The Grey–Taguchi Method, a Statistical Tool to Optimize the Photo-Fenton Process: A Review
Abstract
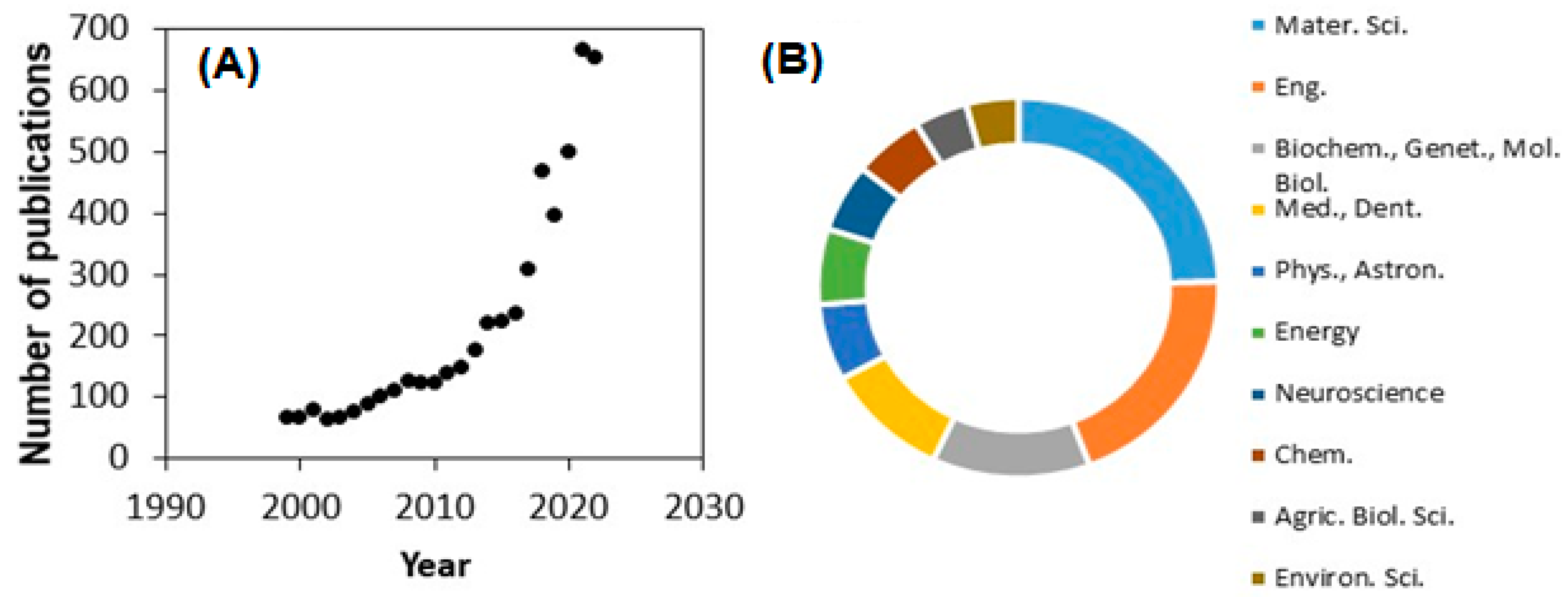
1. Introduction
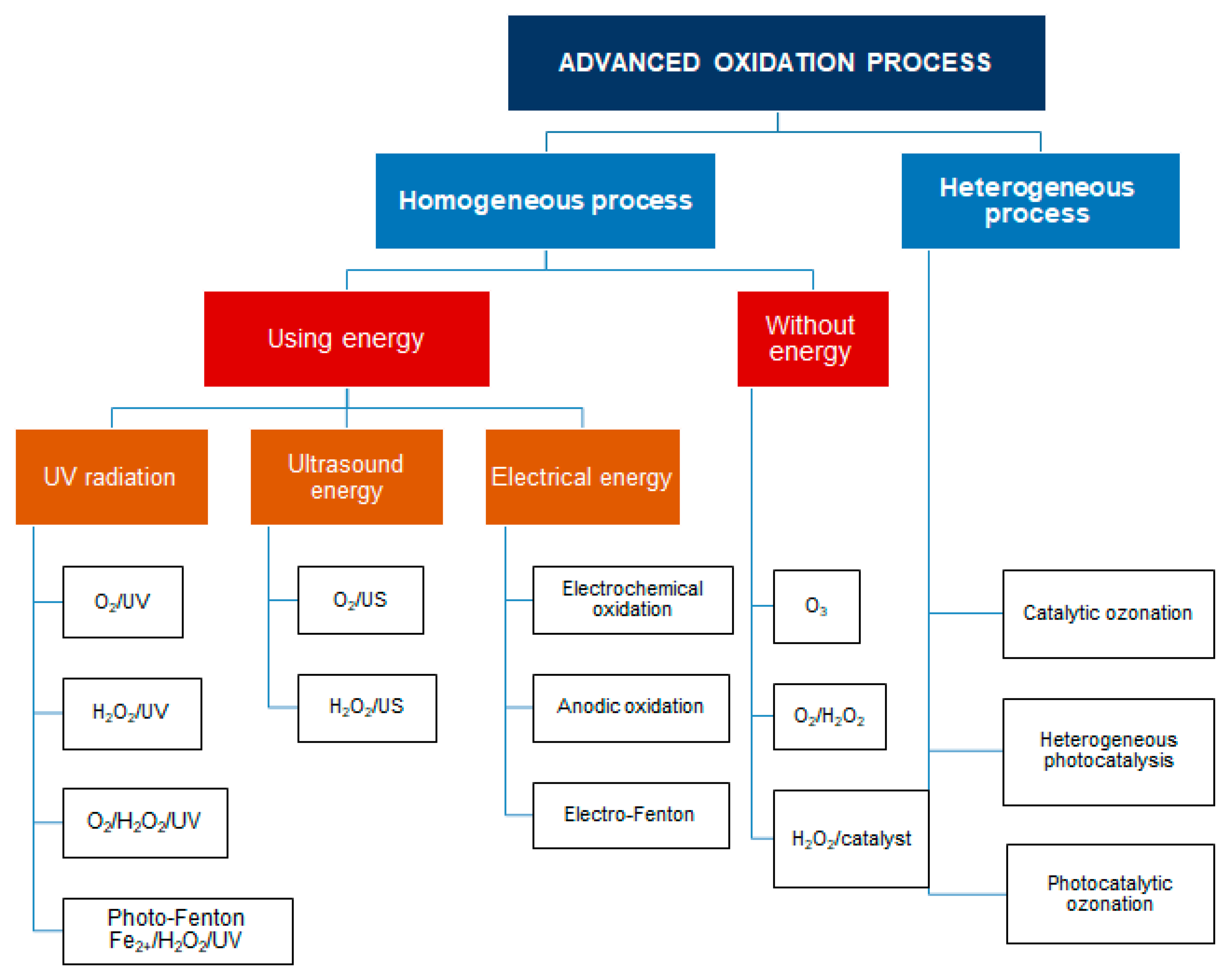
2. Advanced Oxidation Processes
3. Fenton Process
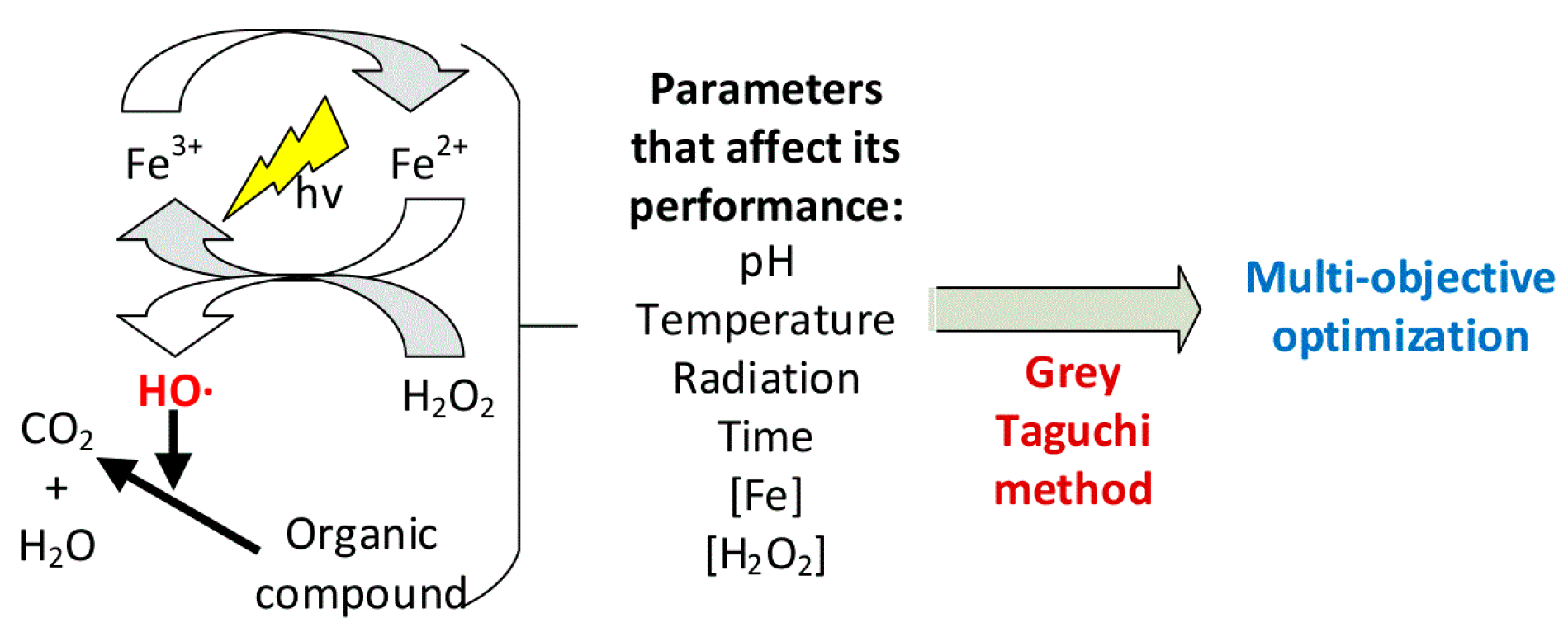
4. Photo-Fenton Process
5. Factors Affecting the Homogeneous Photo-Fenton Process
5.1. Catalyst Concentration
5.2. H2O2 Concentration
5.3. Initial Contaminant Concentration
5.4. pH
5.5. Effect of Radiation: Type and Intensity
5.6. Temperature Effect
5.7. Background Matrix Effect
6. Parameter Optimization
6.1. Design of Experiments
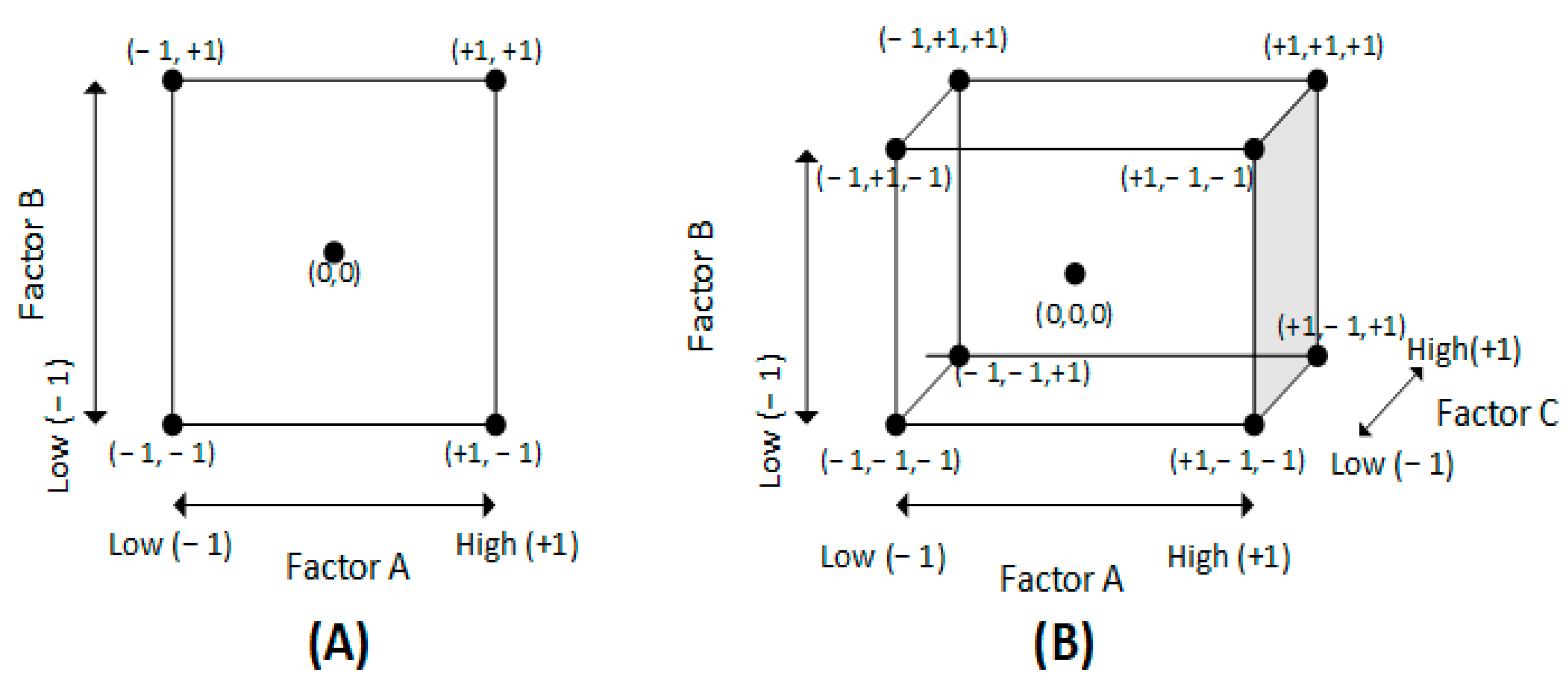
6.1.1. 2k Factorial Design
6.1.2. Central Composite Design (CCD)
6.1.3. Box-Behnken Design (BBD)
6.1.4. Taguchi Method
7. Grey–Taguchi Method
8. Challenges and Perspectives
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kümmerer, K. Pharmaceuticals in the Environment. Annu. Rev. Environ. Resour. 2010, 35, 57–75. [Google Scholar] [CrossRef]
- Halden, R.U. Pharma-Ecology—The Occurrence and Fate of Pharmaceuticals and Personal Care Products in the Environment. Environ. Health Perspect. 2009, 117, A172. [Google Scholar]
- Jjemba, P.K. Excretion and Ecotoxicity of Pharmaceutical and Personal Care Products in the Environment. Ecotoxicol. Environ. Saf. 2006, 63, 113–130. [Google Scholar] [CrossRef]
- Drillia, P.; Stamatelatou, K.; Lyberatos, G. Fate and Mobility of Pharmaceuticals in Solid Matrices. Chemosphere 2005, 60, 1034–1044. [Google Scholar] [CrossRef]
- Bound, J.P.; Voulvoulis, N. Household Disposal of Pharmaceuticals as a Pathway for Aquatic Contamination in the United Kingdom. Environ. Health Perspect. 2005, 113, 1705–1711. [Google Scholar] [CrossRef]
- Escher, B.I.; Baumgartner, R.; Koller, M.; Treyer, K.; Lienert, J.; McArdell, C.S. Environmental Toxicology and Risk Assessment of Pharmaceuticals from Hospital Wastewater. Water Res. 2011, 45, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging Organic Contaminants in Groundwater: A Review of Sources, Fate and Occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive Removal of Antibiotics from Water and Wastewater: Progress and Challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Belhaj, D.; Baccar, R.; Jaabiri, I.; Bouzid, J.; Kallel, M.; Ayadi, H.; Zhou, J.L. Fate of Selected Estrogenic Hormones in an Urban Sewage Treatment Plant in Tunisia (North Africa). Sci. Total Environ. 2015, 505, 154–160. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2014; Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 4 May 2022).
- Bueno, M.J.M.; Gomez, M.J.; Herrera, S.; Hernando, M.D.; Agüera, A.; Fernández-Alba, A.R. Occurrence and Persistence of Organic Emerging Contaminants and Priority Pollutants in Five Sewage Treatment Plants of Spain: Two Years Pilot Survey Monitoring. Environ. Pollut. 2012, 164, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and Removal of Pharmaceutical and Personal Care Products from Aquatic Systems Using Advanced Treatment—A Review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef]
- Ramírez-Morales, D.; Masís-Mora, M.; Montiel-Mora, J.R.; Cambronero-Heinrichs, J.C.; Briceño-Guevara, S.; Rojas-Sánchez, C.E.; Méndez-Rivera, M.; Arias-Mora, V.; Tormo-Budowski, R.; Brenes-Alfaro, L.; et al. Occurrence of Pharmaceuticals, Hazard Assessment and Ecotoxicological Evaluation of Wastewater Treatment Plants in Costa Rica. Sci. Total Environ. 2020, 746, 141200. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, A.-K.; Fischer, F.; Wick, A.; Ternes, T.A. Anaerobic Biodegradation of (Emerging) Organic Contaminants in the Aquatic Environment. Water Res. 2017, 116, 268–295. [Google Scholar] [CrossRef] [PubMed]
- Hom-Diaz, A.; Norvill, Z.N.; Blánquez, P.; Vicent, T.; Guieysse, B. Ciprofloxacin Removal during Secondary Domestic Wastewater Treatment in High Rate Algal Ponds. Chemosphere 2017, 180, 33–41. [Google Scholar] [CrossRef]
- Velázquez, Y.F.; Nacheva, P.M. Biodegradability of Fluoxetine, Mefenamic Acid, and Metoprolol Using Different Microbial Consortiums. Environ. Sci. Pollut. Res. 2017, 24, 6779–6793. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Kim, J.R.; Roh, H.-S.; Jeon, B.-H. Ciprofloxacin Toxicity and Its Co-Metabolic Removal by a Freshwater Microalga Chlamydomonas mexicana. J. Hazard. Mater. 2017, 323, 212–219. [Google Scholar] [CrossRef]
- Prada-Vásquez, M.A.; Estrada-Flórez, S.E.; Serna-Galvis, E.A.; Torres-Palma, R.A. Developments in the Intensification of Photo-Fenton and Ozonation-Based Processes for the Removal of Contaminants of Emerging Concern in Ibero-American Countries. Sci. Total Environ. 2021, 765, 142699. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water. A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Cruz-Alcalde, A.; Esplugas, S.; Sans, C. Continuous versus Single H2O2 Addition in Peroxone Process: Performance Improvement and Modelling in Wastewater Effluents. J. Hazard. Mater. 2020, 387, 121993. [Google Scholar] [CrossRef]
- Roccamante, M.; Salmerón, I.; Ruiz, A.; Oller, I.; Malato, S. New Approaches to Solar Advanced Oxidation Processes for Elimination of Priority Substances Based on Electrooxidation and Ozonation at Pilot Plant Scale. Catal. Today 2020, 355, 844–850. [Google Scholar] [CrossRef]
- Haag, W.R.; Yao, C.C.D. Rate Constants for Reaction of Hydroxyl Radicals with Several Drinking Water Contaminants. Environ. Sci. Technol. 1992, 26, 1005–1013. [Google Scholar] [CrossRef]
- Hoigné, J. Inter-Calibration of OH Radical Sources and Water Quality Parameters. Water Sci. Technol. 1997, 35, 1–8. [Google Scholar] [CrossRef]
- Meyerstein, D. Re-Examining Fenton and Fenton-like Reactions. Nat. Rev. Chem. 2021, 5, 595–597. [Google Scholar] [CrossRef]
- Silva, M.; Baltrusaitis, J. Destruction of Emerging Organophosphate Contaminants in Wastewater Using the Heterogeneous Iron-Based Photo-Fenton-like Process. JHM Lett. 2021, 2, 100012. [Google Scholar] [CrossRef]
- Asghar, A.; Abdul Raman, A.A.; Daud, W.M.A.W. A Comparison of Central Composite Design and Taguchi Method for Optimizing Fenton Process. Sci. World J. 2014, 2014, e869120. [Google Scholar] [CrossRef] [PubMed]
- Torrades, F.; Saiz, S.; García-Hortal, J.A. Using Central Composite Experimental Design to Optimize the Degradation of Black Liquor by Fenton Reagent. Desalination 2011, 268, 97–102. [Google Scholar] [CrossRef]
