Impact of Elevated Atmospheric CO2 in Spartina maritima Rhizosphere Extracellular Enzymatic Activities
Abstract
:1. Introduction
2. Materials and Methods
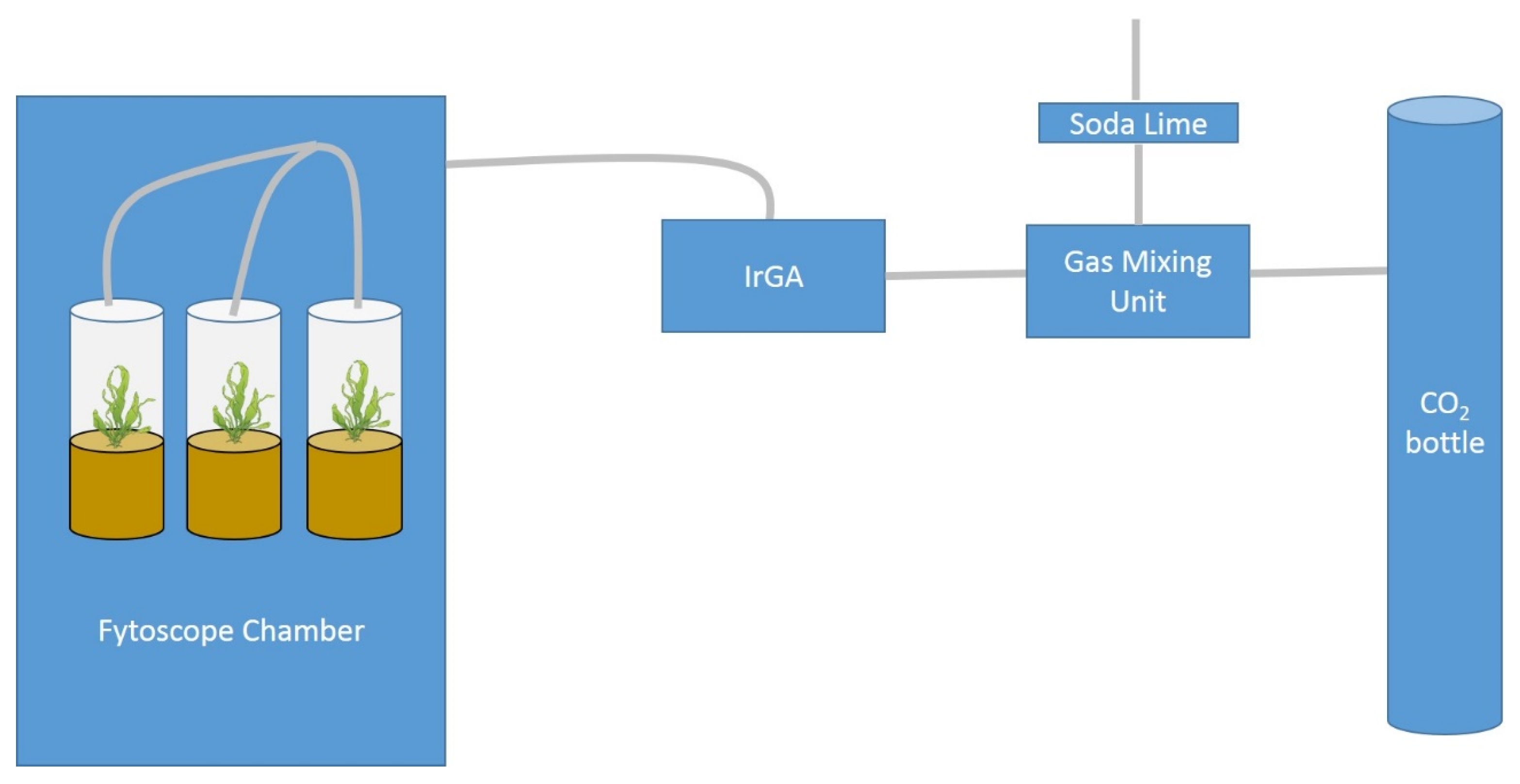
2.1. Study Area, Sampling, and Mesocosms Setup
2.2. Sediment Physicochemical Characterization
2.3. Total Carbon and Nitrogen Content and Stable Isotope Analysis
2.4. Dehydrogenase and Extracellular Enzymatic Activities (EEAs)
2.5. Statistical Analysis
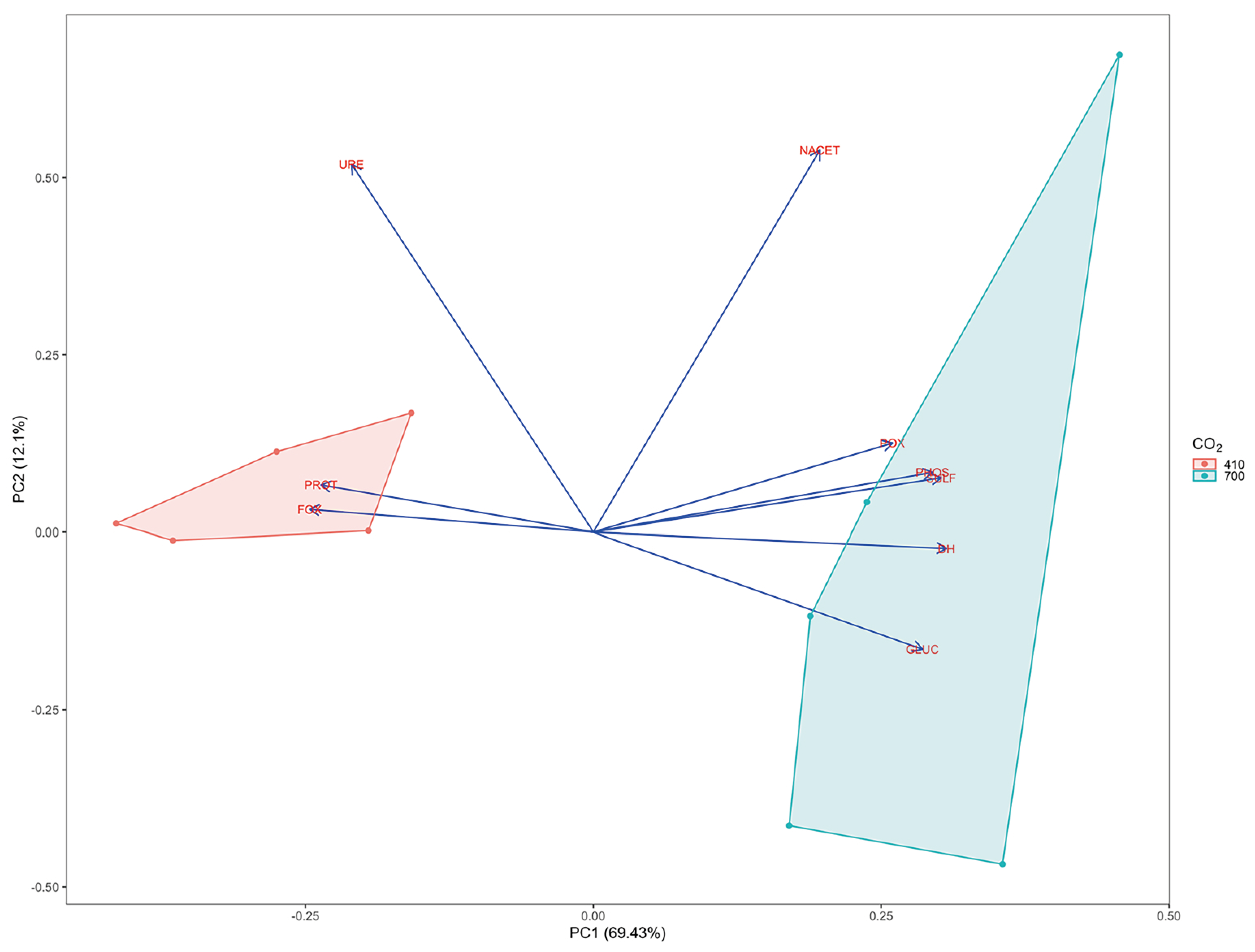
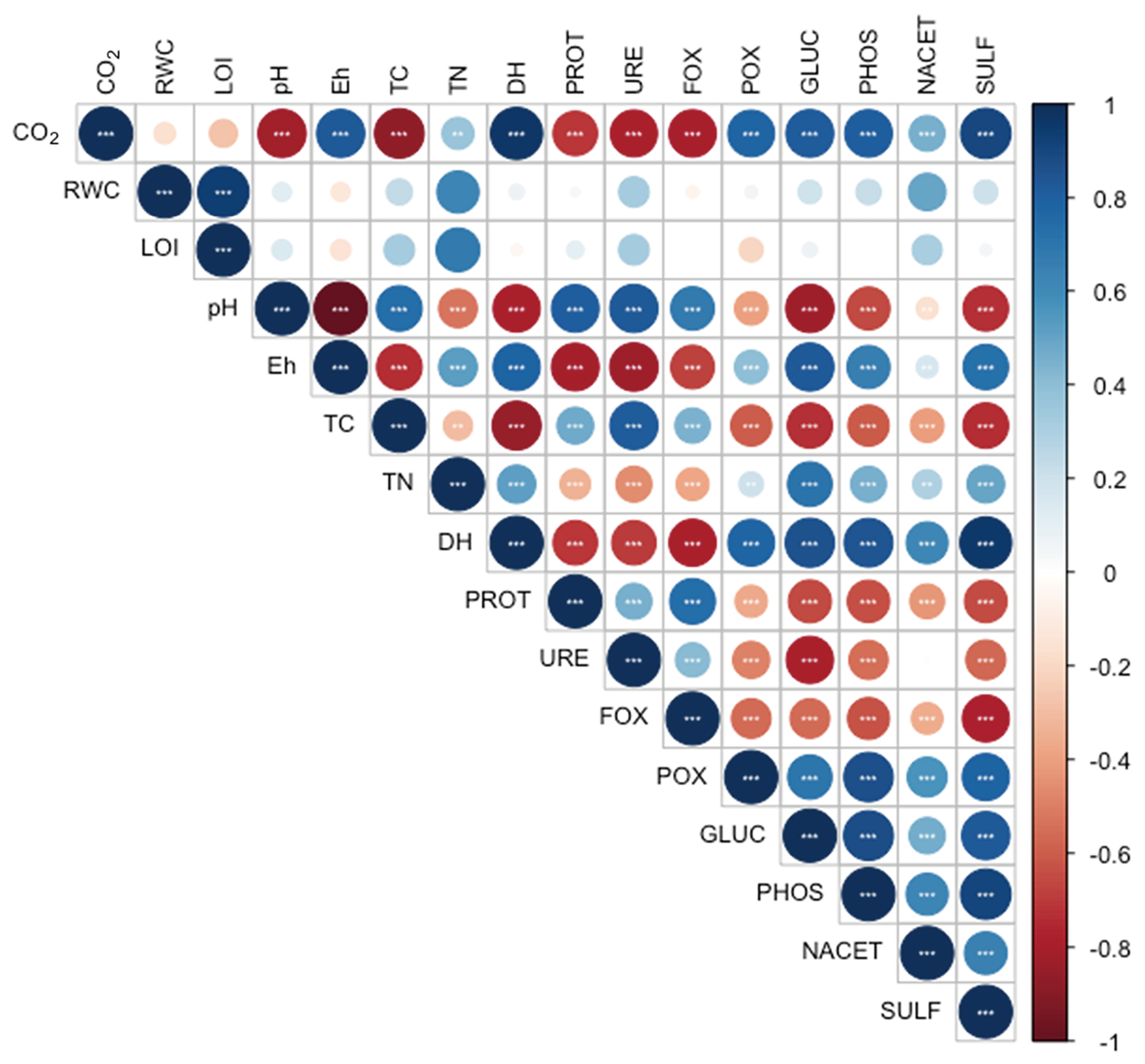
3. Results
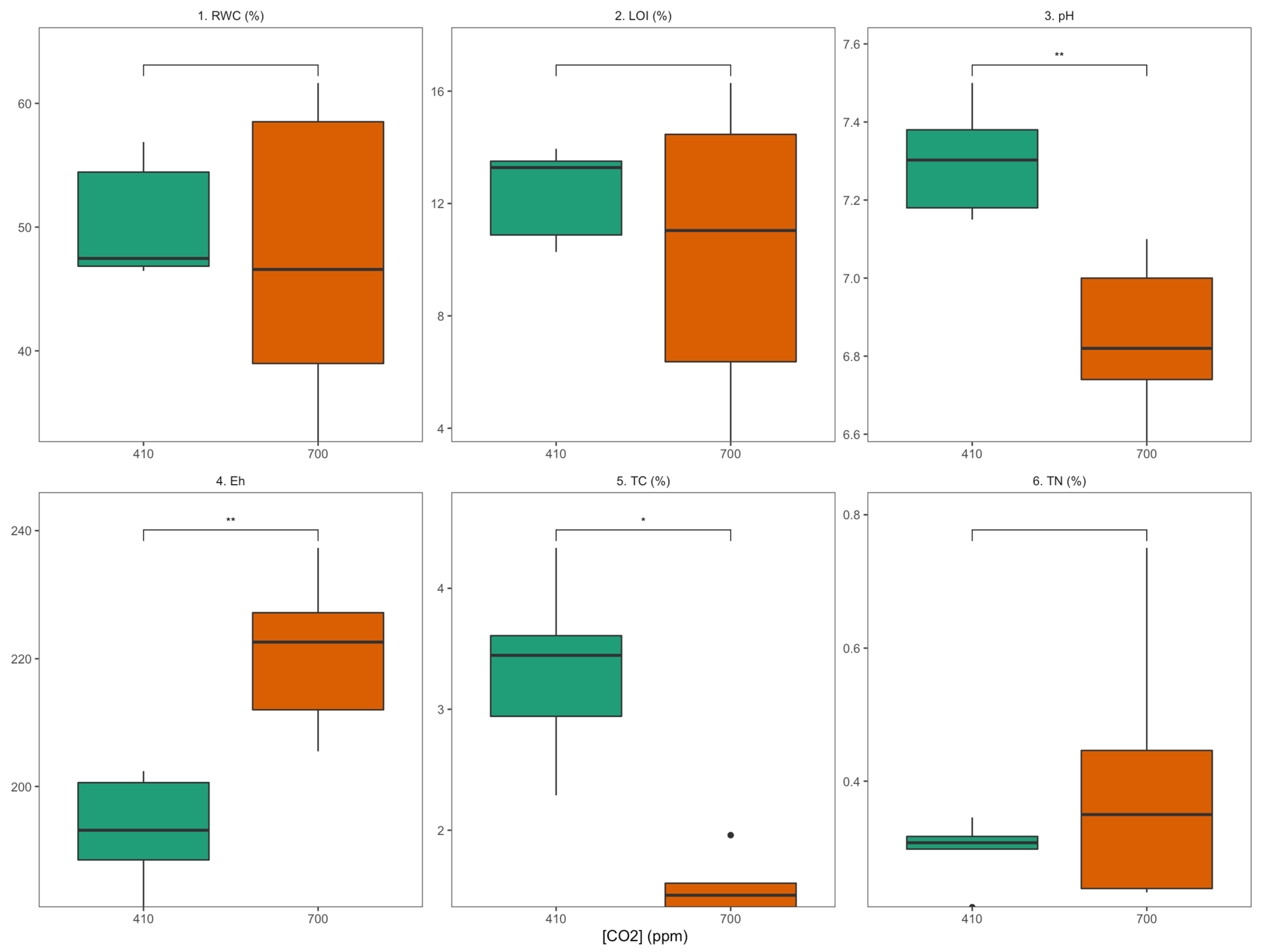
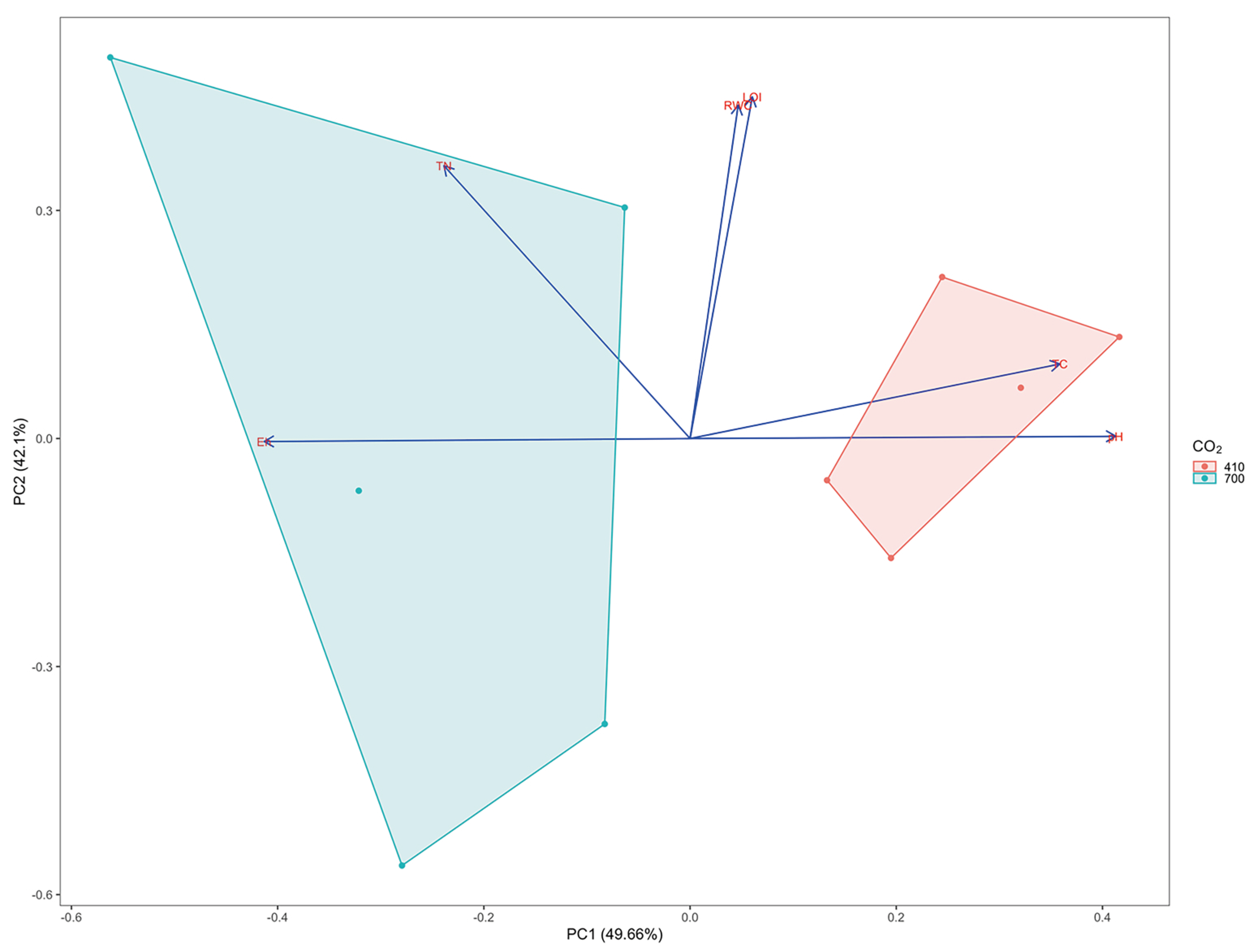
3.1. Physic-Chemical Sediment Characteristics and Stable Isotope Signatures
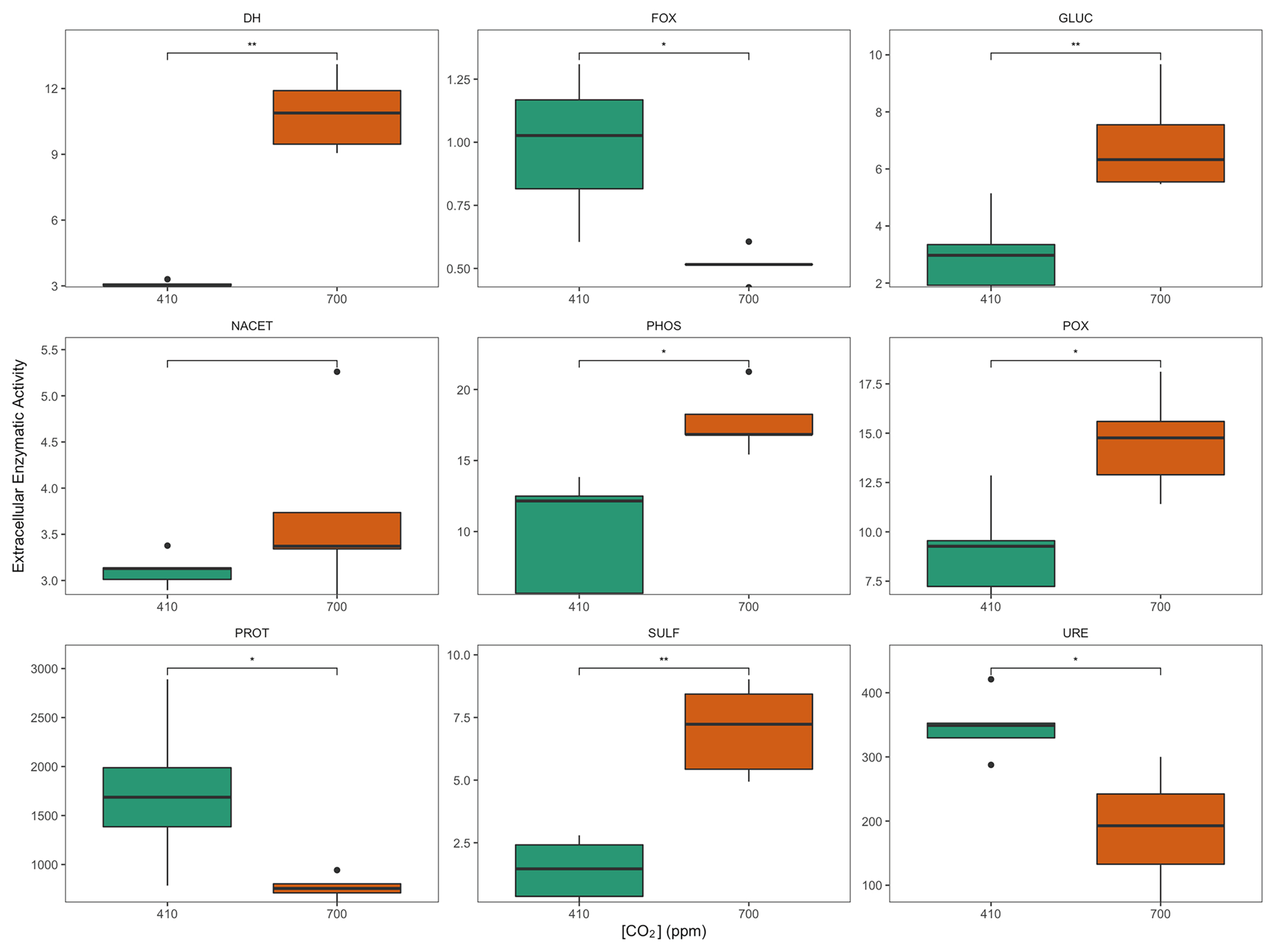
3.2. Microbial Enzymatic Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, H.; Kim, S.-Y.; Fenner, N.; Freeman, C. Shifts of Soil Enzyme Activities in Wetlands Exposed to Elevated CO2. Sci. Total Environ. 2005, 337, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Goldfarb, L.; Gomis, M.I.; Huang, M.; et al. IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Ainsworth, E.A.; Long, S.P. What Have We Learned from 15 Years of Free-Air CO2 Enrichment (FACE)? A Meta-Analytic Review of the Responses of Photosynthesis, Canopy Properties and Plant Production to Rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Baxter, R.; Gantley, M.; Ashenden, T.W.; Farrar, J.F. Effects of Elevated Carbon Dioxide on Three Grass Species from Montane Pasture II. Nutrient Uptake, Allocation and Efficiency of Use. J. Exp. Bot. 1994, 45, 1267–1278. [Google Scholar] [CrossRef]
- Hunt, R.; Hand, D.W.; Hannah, M.A.; Neal, A.M. Response to CO2 Enrichment in 27 Herbaceous Species. Funct. Ecol. 1991, 5, 410–421. [Google Scholar] [CrossRef]
- Kang, H.; Freeman, C.; Ashendon, T.W. Effects of Elevated CO2 on Fen Peat Biogeochemistry. Sci. Total Environ. 2001, 279, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Santos, D.; Silva, H.; Marques, J.C.; Caçador, I. Photochemical and Biophysical Feedbacks of C3 and C4 Mediterranean Halophytes to Atmospheric CO2 Enrichment Confirmed by Their Stable Isotope Signatures. Plant Physiol. Biochem. 2014, 80, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Ineson, P.; Scott, A. Elevated CO2 Reduces the Nitrogen Concentration of Plant Tissues. Glob. Chang. Biol. 1998, 4, 43–54. [Google Scholar] [CrossRef]
