Water Holding Capacity of Some Bryophyta Species from Tundra and North Taiga of the West Siberia
Abstract
:1. Introduction
2. Objects, Methods and Study Sites
2.1. Bryophyta and Their Functional Traits
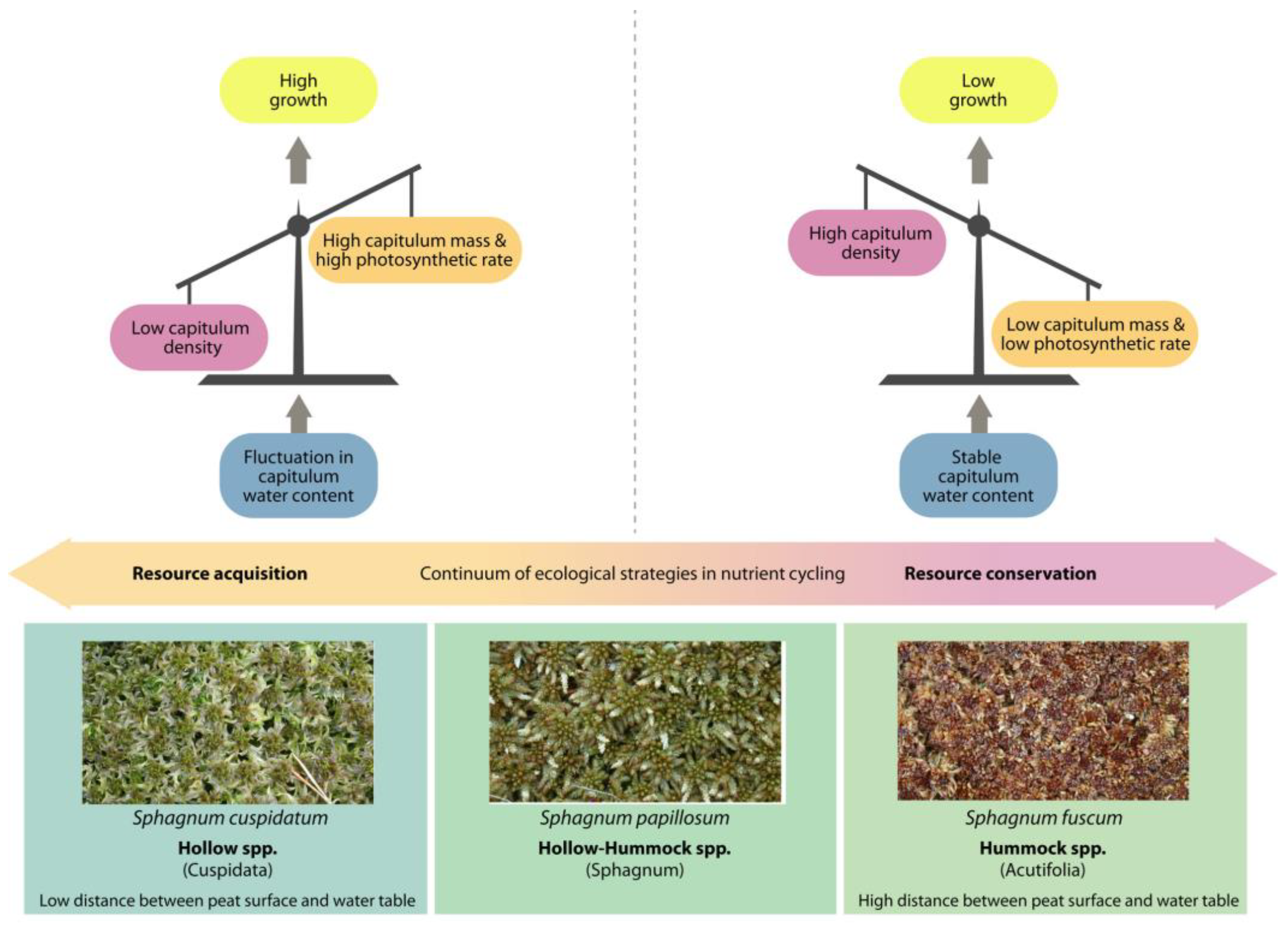
2.2. Water Holding Capacity Supporting Traits on the Example of Sphagnum
- Leaf anatomy: hyaline cells amount and size, amount and size of pores;
- Stem anatomy (hyalodermis);
- Shoot morphology: overall height, branch and leaf density, fuzz on stem and branches, leaf size and shape;
- Colony structure: loose or dense, proximity of growing;
- Confinement to a certain element of the microtopographical element: hummock, loan or hollow at the mire, raised or lowered elements of the tundra microrelief, etc.