- Tayeb, A.M.; Tony, M.A.; Mansour, S.A. Application of Box–Behnken Factorial Design for Parameters Optimization of Basic Dye Removal Using Nano-Hematite Photo-Fenton Tool. Appl. Water Sci. 2018, 8, 138. [Google Scholar] [CrossRef]
- Taguchi, G.; Konishi, S.; Wu, Y. Taguchi Methods: Orthogonal Arrays and Linear Graphs. Tools for Quality Engineering; American Supplier Institute: Dearborn, MI, USA, 1987. [Google Scholar]
- Taguchi, G. Taguchi on Robust Technology Development: Bringing Quality Engineering Upstream; ASME Press: New York, NY, USA, 1993. [Google Scholar]
- Sohrabi, M.R.; Khavaran, A.; Shariati, S.; Shariati, S. Removal of Carmoisine Edible Dye by Fenton and Photo Fenton Processes Using Taguchi Orthogonal Array Design. Arab. J. Chem. 2017, 10, S3523–S3531. [Google Scholar] [CrossRef]
- Asgari, G.; Feradmal, J.; Poormohammadi, A.; Sadrnourmohamadi, M.; Akbari, S. Taguchi Optimization for the Removal of High Concentrations of Phenol from Saline Wastewater Using Electro-Fenton Process. Desalin. Water Treat. 2016, 57, 27331–27338. [Google Scholar] [CrossRef]
- Julong, D. Introduction to Grey System Theory. J. Grey Syst. 1989, 1, 1–24. [Google Scholar]
- Lin, J.L.; Tarng, Y.S. Optimization of the Multi-Response Process by the Taguchi Method with Grey Relational Analysis. J. Grey Syst. 1998, 4, 355–370. [Google Scholar]
- Song, C.; Wang, H.; Sun, Z.; Wei, Z.; Yu, H.; Chen, H.; Wang, Y. Optimization of Process Parameters Using the Grey-Taguchi Method and Experimental Validation in TRIP-Assisted Steel. Mater. Sci. Eng. A 2020, 777, 139084. [Google Scholar] [CrossRef]
- Pahange, H.; Abolbashari, M.H. Mass and Performance Optimization of an Airplane Wing Leading Edge Structure against Bird Strike Using Taguchi-Based Grey Relational Analysis. Chin. J. Aeronaut. 2016, 29, 934–944. [Google Scholar] [CrossRef]
- Shinde, A.B.; Pawar, P.M. Multi-Objective Optimization of Surface Textured Journal Bearing by Taguchi Based Grey Relational Analysis. Tribol. Int. 2017, 114, 349–357. [Google Scholar] [CrossRef]
- Rawat, S.; Zhang, Y.X.; Lee, C.K. Multi-Response Optimization of Hybrid Fibre Engineered Cementitious Composite Using Grey-Taguchi Method and Utility Concept. Constr. Build. Mater. 2022, 319, 126040. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-Merit for the Technical Development and Application of Advanced Oxidation Processes. J. Adv. Oxid. Technol. 1996, 1, 13–17. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I. Electrochemical Remediation Technologies for Waters Contaminated by Pharmaceutical Residues. In Environmental Chemistry for a Sustainable World, 1st ed.; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Environmental Chemistry for a Sustainable World; Springer: Dordrecht, The Netherlands, 2012; Volume 2, pp. 297–346. [Google Scholar]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- Mokhbi, Y.; Korichi, M.; Akchiche, Z. Combined Photocatalytic and Fenton Oxidation for Oily Wastewater Treatment. Appl. Water Sci. 2019, 9, 35. [Google Scholar] [CrossRef]
- Litter, M.I.; Quici, N. Photochemical Advanced Oxidation Processes for Water and Wastewater Treatment. Recent Pat. Eng. 2010, 4, 217–241. [Google Scholar] [CrossRef]
- Fenton, H.J.H. LXXIII.—Oxidation of Tartaric Acid in Presence of Iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. The Catalytic Decomposition of Hydrogen Peroxide by Iron Salts. Proc. Roy. Soc. A Math. Phys. Eng. Sci. 1934, 147, 332–351. [Google Scholar] [CrossRef]
- Bray, W.C.; Gorin, M.H. Ferryl ion, a compound of tetravalent iron. J. Am. Chem. Soc. 1932, 54, 2124–2125. [Google Scholar] [CrossRef]
- Eberson, L. Formation of Hydroxyl Spin Adducts via Nucleophilic Addition--Oxidation to 5,5-Dimethyl-1-Pyrroline N-Oxide (DMPO). Acta Chem. Scand. 1999, 53, 584–593. [Google Scholar] [CrossRef]
- Goldstein, S.; Meyerstein, D.; Czapski, G. The Fenton Reagents. Free Rad. Biol. Med. 1993, 15, 435–445. [Google Scholar] [CrossRef]
- Masarwa, M.; Cohen, H.; Meyerstein, D.; Hickman, D.L.; Bakac, A.; Espenson, J.H. Reactions of Low-Valent Transition-Metal Complexes with Hydrogen Peroxide. Are They” Fenton-like” or Not? 1. The Case of Cu+Aq and Cr2+Aq. J. Am. Chem. Soc. 1988, 110, 4293–4297. [Google Scholar] [CrossRef]
- Rachmilovich-Calis, S.; Masarwa, A.; Meyerstein, N.; Meyerstein, D.; van Eldik, R. New Mechanistic Aspects of the Fenton Reaction. Chem–Eur. J. 2009, 15, 8303–8309. [Google Scholar] [CrossRef] [PubMed]
- Bataineh, H.; Pestovsky, O.; Bakac, A. PH-Induced Mechanistic Changeover from Hydroxyl Radicals to Iron(IV) in the Fenton Reaction. Chem. Sci. 2012, 3, 1594–1599. [Google Scholar] [CrossRef]
- Novikov, A.S.; Kuznetsov, M.L.; Pombeiro, A.J.L.; Bokach, N.A.; Shul’pin, G.B. Generation of HO• Radical from Hydrogen Peroxide Catalyzed by Aqua Complexes of the Group III Metals [M(H2O)n]3+ (M = Ga, In, Sc, Y, or La): A Theoretical Study. ACS Catal. 2013, 3, 1195–1208. [Google Scholar] [CrossRef]
- Patra, S.G.; Mizrahi, A.; Meyerstein, D. The Role of Carbonate in Catalytic Oxidations. Acc. Chem. Res. 2020, 53, 2189–2200. [Google Scholar] [CrossRef]
- Asaithambi, P.; Alemayehu, E.; Sajjadi, B.; Aziz, A.R.A. Electrical Energy per Order Determination for the Removal Pollutant from Industrial Wastewater Using UV/Fe2+/H2O2 Process: Optimization by Response Surface Methodology. Water Resour. Ind. 2017, 18, 17–32. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Damiani, S. Treatments for Color Removal from Wastewater: State of the Art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Bahri, M.; Mahdavi, A.; Mirzaei, A.; Mansouri, A.; Haghighat, F. Integrated Oxidation Process and Biological Treatment for Highly Concentrated Petrochemical Effluents: A Review. Chem. Eng. Process.-Process Intensif. 2018, 125, 183–196. [Google Scholar] [CrossRef]
- Torres, G.F.; Ortega Méndez, J.A.; Tinoco, D.L.; Marin, E.D.; Araña, J.; Herrera-Melián, J.A.; Doña Rodrígez, J.M.; Pérez Peña, J. Detoxification of Synthetic and Real Groundwater Contaminated with Gasoline and Diesel Using Fenton, Photo-Fenton, and Biofilters. Desalin. Water Treat. 2016, 57, 23760–23769. [Google Scholar] [CrossRef]
- Alam, F.; Mobin, S.; Chowdhury, H. Third Generation Biofuel from Algae. Procedia Eng. 2015, 105, 763–768. [Google Scholar] [CrossRef]
