromis niloticus culture systems. Rev. Latinoam. Recur. Nat. 2012, 8, 46–55. [Google Scholar]
- Gross, A.; Boyd, C.E.; Wood, C.W. Nitrogen transformations and balance in channel catfish ponds. Aquac. Eng. 2000, 24, 1–14. [Google Scholar] [CrossRef]
- Alongi, D.M.; McKinnon, A.D.; Brinkman, R.; Trott, L.A.; Undu, M.C.; Muawanah; Rachmansyah, R. The fate of organic matter derived from small–scale fish cage aquaculture in coastal waters of Sulawesi and Sumatra, Indonesia. Aquaculture 2009, 295, 60–75. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, D.G.; Li, Z.F.; Xie, J.; Wang, G.J.; Gong, W.B.; Yu, E.R.; Tian, J.J. Influence of eco–substrate addition on organic carbon, nitrogen and phosphorus budgets of intensive aquaculture ponds of the Pearl River, China. Aquaculture 2002, 520, 734868. [Google Scholar] [CrossRef]
- David, F.S.; Proença, D.C.; Valenti, W.C. Nitrogen budget in integrated aquaculture systems with Nile tilapia and Amazon River prawn. Aquac. Int. 2017, 25, 1733–1746. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.X.; Wu, J.; Wang, J.H. Nitrogen removal in recirculating aquaculture water with high dissolved oxygen conditions using the simultaneous partial nitrification, anammox and denitrification system. Bioresour. Technol. 2020, 305, 123037. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Nitrogen biogeochemistry of aquaculture ponds. Aquaculture 1998, 166, 181–212. [Google Scholar] [CrossRef]
- Hu, B.; Quan, J.; Huang, K.; Zhao, J.; Hu, Y. Effects of C/N ratio and dissolved oxygen on aerobic denitrification process: A mathematical modeling study. Chemosphere 2021, 272, 129521. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.; Pandey, P.K.; Anand, T.; Bhuvaneswari, R.; Kumar, S. Effect of periphyton (aquamat) on water quality, nitrogen budget, microbial ecology, and growth parameters of Litopenaeus vannamei, in a semi-intensive culture system. Aquaculture 2017, 479, 240–249. [Google Scholar] [CrossRef]
- Guo, K.; Zhao, W.; Dong, S.L.; Jiang, Z.Q. Structure of suspended particles and organic carbon storage in jellyfish-shellfish-fish-prawn polyculture ponds. Acta Ecol. Sin. 2016, 36, 1872–1880. [Google Scholar]
- Yang, P.; Lai, D.Y.F.; Jin, B.S.; Bastviken, D.; Tan, L.S.; Tong, C. Dynamics of dissolved nutrients in the aquaculture shrimp ponds of the Min river estuary, China: Concentrations, fluxes and environmental loads. Sci. Total Environ. 2017, 603–604, 256–267. [Google Scholar] [CrossRef]
- Wang, S.; Gao, S.S.; Jiang, L.L.; Shen, Q.Q.; Feng, J.X. A review of budget and ecological impact of nutruents nitroden and phosphorus in an aquaculture ecosystem. Chin. J. Fish. 2018, 31, 50–58. [Google Scholar]
- Li, Y.R.; Wu, X.J.; Huo, S.Y.; Hao, Y.G.; Pang, X.W. Effects of diludin on thegrowth performance and the antioxidant activity of Tilapia nilotia. J. Agric. Unver. Hebei 2003, 26, 11–15. [Google Scholar]
