Assemblage Patterns of Microalgae along the Upstream to Downstream Gradient of the Okavango Delta: Abundance, Taxonomic Diversity, and Functional Diversity
Abstract
1. Introduction
2. Methods
2.1. Study Region
2.2. Sampling Protocols
2.3. Algal Analyses
2.4. Algal Traits
2.5. Statistical Analyses
3. Results
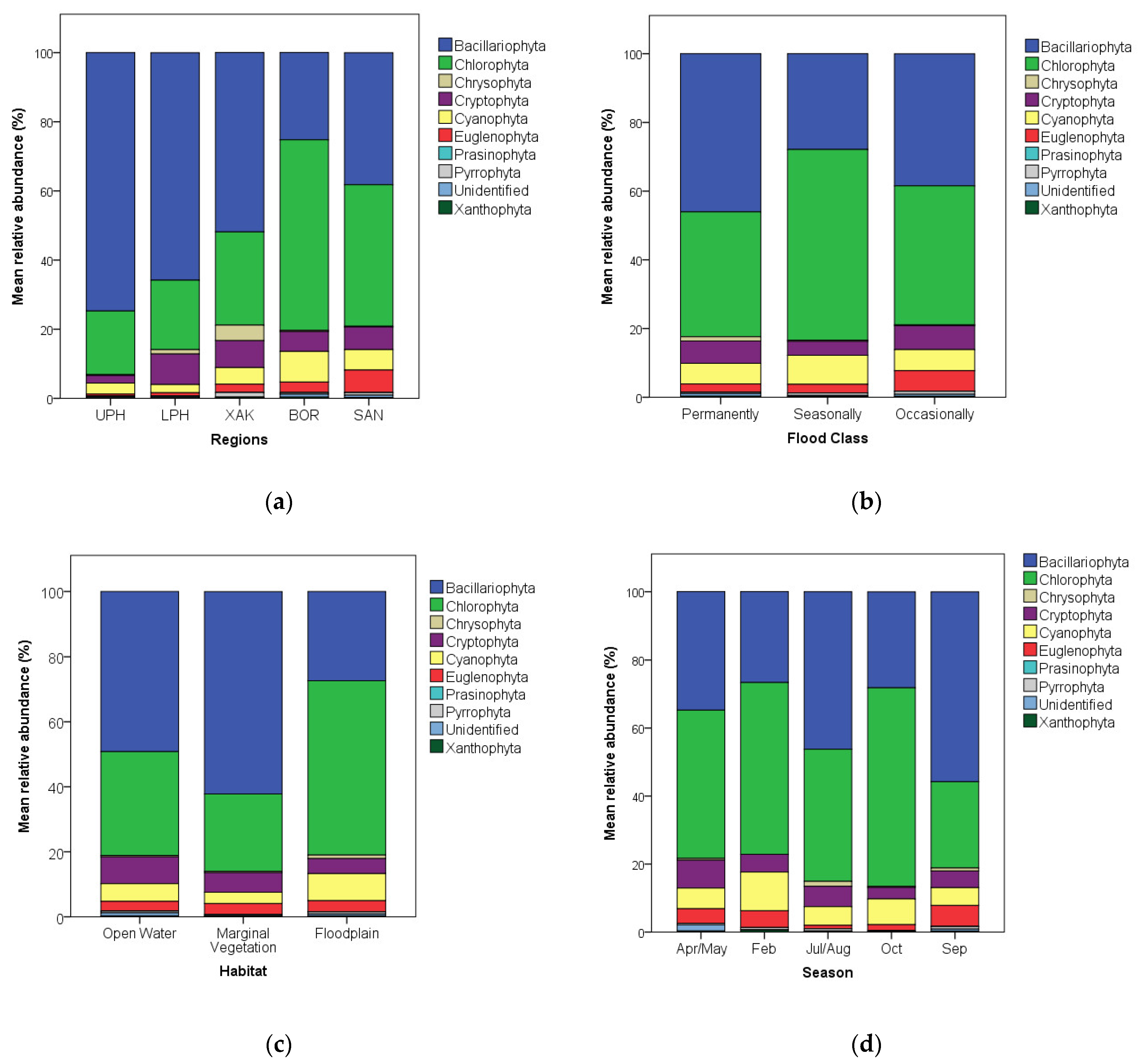
3.1. Algal Relative Abundance Patterns
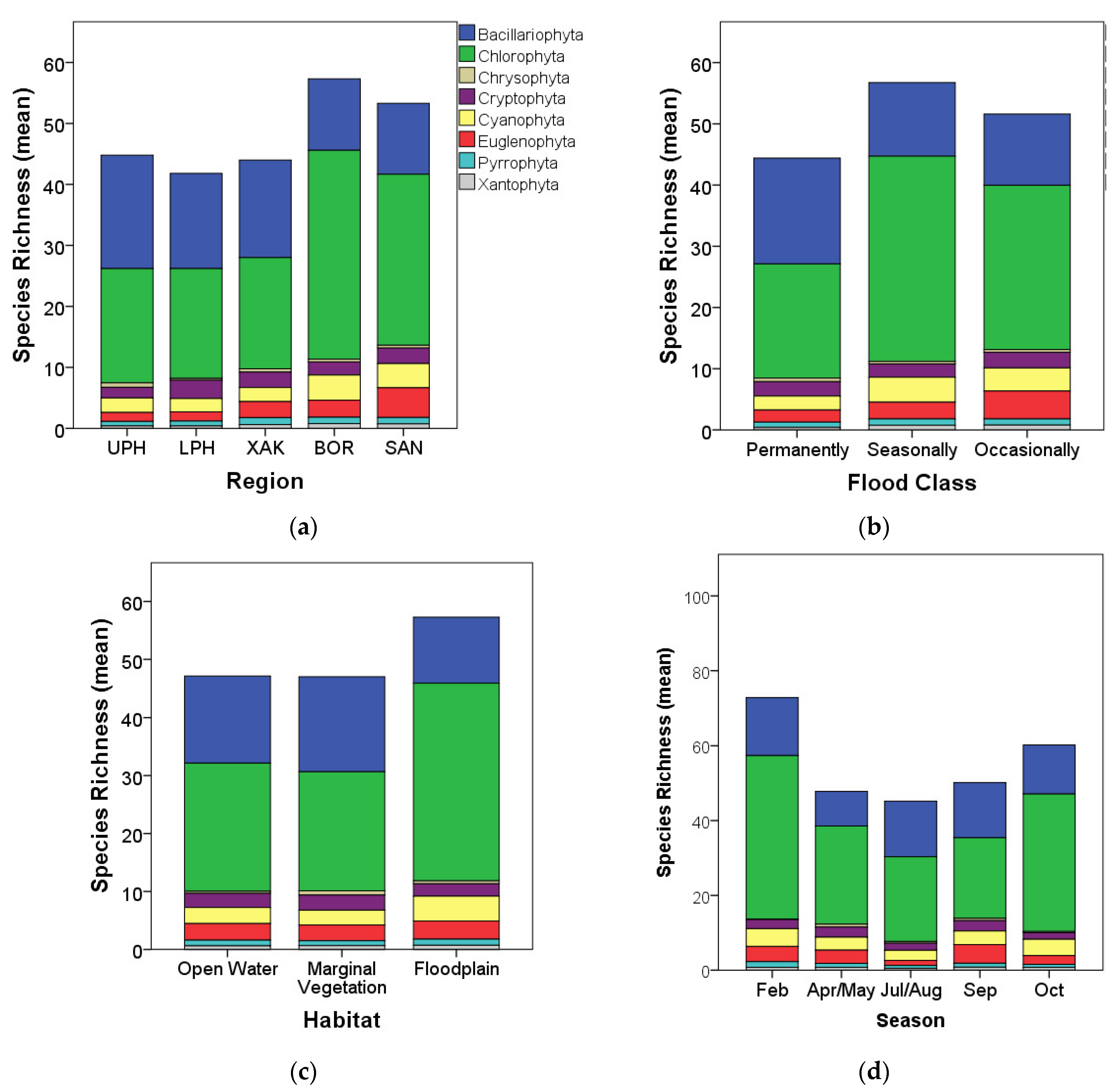
3.2. Algal Species Richness and Diversity Patterns