- De la Cruz, N.; Esquius, L.; Grandjean, D.; Magnet, A.; Tungler, A.; de Alencastro, L.F.; Pulgarín, C. Degradation of Emergent Contaminants by UV, UV/H2O2 and Neutral Photo-Fenton at Pilot Scale in a Domestic Wastewater Treatment Plant. Water Res. 2013, 47, 5836–5845. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced Oxidation Process-Mediated Removal of Pharmaceuticals from Water: A Review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Zapata, A.; Oller, I.; Gallay, R.; Pulgarín, C.; Maldonado, M.I.; Malato, S.; Gernjak, W. Comparison of Photo-Fenton Treatment and Coupled Photo-Fenton and Biological Treatment for Detoxification of Pharmaceutical Industry Contaminants. J. Adv. Oxid. Technol. 2008, 11, 261–269. [Google Scholar] [CrossRef]
- Jeong, J.; Yoon, J. PH Effect on OH Radical Production in Photo/Ferrioxalate System. Water Res. 2005, 39, 2893–2900. [Google Scholar] [CrossRef]
- Zapata, A.; Velegraki, T.; Sánchez-Pérez, J.A.; Mantzavinos, D.; Maldonado, M.I.; Malato, S. Solar Photo-Fenton Treatment of Pesticides in Water: Effect of Iron Concentration on Degradation and Assessment of Ecotoxicity and Biodegradability. Appl. Catal. B Environ. 2009, 88, 448–454. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Kavitha, V.; Palanivelu, K. The Role of Ferrous Ion in Fenton and Photo-Fenton Processes for the Degradation of Phenol. Chemosphere 2004, 55, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, K.; Pillai, S.C. Photo-Fenton Disinfection at near Neutral pH: Process, Parameter Optimization and Recent Advances. J. Environ. Chem. Eng. 2020, 8, 104063. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.; Chen, B. Self-Assembled Nano-FeO(OH)/Reduced Graphene Oxide Aerogel as a Reusable Catalyst for Photo-Fenton Degradation of Phenolic Organics. Environ. Sci. Technol. 2018, 52, 7043–7053. [Google Scholar] [CrossRef]
- Rubio-Clemente, A.; Chica, E.; Peñuela, G.A. Petrochemical Wastewater Treatment by Photo-Fenton Process. Water Air Soil Pollut. 2015, 226, 62. [Google Scholar] [CrossRef]
- Sánchez Pérez, J.A.; Arzate, S.; Soriano-Molina, P.; García Sánchez, J.L.; Casas López, J.L.; Plaza-Bolaños, P. Neutral or Acidic PH for the Removal of Contaminants of Emerging Concern in Wastewater by Solar Photo-Fenton? A Techno-Economic Assessment of Continuous Raceway Pond Reactors. Sci. Total Environ. 2020, 736, 139681. [Google Scholar] [CrossRef]
- Jo, W.-K.; Tayade, R.J. New Generation Energy-Efficient Light Source for Photocatalysis: LEDs for Environmental Applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.-Y.; Kang, C.-L.; Peng, F.; Liu, H.-F.; Yang, T.; Shi, L.; Wang, H.-L. Photo-Fenton Effect of 4-Chlorophenol in Ice. J. Hazard. Mater. 2013, 261, 500–511. [Google Scholar] [CrossRef]
- Pliego, G.; Xekoukoulotakis, N.; Venieri, D.; Zazo, J.A.; Casas, J.A.; Rodriguez, J.J.; Mantzavinos, D. Complete Degradation of the Persistent Anti-Depressant Sertraline in Aqueous Solution by Solar Photo-Fenton Oxidation. J. Chem. Technol. Biotechnol. 2014, 89, 814–818. [Google Scholar] [CrossRef]
- Bautitz, I.R.; Nogueira, R.F.P. Degradation of Tetracycline by Photo-Fenton Process—Solar Irradiation and Matrix Effects. J. Photochem. Photobiol. A Chem. 2007, 187, 33–39. [Google Scholar] [CrossRef]
- Trovó, A.G.; Melo, S.A.S.; Nogueira, R.F.P. Photodegradation of the Pharmaceuticals Amoxicillin, Bezafibrate and Paracetamol by the Photo-Fenton Process—Application to Sewage Treatment Plant Effluent. J. Photochem. Photobiol. A Chem. 2008, 198, 215–220. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Antonopoulou, M.; Sykiotou, M.; Εvgenidou, Ε.; Konstantinou, I.; Lambropoulou, D.A. Photo-Fenton and Fenton-like Processes for the Treatment of the Antineoplastic Drug 5-Fluorouracil under Simulated Solar Radiation. Environ. Sci. Pollut. Res. 2017, 24, 4791–4800. [Google Scholar] [CrossRef]
- Monteoliva-García, A.; Martín-Pascual, J.; Muñío, M.M.; Poyatos, J.M. Removal of Carbamazepine, Ciprofloxacin and Ibuprofen in Real Urban Wastewater by Using Light-Driven Advanced Oxidation Processes. Int. J. Environ. Sci. Technol. 2019, 16, 6005–6018. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Giraldo-Aguirre, A.L.; Silva-Agredo, J.; Flórez-Acosta, O.A.; Torres-Palma, R.A. Removal of Antibiotic Cloxacillin by Means of Electrochemical Oxidation, TiO2 Photocatalysis, and Photo-Fenton Processes: Analysis of Degradation Pathways and Effect of the Water Matrix on the Elimination of Antimicrobial Activity. Environ. Sci. Pollut. Res. Int. 2017, 24, 6339–6352. [Google Scholar] [CrossRef]
- Davididou, K.; Monteagudo, J.M.; Chatzisymeon, E.; Durán, A.; Expósito, A.J. Degradation and Mineralization of Antipyrine by UV-A LED Photo-Fenton Reaction Intensified by Ferrioxalate with Addition of Persulfate. Sep. Purif. Technol. 2017, 172, 227–235. [Google Scholar] [CrossRef]
- Polo, A.M.S.; López-Peñalver, J.J.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Velo-Gala, I.; Salazar-Rábago, J.J. Oxidation of Diatrizoate in Aqueous Phase by Advanced Oxidation Processes Based on Solar Radiation. J. Photochem. Photobiol. A Chem. 2016, 319–320, 87–95. [Google Scholar] [CrossRef]
- Villota, N.; Lomas, J.M.; Camarero, L.M. Study of the Paracetamol Degradation Pathway That Generates Color and Turbidity in Oxidized Wastewaters by Photo-Fenton Technology. J. Photochem. Photobiol. A Chem. 2016, 329, 113–119. [Google Scholar] [CrossRef]
- Marchetti, M.D.; Bessa Azevedo, E. Degradation of NSAIDs by Optimized Photo-Fenton Process Using UV-LEDs at near-Neutral pH. J. Water Process Eng. 2020, 35, 101171. [Google Scholar] [CrossRef]
- Davarnejad, R.; Zangene, K.; Fazlali, A.R.; Behfar, R. Ibuprofen Removal from a Pharmaceutical Wastewater Using Electro-Fenton Process: An Efficient Technique (RESEARCH NOTE). Int. J. Eng. 2017, 30, 1639–1646. [Google Scholar]
- Özcan, A.; Atılır Özcan, A.; Demirci, Y. Evaluation of Mineralization Kinetics and Pathway of Norfloxacin Removal from Water by Electro-Fenton Treatment. Chem. Eng. J. 2016, 304, 518–526. [Google Scholar] [CrossRef]
- Annabi, C.; Fourcade, F.; Soutrel, I.; Geneste, F.; Floner, D.; Bellakhal, N.; Amrane, A. Degradation of Enoxacin Antibiotic by the Electro-Fenton Process: Optimization, Biodegradability Improvement and Degradation Mechanism. J. Environ. Manag. 2016, 165, 96–105. [Google Scholar] [CrossRef]
