Experimental Study on the Application of Sludge from Water Treatment Plant as a Reagent for Phosphate Removal from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
- Analysis of the quality of raw water from the Astana 3 reservoir (Vyacheslav);
- Analysis of the quality of treated water taken from clean-water tanks;
- Analysis of hydration and ash content in WTS;
- Analysis of the chemical composition of the dry sludge residue of WTS;
- Qualitative analyses of wastewater samples mixed with WTS at different contact times.
3. Results and Discussion
3.1. Characteristics of Surface and Treated Water and WTS
- The surface water of the reservoir corresponds to a neutral or slightly alkaline environment;
- The contents of sulfates, chlorides, nitrogen compounds, heavy metals, and microbiological indicators do not exceed the standards for surface water and drinking water.
3.2. Results of Laboratory Tests of Wastewater Samples with WTS Additive
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babatunde, A.O.; Zhao, Y.Q. Constructive Approaches Toward Water Treatment Works Sludge Management: An International Review of Beneficial Reuses. Crit. Rev. Environ. Sci. Technol. 2007, 37, 129–164. [Google Scholar] [CrossRef]
- Carleton, G.; Cutright, T.J. Evaluation of alum-based water treatment residuals used to adsorb reactive phosphorus. Water Sci. Eng. 2020, 13, 181–192. [Google Scholar] [CrossRef]
- Gibbons, M.K.; Gagnon, G.A. Understanding removal of phosphate or arsenate onto water treatment residual solids. J. Hazard. Mater. 2011, 186, 1916–1923. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable management of water treatment sludge through 3‘R’ concept. J. Clean. Prod. 2016, 124, 1–13. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Alam, M. Characterization of water treatment plant’s sludge and its safe disposal options. Procedia Environ. Sci. 2016, 35, 950–955. [Google Scholar] [CrossRef]
- Muisa, N.; Nhapi, I.; Ruziwa, W.; Manyuchi, M.M. Utilization of alum sludge as adsorbent for phosphorus removal in municipal wastewater: A review. J. Water Process Eng. 2020, 35, 101187. [Google Scholar] [CrossRef]
- Keeley, J.; Jarvis, P.; Judd, S.J. Coagulant Recovery from Water Treatment Residuals: A Review of Applicable Technologies. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2675–2719. [Google Scholar] [CrossRef] [Green Version]
- Marguti, A.L.; Filho, S.F.; Piveli, R.P. Full-scale effects of addition of sludge from water treatment stations into processes of sewage treatment by conventional activated sludge. J. Environ. Manag. 2018, 25, 283–293. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Barbarick, K.A.; Elliott, H.A. Drinking Water Treatment Residuals: A Review of Recent Uses. J. Environ. Qual. 2011, 40, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, M.D.; Thomas, M.; Surapaneni, A.; Moon, E.M.; Milne, N.A. Beneficial reuse of water treatment sludge in the context of circular economy. Environ. Technol. Innov. 2022, 28, 102651. [Google Scholar] [CrossRef]
- Guan, X.-H.; Chen, G.-H.; Shang, C. Re-use of water treatment works sludge to enhance particulate pollutant removal from sewage. Water Res. 2005, 39, 3433–3440. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.T.; Ahammed, M.M. The reuse of water treatment sludge as a coagulant for post-treatment of UASB reactor treating urban wastewater. J. Clean. Prod. 2015, 96, 271–281. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.Q.; Babatunde, A.O.; Wang, L.; Ren, Y.X.; Han, Y. Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Sep. Purif. Technol. 2006, 51, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Lee, L.Y.; Lim, F.Y.; Lyu, Z.; Zhu, H.; Ong, S.L.; Hu, J. Water treatment residual: A critical review of its applications on pollutant removal from stormwater runoff and future perspectives. J. Environ. Manag. 2020, 259, 109649. [Google Scholar] [CrossRef]