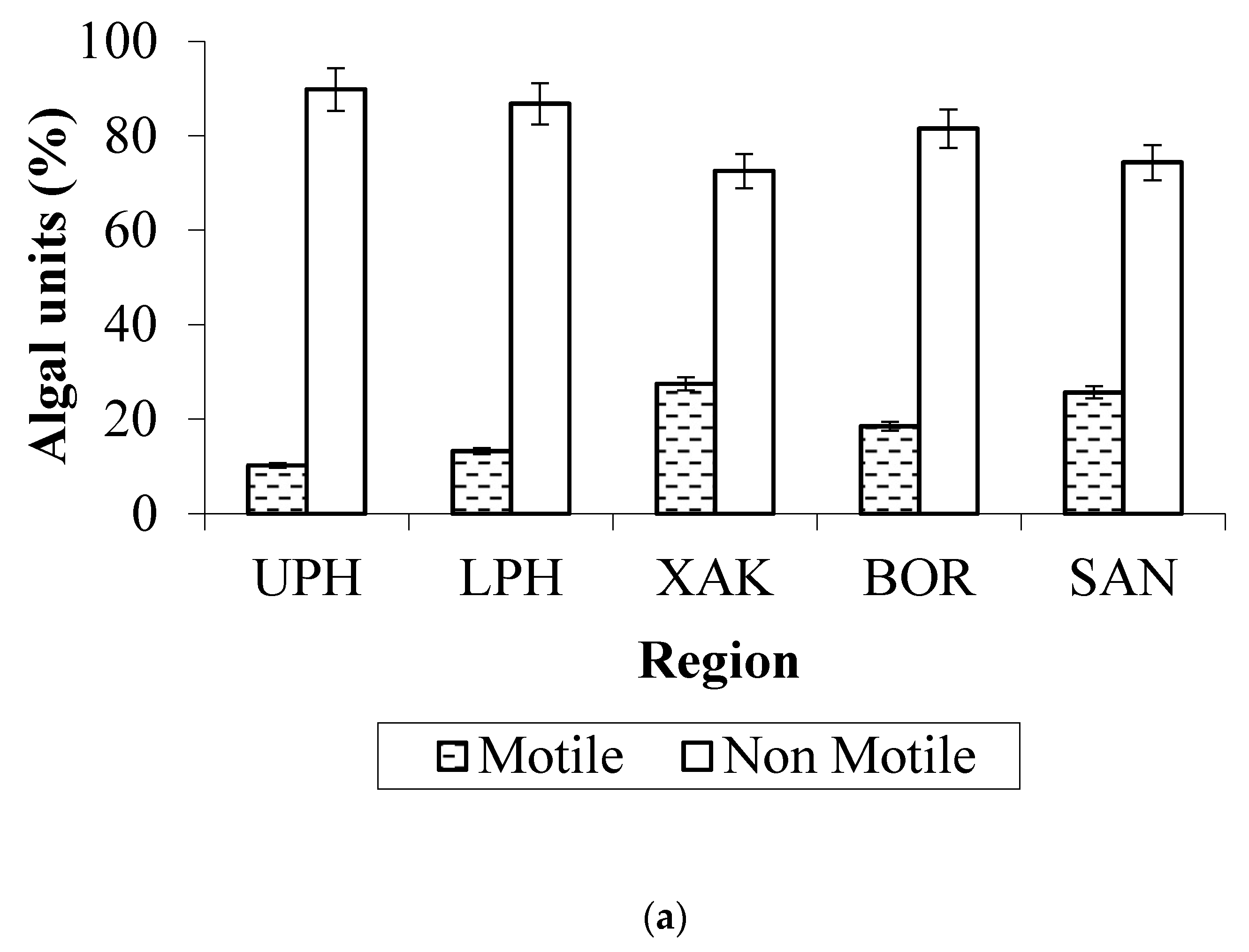
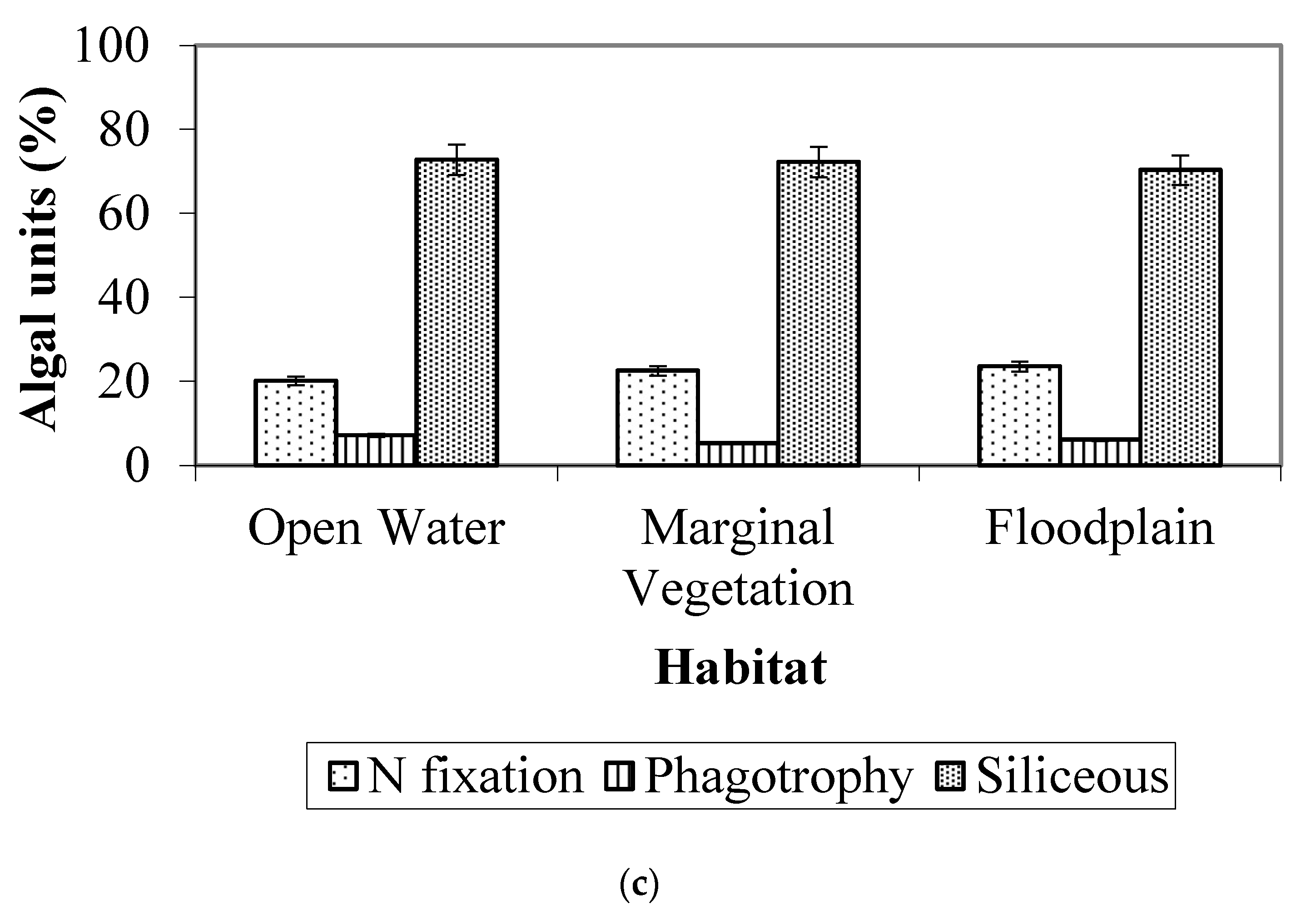
3.3. Algal Traits
4. Discussion
- Abundance and richness: Chlorophyta and Bacillariophyta were the most abundant and diverse algae in the Okavango, with diatoms being most abundant and diverse in deeper, permanently flooded sites (especially upstream) and green algae being most abundant and diverse in shallower, seasonally flooded sites (especially downstream);
- Mode of living: The mean abundance of typically benthic taxa was higher in the low and high-water phases than in the expansion and recession phases;
- Traits: Smaller algae were more abundant in downstream seasonally flooded sites, while medium-sized to larger algae were more abundant in permanently flooded sites. The great majority of the algae observed were non-motile, and the presence of a siliceous exoskeleton (predominantly in Bacillariophyta) was the second most common trait.