- Kadji, H.; Yahiaoui, I.; Garti, Z.; Amrane, A.; Aissani-Benissad, F. Kinetic Degradation of Amoxicillin by Using the Electro-Fenton Process in the Presence of a Graphite Rods from Used Batteries. Chin. J. Chem. Eng. 2021, 32, 183–190. [Google Scholar] [CrossRef]
- Martínez-Costa, J.I.; Rivera-Utrilla, J.; Leyva-Ramos, R.; Sánchez-Polo, M.; Velo-Gala, I.; Mota, A.J. Individual and Simultaneous Degradation of the Antibiotics Sulfamethoxazole and Trimethoprim in Aqueous Solutions by Fenton, Fenton-like and Photo-Fenton Processes Using Solar and UV Radiations. J. Photochem. Photobiol. A Chem. 2018, 360, 95–108. [Google Scholar] [CrossRef]
- Gupta, A.; Garg, A. Degradation of Ciprofloxacin Using Fenton’s Oxidation: Effect of Operating Parameters, Identification of Oxidized by-Products and Toxicity Assessment. Chemosphere 2018, 193, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Monsalvo, V.M.; Lopez, J.; Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Mohedano, A.F.; Rodriguez, J.J. Application of Fenton-like Oxidation as Pre-Treatment for Carbamazepine Biodegradation. Chem. Eng. J. 2015, 264, 856–862. [Google Scholar] [CrossRef]
- Mackuľak, T.; Mosný, M.; Grabic, R.; Golovko, O.; Koba, O.; Birošová, L. Fenton-like Reaction: A Possible Way to Efficiently Remove Illicit Drugs and Pharmaceuticals from Wastewater. Environ. Toxicol. Pharmacol. 2015, 39, 483–488. [Google Scholar] [CrossRef]
- Gamarra-Güere, C.D.; Dionisio, D.; Santos, G.O.S.; Vasconcelos Lanza, M.R.; de Jesus Motheo, A. Application of Fenton, Photo-Fenton and Electro-Fenton Processes for the Methylparaben Degradation: A Comparative Study. J. Environ. Chem. Eng. 2022, 10, 106992. [Google Scholar] [CrossRef]
- Martins, R.C.; Gmurek, M.; Rossi, A.F.; Corceiro, V.; Costa, R.; Quinta-Ferreira, M.E.; Ledakowicz, S.; Quinta-Ferreira, R.M. Application of Fenton Oxidation to Reduce the Toxicity of Mixed Parabens. Water Sci. Technol. 2016, 74, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Benítez, H.; Aristizábal-Ciro, C.; Peñuela, G.A. Photodegradation of the Endocrine-Disrupting Chemicals Benzophenone-3 and Methylparaben Using Fenton Reagent: Optimization of Factors and Mineralization/Biodegradability Studies. J. Taiwan Inst. Chem. Eng. 2016, 59, 380–388. [Google Scholar] [CrossRef]
- De Luca, A.; Dantas, R.F.; Esplugas, S. Assessment of Iron Chelates Efficiency for Photo-Fenton at Neutral pH. Water Res. 2014, 61, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Molkenthin, M.; Olmez-Hanci, T.; Jekel, M.R.; Arslan-Alaton, I. Photo-Fenton-like Treatment of BPA: Effect of UV Light Source and Water Matrix on Toxicity and Transformation Products. Water Res. 2013, 47, 5052–5064. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Huang, Y.-J.; Tsai, H.-C.; Chen, H.-T. Degradation of Phenol Using Low Concentration of Ferric Ions by the Photo-Fenton Process. J. Taiwan Inst. Chem. Eng. 2010, 41, 699–704. [Google Scholar] [CrossRef]
- Kavitha, V.; Palanivelu, K. Degradation of Nitrophenols by Fenton and Photo-Fenton Processes. J. Photochem. Photobiol. A Chem. 2005, 170, 83–95. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Angelidaki, I.; Zhang, Y. Bio-Electro-Fenton Processes for Wastewater Treatment: Advances and Prospects. Chem. Eng. J. 2018, 354, 492–506. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Ning, P. Degradation Mechanism of Methylene Blue in a Heterogeneous Fenton-like Reaction Catalyzed by Ferrocene. Ind. Eng. Chem. Res. 2014, 53, 643–649. [Google Scholar] [CrossRef]
- Salari, M.; Rakhshandehroo, G.R.; Nikoo, M.R. Multi-Objective Optimization of Ciprofloxacin Antibiotic Removal from an Aqueous Phase with Grey Taguchi Method. J. Water Health 2018, 16, 530–541. [Google Scholar] [CrossRef]
- Levchuk, I.; Bhatnagar, A.; Sillanpää, M. Overview of Technologies for Removal of Methyl Tert-Butyl Ether (MTBE) from Water. Sci. Total Environ. 2014, 476–477, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.; Meng, D. A Review on Fenton Process for Organic Wastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Iron Metabolism and Toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef]
- Ramirez, J.H.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Moreno-Castilla, C.; Costa, C.A.; Madeira, L.M. Azo-Dye Orange II Degradation by Heterogeneous Fenton-like Reaction Using Carbon-Fe Catalysts. Appl. Catal. B Environ. 2007, 75, 312–323. [Google Scholar] [CrossRef]
- Sun, J.-H.; Sun, S.-P.; Fan, M.-H.; Guo, H.-Q.; Lee, Y.-F.; Sun, R.-X. Oxidative Decomposition of P-Nitroaniline in Water by Solar Photo-Fenton Advanced Oxidation Process. J. Hazard. Mater. 2008, 153, 187–193. [Google Scholar] [CrossRef]
- Arefieva, O.D.; Vasilyeva, M.S.; Ermolenko, E.V.; Bychkova, A.V. Photo-Fenton Process of Oxidative Destruction of Rice Husk Alkaline Hydrolysates Lignin. Water Pract. Technol. 2019, 14, 391–398. [Google Scholar] [CrossRef]
- Gou, Y.; Chen, P.; Yang, L.; Li, S.; Peng, L.; Song, S.; Xu, Y. Degradation of Fluoroquinolones in Homogeneous and Heterogeneous Photo-Fenton Processes: A Review. Chemosphere 2021, 270, 129481. [Google Scholar] [CrossRef] [PubMed]
- Rafin, C.; Veignie, E.; Fayeulle, A.; Surpateanu, G. Benzo[a]Pyrene Degradation Using Simultaneously Combined Chemical Oxidation, Biotreatment with Fusarium Solani and Cyclodextrins. Bioresour. Technol. 2009, 100, 3157–3160. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Castro, A.V.; Dezotti, M.; Sant’Anna, G.L. Treatment of Petroleum Refinery Sourwater by Advanced Oxidation Processes. J. Hazard. Mater. 2006, 137, 178–184. [Google Scholar] [CrossRef]
- Da Silva, S.S.; Chiavone-Filho, O.; de Barros Neto, E.L.; Nascimento, C.A.O. Integration of Processes Induced Air Flotation and Photo-Fenton for Treatment of Residual Waters Contaminated with Xylene. J. Hazard. Mater. 2012, 199–200, 151–157. [Google Scholar] [CrossRef]
- Ammar, H.B. Sono-Fenton Process for Metronidazole Degradation in Aqueous Solution: Effect of Acoustic Cavitation and Peroxydisulfate Anion. Ultrason. Sonochem. 2016, 33, 164–169. [Google Scholar] [CrossRef]
- Nieto, L.M.; Hodaifa, G.; Rodríguez, S.; Giménez, J.A.; Ochando, J. Degradation of Organic Matter in Olive-Oil Mill Wastewater through Homogeneous Fenton-like Reaction. Chem. Eng. J. 2011, 173, 503–510. [Google Scholar] [CrossRef]