- Hirschel, G.; Körner, C.; Arnone, J.A., III. Will Rising Atmospheric CO2 Affect Leaf Litter Quality and in Situ Decomposition Rates in Native Plant Communities? Oecologia 1997, 110, 387–392. [Google Scholar] [CrossRef]
- Jung, S.-H.; Lee, S.-H.; Park, S.-S.; Kang, H.-J. Effects of Elevated CO2 on Organic Matter Decomposition Capacities and Community Structure of Sulfate-Reducing Bacteria in Salt Marsh Sediment. J. Ecol. Environ. 2010, 33, 261–270. [Google Scholar] [CrossRef]
- Caçador, I.; Costa, J.L.; Duarte, B.; Silva, G.; Medeiros, J.P.; Azeda, C.; Castro, N.; Freitas, J.; Pedro, S.; Almeida, P.R.; et al. Macroinvertebrates and Fishes as Biomonitors of Heavy Metal Concentration in the Seixal Bay (Tagus Estuary): Which Species Perform Better? Ecol. Indic. 2012, 19, 184–190. [Google Scholar] [CrossRef]
- Caçador, I.; Caetano, M.; Duarte, B.; Vale, C. Stock and Losses of Trace Metals from Salt Marsh Plants. Mar. Environ. Res. 2009, 67, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, B.; Carreiras, J.; Caçador, I. Climate Change Impacts on Salt Marsh Blue Carbon, Nitrogen and Phosphorous Stocks and Ecosystem Services. Appl. Sci. 2021, 11, 1969. [Google Scholar] [CrossRef]
- Teixeira, A.; Duarte, B.; Caçador, I. Salt Marshes and Biodiversity. In Sabkha Ecosystems: Volume IV: Cash Crop Halophyte and Biodiversity Conservation; Khan, M.A., Böer, B., Öztürk, M., Al Abdessalaam, T.Z., Clüsener-Godt, M., Gul, B., Eds.; Tasks for Vegetation Science; Springer: Dordrecht, The Netherlands, 2014; Volume 47, pp. 283–298. ISBN 978-94-007-7410-0. [Google Scholar]
- Duarte, B.; Freitas, J.; Caçador, I. Sediment Microbial Activities and Physic-Chemistry as Progress Indicators of Salt Marsh Restoration Processes. Ecol. Indic. 2012, 19, 231–239. [Google Scholar] [CrossRef]