- Awual, M.R. Efficient phosphate removal from water for controlling eutrophication using novel composite adsorbent. J. Cle. Pro. 2019, 228, 1311–1319. [Google Scholar] [CrossRef]
- Awual, M.R.; Jyo, A.; Ihara, T.; Seko, N.; Tamada, M.; Lim, K.T. Enhanced trace phosphate removal from water by zirconium(IV) loaded fibrous adsorbent. Water Res. 2011, 45, 4592–4600. [Google Scholar] [CrossRef]
- Hou, Q.; Meng, P.; Pei, P.; Hu, W.; Chen, Y. Phosphorus adsorption characteristics of alum sludge: Adsorption capacity and the forms of phosphorus retained in alum sludge. Mater. Lett. 2018, 229, 31–35. [Google Scholar] [CrossRef]
- Kim, J.G.; Kim, J.H.; Moon, H.S.; Chon, C.M.; Ahn, J.S. Removal capacity of water plant alum sludge for phosphorus in aqueous solutions. Chem. Speciat. Bioavailab. 2002, 14, 67–73. [Google Scholar] [CrossRef]
- Lee, L.Y.; Wang, B.; Guo, H.; Hu, J.Y.; Ong, S.L. Aluminum-Based Water Treatment Residue Reuse for Phosphorus Removal. Water 2015, 7, 1480–1496. [Google Scholar] [CrossRef] [Green Version]
- Chu, W. Dye Removal from Textile Dye Wastewater Using Recycled Alum Sludge. Water Res. 2001, 35, 3147–3152. [Google Scholar] [CrossRef]
- Abba, A.B.; Saggai, S.; Touil, Y.; Al-Ansari, N.; Kouadri, S.; Nouasria, F.Z.; Najm, H.M.; Mashaan, N.S.; Eldirderi, M.M.A.; Khedher, K.M. Copper and Zinc Removal from Wastewater Using Alum Sludge Recovered from Water Treatment Plant. Sustainability 2022, 14, 9806. [Google Scholar] [CrossRef]
- Makris, K.C.; Sarkar, D.; Datta, R. Evaluating a drinking-water waste by-product as a novel sorbent for arsenic. Chemosphere 2006, 64, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Nagar, R.; Sarkar, D.; Makris, K.C.; Datta, R. Effect of solution chemistry on arsenic sorption by fe- and al-based drinking-water treatment residuals. Chemosphere 2010, 78, 1028–1035. [Google Scholar] [CrossRef]
- Gomes, S.D.-C.; Zhou, J.L.; Li, W.; Long, G. Progress in manufacture and properties of construction materials incorporating water treatment sludge: A review. Resour. Conserv. Recycl. 2019, 145, 148–159. [Google Scholar] [CrossRef]
- Huang, C.; Pan, J.R.; Liu, Y. Mixing water treatment residual with excavation waste soil in brick and artificial aggregate making. J. Environ. Eng. 2005, 131, 272–277. [Google Scholar] [CrossRef]
- Zamora, R.M.R.; Alfaro, O.C.; Cabirol, N.; Ayala, F.E.; Moreno, A.D. Valorization of drinking water treatment sludges as raw materials to produce concrete and mortar. Am. J. Environ. Sci. 2008, 4, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Mab, X.; Dai, H. Reuse of water purification sludge as raw material in cement production. Cem. Concr. Compos. 2010, 32, 436–439. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, S.-Y. Application of water treatment sludge in the manufacturing of lightweight aggregate. Constr. Build. Mater. 2013, 43, 174–183. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, R.; Awe, O.W.; Yang, Y.; Shen, C. Acceptability of land application of alum-based water treatment residuals—An explicit and comprehensive review. Chem. Eng. J. 2018, 353, 717–726. [Google Scholar] [CrossRef]
- Andraka, D.; Ospanov, K.; Myrzakhmetov, M. Current state of communal sewage treatment in the Republic of Kazakhstan. J. Ecol. Eng. 2015, 16, 101–109. [Google Scholar] [CrossRef] [Green Version]
- ISO 5667-6:2017; Water quality—Sampling—Part 6: Guidance on Sampling of Rivers and Streams. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 5667-1:2006; Water quality—Sampling—Part 1: Guidance on the design of sampling programmes and sampling techniques. International Organization for Standardization: Geneva, Switzerland, 2006.