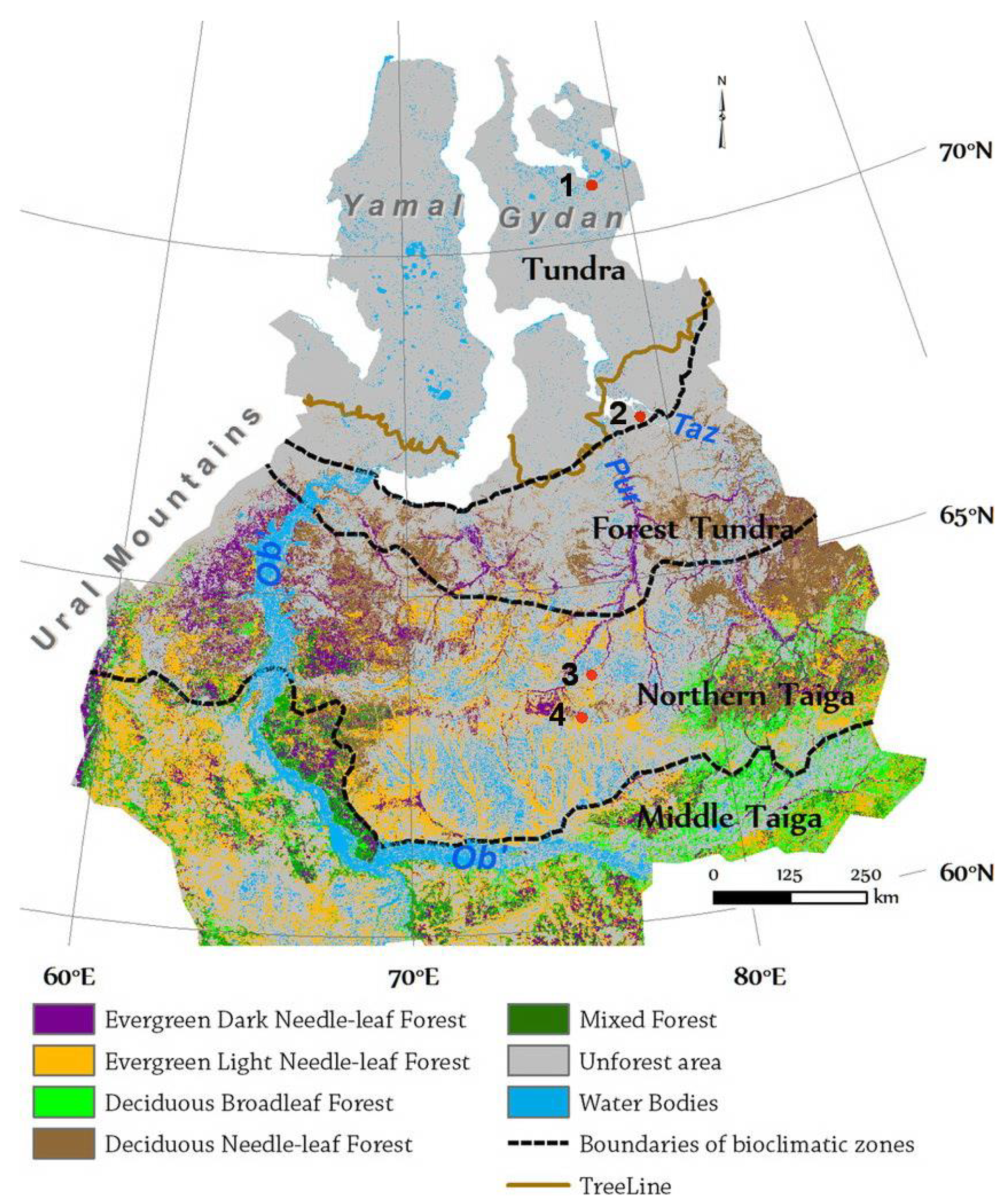
2.3. Study Area
- Gydan Peninsula: dryas tundra flat uplands—herb-dwarf shrub-moss tundra plant community;
- Taz Peninsula south (shrubby) tundra: mossy frozen mounds and hollows in palsa mire, palsa mound slopes, high terrace of Taz River—shrub-dwarf shrub-moss tundra plant community;
- Khanymey Field Research Station: bogs, palsa mire, tall frozen mounds, coniferous forest—shrub-moss-lichen communities;
- Vicinities of Noyabrsk: small mounds in palsa mire—herb-dwarf shrub-moss plant community.