- Duarte, B.; Almeida, P.R.; Cacador, I. Spartina Maritima (Cordgrass) Rhizosediment Extracellular Enzymatic Activity and Its Role in Organic Matter Decomposition Processes and Metal Speciation. Mar. Ecol. 2009, 30, 65–73. [Google Scholar] [CrossRef]
- Duarte, B.; Reboreda, R.; Caçador, I. Seasonal Variation of Extracellular Enzymatic Activity (EEA) and Its Influence on Metal Speciation in a Polluted Salt Marsh. Chemosphere 2008, 73, 1056–1063. [Google Scholar] [CrossRef]
- Freitas, J.; Duarte, B.; Caçador, I. Biogeochemical Drivers of Phosphatase Activity in Salt Marsh Sediments. J. Sea Res. 2014, 93, 57–62. [Google Scholar] [CrossRef]
- Duarte, B.; Santos, D.; Marques, J.C.; Caçador, I. Biophysical Probing of Spartina Maritima Photo-System II Changes during Prolonged Tidal Submersion Periods. Plant Physiol. Biochem. 2014, 77, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Duarte, B.; Freitas, J.; Caçador, I. The Role of Organic Acids in Assisted Phytoremediation Processes of Salt Marsh Sediments. Hydrobiologia 2011, 674, 169–177. [Google Scholar] [CrossRef]
- Duarte, B.; Caetano, M.; Almeida, P.R.; Vale, C.; Caçador, I. Accumulation and Biological Cycling of Heavy Metal in Four Salt Marsh Species, from Tagus Estuary (Portugal). Environ. Pollut. 2010, 158, 1661–1668. [Google Scholar] [CrossRef]
- Pereira, P.; Caçador, I.; Vale, C.; Caetano, M.; Costa, A.L. Decomposition of Belowground Litter and Metal Dynamics in Salt Marshes (Tagus Estuary, Portugal). Sci. Total Environ. 2007, 380, 93–101. [Google Scholar] [CrossRef]
- Duarte, B.; Valentim, J.M.; Dias, J.M.; Silva, H.; Marques, J.C.; Caçador, I. Modelling Sea Level Rise (SLR) Impacts on Salt Marsh Detrital Outwelling C and N Exports from an Estuarine Coastal Lagoon to the Ocean (Ria de Aveiro, Portugal). Ecol. Model. 2014, 289, 36–44. [Google Scholar] [CrossRef]
- Tobias, C.R.; Macko, S.A.; Anderson, I.C.; Canuel, E.A.; Harvey, J.W. Tracking the Fate of a High Concentration Groundwater Nitrate Plume through a Fringing Marsh: A Combined Groundwater Tracer and in Situ Isotope Enrichment Study. Limnol. Oceanogr. 2001, 46, 1977–1989. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Thornton, B.; Paterson, E. Incorporation of 13C-Labelled Rice Rhizodeposition Carbon into Soil Microbial Communities under Different Water Status. Soil Biol. Biochem. 2012, 53, 72–77. [Google Scholar] [CrossRef]