- GOST 26449.1-85; Stationary Distillation Desalting Units. Methods of Saline Water Chemical Analysis. Standards Publishing House: Colorado Springs, CO, USA, 1985.
- Drugov, Y.S. Analysis of Polluted Water: A Practical Guide; Publishing House “Laboratoriya znaniy”: Moscow, Russia, 2015; ISBN 978-5-9963-2653-2. (In Russian) [Google Scholar]
- FR 1.31.2008.04399; Methodology for Measuring the Ash Content of Raw Sludge, Activated Sludge. AKVAROS: Moscow, Russia, 2008.
- ISO 6878:2004; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 12020:1997; Water Quality—Determination of Aluminum—Atomic Absorption Spectrometric Methods. International Organization for Standardization: Geneva, Switzerland, 1997.
- PND F 14.1:2:4.50-96; Quantitative Chemical Analysis of Water. Methods of Measuring the Mass Concentration of Total Iron in Drinking, Surface and Wastewater Photometric Method with Sulfosalicylic Acid Publisher: Moscow, Russia, 1996.
- RD 52.24.468-2019; Mass Concentration of Suspended Solids and Solids in Water. Gravimetric Measurement Procedure. Rosgidromet: Rostov-on-Don, Russia, 2020.
- Vorob’yeva, L.A. (Ed.) Theory and Practice of the Chemical Analysis of Soils; GEOS: Moscow, Russia, 2006. (In Russian) [Google Scholar]
- Chittoo, B.S.; Sutherland, C. Adsorption of Phosphorus Using Water Treatment Sludge. J. Appl. Sci. 2014, 14, 3455–3463. [Google Scholar] [CrossRef] [Green Version]
- Babatunde, A.O.; Zhao, Y.Q.; Yang, Y.; Kearney, P. Reuse of dewatered aluminium-coagulated water treatment residual to immobilize phosphorus: Batch and column trials using a condensed phosphate. Chem. Eng. J. 2008, 136, 108–115. [Google Scholar] [CrossRef] [Green Version]




| Parameter | Unit | Surface Water | Treated Water | National Standard for Drinking Water |
|---|---|---|---|---|
| turbidity | NTU | 0.8 | 0.5 | 1.5 |
| color | Pt Co | 10 | 5 | 20 |
| odor | - | very weak | very weak | weak |
| taste | - | - | no taste | very weak |
| pH | - | 7.65 | 7.92 | 6.0–9.0 |
| alkalinity as CaCO3 | mg/L | 150 | 145 | - |
| oxidizability | mg/L | 2.8 | 2.8 | 5.0 |
| hardness as CaCO3 | mg/L | 225 | 250 | 350 |