4.1. Algal Abundance Patterns
4.2. Algal Richness Patterns
4.3. Algal Traits
4.4. Summary of Scientific Contribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fluet-Chouinard, E.; Lehner, B.; Rebelo, L.-M.; Papa, F.; Hamilton, S.K. Development of a global inundation map at high spatial resolution from topographic downscaling of coarse-scale remote sensing data. Remote Sens. Environ. 2015, 158, 348–361. [Google Scholar] [CrossRef]
- Davidson, N.C.; Fluet-Chouinard, E.; Finlayson, C.M. Global extent and distribution of wetlands: Trends and issues. Mar. Freshw. Res. 2018, 69, 620–627. [Google Scholar] [CrossRef]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river–floodplain systems. Can. J. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Maltby, E.; Acreman, M.C. Ecosystem services of wetlands: Pathfinder for a new paradigm. Hydrol. Sci. J. 2011, 56, 1341–1359. [Google Scholar] [CrossRef]
- Junk, W.J.; An, S.; Finlayson, C.M.; Gopal, B.; Květ, J.; Mitchell, S.A.; Mitsch, W.J.; Robarts, R.D. Current state of knowledge regarding the world’s wetlands and their future under global climate change: A synthesis. Aquat. Sci. 2013, 75, 151–167. [Google Scholar] [CrossRef]
- Junk, W.J. Long-term environmental trends and the future of tropical wetlands. Environ. Conserv. 2002, 29, 414–435. [Google Scholar] [CrossRef]
- Junk, W.J.; Da Cunha, C.N.; Wantzen, K.M.; Petermann, P.; Strüssmann, C.; Marques, M.I.; Adis, J. Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat. Sci. 2006, 68, 278–309. [Google Scholar] [CrossRef]
- Erwin, K.L. Wetlands and global climate change: The role of wetland restoration in a changing world. Wetl. Ecol. Manag. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- Adis, J.; Junk, W.J. Terrestrial invertebrates inhabiting lowland river floodplains of Central Amazonia and Central Europe: A review. Freshw. Biol. 2002, 47, 711–731. [Google Scholar] [CrossRef]
- Reynolds, C.S. Vegetation Processes in the Pelagic: A Model for Ecosystem Theory; Ecology Institute: Oldendorf, Germany, 1997. [Google Scholar]
- Reynolds, C.S. Ecology of Phytoplankton, 3rd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Law, R.J. A review of the function and uses of, and factors affecting, stream phytobenthos. Freshw. Rev. 2013, 4, 135–166. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T.F.; Lourival, R.; Wittmann, F.; Kandus, P.; Lacerda, L.D.; Bozelli, R.L.; Esteves, F.A.; Nunes da Cunha, C.; Maltchik, L.; et al. Brazilian wetlands: Their definition, delineation, and classification for research, sustainable management, and protection. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 5–22. [Google Scholar] [CrossRef]
- Wittmann, F.; Householder, E.; Piedade, M.T.F.; Assis, R.L.; Schöngart, J.; Parolin, P.; Junk, W.J. Habitat specificity, endemism and the neotropical distribution of Amazonian white-water floodplain trees. Ecography 2013, 36, 690–707. [Google Scholar] [CrossRef]
- McCarthy, T.S.; Ellery, W.N. The effect of vegetation on soil and ground water chemistry and hydrology of islands in the seasonal swamps of the Okavango Fan, Botswana. J. Hydrol. 1994, 154, 169–193. [Google Scholar] [CrossRef]
- Mackay, A.W.; Davidson, T.; Wolski, P.; Woodward, S.; Mazebedi, R.; Masamba, W.R.L.; Todd, M. Diatom sensitivity to hydrological and nutrient variability in a subtropical, flood-pulse wetland. Ecohydrology 2012, 5, 491–502. [Google Scholar] [CrossRef]
- Cronberg, G.; Gieske, A.; Martins, E.; Nengu, J.P.; Stenstrom, I.M. Major ion chemistry, plankton, and bacterial assemblages of the Jao/Boro River, Okavango Delta, Botswana: The swamps and flood plains. Arch. Für Hydrobiol. Supplementband. Monogr. Beiträge 1996, 107, 335–407. [Google Scholar]
- West, D.T.; van As, J.G.; van As, L.L. Surface water quality in the Okavango Delta panhandle, Botswana. Afr. J. Aquat. Sci. 2015, 40, 359–372. [Google Scholar] [CrossRef]
- Mosimane, K.; Struyf, E.; Gondwe, M.J.; Frings, P.; van Pelt, D.; Wolski, P.; Schoelynck, J.; Schaller, J.; Conley, D.J.; Murray-Hudson, M. Variability in chemistry of surface and soil waters of an evapotranspiration-dominated flood-pulsed wetland: Solute processing in the Okavango Delta, Botswana. Water SA 2017, 43, 104–115. [Google Scholar] [CrossRef]
- Masamba, W.R.; Mazvimavi, D. Impact on water quality of land uses along Thamalakane-Boteti River: An outlet of the Okavango Delta. Phys. Chem. Earth 2008, 33, 687–694. [Google Scholar] [CrossRef]
- Pinheiro, I.; Gabaake, G.; Heyns, P. Cooperation in the Okavango River Basin: The OKACOM Perspective; Turton, A.R., Ashton, P., Cloete, E., Eds.; African Water Issues Research Unit (AWIRU): Pretoria, South Africa; Green Cross International (GCI): Geneva, Switzerland, 2003. [Google Scholar]
- Mendelsohn, J.M.; Vanderpost, C.; Ramberg, L.; Murray-Hudson, M.; Wolski, P.; Mosepele, K. Okavango Delta: Floods of Life; Raison: Windhoek, Namibia, 2010. [Google Scholar]
- Quammen, D. Inside the Mission to Save Africa’s Okavango Delta. National Geographic Magazine November 2017. Available online: https://www.nationalgeographic.com/magazine/article/africa-expedition-conservation-okavango-delta-cuito (accessed on 10 June 2014).
- Milzow, C.; Kgotlhang, L.; Bauer-Gottwein, P.; Meier, P.; Kinzelbach, W. Regional review: The hydrology of the Okavango Delta, Botswana—Processes, data and modelling. Hydrogeol. J. 2009, 17, 1297–1328. [Google Scholar] [CrossRef]
- Musora, O.; Mbaiwa, J.E.; Murray-Hudson, M. Tourists’ perceptions of environmental impacts of tourism development on water resources in the Okavango Delta, Botswana. Afr. J. Hosp. Tour. Leis. 2017, 6, 1–10. [Google Scholar]
- Statistics Botswana. Population and Housing Census Preliminary Results. Gaborone, Botswana, 2022. Available online: https://www.statsbots.org.bw/sites/default/files/2022%20Population%20and%20Housing%20Census%20Preliminary%20Results.pdf (accessed on 15 January 2023).