- Batista, A.P.S.; Nogueira, R.F.P. Parameters Affecting Sulfonamide Photo-Fenton Degradation–Iron Complexation and Substituent Group. J. Photochem. Photobiol. A Chem. 2012, 232, 8–13. [Google Scholar] [CrossRef]
- Rubio, D.; Nebot, E.; Casanueva, J.F.; Pulgarin, C. Comparative Effect of Simulated Solar Light, UV, UV/H2O2 and Photo-Fenton Treatment (UV–Vis/H2O2/Fe2+,3+) in the Escherichia coli Inactivation in Artificial Seawater. Water Res. 2013, 47, 6367–6379. [Google Scholar] [CrossRef]
- Simunovic, M.; Kusic, H.; Koprivanac, N.; Bozic, A.L. Treatment of Simulated Industrial Wastewater by Photo-Fenton Process: Part II. The Development of Mechanistic Model. Chem. Eng. J. 2011, 173, 280–289. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A Review on Fenton and Improvements to the Fenton Process for Wastewater Treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Kušić, H.; Koprivanac, N.; Božić, A.L.; Selanec, I. Photo-Assisted Fenton Type Processes for the Degradation of Phenol: A Kinetic Study. J. Hazard. Mater. 2006, 136, 632–644. [Google Scholar] [CrossRef]
- Dopar, M.; Kusic, H.; Koprivanac, N. Treatment of Simulated Industrial Wastewater by Photo-Fenton Process. Part I: The Optimization of Process Parameters Using Design of Experiments (DOE). Chem. Eng. J. 2011, 173, 267–279. [Google Scholar] [CrossRef]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Degradation of Four Pharmaceuticals by Solar Photo-Fenton Process: Kinetics and Costs Estimation. J. Environ. Chem. Eng. 2015, 3, 46–51. [Google Scholar] [CrossRef]
- Krutzler, T.; Bauer, R. Optimization of a Photo-Fenton Prototype Reactor. Chemosphere 1999, 38, 2517–2532. [Google Scholar] [CrossRef]
- Sá, R.D.; Rodríguez-Pérez, A.P.; Rodrigues-Silva, F.; de Paula, V.d.C.S.; Prola, L.D.T.; de Freitas, A.M.; de Carvalho, K.Q.; de Liz, M.V. Treatment of a Clinical Analysis Laboratory Wastewater from a Hospital by Photo-Fenton Process at Four Radiation Settings and Toxicity Response. Environ. Sci. Pollut. Res. 2021, 28, 24180–24190. [Google Scholar] [CrossRef]
- Villota, N.; Lomas, J.M.; Camarero, L.M. Kinetic Modelling of Water-Color Changes in a Photo-Fenton System Applied to Oxidate Paracetamol. J. Photochem. Photobiol. A Chem. 2018, 356, 573–579. [Google Scholar] [CrossRef]
- Conte, L.O.; Schenone, A.V.; Alfano, O.M. Photo-Fenton Degradation of the Herbicide 2,4-D in Aqueous Medium at pH Conditions Close to Neutrality. J. Environ. Manag. 2016, 170, 60–69. [Google Scholar] [CrossRef]
- Khamaruddin, P.F.; Bustam, M.A.; Omar, A.A. Using Fenton’s Reagents for the Degradation of Diisopropanolamine: Effect of Temperature and pH. In Proceedings of the International Conference on Environment and Industrial Innovation IPCBEE, Kuala Lumpur, Malaysia, 17–19 June 2011. [Google Scholar]
- Zazo, J.A.; Pliego, G.; Blasco, S.; Casas, J.A.; Rodriguez, J.J. Intensification of the Fenton Process by Increasing the Temperature. Ind. Eng. Chem. Res. 2011, 50, 866–870. [Google Scholar] [CrossRef]
- Carbajo, J.; Silveira, J.E.; Pliego, G.; Zazo, J.A.; Casas, J.A. Increasing Photo-Fenton Process Efficiency: The Effect of High Temperatures. Sep. Purif. Technol. 2021, 271, 118876. [Google Scholar] [CrossRef]
- De Laat, J.; Truong Le, G.; Legube, B. A Comparative Study of the Effects of Chloride, Sulfate and Nitrate Ions on the Rates of Decomposition of H2O2 and Organic Compounds by Fe(II)/H2O2 and Fe(III)/H2O2. Chemosphere 2004, 55, 715–723. [Google Scholar] [CrossRef]
- Siedlecka, E.M.; Więckowska, A.; Stepnowski, P. Influence of Inorganic Ions on MTBE Degradation by Fenton’s Reagent. J. Hazard. Mater. 2007, 147, 497–502. [Google Scholar] [CrossRef]
- Vallés, I.; Santos-Juanes, L.; Amat, A.M.; Moreno-Andrés, J.; Arques, A. Effect of Salinity on UVA-Vis Light Driven Photo-Fenton Process at Acidic and Circumneutral pH. Water 2021, 13, 1315. [Google Scholar] [CrossRef]
- Mack, J.; Bolton, J.R. Photochemistry of Nitrite and Nitrate in Aqueous Solution: A Review. J. Photochem. Photobiol. A Chem. 1999, 128, 1–13. [Google Scholar] [CrossRef]
- Marussi, G.; Vione, D. Secondary Formation of Aromatic Nitroderivatives of Environmental Concern: Photonitration Processes Triggered by the Photolysis of Nitrate and Nitrite Ions in Aqueous Solution. Molecules 2021, 26, 2550. [Google Scholar] [CrossRef] [PubMed]
- Gomis, J.; Bianco Prevot, A.; Montoneri, E.; Gonzalez, M.C.; Amat, A.M.; Martire, D.O.; Arques, A.; Carlos, L. Waste Sourced Bio-Based Substances for Solar-Driven Wastewater Remediation: Photodegradation of Emerging Pollutants. Chem. Eng. J. 2014, 235, 236–243. [Google Scholar] [CrossRef]
- Silva, J.O.; Silva, V.M.; Cardoso, V.L.; Machado, A.E.H.; Trovó, A.G. Treatment of Sanitary Landfill Leachate by Photo-Fenton Process: Effect of the Matrix Composition. J. Braz. Chem. Soc. 2016, 27, 2264–2272. [Google Scholar] [CrossRef]
- Fukushima, M.; Tatsumi, K. Degradation Pathways of Pentachlorophenol by Photo-Fenton Systems in the Presence of Iron(III), Humic Acid, and Hydrogen Peroxide. Environ. Sci. Technol. 2001, 35, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.H.O.S.; Queirós, D.B.; Reis, A.C.; Nunes, O.C.; Borges, M.T.; Boaventura, R.A.R.; Vilar, V.J.P. Process Enhancement at near Neutral pH of a Homogeneous Photo-Fenton Reaction Using Ferricarboxylate Complexes: Application to Oxytetracycline Degradation. Chem. Eng. J. 2014, 253, 217–228. [Google Scholar] [CrossRef]
- López-Vinent, N.; Cruz-Alcalde, A.; Giménez, J.; Esplugas, S. Mixtures of Chelating Agents to Enhance Photo-Fenton Process at Natural pH: Influence of Wastewater Matrix on Micropollutant Removal and Bacterial Inactivation. Sci. Total Environ. 2021, 786, 147416. [Google Scholar] [CrossRef] [PubMed]
- Diya’uddeen, B.H.; Abdul Aziz, A.R.; Daud, W.W. On the Limitation of Fenton Oxidation Operational Parameters: A Review. Int. J. Chem. React. Eng. 2012, 10. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- De Castro Peixoto, A.L.; Costalonga, A.G.C.; Esperança, M.N.; dos Santos Salazar, R.F. Design of Experiments Applied to Antibiotics Degradation by Fenton’s Reagent; IntechOpen: Rijeka, Croatia, 2017; ISBN 978-953-51-3878-5. [Google Scholar]