- Bortolini, J.L.; Alvarez, F.; Rodríguez-Almaraz, G. On the presence of the Australian redclaw crayfifish, Cherax quadricarinatus, in Mexico. Biol. Invasions 2007, 9, 615–620. [Google Scholar] [CrossRef]
- Tian, X.L.; Li, D.S.; Dong, S.L.; Yan, X.Z.; Qi, Z.X.; Liu, G.C.; Lu, J. An experimental study on closed-polyculture of penaeid shrimp with tiapia and constricted tagelus. Aquaculture 2001, 202, 57–77. [Google Scholar] [CrossRef]
- Li, Z.J.; Yu, W.; Zhu, C.B.; Wen, G.L.; Ma, G.Z. Study on nitrogen and phosphorus budgets of experimental enclosures with shrimp monoculture and shrimp-tilapia polyculture. J. Saf. Environ. 2012, 122, 50–56. [Google Scholar]
- Xia, X.J.; Xu, Z.N.; Lin, X.T.; Xie, Z.Y.; Jin, Z.L. Environmental nitrogen and phosphorus loading and water quality of three culture methods in ponds. Mar. Sci. 2012, 36, 87–92. [Google Scholar]
- Yang, Y.P.; Wang, Z.H.; Sun, J.; Hu, M.H. The variation law of aqueous chemical factors and the buddets od nitrogen in the intensive shrimp ponds. Mar. Sci. 1999, 1, 15–17. [Google Scholar]
| Oreochromis niloticus | Aristichthys nobilis | Cherax quadricarinatus | ||||
|---|---|---|---|---|---|---|
| Weight (g) | Density (ind/m2) | Weight (g) | Density (ind/m2) | Weight (g) | Density (ind/m2) | |
| CH | 6.22 ± 0.48 | 4 | 21.04 ± 3.56 | 0.15 | ||
| CHC1 | 6.21 ± 0.52 | 4 | 20.27 ± 6.28 | 0.15 | 1.26 ± 0.09 | 1.5 |
| CHC2 | 6.18 ± 0.53 | 4 | 22.85 ± 4.37 | 0.15 | 1.26 ± 0.23 | 3 |
| CHC3 | 6.19 ± 0.48 | 4 | 21.14 ± 5.15 | 0.15 | 1.25 ± 0.21 | 4.5 |
| Environmental Compartment | Parameter | CH | CHC1 | CHC2 | CHC3 |
|---|---|---|---|---|---|
| Water | T (°C) | 23.5–33.4 (27.2 ± 0.4) | 23.5–33.4 (27.2 ± 0.4) | 23.5–33.4 (27.2 ± 0.4) | 23.5–33.4 (27.2 ± 0.4) |
| DO (mg/L) | 4.83–8.54 (4.98 ± 0.21) | 4.97–10.2 (5.17 ± 0.2) | 5.35–12.4 (5.72 ± 0.75) | 4.98–13.1 (5.58 ± 0.54) | |
| pH | 7.07–8.04 (7.27 ± 0.02) | 7.14–8.31 (7.36 ± 0.01) | 7.54–8.39 (7.58 ± 0.06) | 7.32–8.15 (7.41 ± 0.03) | |
| TAN (mg/L) | 0.043–0.586 (0.331 ± 0.036) | 0.024–1.285 (0.348 ± 0.031) | 0.036–1.24 (0.369 ± 0.037) | 0.028–1.49 (0.368 ± 0.029) | |
| NO3−-N (mg/L) | 0.019–0.087 (0.081 ± 0.038) | 0.067–1.12 (0.083 ± 0.052) | 0.033–1.08 (0.084 ± 0.041) | 0.124–1.46 (0.083 ± 0.043) | |
| NO2−-N (mg/L) | 0.018–0.059 (0.003 ± 0.001) | 0.002–0.044 (0.003 ± 0.001) | 0.004–0.171 (0.004 ± 0.001) | 0.003–0.129 (0.003 ± 0.001) | |
| PO43−-P (mg/L) | 0.005–0.131 (0.091 ± 0.04) | 0.035–0.179 (0.093 ± 0.007) | 0.037–0.202 (0.097 ± 0.010) | 0.027–0.219 (0.107 ± 0.012) | |
| TN (mg/L) | 0.69–1.731 (1.29 ± 0.041) | 0.504–1.98 (1.304 ± 0.068) | 0.815–2.23 (1.32 ± 0.053) | 0.607–2.16 (1.21 ± 0.073) | |
| TP (mg/L) | 0.009–0.217 (0.091 ± 0.007) | 0.042–0.201 (0.122 ± 0.021) | 0.064–0.254 (0.112 ± 0.013) | 0.043–0.217 (0.143 ± 0.017) | |
| Sediment | TN (g/L) | (0.740 ± 0.114) | (0.792 ± 0.107) | (0.793 ± 0.078) | (0.762 ± 0.087) |
| TP (g/L) | (0.683 ± 0.067) | (0.629 ± 0.054) | (0.617 ± 0.063) | (0.621 ± 0.058) |