- Rauch, M.; Denis, L. Spatio-Temporal Variability in Benthic Mineralization Processes in the Eastern English Channel. Biogeochemistry 2008, 89, 163–180. [Google Scholar] [CrossRef]
- Duarte, B.; Freitas, J.; Couto, T.; Valentim, J.; Dias, J.M.; Silva, H.; Marques, J.C.; Caçador, I. New Multi-Metric Salt Marsh Sediment Microbial Index (SSMI) Application to Salt Marsh Sediments Ecological Status Assessment. Ecol. Indic. 2013, 29, 390–397. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. (Eds.) 7—Enzyme Activities. In Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; pp. 311–373. ISBN 978-0-12-513840-6. [Google Scholar]
- Couto, T.; Duarte, B.; Caçador, I.; Baeta, A.; Marques, J.C. Salt Marsh Plants Carbon Storage in a Temperate Atlantic Estuary Illustrated by a Stable Isotopic Analysis Based Approach. Ecol. Indic. 2013, 32, 305–311. [Google Scholar] [CrossRef]
- Lynch, J.M.; Whipps, J.M. Substrate Flow in the Rhizosphere. Plant Soil 1990, 129, 1–10. [Google Scholar] [CrossRef]
- Duarte, B.; Freitas, J.; Valentim, J.; Medeiros, J.P.; Costa, J.L.; Silva, H.; Dias, J.M.; Costa, M.J.; Marques, J.C.; Caçador, I. Abiotic Control Modelling of Salt Marsh Sediments Respiratory CO2 Fluxes: Application to Increasing Temperature Scenarios. Ecol. Indic. 2014, 46, 110–118. [Google Scholar] [CrossRef]
- Dalenberg, J.W.; Jager, G. Priming Effect of Some Organic Additions to 14C-Labelled Soil. Soil Biol. Biochem. 1989, 21, 443–448. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of Soil Enzyme Activity at Global Scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [Green Version]
- Sinsabaugh, R.L.; Moorhead, D.L. Resource Allocation to Extracellular Enzyme Production: A Model for Nitrogen and Phosphorus Control of Litter Decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Mooney, H.A.; Drake, B.G.; Luxmoore, R.J.; Oechel, W.C.; Pitelka, L.F. Predicting Ecosystem Responses to Elevated CO2 Concentrations: What Has Been Learned from Laboratory Experiments on Plant Physiology and Field Observations? BioScience 1991, 41, 96–104. [Google Scholar] [CrossRef]