2.3.1. Northern Taiga Bioclimatic Subzone (Khanymey, Noyabrsk)
2.3.2. Tundra Bioclimatic Subzone (Taz, Gyda)
2.4. WHC Data Collection and Analysis
3. Results and Discussion
3.1. The Comparison of the Water Holding Capacity between the Species
3.2. The Comparison of Average Water Holding Capacity Inside the Species
- Overall, the taiga samples showed a higher water holding capacity both with and without external water, sometimes reaching a number almost twice the measurement of the sample taken from the tundra area. This does not come as a surprise—moss colonies tend to hold more water in moister habitats, and the taiga is moister than the tundra. The other reason is that shoots grow higher and bigger in moister habitats and, the size of the shoot is a trait that supports WHC.
- Sphagnum fuscum showed a striking consistency between both WHC measurements of all samples. This species is expected to vary in height, but the researched samples did not demonstrate any variety.
- The difference in WHCe tends to manifest in numbers equal or proportional to WHC.
3.3. The Standard Deviation
4. Conclusions
- On average, the northern taiga samples showed slightly higher WHC than tundra samples, probably due to environmental specifics of the habitat—taiga habitats were moister, while the tundra was drier.
- In regard to comparisons inside the species, overall, the northern taiga samples showed higher water holding capacity both with and without water, sometimes reaching a number almost twice the measurement of the sample taken from the tundra area. The difference in WHCe tends to manifest in numbers equal or proportional to WHC.
- The variability of WHCe within moss samples is significantly higher than WHC in the majority of species, with the standard deviation of WHCe rising up to 18.27, while the WHC standard deviation is 5.06 at maximum. Such high variability in WHCe may be explained by the morphological features of each individual considerably shifting between the samples of the same species while the anatomical features retain more stable results. The worldwide level of WHC is lower, but the final comparison remains unsure.
- The highest WHCe and WHC were demonstrated by Sphagnum magellanicum, while the lowest WHCe and WHC were split between Sphagnum lenense and Dicranum elongatum. Sphagnum fuscum showed a striking consistency between both WHC measurements of all samples. This can be explained by shoot morphology: topping samples species tend to grow higher and form dense turf, while the leaf anatomy supports the WHC with curved leaf forms and big amounts of hyaline cells and many pores. The samples that showed those with lesser WHC tend to be species with lesser growth size, plainer leaf forms and lesser pores in concave and convex cells.
- Based on the ratio of external and internal water, two groups of mosses can be distinguished: 1—mosses in which the amount of internal water is equal to or greater than external water (Sphagnum magellanicum and Sphagnum warnstorfii), and 2—mosses in which the amount of internal water is less than external. Mosses of the first group are able to hold the water better and theoretically will gain a certain stability and competitive advantage in the conditions of habitats’ wetness reduction under climate change. At the same time, this advantage is likely to be less applicable in the tundra, where the thawing of permafrost at first is likely to compensate for the increased evaporation and the aridization of the climate. On the contrary, in the conditions of the taiga zone, where permafrost is absent or sporadic and lies more deeply, a change in the water balance can give a sharp advantage to these species. This is especially important in relation to raised bogs, which occupy a larger area of Western Siberia and in which Sphagnum magellanicum is one of the main edificators and engineering species. Therefore, the ability of this species to hold internal water may play a key role in the stability of the peatlands as one of the main carbon sinks in the context of global climate change.
- The collected WHC measurements of Bryophyta may serve as an environmental change indication tool and contribute to worldwide WHC research. Our results confirm that the water holding capacity, being the key bryophyte functional trait, shows the potential for the identification and clarification of bryophyte functional groups (as was demonstrated by Lett et al. [27]), and the quantitative data on the WHC in areas with a high abundance of mosses can be used to assess the ecosystem water economy [29] and to predict the ecosystem dynamics in the tundra and north taiga under various climate change scenarios.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reich, P.B. The world-wide “fast-slow” plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Proctor, M.C.; Tuba, Z. Poikilohydry and homoihydry: Antithesis or spectrum of possibilities? New Phytol. 2002, 156, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.; Fenner, N.; Ostle, N.J.; Kang, H.; Dowrick, D.J.; Reynolds, B.; Hudson, J. Export of dissolved organic carbon from peatlands under elevated carbon dioxide levels. Nature 2004, 430, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Glime, J.M. Physiological ecology. In Bryophyte Ecology; Glime, J.M., Ed.; Springer Science & Business Media: Berlin, Germany, 2017; Volume 1, Available online: http://digitalcommons.mtu.edu/bryophyte-ecology1/ (accessed on 25 March 2020).