- Maslahati Roudi, A.; Chelliapan, S.; Wan Mohtar, W.H.M.; Kamyab, H. Prediction and Optimization of the Fenton Process for the Treatment of Landfill Leachate Using an Artificial Neural Network. Water 2018, 10, 595. [Google Scholar] [CrossRef]
- Schenone, A.V.; Conte, L.O.; Botta, M.A.; Alfano, O.M. Modeling and Optimization of Photo-Fenton Degradation of 2,4-D Using Ferrioxalate Complex and Response Surface Methodology (RSM). J. Environ. Manag. 2015, 155, 177–183. [Google Scholar] [CrossRef]
- Speck, F.; Raja, S.; Ramesh, V.; Thivaharan, V. Modelling and Optimization of Homogenous Photo-Fenton Degradation of Rhodamine B by Response Surface Methodology and Artificial Neural Network. Int. J. Environ. Res. 2016, 10, 543–554. [Google Scholar] [CrossRef]
- Gunst, R.F. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. Technometrics 1996, 38, 284–286. [Google Scholar] [CrossRef]
- Kasiri, M.B.; Aleboyeh, H.; Aleboyeh, A. Modeling and Optimization of Heterogeneous Photo-Fenton Process with Response Surface Methodology and Artificial Neural Networks. Environ. Sci. Technol. 2008, 42, 7970–7975. [Google Scholar] [CrossRef]
- Portela-Marcelino, R. Aplicação de Processos Oxidativos Avançados Para o Tratamento de Efluente Da Produção de Antibióticos. Master’s Thesis, Federal University of Minas Gerais, Belo Horizonte, Brazil, 2014. [Google Scholar]
- Vargas da Silva, V. Degradação de Amoxicilina Por Fenton e Foto-Fenton; Federal University of Rio Grande do Sul: Porto Alegre, Brazil, 2015. [Google Scholar]
- Mitsika, E.E.; Christophoridis, C.; Kouinoglou, N.; Lazaridis, N.; Zacharis, C.K.; Fytianos, K. Optimized Photo-Fenton Degradation of Psychoactive Pharmaceuticals Alprazolam and Diazepam Using a Chemometric Approach—Structure and Toxicity of Transformation Products. J. Hazard. Mater. 2021, 403, 123819. [Google Scholar] [CrossRef]
- Pérez-Moya, M.; Graells, M.; Castells, G.; Amigó, J.; Ortega, E.; Buhigas, G.; Pérez, L.M.; Mansilla, H.D. Characterization of the Degradation Performance of the Sulfamethazine Antibiotic by Photo-Fenton Process. Water Res. 2010, 44, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Arslan-Alaton, I.; Ayten, N.; Olmez-Hanci, T. Photo-Fenton-like Treatment of the Commercially Important H-Acid: Process Optimization by Factorial Design and Effects of Photocatalytic Treatment on Activated Sludge Inhibition. Appl. Catal. B Environ. 2010, 96, 208–217. [Google Scholar] [CrossRef]
- Al-Khafaji, R.Q.; Mohammed, A.H.A.-K. Optimization of Continuous Electro-Fenton and Photo Electro-Fenton Processes to Treat Iraqi Oilfield Produced Water Using Surface Response Methodology. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 518, p. 062007. [Google Scholar] [CrossRef]
- Hadavifar, M.; Zinatizadeh, A.A.; Younesi, H.; Galehdar, M. Fenton and Photo-Fenton Treatment of Distillery Effluent and Optimization of Treatment Conditions with Response Surface Methodology. Asia-Pac. J. Chem. Eng. 2010, 5, 454–464. [Google Scholar] [CrossRef]
- Lu, L.A.; Ma, Y.S.; Daverey, A.; Lin, J.G. Optimization of Photo-Fenton Process Parameters on Carbofuran Degradation Using Central Composite Design. J. Environ. Sci. Health B 2012, 47, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Ay, F.; Kargi, F. Advanced Oxidation of Amoxicillin by Fenton’s Reagent Treatment. J. Hazard. Mater. 2010, 179, 622–627. [Google Scholar] [CrossRef]
- Sangle, S.V.; Jadhav, D.M.V. Study of Fenton Reagent for the Removal of Chemical Oxygen Demand from Dairy Wastewater Using Taguchi Orthogonal Array for Design. Int. J. Sci. Adv. Res. Technol. 2017, 3, 23–30. [Google Scholar]
- De Freitas, A.P.B.R.; de Freitas, L.V.; Loures, C.C.A.; dos Santos, M.A.R.; Ricardo, G.D.; Marins, F.A.S.; dos Santos, H.T.L.; Samanamud, G.L.; SouzaAmaral, M.; Silva, M.B. Taguchi Method Applied to Environmental Engineering; IntechOpen: Rijeka, Croatia, 2013; ISBN 978-953-51-1168-9. [Google Scholar]
- Homem, V.; Alves, A.; Santos, L. Amoxicillin Degradation at Ppb Levels by Fenton’s Oxidation Using Design of Experiments. Sci. Total Environ. 2010, 408, 6272–6280. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Tureli, G.; Olmez-Hanci, T. Optimization of the Photo-Fenton-like Process for Real and Synthetic Azo Dye Production Wastewater Treatment Using Response Surface Methodology. Photochem. Photobiol. Sci. 2009, 8, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Santos da Silva, S.; Chiavone-Filho, O.; de Barros Neto, E.L.; Foletto, E.L.; Mota, A.L.N. Effect of Inorganic Salt Mixtures on Phenol Mineralization by Photo-Fenton-Analysis via an Experimental Design. Water Air Soil Pollut. 2013, 225, 1784. [Google Scholar] [CrossRef]
- Marques, L. Avaliação Do Reagente de Fenton e Foto-Fenton Na Remoção de Matéria Orgânica e Toxicidade Em Um Efluente Hospitalar. Master’s Thesis, Federal University of Minas Gerais, Belo Horizonte, Brazil, 2015. [Google Scholar]
- Morshed, M.N.; Pervez, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Nierstrasz, V.A. Statistical Modeling and Optimization of Heterogeneous Fenton-like Removal of Organic Pollutant Using Fibrous Catalysts: A Full Factorial Design. Sci. Rep. 2020, 10, 16133. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Ay, F.; Kargi, F. Effects of Reagent Concentrations on Advanced Oxidation of Amoxicillin by Photo-Fenton Treatment. J. Environ. Eng. 2011, 137, 472–480. [Google Scholar] [CrossRef]
- Lin, C.L. Use of the Taguchi Method and Grey Relational Analysis to Optimize Turning Operations with Multiple Performance Characteristics. Mater. Manuf. Process. 2004, 19, 209–220. [Google Scholar] [CrossRef]
- Das, A.; Majumder, A.; Das, P.K. Detection of Apposite PSO Parameters Using Taguchi Based Grey Relational Analysis: Optimization and Implementation Aspects on Manufacturing Related Problem. Procedia Mater. Sci. 2014, 6, 597–604. [Google Scholar] [CrossRef][Green Version]
- Achuthamenon Sylajakumari, P.; Ramakrishnasamy, R.; Palaniappan, G. Taguchi Grey Relational Analysis for Multi-Response Optimization of Wear in Co-Continuous Composite. Materials 2018, 11, 1743. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Jadhav, V.S.; Shrivastava, R. Review on Single and Multi-Objective Optimization Process Parameters of EDM Using Taguchi Method and Grey Relational Analysis. Mater. Today Proc. 2019, 18, 3856–3866. [Google Scholar] [CrossRef]