| chlorides | mg/L | 74.0 | 97.0 | 350.0 |
| sulfides | mg/L | 65.8 | 84.0 | 500.0 |
| dry residue | mg/L | 444.2 | 498.0 | 1000.0 |
| fluorides | mg/L | 0.24 | 0.30 | 1.2 |
| polyphosphates | mg/L | <0.05 | <0.05 | 3.5 |
| nitrates | mg/L | 0.49 | 0.86 | 45.0 |
| nitrites | mg/L | 0.021 | 0.005 | 3.0 |
| aluminum | mg/L | - | 0.02 | 0.5 |
| ammonia | mg/L | 0.05 | <0.05 | 2.0 |
| sodium | mg/L | 48.4 | 71.4 | 200.0 |
| potassium | mg/L | 3.3 | 3.8 | - |
| magnesium | mg/L | 14.8 | 17.3 | - |
| calcium | mg/L | 52.2 | 40.0 | - |
| iron | mg/L | 0.045 | 0.066 | 0.3 |
| manganese | mg/L | 0.008 | 0.009 | 0.1 |
| lead | mg/L | - | 0.004 | 0.03 |
| copper | mg/L | - | 0.0046 | 1.0 |
| zinc | mg/L | - | 0.015 | 5.0 |
| residual free chlorine | mg/L | - | 0.80 | 0.3–0.5 |
| residual fixed chlorine | mg/L | - | 0.30 | 0.8–1.2 |
| BOD5 | mg/L | 1.3 | - | - |
| dissolved oxygen | mg/L | 7.4 | - | - |
| saprophytic microorganisms, T = 22 °C | number/L | >300 | ||
| saprophytic microorganisms, T = 37 °C | number/L | >300 |
| Parameter | Percentage |
|---|---|
| aluminum oxide (Al2O3) | 10.8–14.6 |
| iron oxide (Fe2O3) | 4.58–5.31 |
| potassium oxide (K2O) | 1.64–1.98 |
| silicon oxide (SiO2) | 49.86–53.3 |
| phosphorus oxide (P2O5) | 0.2–0.3 |
| magnesium oxide (MgO) | 1.74–2.16 |
| Parameter | Control Sample | Contact Time—30 min Volume of WTS Added | Contact Time—60 min Volume of WTS Added | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 mL | 10 mL | 20 mL | 50 mL | 5 mL | 10 mL | 20 mL | 50 mL | ||
| WTS collected on 26 August 2022; WTS moisture content—95.6% | |||||||||
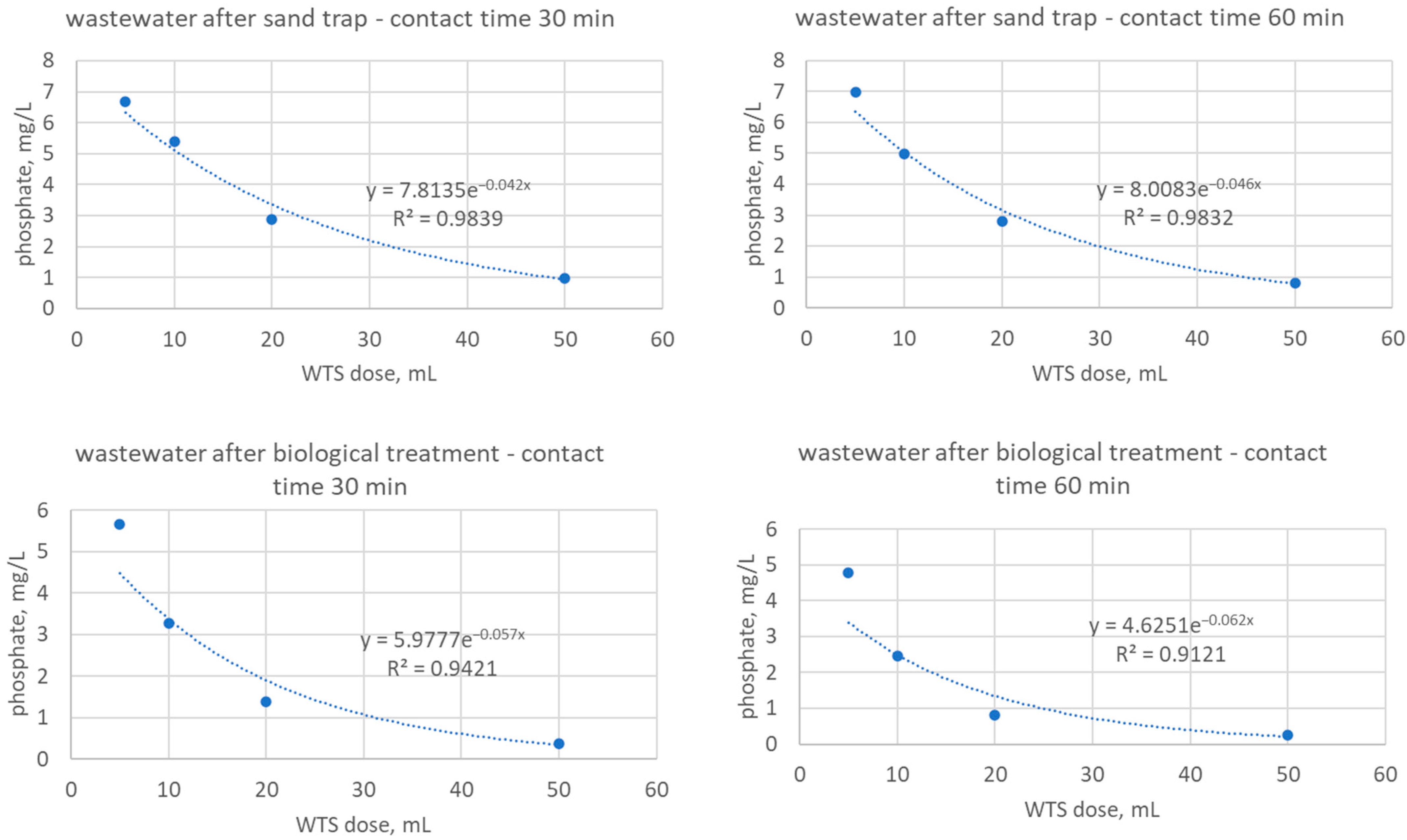
| phosphate, mg/L | 11.09 | - | 6.30 | 3.97 | 1.45 | - | 5.61 | 3.53 | 1.07 |
| iron, mg/L | 3.06 | - | 8.82 | 15.88 | 29.99 | - | 9.41 | 19.11 | 33.52 |
| iron(2) *, mg/L | 1.35 | - | 0.71 | 0.82 | 0.94 | - | 0.94 | 1.06 | 1.76 |
| aluminum, mg/L | 1177 | - | 1170 | 1140 | 1143 | - | 1157 | 1153 | |
| dry residue, mg/L | 280 | - | 921 | 1196 | 2184 | - | - | - | - |
| suspended solids, mg/L | 3.06 | - | 8.82 | 15.88 | 29.99 | - | 9.41 | 19.11 | 33.52 |
| WTS collected on 31 August 2022, WTS moisture content—95.6% | |||||||||