- Wang, Z.; Bader, M.Y. Associations between shoot-level water relations and photosynthetic responses to water and light in 12 moss species. AoB Plants 2018, 10, ply034. [Google Scholar] [CrossRef] [Green Version]
- He, X.; He, K.S.; Hyvönen, J. Will bryophytes survive in a warming world? Perspect. Plant. Ecol. Evol. Syst. 2016, 19, 49–60. [Google Scholar] [CrossRef]
- Oishi, Y. Urban heat island effects on moss gardens in Kyoto, Japan. Lands Ecol. Eng. 2019, 15, 177–184. [Google Scholar] [CrossRef]
- Elumeeva, T.G.; Soudzilovskaia, N.A.; During, H.J.; Cornelissen, J.H. The importance of colony structure versus shoot morphology for the water balance of 22 subarctic bryophyte species. J. Veg. Sci. 2011, 22, 152–164. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Zagt, R.; Van Leerdam, A.; Van Ek, R.; Broekhoven, A.; Van Genderen, M. Hydrological properties of the epiphyte mass of a montane tropical rain forest, Colombia. Vegetatio 1990, 89, 183–192. [Google Scholar] [CrossRef]
- Laing, C.G.; Granath, G.; Belyea, L.R.; Allton, K.E.; Rydin, H. Tradeoffs and scaling of functional traits in Sphagnum as drivers of carbon cycling in peatlands. Oikos 2014, 123, 817–828. [Google Scholar] [CrossRef]
- Hayward, P.M.; Clymo, R.S. Profiles of water content and pore size in Sphagnum and peat, and their relation to peat bog ecology. J. Ecol. 1982, 71, 845–863. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.K.; Waddington, J.M. Sphagnum under pressure: Towards an ecohydrological approach to examining Sphagnum productivity. Ecohydrology 2008, 1, 299–308. [Google Scholar] [CrossRef]