- Kelley, A.M.; Fay, P.A.; Polley, H.W.; Gill, R.A.; Jackson, R.B. Atmospheric CO2 and Soil Extracellular Enzyme Activity: A Meta-Analysis and CO2 Gradient Experiment. Ecosphere 2011, 2, art96. [Google Scholar] [CrossRef]
- Paterson, E.; Hall, J.M.; Rattray, E.A.S.; Griffiths, B.S.; Ritz, K.; Killham, K. Effect of Elevated CO2 on Rhizosphere Carbon Flow and Soil Microbial Processes. Glob. Chang. Biol. 1997, 3, 363–377. [Google Scholar] [CrossRef]
- Larson, J.L.; Zak, D.R.; Sinsabaugh, R.L. Extracellular Enzyme Activity Beneath Temperate Trees Growing Under Elevated Carbon Dioxide and Ozone. Soil Sci. Soc. Am. J. 2002, 66, 1848–1856. [Google Scholar] [CrossRef] [Green Version]
- Zak, D.R.; Pregitzer, K.S.; Curtis, P.S.; Holmes, W.E. Atmospheric CO2 and the Composition and Function of Soil Microbial Communities. Ecol. Appl. 2000, 10, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Zak, D.R.; Ringelberg, D.B.; Pregitzer, K.S.; Randlett, D.L.; White, D.C.; Curtis, P.S. Soil Microbial Communities Beneath Populus Grandidentata Grown Under Elevated Atmospheric CO2. Ecol. Appl. 1996, 6, 257–262. [Google Scholar] [CrossRef]
- Caçador, I.; Tibério, S.; Cabral, H.N.N. Species Zonation in Corroios Salt Marsh in the Tagus Estuary (Portugal) and Its Dynamics in the Past Fifty Years. Hydrobiologia 2007, 587, 205–211. [Google Scholar] [CrossRef]
- Sousa, A.I.; Lillebø, A.I.; Risgaard-Petersen, N.; Pardal, M.A.; Caçador, I. Denitrification: An Ecosystem Service Provided by Salt Marshes. Mar. Ecol. Prog. Ser. 2012, 448, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Gómez, S. Bioaccumulation of Heavy Metals in Spartina. Funct. Plant Biol. 2013, 40, 913–921. [Google Scholar] [CrossRef]
- Valiela, I.; Cole, M.L.; Mcclelland, J.; Hauxwell, J.; Cebrian, J.; Joye, S.B. Role of Salt Marshes as Part of Coastal Landscapes. In Concepts and Controversies in Tidal Marsh Ecology; Weinstein, M.P., Kreeger, D.A., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 23–36. ISBN 978-0-306-47534-4. [Google Scholar]