| phosphate, mg/L | 8.95 | 5.36 | 4.60 | 1.83 | 0.88 | 6.05 | 4.16 | 2.14 | 0.76 |
| iron, mg/L | 2.12 | 6.00 | 8.94 | 16.46 | 37.04 | 7.53 | 8.11 | 15.58 | 31.75 |
| iron (2) *, mg/L | 1.18 | 1.06 | 1.41 | 1.52 | 1.52 | 1.06 | 1.18 | 1.29 | 1.41 |
| aluminum, mg/L | 0.07 | 4.80 | 25.10 | 32.30 | 38.40 | 10.50 | 29.40 | 31.90 | 39.90 |
| dry residue, mg/L | 1040 | 1050 | 1053 | 1013 | 1027 | 1033 | 1043 | 1030 | 1037 |
| suspended solids, mg/L | 215 | 492 | 1020 | 1102 | 2267 | - | - | - | - |
| WTS collected on 02 September 2022, WTS moisture content—90.5% | |||||||||
| phosphate, mg/L | 11.59 | 8.00 | 5.29 | 2.84 | 0.63 | 7.88 | 5.17 | 2.71 | 0.63 |
| iron, mg/L | 5.94 | 8.11 | 15.29 | 24.70 | 31.75 | 16.22 | 31.40 | 37.34 | 81.14 |
| iron(2) *, mg/L | 0.71 | 0.71 | 0.71 | 0.59 | 0.82 | 0.88 | 1.30 | 1.76 | 1.12 |
| aluminum, mg/L | 0.08 | 23.6 | 30.6 | 38.6 | 45.0 | 25.0 | 28.2 | 38.8 | 48.8 |
| dry residue, mg/L | 1113 | 1077 | 1047 | 1043 | 1000 | 1063 | 1087 | 1067 | 1067 |
| suspended solids, mg/L | 150 | 602 | 1003 | 1734 | 4011 | - | - | - | - |
| Parameter | Control Sample | Contact Time—30 min Volume of WTS Added | Contact Time—60 min Volume of WTS Added | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 mL | 10 mL | 20 mL | 50 mL | 5 mL | 10 mL | 20 mL | 50 mL | ||
| WTS collected on 14 September 2022; WTS moisture content—94.4%; ash content—63.76% | |||||||||
| phosphate, mg/L | 9.89 | 5.67 | 3.28 | 1.39 | 0.38 | 4.79 | 2.46 | 0.82 | 0.25 |
| iron, mg/L | 0.51 | 4.12 | 9.17 | 24.11 | 71.15 | 5.17 | 13.05 | 24.11 | 80.56 |
| iron(2) *, mg/L | 0.38 | 0.47 | 0.47 | 0.47 | 0.68 | 0.45 | 0.45 | 0.66 | 0.71 |
| aluminum, mg/L | 0.18 | 13.75 | 35.15 | 41.25 | 46.00 | 26.20 | 39.85 | 44.35 | 46.90 |
| dry residue, mg/L | 1073 | 1100 | 1100 | 1100 | 1100 | 1113 | 1073 | 1120 | 1106 |
| suspended solids, mg/L | 18.2 | 446 | 863 | 1433 | 3524 | - | - | - | - |
| Date of WTS Collection | Contact Time—30 min Volume of WTS Added | Contact Time—60 min Volume of WTS Added | ||||||
|---|---|---|---|---|---|---|---|---|
| 5 mL | 10 mL | 20 mL | 50 mL | 5 mL | 10 mL | 20 mL | 50 mL | |