- Waddington, J.M.; Morris, P.J.; Kettridge, N.; Granath, G.; Thompson, D.K.; Moore, P.A. Hydrological feedbacks in northern peatlands. Ecohydrology 2015, 8, 113–127. [Google Scholar] [CrossRef]
- Schipperges, B.; Rydin, H. Response of photosynthesis of Sphagnum species from contrasting microhabitats to tissue water content and repeated desiccation. New Phytol. 1998, 140, 677–684. [Google Scholar] [CrossRef]
- Rydin, H. Mechanisms of interactions among Sphagnum species along water level gradients. Adv. Bryol. 1993, 5, 153–185. [Google Scholar]
- Hájek, T. Physiological ecology of peatland bryophytes. In Photosynthesis in Early Land Plants; Hanson, D.T., Rice, S.K., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 233–252. [Google Scholar] [CrossRef]
- Marschall, M.; Proctor, M.C.F. Are bryophytes shade plants? Photosynthetic light responses and proportions of chlorophyll a, chlorophyll b and total carotenoids. Ann. Bot. 2004, 94, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Glime, M.; Liao, C. Responses of two interacting Sphagnum species to water level. J. Bryol. 1992, 17, 59–70. [Google Scholar] [CrossRef]
- Hajek, T.; Vicherova, E. Desiccation tolerance of Sphagnum revisited: A puzzle resolved. Plant Biol. 2013, 16, 765–773. [Google Scholar] [CrossRef]
- Såstad, S.M.; Flatberg, K.I. Leaf morphology of Sphagnum strictum in Norway, related to habitat characteristics. Lindbergia 1993, 18, 71–77. [Google Scholar]
- Malcolm, J.E. Relationships between Sphagnum Morphology and Absorbency of Commercial Sphagnum Board. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 1996. [Google Scholar] [CrossRef]