- Caçador, I.; Neto, J.M.; Duarte, B.; Barroso, D.V.; Pinto, M.; Marques, J.C. Development of an Angiosperm Quality Assessment Index (AQuA-Index) for Ecological Quality Evaluation of Portuguese Water Bodies—A Multi-Metric Approach. Ecol. Indic. 2013, 25, 141–148. [Google Scholar] [CrossRef]
- Bortolus, A.; Adam, P.; Adams, J.B.; Ainouche, M.L.; Ayres, D.; Bertness, M.D.; Bouma, T.J.; Bruno, J.F.; Caçador, I.; Carlton, J.T.; et al. Supporting Spartina: Interdisciplinary Perspective Shows Spartina as a Distinct Solid Genus. Ecology 2019, 100, e02863. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Dan, L.; Deng, X.; Feng, J.; Wang, Y.; Peng, J.; Tian, J.; Qi, W.; Liu, Z.; Zheng, X.; et al. Global Monthly Gridded Atmospheric Carbon Dioxide Concentrations under the Historical and Future Scenarios. Sci. Data 2022, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, K.; Zhang, B.; Lye, L.M. Assessment of Microbial Communities and Their Relationship with Enzymatic Activity during Composting. World J. Eng. Technol. 2017, 5, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Aşkın, T.; Kızılkaya, R. Assessing Spatial Variability of Soil Enzyme Activities in Pasture Topsoils Using Geostatistics. Eur. J. Soil Biol. 2006, 42, 230–237. [Google Scholar] [CrossRef]
- Aminot, Y.; Fuster, L.; Pardon, P.; Le Menach, K.; Budzinski, H. Suspended Solids Moderate the Degradation and Sorption of Waste Water-Derived Pharmaceuticals in Estuarine Waters. Sci. Total Environ. 2018, 612, 39–48. [Google Scholar] [CrossRef]
- Ravit, B.; Ehrenfeld, J.G.; Haggblom, M.M. A Comparison of Sediment Microbial Communities Associated with Phragmites Australis and Spartina Alterniflora in Two Brackish Wetlands of New Jersey. Estuaries 2003, 26, 465–474. [Google Scholar] [CrossRef]
- Ladd, J.N.; Brisbane, P.G.; Butler, J.H.A.; Amato, M. Studies on Soil Fumigation—III: Effects on Enzyme Activities, Bacterial Numbers and Extractable Ninhydrin Reactive Compounds. Soil Biol. Biochem. 1976, 8, 255–260. [Google Scholar] [CrossRef]