| wastewater samples after the sand trap | ||||||||
| 26 August 2022 | 43.2% | 64.2% | 86.9% | 49.4% | 68.2% | 90.4% | ||
| 31 August 2022 | 40.1% | 48.6% | 79.6% | 90.2% | 32.4% | 53.5% | 76.1% | 91.5% |
| 2 September 2022 | 31.0% | 54.4% | 75.5% | 94.6% | 32.0% | 55.4% | 76.6% | 94.6% |
| average | 35.5% | 48.7% | 73.1% | 90.6% | 32.3% | 52.8% | 73.6% | 92.1% |
| wastewater samples after biological treatment | ||||||||
| 2 September 2022 | 42.7% | 66.8% | 85.9% | 96.2% | 51.6% | 75.1% | 91.7% | 97.5% |
| Removal Efficiency | P-Concentration, mg/L | Test Conditions | Reference | |
|---|---|---|---|---|
| Initial | Final | |||
| 86.9–94.6% | 8.95–11.59 | 0.25–1.45 | naturally dried WTS added to wastewater from WWTP, batch jar test | this study |
| >90% | 30 | <1.0 | air-dried alum sludge mixed with synthetic P, batch column | [18] |
| up to 85% | 10 | not specified | oven-dried alum sludge, synthetic P and wastewater, continuous column | [41] |
| 90% | not specified | dewatered WTS and condensed phosphate, batch column | [42] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuldeyev, E.; Ospanov, K.; Andraka, D.; Merkýreva, S. Experimental Study on the Application of Sludge from Water Treatment Plant as a Reagent for Phosphate Removal from Wastewater. Water 2023, 15, 2691. https://doi.org/10.3390/w15152691
Kuldeyev E, Ospanov K, Andraka D, Merkýreva S. Experimental Study on the Application of Sludge from Water Treatment Plant as a Reagent for Phosphate Removal from Wastewater. Water. 2023; 15(15):2691. https://doi.org/10.3390/w15152691
Chicago/Turabian StyleKuldeyev, Erzhan, Kairat Ospanov, Dariusz Andraka, and Snejanna Merkýreva. 2023. "Experimental Study on the Application of Sludge from Water Treatment Plant as a Reagent for Phosphate Removal from Wastewater" Water 15, no. 15: 2691. https://doi.org/10.3390/w15152691
APA StyleKuldeyev, E., Ospanov, K., Andraka, D., & Merkýreva, S. (2023). Experimental Study on the Application of Sludge from Water Treatment Plant as a Reagent for Phosphate Removal from Wastewater. Water, 15(15), 2691. https://doi.org/10.3390/w15152691