- Pai, T.-Y.; Hanaki, K.; Chiou, R.-J. Forecasting Hourly Roadside Particulate Matter in Taipei County of Taiwan Based on First-Order and One-Variable Grey Model. CLEAN–Soil Air Water 2013, 41, 737–742. [Google Scholar] [CrossRef]
- Li, X.; Zheng, W.; Yin, L.; Yin, Z.; Song, L.; Tian, X. Influence of Social-Economic Activities on Air Pollutants in Beijing, China. Open Geosci. 2017, 9, 314–321. [Google Scholar] [CrossRef]
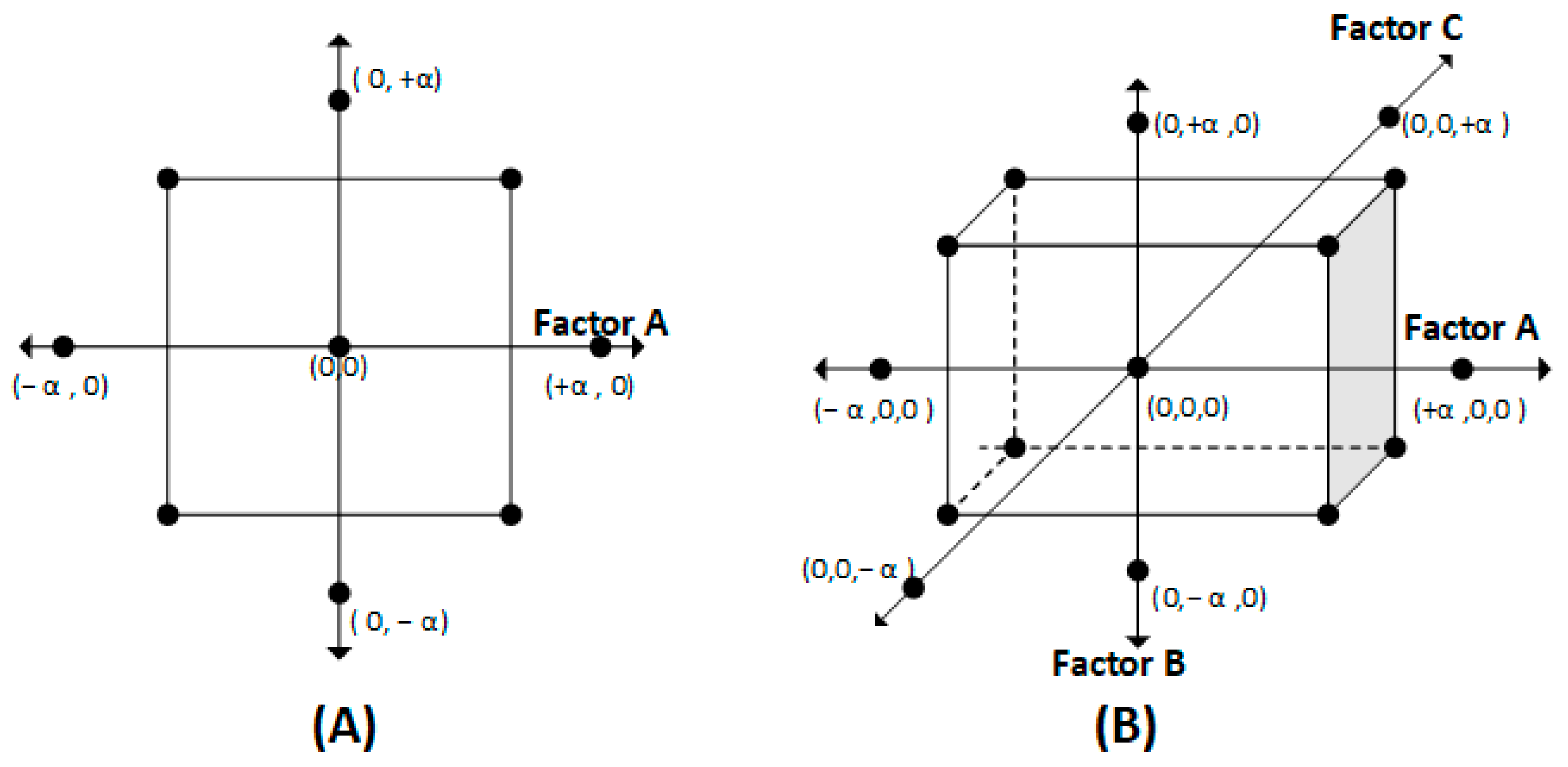
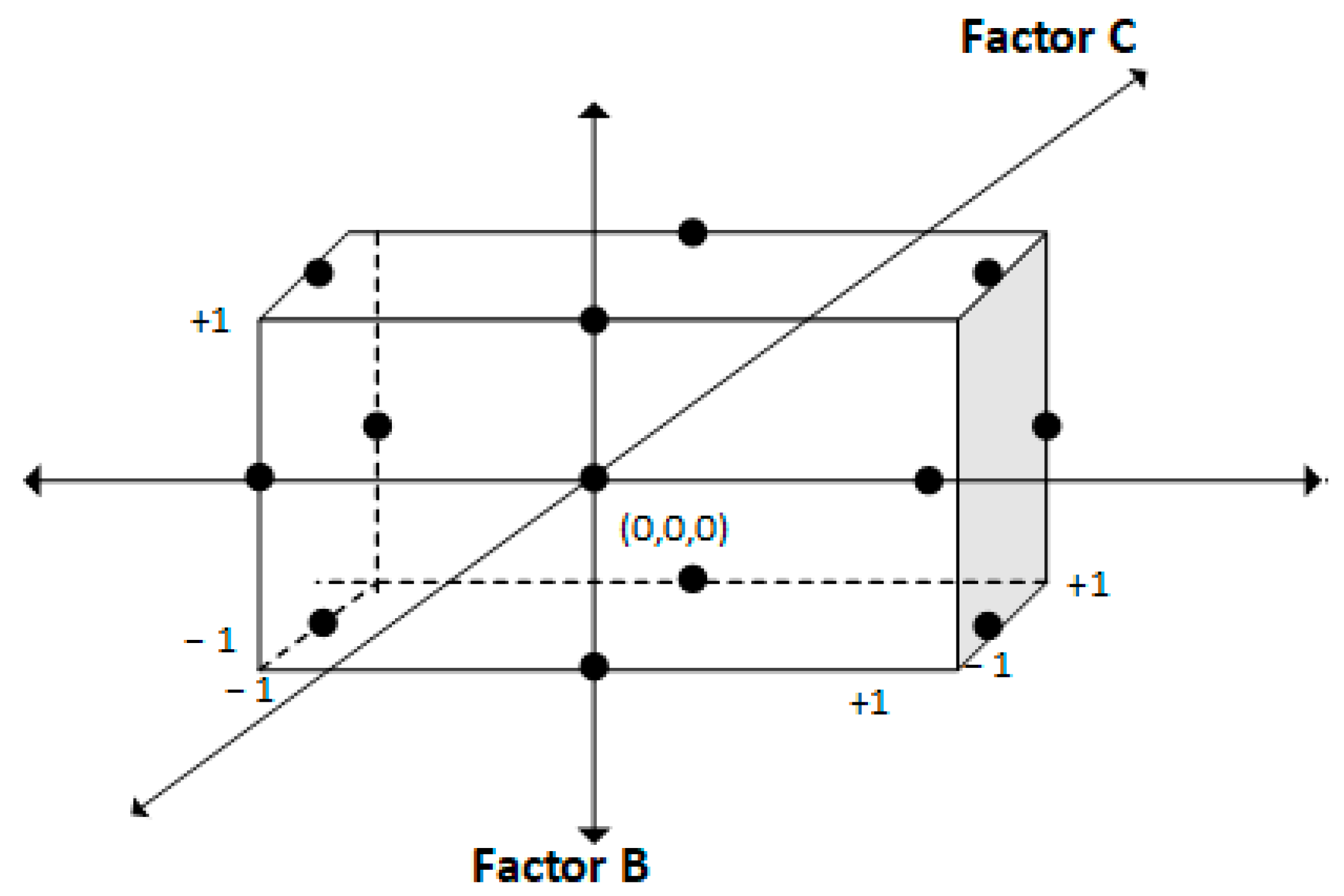
| Redox Couple | Name | Reduction Reaction | E° (V vs. SHE) |
|---|---|---|---|
| F2/F− | Fluorine | F2(g) + 2H+ + 2e− → 2HF | 3.05 |
| ·OH/H2O | Hydroxyl radical | ·OH + H+ + e− → H2O | 2.81 |
| SO4−/SO42− | Sulfate radical | SO4− + e− → SO42− | 2.60 |
| Fe6+/Fe3+ | Ferrate | FeO42− + 8H+ + 3e → Fe3+ + 4H2O | 2.20 |
| O3/O2 | Ozone | O3+2H+ + 2e− → O2 + H2O | 2.08 |
| S2O82−/SO42− | Peroxodisulfate ion | S2O32− + 2e− → 2SO42− | 2.01 |
| H2O2/H2O | Hydrogen peroxide | H2O2 + 2H+ + 2e− → 2H2O | 1.76 |
| Mn7+/Mn4+ (a) | Permanganate | MnO4− + 4H+ + 3e− → MnO2(s) + 2H2O | 1.67 |
| ·O2H/H2O (a) | Hydroperoxyl radical | ·O2H + 3H+ + 3e− → 2H2O | 1.65 |
| Mn7+/Mn2+ (b) | Permanganate | MnO4− + 8H+ + 5e− → Mn2+ + 4H2O | 1.51 |
| ·O2H/H2O2 (b) | Hydroperoxyl radical | ·O2H + H++e− → H2O2 | 1.44 |
| HClO/Cl− | Hypochlorous acid | HClO +H++2e− → Cl− + H2O | 1.49 |
| Cr6+/Cr3+ | Dichromate | Cr2O72− + 14H+ + 6e− → 2Cr3+ + 7H2O | 1.36 |
| Cl2/Cl− | Chlorine | Cl2(g)+2e− → 2Cl− | 1.36 |
| Mn4+/Mn2+ | Manganese dioxide | MnO2 + 4H+ + 2e− → Mn2+ + 2H2O | 1.23 |
| O2/H2O | Oxygen | O2(g) + 4H+ + 4e− → 2H2O | 1.23 |
| Br2/Br− | Bromine | Br2(l)+2e− → 2Br− | 1.06 |
| ClO2/ClO2− | Chlorite | ClO2 + e− → ClO2− | 0.95 |
| Pollutant | AOP | Reactor Volume | Aqueous Matrix | Reaction Conditions | Significant Findings | Reference |
|---|---|---|---|---|---|---|
| Tetracycline | Photo-Fenton | 500 mL | Sewage treatment plant effluent | [TC] = 24 mg/L; 5 min; [Fe(NO3)3] = 0.20 mM; [H2O2] = 3.0 mM. Solar and black light irradiation (15 W, 365 nm). | Total mineralization of total carbon. | [75] |
| Amoxicilin (AMX), bezafibrate (BZF) and paracetamol (PCT) | Photo-Fenton | 800 mL | Sewage treatment plant effluent | [AMX] = 42 mg/L; [PCT] = 15 mg/L; [BZF] = 20 mg/L; [FeOx] = 0.2 mM; [H2O2] = 5.0 mM; Solar and black light irradiation (15 W, 365 nm). | Up to 98% removal of BZF and PCT and 84% removal of AMX using FeOx. | [76] |
| 5-fluorouracil (5-FU) | Photo-Fenton | 100 mL | Ultrapure water | [5-FU] = 30 mg/L; pH = 2.8; [Fe3+] = 0.08 mM; [C2K2O4·H2O] = 0.24 mM; [H2O2] = 2.9 mM or [S2O82−] = 0.42 mM; simulated solar light (SSL) 500 W/m2; 30 min | Of the three processes tested (SSL/Fe3+/H2O2, SSL/Fe3+/S2O82−, SSL/[Fe(C2O4)3]3−/H2O2) the SSL/[Fe(C2O4)3]3−/H2O2 process was the most efficient. | [77] |
| Ibuprofen (IBU), Carbamazepine and ciprofloxacin (CIP) | Photo-Fenton | 700 mL | Real urban wastewater | [Fe3+] = 0.71 mM; [H2O2] = 0.73, 1.47 or 2.94 mM; [IBU] = 59.83 μg/L; [CIP] = 54.60 μg/L; [carbamazepine] = 17.17 μg/L; 150 W (190 nm). | Complete degradation of all drugs after 20 min of reaction. | [78] |
| Cloxacillin | Photo-Fenton | 100 mL | Synthetic pharmaceutical wastewater | [Fe3+] = 90 μM; [H2O2] = 10 mM; 30 W | Total degradation for antibiotic after 240 min | [79] |
| Antipyrine | Photo-Fenton (UV-A LED photocatalytic reactor) | 150 mL | Aqueous solution | [H2O2] = 2.9 mM; [Fe2+] = 0.35 mM; [C2H2O4] = 1.1 mM; 24–26 °C; pH = 2.8. | Complete degradation of antipyrine and 93% mineralization was reached in 2.5 and 60 min. | [80] |
| Diatrizoate | Photo-Fenton | 100 mL | Aqueous solution | [Diatrizoate] = 25 mg/L; [Fe3+] = 0.09 mM; [H2O2] = 0.74 mM; pH 2.8, 90 min and Solar radiation (the best condition). | Total degradation of diatrizoate | [81] |
| Paracetamol | Photo-Fenton | 500 mL | Aqueous solution | [Paracetamol] = 100 mg/L; [H2O2] = 6.5 mM; [Fe2+] = 0.36 mM; pH =3.0; 15W (300–570 nm) room temperature; 60 min. | 100% removal | [82] |
| Salicylic acid, ketoprofen, diclofenac, paracetamol and caffeine | Photo-Fenton | 200 mL | Aqueous solution | [Drug] = 10 mg/L; LEDs power: 1.768 W; [Fe3+] = 0.27 mM; [H2O2] = 0.82 mM; [oxalate] = 0.81 mM; pH = 6.5; 25 min. | >80% removal of all drugs and 30–40% mineralization | [83] |
| Ibuprofen | Electro-Fenton | 400 mL | Synthetic wastewater (SW) | [Ibuprofen] = 400 mg/L; pH = 2.43; 28 min; current density of 23.08 mA/cm; H2O2/Fe2+ molar ratio of 2.69, volume ratio of H2O2/SW of 1.84 mL/L. | 98.29% COD removal | [84] |
| Norfloxacin | Electro-Fenton | 175 mL | Aqueous solution | [Norfloxacin] = 0.25 mM; 25 °C; pH = 3.0; 5 h; Fe3+ = 0.1 mM; I = 60 mA | 97.7% mineralization after 5 h | [85] |
| Enoxacin | Electro-Fenton | 250 mL | Aqueous solution | [Enoxacin] = 50 mg/L; pH = 3.0, 60 min, Fe2+ = 0.2 mM; [Na2SO4] = 50 mM; I = 300 mA; room temperature. | 97% drug removal and 43% mineralization (measured as TOC removal). | [86] |
| Amoxicillin | Electro-Fenton | 500 mL | Aqueous solution | [AMX] = 0.082 mM; 25 °C, pH = 3; I = 600 mA, [Fe2+] = 1 mM; graphite electrode. | 95% degradation and 74% mineralization | [87] |
| Sulfamethoxazole (SMX) | Fenton or Fenton-like | 30 mL | Aqueous solution | [SMX] = 158 μM; 25 °C, pH = 3.0, 50 min, [Fe2+] = 179 μM, [H2O2] = 100 uM; UV (254 nm) or sun light | 97% and 100% removal of SMX for the process with sunlight and UV, respectively. | [88] |
| Trimethoprim (TMP) | Fenton or Fenton-like | 30 mL | Aqueous solution | [TMP] = 138 μM; 25 °C, pH = 3.0, 50 min, [Fe2+] = 179 μM, [H2O2] = 100 uM; UV (254 nm) or sun light | 52% and 79% removal of SMX for the process with sunlight and UV, respectively. | [88] |
| Ciprofloxacin (CIP) | Fenton | 100 mL | Synthetic wastewater | [CIP] = 100 mg/L; [H2O2]:[Fe2+] = 10; stoichiometric H2O2 concentration = 14.2 mM; pH = 3.0; 60 min | 70% and 55% removal of CIP and TOC, respectively | [89] |
| Carbamazepine | Fenton-like | 50 mL | Aqueous solution | [ carbamazepine] = 10 mg/L; [H2O2] = 1.47 mM; [Fe3+] = 0.035 mM; 50 °C, pH = 3.0, 60 min. | 100% removal and 73% mineralization of the drug | [90] |
| Amphetamine | Fenton-like | 300 mL | Wastewater | [amphetamine] = 14–233 mg/L; 20 °C; pH 3.0; 60 min; [Fe2+] = 1 mM; [H2O2] = 1.75 mM | >80% amphetamine removal | [91] |
| Methylparaben (MeP) | Fenton | 300 mL | Ultrapure water | [MeP] = 100 mg/L; [Fe2+] = 0.29 mM; [H2O2] = 1.82 mM; pH 3; 30 °C. | 33.3% TOC removal | [92] |