- Kang, E.J.; Kim, K.Y. Effects of Future Climate Conditions on Photosynthesis and Biochemical Component of Ulva Pertusa (Chlorophyta). Algae 2016, 31, 49–59. [Google Scholar] [CrossRef]
- Allard, V.; Robin, C.; Newton, P.C.D.; Lieffering, M.; Soussana, J.F. Short and Long-Term Effects of Elevated CO2 on Lolium perenne Rhizodeposition and Its Consequences on Soil Organic Matter Turnover and Plant N Yield. Soil Biol. Biochem. 2006, 38, 1178–1187. [Google Scholar] [CrossRef]
- Shackle, V.; Freeman, C.; Reynolds, B. Exogenous Enzyme Supplements to Promote Treatment Efficiency in Constructed Wetlands. Sci. Total Environ. 2006, 361, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Domanski, G.; Kuzyakov, Y.; Siniakina, S.V.; Stahr, K. Carbon Flows in the Rhizosphere of Ryegrass (Lolium perenne). J. Plant Nutr. Soil Sci. 2001, 164, 381–387. [Google Scholar] [CrossRef]
- Hungate, B.A.; Van GROENIGEN, K.-J.; Six, J.; Jastrow, J.D.; Luo, Y.; De GRAAFF, M.-A.; Van KESSEL, C.; Osenberg, C.W. Assessing the Effect of Elevated Carbon Dioxide on Soil Carbon: A Comparison of Four Meta-Analyses. Glob. Chang. Biol. 2009, 15, 2020–2034. [Google Scholar] [CrossRef]
- Bergstrom, D.W.; Monreal, C.M.; Tomlin, A.D.; Miller, J.J. Interpretation of Soil Enzyme Activities in a Comparison of Tillage Practices along a Topographic and Textural Gradient. Can. J. Soil. Sci. 2000, 80, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Moorhead, D.L.; Linkins, A.E. Elevated CO2 Alters Belowground Exoenzyme Activities in Tussock Tundra. Plant Soil 1997, 189, 321–329. [Google Scholar] [CrossRef]
- Dhillion, S.S.; Roy, J.; Abrams, M. Assessing the Impact of Elevated CO2 on Soil Microbial Activity in a Mediterranean Model Ecosystem. Plant Soil 1996, 187, 333–342. [Google Scholar] [CrossRef]
- Ebersberger, D.; Niklaus, P.A.; Kandeler, E. Long Term CO2 Enrichment Stimulates N-Mineralisation and Enzyme Activities in Calcareous Grassland. Soil Biol. Biochem. 2003, 35, 965–972. [Google Scholar] [CrossRef]
- Barrett, D.J.; Richardson, A.E.; Gifford, R.M. Elevated Atmospheric CO2 Concentrations Increase Wheat Root Phosphatase Activity When Growth Is Limited by Phosphorus. Funct. Plant Biol. 1998, 25, 87–94. [Google Scholar] [CrossRef]