| Group | Oreochromis niloticus | Aristichthys nobilis | Cherax quadricarinatus | Total Yield (Ton/hm2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (kg) | Survival Rate (%) | Yield (Ton/hm2) | Feed (Ton/hm2) | FCR | Size (kg) | Survival Rate (%) | Yield (Ton/hm2) | Size (kg) | Survival Rate (%) | Yield (Ton/hm2) | ||
| CH | 0.654 ± 0.017 a | 89.4 ± 2.23 a | 23.3 ± 0.734 a | 30.4 ± 1.15 | 1.30 ± 0.11 | 0.515 ± 0.011 | 100 | 0.772 ± 0.043 | - | - | - | 24.1 ± 0.654 a |
| CHC1 | 0.670 ± 0.011 ab | 90.5 ± 1.72 ab | 24.2 ± 0.759 ab | 30.3 ± 1.04 | 1.25 ± 0.70 | 0.516 ± 0.011 | 100 | 0.774 ± 0.058 | 0.059 ± 0.007 | 52.1 ± 6.76 a | 0.458 ± 0.009 a | 25.4 ± 0.749 a |
| CHC2 | 0.684 ± 0.009 b | 93.2 ± 2.16 b | 25.5 ± 0.803 b | 30.6 ± 1.10 | 1.20 ± 0.05 | 0.517 ± 0.012 | 100 | 0.776 ± 0.103 | 0.057 ± 0.007 | 50.3 ± 8.13 a | 0.865 ± 0.017 b | 27.1 ± 0.804 b |
| CHC3 | 0.665 ± 0.014 ab | 92.5 ± 1.45 ab | 24.6± 0.784 ab | 30.3 ± 0.989 | 1.23 ± 0.06 | 0.511 ± 0.014 | 100 | 0.766 ± 0.089 | 0.050 ± 0.006 | 32.1 ± 7.85 b | 0.721 ± 0.012 c | 26.1 ± 0.814 b |
| Item | Sampling Time | Dry Matter (%) | N (Dry Matter) | P (Dry Matter) |
|---|---|---|---|---|
| Oreochromis niloticus | Stocked | 21.8 ± 1.34 a | 14.4 ± 0.69 a | 1.46 ± 0.05 a |
| Harvested | 22.6 ± 1.64 a | 15.6 ± 0.77 b | 1.78 ± 0.02 b | |
| Aristichthys nobilis | Stocked | 20.7 ± 1.94 a | 11.3 ± 0.48 a | 1.06 ± 0.009 a |
| Harvested | 21.3 ± 1.63 b | 12.3 ± 0.55 b | 1.17 ± 0.024 b | |
| Cherax quadricarinatus | Stocked | 21.4 ± 1.82 a | 10.8 ± 0.32 a | 0.95 ± 0.08 a |
| Harvested | 22.8 ± 2.07 b | 11.7 ± 1.05 b | 1.15 ± 0.06 b | |
| Feed | 93.7 | 6.94 | 1.31 |
| Item | Sampling Time | Treatments | |||
|---|---|---|---|---|---|
| CH | CH1 | CH2 | CH3 | ||
| Dry matter % | Before | 63.8 ± 3.05 a | 63.8 ± 3.05 a | 63.8 ± 3.05 a | 63.8 ± 3.05 a |
| After | 40.3 ± 2.68 b | 38.3 ± 2.61 b | 37.5 ± 3.01 b | 40.5 ± 2.46 b | |
| Density/g.cm−3 | Before | 1.83 ± 0.06 a | 1.83 ± 0.06 a | 1.83 ± 0.06 a | 1.83 ± 0.06 a |
| After | 1.31 ± 0.03 b | 1.38 ± 0.04 b | 1.47 ± 0.03 b | 1.56 ± 0.05 b | |
| N (Dry matter) | Before | 0.27 ± 0.02 a | 0.27 ± 0.02 a | 0.27 ± 0.02 a | 0.27 ± 0.02 a |
| After | 1.56 ± 0.08 b | 1.44 ± 0.06 b | 1.31 ± 0.02 b | 1.12 ± 0.03 b | |
| P Dry matter | Before | 0.019 ± 0.001 a | 0.019 ± 0.001 a | 0.019 ± 0.001 a | 0.019 ± 0.001 a |
| After | 0.094 ± 0.006 b | 0.103 ± 0.003 b | 0.097 ± 0.002 b | 0.104 ± 0.002 b | |
| Systems | Treatments | ||||
|---|---|---|---|---|---|
| CH | CH1 | CH2 | CH3 | ||
| N (g/m2) | Feed | 203.6 ± 8.43 | 211.6 ± 13.03 | 204.9 ± 11.65 | 211.7 ± 14.37 |
| Animals | 0.490 | 0.520 | 0.545 | 0.558 | |
| Water | 1.98 ± 0.09 | 2.03 ± 0.11 | 2.12 ± 0.15 | 2.00 ± 0.09 | |
| Rain | 1.04 | 1.04 | 1.04 | 1.04 | |
| Total | 207.1 ± 8.78 | 215.2 ± 13.04 | 208.6 ± 11.38 | 215.3 ± 14.28 | |
| P (g/m2) | Feed | 47.0 ± 3.22 | 46.5 ± 2.89 | 47.0 ± 2.98 | 46.5 ± 2.16 |