- Mitchell, S.A. The status of wetlands, threats and the predicted effect of global climate change: The situation in Sub-Saharan Africa. Aquat. Sci. 2013, 75, 95–112. [Google Scholar] [CrossRef]
- Niang, I.; Ruppel, O.C.; Abdrabo, M.A.; Essel, A.; Lennard, C.; Padgham, J.; Urquhart, P. Africa. In Climate Change 2014, Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Barros, V.R., Field, C.B., Dokken, D.J., Mastrandrea, K.J., Mach, T.E., Bilir, M., Chatterjee, K.L., Ebi, Y.O., Estrada, R.C., Genova, B., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Kgathi, D.L.; Mmopelwa, G.; Vanderpost, C.; Wolski, P.; Motsholapheko, M.R. Impacts of desiccation on ecosystem services and household adaptation in the Okavango Delta, Botswana. Biodivers. Ecol. 2013, 5, 263–278. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R.; et al. Technical Summary. In Climate Change 2022, Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change 2022; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; pp. 37–118. [Google Scholar]
- Stevenson, R.J.; Bothwell, M.L.; Lowe, R.L.; Thorp, J.H. Algal Ecology: Freshwater Benthic Ecosystem; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Lewis, W.M., Jr.; Hamilton, S.K.; Rodríguez, M.A.; Saunders, J.F., III; Lasi, M.A. Foodweb analysis of the Orinoco floodplain based on production estimates and stable isotope data. J. N. Am. Benthol. Soc. 2001, 20, 241–254. [Google Scholar] [CrossRef]
- Douglas, M.M.; Bunn, S.E.; Davies, P.M. River and wetland food webs in Australia’s wet–dry tropics: General principles and implications for management. Mar. Freshw. Res. 2005, 56, 329–342. [Google Scholar] [CrossRef]
- Thompson, R.M.; Dunne, J.A.; Woodward, G. Freshwater food webs: Towards a more fundamental understanding of biodiversity and community dynamics. Freshw. Biol. 2012, 57, 1329–1341. [Google Scholar] [CrossRef]
- Brett, M.T.; Bunn, S.E.; Chandra, S.; Galloway, A.W.; Guo, F.; Kainz, M.J.; Kankaala, P.; Lau, D.C.; Moulton, T.P.; Power, M.E.; et al. How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems? Freshw. Biol. 2017, 62, 833–853. [Google Scholar] [CrossRef]
- McCormick, P.V.; Cairns, J. Algae as indicators of environmental change. J. Appl. Phycol. 1994, 6, 509–526. [Google Scholar] [CrossRef]
- Stevenson, J. Ecological assessments with algae: A review and synthesis. J. Phycol. 2014, 50, 437–461. [Google Scholar] [CrossRef]
- Wu, N.; Dong, X.; Liu, Y.; Wang, C.; Baattrup-Pedersen, A.; Riis, T. Using river microalgae as indicators for freshwater biomonitoring: Review of published research and future directions. Ecol. Indic. 2017, 81, 124–131. [Google Scholar] [CrossRef]
- Passy, S.I. Continental diatom biodiversity in stream benthos declines as more nutrients become limiting. Proc. Natl. Acad. Sci. USA 2008, 105, 9663–9667. [Google Scholar] [CrossRef]
- Finlay, B.J. Global dispersal of free-living microbial eukaryote species. Science 2002, 296, 1061–1063. [Google Scholar] [CrossRef]
- Lachance, M.A. Here and there or everywhere? BioScience 2004, 54, 884. [Google Scholar] [CrossRef][Green Version]
- Vyverman, W.; Verleyen, E.; Sabbe, K.; Vanhoutte, K.; Sterken, M.; Hodgson, D.A.; Mann, D.G.; Juggins, S.; Vijver, B.V.D.; Jones, V.; et al. Historical processes constrain patterns in global diatom diversity. Ecology 2007, 88, 1924–1931. [Google Scholar] [CrossRef]
- Bolgovics, Á.; Ács, É.; Várbíró, G.; Görgényi, J.; Borics, G. Species area relationship (SAR) for benthic diatoms: A study on aquatic islands. Hydrobiologia 2016, 764, 91–102. [Google Scholar] [CrossRef][Green Version]
- Cholnoky, B.J. Die Diatomeen im Unterlaufe des Okawango Flusses. Beih. Nova Hedwig. 1966, 21, 1–102. [Google Scholar]
- Grönblad, R.; Croasdale, H. Desmids from Namibia. Acta Bot. Fenn. 1971, 93, 1–40. [Google Scholar]
- Coesel, P.F.M.; van Geest, A. Taxonomic and biogeographical notes on Okavango desmids (Zygnematophyceae, Streptophyta). Syst. Geogr. Plants 2008, 78, 27–46. [Google Scholar]
- Coesel, P.F.M.; van Geest, A. Taxonomic and biogeographical notes on Okavango desmids (Zygnematophyceae, Streptophyta) II: Genera Cosmarium, Xanthidium and Staurastrum. Syst. Geogr. Plants 2009, 79, 15–31. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Davidson, T.A.; Mackay, A.W.; Wolski, P.; Mazebedi, R.; Murray-Hudson, M.; Todd, M. Seasonal and spatial hydrological variability drives aquatic biodiversity in a flood-pulsed, sub-tropical wetland. Freshw. Biol. 2012, 57, 1253–1265. [Google Scholar] [CrossRef]
- Marazzi, L.; Gaiser, E.E.; Jones, V.J.; Tobias, F.A.; Mackay, A.W. Algal richness and life-history strategies are influenced by hydrology and phosphorus in two major subtropical wetlands. Freshw. Biol. 2017, 62, 274–290. [Google Scholar] [CrossRef]
- Weithoff, G. The concepts of ‘plant functional types’ and ‘functional diversity’ in lake phytoplankton–a new understanding of phytoplankton ecology? Freshw. Biol. 2003, 48, 1669–1675. [Google Scholar] [CrossRef]
- Passy, S.I. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat. Bot. 2007, 86, 171–178. [Google Scholar] [CrossRef]
- Rimet, F.; Bouchez, A. Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowl. Manag. Aquat. Ecosyst. 2012, 406, 1–12. [Google Scholar] [CrossRef]
- Lange, K.; Townsend, C.R.; Matthaei, C.D. A trait-based framework for stream algal communities. Ecol. Evol. 2016, 6, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Tapolczai, K.; Bouchez, A.; Stenger-Kovács, C.; Padisák, J.; Rimet, F. Trait-based ecological classifications for benthic algae: Review and perspectives. Hydrobiologia 2016, 776, 1–17. [Google Scholar] [CrossRef]
- Mosepele, K. Preliminary Length-Based Stock Assessment of the Main Exploited Stocks of the Okavango Delta Fishery. Master’s Thesis, University of Bergen, Bergen, Norway, 2000. [Google Scholar]
- Secretariat of the Convention on Wetlands. The List of Wetlands of International Importance, Convention on Wetlands Secretariat. Gland, Switzerland, 2023. Available online: https://www.ramsar.org/sites/default/files/documents/library/sitelist.pdf (accessed on 26 December 2017).