- Freeman, C.; Baxter, R.; Farrar, J.F.; Jones, S.E.; Plum, S.; Ashendon, T.W.; Stirling, C. Could Competition between Plants and Microbes Regulate Plant Nutrition and Atmospheric CO2 Concentrations? Sci. Total Environ. 1998, 220, 181–184. [Google Scholar] [CrossRef]
- Kampichler, C.; Kandeler, E.; Bardgett, R.D.; Jones, T.H.; Thompson, L.J. Impact of Elevated Atmospheric CO2 Concentration on Soil Microbial Biomass and Activity in a Complex, Weedy Field Model Ecosystem. Glob. Chang. Biol. 1998, 4, 335–346. [Google Scholar] [CrossRef]
- Langley, J.A.; Megonigal, J.P. Ecosystem Response to Elevated CO2 Levels Limited by Nitrogen-Induced Plant Species Shift. Nature 2010, 466, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Naranjo, E.; Redondo-Gómez, S.; Andrades-Moreno, L.; Davy, A.J. Growth and Photosynthetic Responses of the Cordgrass Spartina Maritima to CO2 Enrichment and Salinity. Chemosphere 2010, 81, 725–731. [Google Scholar] [CrossRef]
- Dai, T.; Wiegert, R.G. A Field Study of Photosynthetic Capacity and Its Response to Nitrogen Fertilization In Spartina Alterniflora. Estuar. Coast. Shelf Sci. 1997, 45, 273–283. [Google Scholar] [CrossRef]
- Bradley, P.M.; Morris, J.T. Effect of Salinity on the Critical Nitrogen Concentration of Spartina Alterniflora Loisel. Aquat. Bot. 1992, 43, 149–161. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, B.; Baeta, A.; Marques, J.C.; Caçador, I. Impact of Elevated Atmospheric CO2 in Spartina maritima Rhizosphere Extracellular Enzymatic Activities. Water 2023, 15, 2667. https://doi.org/10.3390/w15142667
Duarte B, Baeta A, Marques JC, Caçador I. Impact of Elevated Atmospheric CO2 in Spartina maritima Rhizosphere Extracellular Enzymatic Activities. Water. 2023; 15(14):2667. https://doi.org/10.3390/w15142667
Chicago/Turabian StyleDuarte, Bernardo, Alexandra Baeta, João Carlos Marques, and Isabel Caçador. 2023. "Impact of Elevated Atmospheric CO2 in Spartina maritima Rhizosphere Extracellular Enzymatic Activities" Water 15, no. 14: 2667. https://doi.org/10.3390/w15142667