| Animals | 0.057 | 0.06 | 0.065 | 0.068 | |
| Water | 0.13 ± 0.01 | 0.14 ± 0.04 | 0.16 ± 0.05 | 0.18 ± 0.03 | |
| Rain | 0.02 | 0.02 | 0.02 | 0.02 | |
| Total | 47.2 ± 0.11 a | 46.7 ± 0.07 b | 47.2 ± 0.02 c | 46.8 ± 0.03 c | |
| Systems | Treatments | ||||
|---|---|---|---|---|---|
| CH | CH1 | CH2 | CH3 | ||
| N (g/m2) | Sediment accumulatio | 123.4 ± 8.43 a | 94.8 ± 7.54 b | 65.6 ± 6.36 c | 90.5 ± 6.19 b |
| Harvested animals | 65.8 ± 2.28 a | 69.4 ± 2.67 ab | 74.0 ± 2.70 b | 71.1 ± 2.36 b | |
| Water accumulation | 0.620 ± 0.09 a | 0.410 ± 0.116 b | 0.080 ± 0.05 c | 0.250 ± 0.04 d | |
| Absorption | 1.01 ± 0.17 a | 0.96 ± 0.09 a | 0.71 ± 0.04 b | 0.76 ± 0.11 b | |
| Seepage | 2.52 ± 0.21 a | 1.65 ± 025 b | 0.37 ± 0.12 c | 0.34 ± 0.16 c | |
| Volatilization | 1.28 ± 0.06 a | 1.18 ± 0.28 a | 1.06 ± 0.19 ab | 1.04 ± 0.25 b | |
| Total | 194.6 ± 9.61 a | 168.4 ± 7.27 b | 141.8 ± 6.68 c | 164 ± 7.12 b | |
| P (g/m2) | Sediment accumulation | 37.0 ± 3.22 a | 36.6 ± 3.35 a | 34.9 ± 3.90 a | 35.1 ± 2.47 a |
| Harvested animals | 6.50 ± 0.022 a | 6.85 ± 0.024 b | 7.31 ± 0.026 c | 7.02 ± 0.026 d | |
| Water accumulation | 0.14 ± 0.01 a | 0.10 ± 0.01 a | 0.08 ± 0.01 b | 0.90 ± 0.02 b | |
| Absorption | 0.27 ± 0.05 a | 0.18 ± 0.03 a | 0.11 ± 0.02 b | 0.15 ± 0.04 b | |
| Seepage | 0.48 ± 0.11 a | 0.35 ± 0.07 b | 0.14 ± 0.02 c | 0.17 ± 0.03 c | |
| Volatilization | - | - | - | - | |
| Total | 44.4 ± 3.23 a | 44.1 ± 3.31 a | 42.5 ± 2.73 a | 43.34 ± 2.26 a | |
| Group | PPCP (g/m2) | PPCSD (g/m2) | ||
|---|---|---|---|---|
| KTN | KTP | KTN | KTP | |
| CH | 6.26 ± 0.45 a | 1.17 ± 0.02 a | 14.8 ± 0.41 a | 4.64 ± 0.29 a |
| CH1 | 6.01 ± 0.36 a | 1.61 ± 0.03 b | 13.5 ± 0.22 b | 4.57 ± 0.32 a |
| CH2 | 5.35 ± 0.26 b | 1.52 ± 0.01 b | 12.5 ± 0.14 c | 4.49 ± 0.09 a |
| CH3 | 5.41 ± 0.32 b | 1.56 ± 0.04 b | 11.9 ± 1.02 c | 4.36 ± 0.11 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhang, K.; Wang, G.; He, X. A Study on Nitrogen and Phosphorus Budgets in a Polyculture System of Oreochromis niloticus, Aristichthys nobilis, and Cherax quadricarinatus. Water 2023, 15, 2699. https://doi.org/10.3390/w15152699
Liu B, Zhang K, Wang G, He X. A Study on Nitrogen and Phosphorus Budgets in a Polyculture System of Oreochromis niloticus, Aristichthys nobilis, and Cherax quadricarinatus. Water. 2023; 15(15):2699. https://doi.org/10.3390/w15152699
Chicago/Turabian StyleLiu, Banghui, Kai Zhang, Guangjun Wang, and Xugang He. 2023. "A Study on Nitrogen and Phosphorus Budgets in a Polyculture System of Oreochromis niloticus, Aristichthys nobilis, and Cherax quadricarinatus" Water 15, no. 15: 2699. https://doi.org/10.3390/w15152699
APA StyleLiu, B., Zhang, K., Wang, G., & He, X. (2023). A Study on Nitrogen and Phosphorus Budgets in a Polyculture System of Oreochromis niloticus, Aristichthys nobilis, and Cherax quadricarinatus. Water, 15(15), 2699. https://doi.org/10.3390/w15152699