- United Nations Educational, Scientific and Cultural Organisation (UNESCO). Okavango Delta. Available online: http://whc.unesco.org/en/list/1432/ (accessed on 27 December 2017).
- Marazzi, L. Biodiversity and Biomass of Algae in the Okavango Delta (Botswana), A Subtropical Flood-Pulsed Wetland. Ph.D. Thesis, UCL (University College London), London, UK, 2014. [Google Scholar]
- McCarthy, J.M.; Gumbricht, T.; McCarthy, T.; Frost, P.; Wessels, K.; Seidel, F. Flooding patterns of the Okavango Wetland in Botswana between 1972 and 2000. Ambio A J. Hum. Environ. 2003, 32, 453–457. [Google Scholar] [CrossRef]
- Kurugundla, C.N.; Parida, B.P.; Buru, J.C. Revisiting Hydrology of Lake Ngami in Botswana. Hydrol. Curr. Res. 2018, 9, 1–11. [Google Scholar]
- Mackay, A.W.; Davidson, T.; Wolski, P.; Mazebedi, R.; Masamba, W.R.L.; Huntsman-Mapila, P.; Todd, M. Spatial and seasonal variability in surface water chemistry in the Okavango Delta, Botswana: A multivariate approach. Wetlands 2011, 31, 815–829. [Google Scholar] [CrossRef]
- Siziba, N.; Chimbari, M.J.; Masundire, H.; Mosepele, K. Spatial and temporal variations of microinvertebrates across temporary floodplains of the Lower Okavango Delta, Botswana. Phys. Chem. Earth 2011, 36, 939–948. [Google Scholar] [CrossRef]
- Siziba, N.; Chimbari, M.J.; Masundire, H.; Mosepele, K. Spatial variations of microinvertebrates across different microhabitats of temporary floodplains of Lower Okavango Delta, Botswana. Afr. J. Ecol. 2012, 50, 43–52. [Google Scholar] [CrossRef]
- Willén, T. Studies on the phytoplankton of some lakes connected with or recently isolated from the Baltic. Oikos 1962, 13, 169–199. [Google Scholar] [CrossRef]
- European Standard. Water quality—Guidance standard for the routine analysis of phytoplankton abundance and composition using inverted microscopy (Utermöhl technique) prEN 15204:2005. European Committee for Standardization (CEN), 2005.
- Utermohl, H. Zur Vervollkommung der quantitativen phytoplankton-methodik. Mitt Int. Ver Limnol. 1958, 9, 38. [Google Scholar]
- Thomasson, K. Notes on the plankton of Lake Bangwelu. Nova Acta Regiae Soc. Sci. Ups. 1957, 17, 1–18. [Google Scholar]
- Thomasson, K. Notes on the plankton of Lake Bangwelu. Nova Acta Regiae Soc. Sci. Ups. 1960, 17, 1–43. [Google Scholar]
- Thomasson, K. Notes on algal vegetation of Lake Kariba. Nova Acta Regiae Soc. Sci. Ups. 1965, 19, 1. [Google Scholar]
- Thomasson, K. Amazonian algae. Inst. R. Sci. Nat. Belg. 1971, 2, 1–57. [Google Scholar]
- Coesel, P.F.M. The relevance of desmids in the biological typology and evaluation of fresh waters. Aquat. Ecol. 1975, 9, 93–101. [Google Scholar]
- Coesel, P.F.M. Structural characteristics and adaptations of desmid assemblages. J. Ecol. 1982, 70, 163–177. [Google Scholar] [CrossRef]
- Ramberg, L. Compendium on Phytoplankton; Unpublished manuscript; Uppsala University: Uppsala, Sweden, 1986. [Google Scholar]
- Gerrath, J.F.; John, D.M. The Desmids of Ghana, West Africa, I. Nova Hedwig 1988, 46, 187–230. [Google Scholar]
- Ricci, S.; Alfinito, S.; Fumanti, B. Desmids from Guma Valley (Sierra Leone, West Africa). Hydrobiologia 1990, 208, 235–243. [Google Scholar] [CrossRef]
- Cox, E.J. Identification of Freshwater Diatoms from Live Material; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Carter, C.F. Desmid Images in Three Dimensions—An Aid to Identification; Unpublished material—Advanced Course on Blue-Green and Green Algae; Durham University: Durham, UK, 2011. [Google Scholar]
- Van Dam, H.; Mertensen, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar]
- Porter, K.G. Phagotrophic phytoflagellates in microbial food webs. Hydrobiologia 1988, 159, 89–97. [Google Scholar] [CrossRef]
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). R package. 2014. Available online: https://CRAN.R-project.org/package=PMCMR (accessed on 26 December 2017).