| Methylparaben (MeP) | Photo-Fenton | 300 mL | Ultrapure water | [MeP] = 100 mg/L; [Fe2+] = 0.07 mM; [H2O2] = 1.53 mM; pH 3; 30 °C; UVC lamp 4 W | 34.9% TOC removal | [92] |
| Methylparaben (MeP) | Fenton | 300 mL | Tap water | [MeP] = 10 mg/L; [Fe2+] = 0.29 mM; [H2O2] = 1.82 mM; pH 3; 30 °C. | 20.6% TOC removal | [92] |
| Methylparaben (MeP) | Photo-Fenton | 300 mL | Tap water | [MeP] = 10 mg/L; [Fe2+] = 0.07 mM; [H2O2] = 1.53 mM; pH 3; 30 °C; UVC lamp 4 W | 23.8% TOC removal | [92] |
| Methylparaben (MeP) | Fenton | Not specified | Ultrapure water | [MeP] = 10 mg/L; [H2O2]: 1.5 mM; [Fe2+]: 0.18 mM; 60 min; pH: 3.6; room temperature. | 82% COD removal | [93] |
| Methylparaben (MeP) | Photo-Fenton | 200 mL | Ultrapure water | [MeP] = 10 mg/L; [H2O2]: 4.57 mM; [Fe2+]: 0.38 mM; 300 min; pH: 3.0; 35 °C; Xenon lamp 350 W/m2. | >60% TOC removal | [94] |
| Sulfamethoxazole (SMX) | Photo-Fenton | 2.0 L | Aqueous solution | [SMX] = 0.079 mM; [H2O2]: 0.294 mM; [Fe3+-EDTA]: 0.089 mM; 75 min; pH: 7.0; 25 °C; UV lamp (8 W, 350–400 nm). | 77.3% of SMX degradation | [95] |
| Bisphenol A (BPA) | Photo-Fenton | 500 mL | Synthetic water | [BPA] = 50 mg/L; [H2O2]: 1.5 mM; [Fe3+]: 4 mM; 90 min; pH: 5; UV-A lamp (8W, 350 nm). | Complete removal of BPA and COD | [96] |
| Phenol | Photo-Fenton | Not specified | Aqueous solution | [Phenol] = 200 mg/L; [H2O2]: 31.8 mM; [Fe3+]: 0.09 mM; 120 min; pH: 3; UV lamp (3 × 15 W, 365 nm). | Removal of 98% of the COD | [97] |
| 2-Nitrophenol, 4-Nitrophenol, 2,4-Dinitrophenol, 2,4,6-Trinitrophenol | Photo-Fenton | 500 mL | Aqueous solution | [Pollutans] = 200 mg/L; [H2O2]: 10.4–17.6 mM; [Fe3+]: 0.36–0.45 mM; 120 min; pH: 3; UV lamp (150 W, 254 nm) or sunlight. | Mineralization of more than 92% of the nitrophenols, using sunlight or UV light. | [98] |
| Organic Contaminant | Applied Process | DOE Used | Evaluated Factors | Reference |
|---|---|---|---|---|
| Amoxicillin and cephalexin | Fenton | Factorial design (22) | [Fe2+]: 1.79–8.95 mM [H2O2]: 2.94–44.12 mM | [147] |
| Amoxicillin and cephalexin | Photo-Fenton | Factorial design (22) | [Fe2+]: 1.79–8.95 mM [H2O2]: 2.94–44.12 mM | [147] |
| Amoxicillin | Photo-Fenton | Factorial design (24) | Amoxicillin: 20–60 mg/L [Fe2+]: 0.09–0.27 mM [H2O2]: 1.47–4.41 mM UV light intensity: 0–96 W | [148] |
| Crystal violet dye | Heterogeneous Fenton-like | Full factorial design | pH: 5–9 [H2O2]: 0.65–3.26 Type of catalysts (PET–NH2–Fe, PET– Si–NH2–Fe, and PET–SH–Fe) | [129] |
| Alprazolam and diazepam | Photo-Fenton | CCD | [Fe2+]: 9 × 10−3–7.2 × 10−2 mM [H2O2]: 8.8 × 10−2–0.88 mM | [149] |
| Sulfamethazine | Photo-Fenton | CCD | [Fe2+]: 0.25–1.22 mM [H2O2]: 5.18–30.12 mM | [150] |
| Acid Blue 193 | Photo-Fenton–like | CCD | Reaction time: 15–60 min [COD]: 0.10–0.30 g/L [H2O2]: 25–65 mM [Fe3+]: 0.5–4.5 mM | [151] |
| Oilfield produced water | Electro-Fenton | CCD | [Fe3+]: 0.1–0.5 mM Reaction time: 22–81 min Current intensity (100–500 mA) | [152] |
| Distillery effluent | Photo-Fenton | CCD | [COD]: 3–39 g/L pH: 2.5–8.5 [H2O2]: 0.09–0.21 mM [Fe2+]: 13.17–65.83 mM | [153] |
| Carbofuran | Photo-Fenton | CCD | [Carbofuran]: 1–100 mg/L H2O2 dosage rate: 7 × 10−3–0.1746 mM/min [Fe3+]: 0.02–0.90 mM | [154] |
| 2,4–Dichlorophenoxyacetic acid (2,4–D) | Photo-Fenton | CCD | Temperature: 25–50 H2O2 to 2,4–D ratio: 7–50 | [143] |
| Rhodamine B | Photo-Fenton | CCD | Reaction time: 3–39 min [Fe2+]: 0.36–1.07 mM H2O2]: 2.2–11.0 mM [Dye]: 50–250 mg/L | [144] |
| Acid Blue 113 | Fenton | CCD | [Dye]: 100–300 mg/L H2O2: Fe2+: 5–25 (w/w) Dye: Fe2+: 10–50 (w/w) pH: 2–9 | [27] |
| Black liquor effluent from the pulp and paper industry | Fenton | CCD | Temperature: 28–60 °C [H2O2]: 29.4–58.8 mM [Fe2+]: 0.36–8.95 mM | [28] |
| Methylene blue dye | Photo-Fenton | BBD | [Fe3+]: 0.36–1.07 mM [H2O2]: 5.9–17.6 mM pH: 2.5–3.5 | [29] |
| Simulated industrial wastewater | Photo-Fenton | BBD | pH: 1.9–7.0 [Fe2+]: 1.0–12 mM [H2O2]: 30–400 mM | [120] |
| Amoxicillin | Photo-Fenton | BBD | [Amoxicilin]: 10–200 mg/L [H2O2]: 0.3–14.7 mM [Fe2+]: 0–0.9 mM | [155] |
| Carmoisine edible dye | Fenton | Taguchi method | pH: 2–6 [Fe2+]: 0.1–0.3 mM [H2O2]: 2–6 mM [Dye]: 10–30 mg/L | [32] |
| Carmoisine edible dye | Photo-Fenton | Taguchi method | pH: 2–6 [Fe2+]: 0.1–0.3 mM [H2O2]: 2–6 mM [Dye]: 10–30 mg/L | [32] |
| Ciprofloxacin | Fenton | Taguchi method | pH: 2–5 [Fe2+]: 5–50 mM [H2O2]: 10–100 mM [Ciprofloxacin]: 10–200 mg/L Reaction time: 10–30 min | [101] |
| Phenol | Electro-Fenton | Taguchi method | Current density: 0–16 mA/cm2 pH: 2–8 [H2O2]: 0–8.8 mM Reaction time: 20–100 min Salinity: 0–4% [Phenol]: 0.25–2.0 g/L | [33] |
| Acid Blue 113 | Fenton | Taguchi method | [Dye]: 100–300 mg/L H2O2:Fe2+: 5–25 (w/w) Dye:Fe2+: 10–50 (w/w) pH: 2–9 | [27] |
| Dairy wastewater | Fenton | Taguchi method | pH: 3–4.5 Reaction time: 50–70 min [H2O2]: 20.6–32.4 mM | [156] |
| Polyester resin effluent | Heterogeneous photocatalysis | Taguchi method | UV: 0–21 W pH: 3–7 [H2O2]: 3.53–5.35 mM [TiO2]: 1.04–2.28 mM | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barragán-Trinidad, M.; Guadarrama-Pérez, O.; Guillén-Garcés, R.A.; Bustos-Terrones, V.; Trevino-Quintanilla, L.G.; Moeller-Chávez, G. The Grey–Taguchi Method, a Statistical Tool to Optimize the Photo-Fenton Process: A Review. Water 2023, 15, 2685. https://doi.org/10.3390/w15152685
Barragán-Trinidad M, Guadarrama-Pérez O, Guillén-Garcés RA, Bustos-Terrones V, Trevino-Quintanilla LG, Moeller-Chávez G. The Grey–Taguchi Method, a Statistical Tool to Optimize the Photo-Fenton Process: A Review. Water. 2023; 15(15):2685. https://doi.org/10.3390/w15152685
Chicago/Turabian StyleBarragán-Trinidad, Martín, Oscar Guadarrama-Pérez, Rosa Angélica Guillén-Garcés, Victoria Bustos-Terrones, Luis Gerardo Trevino-Quintanilla, and Gabriela Moeller-Chávez. 2023. "The Grey–Taguchi Method, a Statistical Tool to Optimize the Photo-Fenton Process: A Review" Water 15, no. 15: 2685. https://doi.org/10.3390/w15152685
APA StyleBarragán-Trinidad, M., Guadarrama-Pérez, O., Guillén-Garcés, R. A., Bustos-Terrones, V., Trevino-Quintanilla, L. G., & Moeller-Chávez, G. (2023). The Grey–Taguchi Method, a Statistical Tool to Optimize the Photo-Fenton Process: A Review. Water, 15(15), 2685. https://doi.org/10.3390/w15152685