- Marazzi, L. Okavango Delta—Biodiversity of Microalgae, precious invisible plants. Biodiv. Ecol. 2013, 5, 161–163. [Google Scholar] [CrossRef]
- Williamson, D.B.; Marazzi, L. A new Cosmarium (Chlorophyta, Desmidiaceae) variety from the Okavango Delta, Botswana. Quekett J. Microsc. 2013, 42, 35–37. [Google Scholar]
- Butterwick, C.; Heaney, S.; Talling, J. Diversity in the influence of temperature on the growth rates of freshwater algae, and its ecological relevance. Freshw. Biol. 2005, 50, 291–300. [Google Scholar] [CrossRef]
- Martin-Jézéquel, V.; Hildebrand, M.; Brzezinski, M.A. Silicon metabolism in diatoms: Implications for growth. J. Phycol. 2000, 36, 821–840. [Google Scholar] [CrossRef]
- Sala, S.E.; Duque, S.R.; Núñez-Avellaneda, M.; Lamaro, A.A. Diatoms from the Colombian Amazon: Some species of the genus Eunotia (Bacillariophyceae). Acta Amaz. 2002, 3, 589–603. [Google Scholar] [CrossRef][Green Version]
- Hagerthey, S.E.; Bellinger, B.J.; Wheeler, K.; Gantar, M.; Gaiser, E. Everglades periphyton: A biogeochemical perspective. Crit. Rev. Environ. Sci. Technol. 2011, 41, 309–343. [Google Scholar] [CrossRef]
- Engle, D.L.; Melack, J.M. Consequences of riverine flooding for seston and the periphyton of floating meadows in an Amazon floodplain lake. Limnol. Oceanogr. 1993, 38, 1500–1520. [Google Scholar] [CrossRef]
- Weilhoefer, C.L.; Pan, Y.; Eppard, S. The effects of river floodwaters on floodplain wetland water quality and diatom assemblages. Wetlands 2008, 28, 473–486. [Google Scholar] [CrossRef]
- Squires, M.M.; Lesack, L.F.W. Benthic algal response to pulsed versus distributed inputs of sediments and nutrients in a Mackenzie Delta lake. J. N. Am. Benthol. Soc. 2001, 20, 369–384. [Google Scholar] [CrossRef]
- Thomas, D.P. A Limnological Survey of the Alligator Rivers Region, I. Diatoms (Bacillariophyceae) of the Region; Research Report 3; Supervising Scientist for the Alligator Rivers Region, AGPS: Canberra, Australia, 1983. [Google Scholar]
- Ling, H.; Tyler, P.A. A Limnological Survey of the Alligator Rivers Region. II Freshwater Algae, Exclusive of Diatoms; Australian Government Publishing Service Research Report 3; Australian Government Publishing Services: Canberra, Australia, 1986. [Google Scholar]
- Finlayson, C.M.; Lowry, J.; Bellio, M.G.; Nou, S.; Pidgeon, R.; Walden, D.; Humphrey, C.; Fox, G. Biodiversity of the wetlands of the Kakadu region, Northern Australia. Aquat. Sci. 2006, 68, 374–399. [Google Scholar] [CrossRef]
- De-Lamonica-Freire, E.M.; Heckman, C.W. The seasonal succession of biotic assemblages in wetlands of the tropical wet and dry climatic zone: III. The algal assemblages in the Pantanal of Mato Grosso, Brazil, with a comprehensive list of the known species and revision of two desmid taxa. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1996, 81, 253–280. [Google Scholar] [CrossRef]
- Zalocar de Domitrovic, Y.Z. Structure and variation of the Paraguay River phytoplankton in two periods of its hydrological cycle. Hydrobiologia 2002, 472, 177–196. [Google Scholar] [CrossRef]
- Jardine, T.D.; Hunt, R.; Faggotter, S.; Valdez, D.; Burford, M.; Bunn, S. Carbon from periphyton supports fish biomass in waterholes of a wet–dry tropical river. River Res. Appl. 2013, 29, 560–573. [Google Scholar] [CrossRef]
- Molinari, B.; Stewart-Koster, B.; Adame, M.F.; Campbell, M.D.; McGregor, G.; Schulz, C.; Malthus, T.J.; Bunn, S. Relationships between algal primary productivity and environmental variables in tropical floodplain wetlands. Inland Waters 2021, 11, 180–190. [Google Scholar] [CrossRef]
- Biggs, B.J.F.; Stevenson, R.J.; Lowe, R.L. A habitat matrix conceptual model for stream periphyton. Arch. Hydrobiol. 1998, 143, 21–56. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [Google Scholar] [CrossRef]





| Region/Flooding Frequency | Habitat | Total | ||
|---|---|---|---|---|
| Open Water | Marginal Vegetation | Floodplain | ||
| UPH | ||||
| Permanently flooded | 9 | 4 | 2 | 15 |
| LPH | ||||
| Permanently flooded | 3 | 6 | 9 | |
| Seasonally flooded | 1 | 1 | ||
| XAK | ||||
| Permanently flooded | 2 | 4 | 2 | 8 |
| Seasonally flooded | 2 | 2 | ||
| Occasionally flooded | 3 | 3 | ||
| BOR | ||||
| Seasonally flooded | 13 | 4 | 48 | 65 |
| SAN | ||||
| Occasionally flooded | 12 | 15 | 27 | |
| Total | 41 | 18 | 71 | 130 |
| Taxon | Taxonomic Resolution | Phylum | Total Counts | Occurrence Frequency | Mean % Abundance |
|---|---|---|---|---|---|
| Eunotia sp. | Genus | Bacillariophyta | 3535 | 105 | 7.17 |
| Mougeotia sp. | Genus | Chlorophyta | 3161 | 116 | 6.32 |
| Pennate Diatom | Other groups | Bacillariophyta | 2045 | 126 | 4.67 |
| Cosmarium sp. | Genus | Chlorophyta | 2029 | 128 | 4.07 |
| Gomphonema sp. | Genus | Bacillariophyta | 1860 | 111 | 3.72 |
| Scenedesmus sp. | Genus | Chlorophyta | 1792 | 127 | 3.62 |
| Synedra sp. | Genus | Bacillariophyta | 1768 | 120 | 3.67 |
| Monoraphidium arcuatum | Species | Chlorophyta | 1443 | 119 | 2.88 |
| Synedra ulna | Genus | Bacillariophyta | 1426 | 92 | 2.97 |
| Cryptomonas sp. | Genus | Cryptophyta | 1327 | 103 | 2.84 |
| Chroococcales | Order | Cyanophyta | 1110 | 105 | 2.20 |
| Monoraphidium griffithii | Species | Chlorophyta | 1077 | 121 | 2.14 |
| Aulacoseira sp. | Genus | Bacillariophyta | 851 | 71 | 1.79 |
| Nitzschia sp. | Genus | Bacillariophyta | 848 | 96 | 1.69 |
| Chroomonas sp. | Genus | Cryptophyta | 832 | 104 | 1.70 |
| Staurastrum sp. | Genus | Chlorophyta | 819 | 111 | 1.60 |
| Navicula sp. | Genus | Bacillariophyta | 809 | 101 | 1.57 |
| Oedogonium sp. | Genus | Chlorophyta | 796 | 103 | 1.63 |
| Eunotia pectinalis | Species | Bacillariophyta | 752 | 49 | 1.51 |
| Eunotia rhomboidea | Species | Bacillariophyta | 663 | 83 | 1.36 |
| Pediastrum tetras | Species | Chlorophyta | 632 | 117 | 1.28 |
| Oscillatoriales | Order | Cyanophyta | 571 | 92 | 1.24 |
| Euglena sp. | Genus | Euglenophyta | 511 | 94 | 1.05 |
| Euastrum sp. | Genus | Chlorophyta | 509 | 93 | 1.06 |
| Chlorococcales | Order | Chlorophyta | 495 | 94 | 1.01 |
| Closterium sp. | Genus | Chlorophyta | 476 | 109 | 0.98 |
| Chlorella sp. | Genus | Chlorophyta | 462 | 50 | 0.86 |
| Pinnularia sp. | Genus | Bacillariophyta | 459 | 99 | 0.96 |
| Amphora sp. | Genus | Bacillariophyta | 450 | 75 | 0.94 |
| Monoraphidium irregulare | Species | Chlorophyta | 417 | 78 | 0.81 |
| Eunotia bilunaris | Species | Bacillariophyta | 398 | 74 | 0.79 |
| Oscillatoria sp. | Genus | Cyanophyta | 392 | 68 | 0.78 |
| Chroococcus sp. | Genus | Cyanophyta | 384 | 71 | 0.74 |
| Eunotia flexuosa | Species | Bacillariophyta | 375 | 75 | 0.75 |
| Oocystis sp. | Genus | Chlorophyta | 357 | 87 | 0.72 |
| Staurodesmus sp. | Genus | Chlorophyta | 359 | 85 | 0.66 |
| Phacus sp. | Genus | Euglenophyta | 308 | 79 | 0.63 |
| Dinobryon sp. | Genus | Chrysophyta | 296 | 22 | 0.61 |
| Anabaena sp. | Genus | Cyanophyta | 295 | 43 | 0.53 |
| Gomphonema gracile | Species | Bacillariophyta | 294 | 68 | 0.58 |
| Scenedesmus arcuatus | Species | Chlorophyta | 279 | 76 | 0.54 |
| Average | 897 | 90 | 1.84 |
| Flood Phase | Benthic | Planktonic | Planktonic/Benthic |
|---|---|---|---|
| Permanently | 34 | 37 | 28 |
| Seasonally | 22 | 45 | 33 |
| Occasionally | 29 | 45 | 33 |
| Total | 29 | 40 | 30 |
| Flood Phase | Benthic | Planktonic | Planktonic/Benthic |
|---|---|---|---|
| Expansion (Apr/May) | 26 | 41 | 31 |
| High (Jul) | 30 | 39 | 30 |
| Low (Feb) | 32 | 40 | 28 |
| Recession (Sep) | 28 | 40 | 32 |
| Total | 29 | 40 | 30 |
| Phylum | N Species | % | N Genera | % |
|---|---|---|---|---|
| Bacillariophyta | 113 | 22.9 | 37 | 21.6 |
| Chlorophyta | 283 | 57.3 | 86 | 50.3 |
| Chrysophyta | 8 | 1.6 | 7 | 4.1 |
| Cryptophyta | 11 | 2.2 | 3 | 1.8 |
| Cyanophyta | 28 | 5.7 | 22 | 12.9 |
| Euglenophyta | 36 | 7.3 | 5 | 2.9 |
| Prasinophyta | 1 | 0.2 | 1 | 0.6 |
| Pyrrophyta | 8 | 1.6 | 7 | 4.1 |
| Xanthophyta | 6 | 1.2 | 3 | 1.8 |
| Total | 494 | 100.0 | 171 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marazzi, L.; Mackay, A.W.; Mazebedi, R.; Jones, V.J. Assemblage Patterns of Microalgae along the Upstream to Downstream Gradient of the Okavango Delta: Abundance, Taxonomic Diversity, and Functional Diversity. Water 2023, 15, 2692. https://doi.org/10.3390/w15152692
Marazzi L, Mackay AW, Mazebedi R, Jones VJ. Assemblage Patterns of Microalgae along the Upstream to Downstream Gradient of the Okavango Delta: Abundance, Taxonomic Diversity, and Functional Diversity. Water. 2023; 15(15):2692. https://doi.org/10.3390/w15152692
Chicago/Turabian StyleMarazzi, Luca, Anson W. Mackay, Richard Mazebedi, and Vivienne J. Jones. 2023. "Assemblage Patterns of Microalgae along the Upstream to Downstream Gradient of the Okavango Delta: Abundance, Taxonomic Diversity, and Functional Diversity" Water 15, no. 15: 2692. https://doi.org/10.3390/w15152692
APA StyleMarazzi, L., Mackay, A. W., Mazebedi, R., & Jones, V. J. (2023). Assemblage Patterns of Microalgae along the Upstream to Downstream Gradient of the Okavango Delta: Abundance, Taxonomic Diversity, and Functional Diversity. Water, 15(15), 2692. https://doi.org/10.3390/w15152692