- Lewis, A.M. A test of the air-seeding hypothesis using Sphagnum hyalocysts. Plant Physiol. 1988, 87, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Miles, V.V.; Esau, I. Spatial heterogeneity of greening and browning between and within bioclimatic zones in northern WestSiberia. Environ. Res. Lett. 2016, 11, 115002. [Google Scholar] [CrossRef]
- Pomus, M.I. Western Siberia; Academy of Sciences Publishing house of USSR: Moscow, Russia, 1963; 488p. [Google Scholar]
- Wilmking, M. Coincidence and Contradiction in the Warming Boreal Forest. Geophys. Res. Lett. 2009, 32, L15715. [Google Scholar] [CrossRef]
- Hassel, K.; Kyrkjeeide, M.; Yousefi, N.; Prestø, T.; Stenøien, H.; Shaw, J.; Flatberg, K. Sphagnum divinum (sp. nov.) and S. medium Limpr. and their relationship to S. magellanicum Brid. J. Bryol. 2018, 40, 191–222. [Google Scholar] [CrossRef]
- Lett, S.; Jónsdóttir, I.S.; Becker-Scarpitta, A.; Christiansen, C.T.; During, H.; Ekelund, F.; Zuijlen, K.V. Can bryophyte groups increase functional resolution in tundra ecosystems? Arct. Sci. 2021, 8, 609–637. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistics notes: Measurement error. BMJ 1996, 312, 1654. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.C.M.; Gabriel, R.; Ah-Peng, C. Characterizing and quantifying water content in 14 species of bryophytes present in Azorean native vegetation. Diversity 2023, 15, 295. [Google Scholar] [CrossRef]



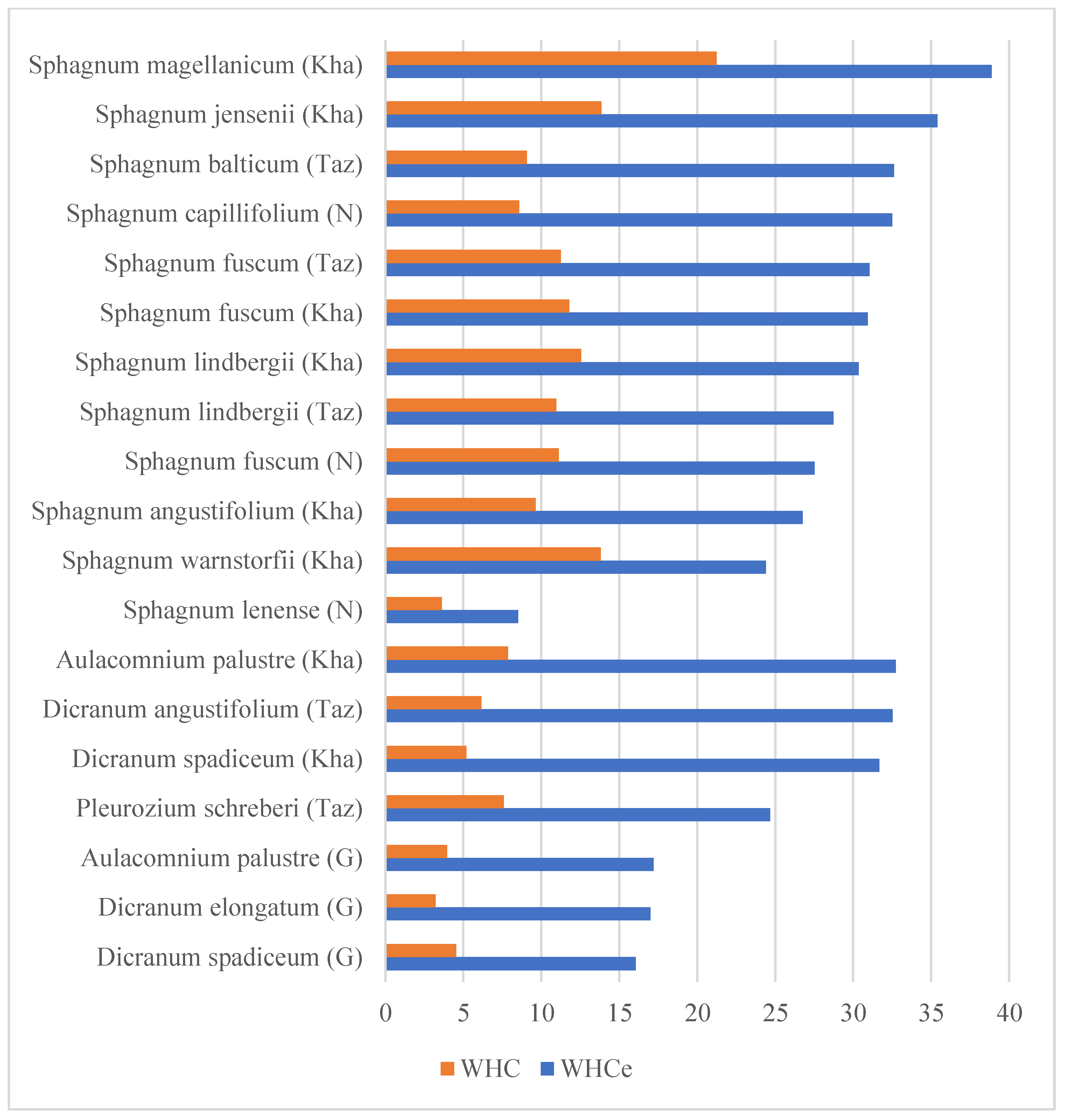
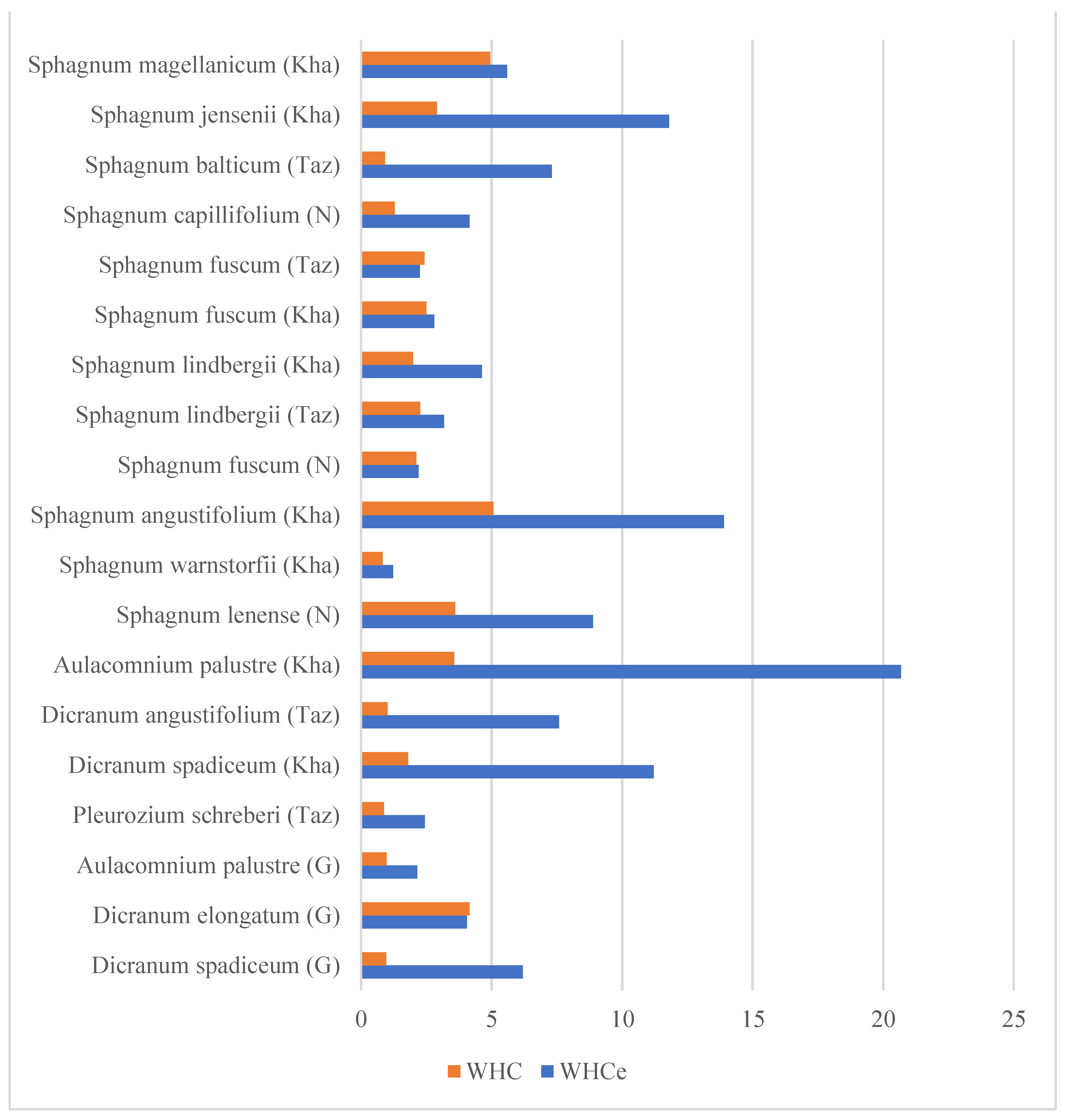
| Species | Average WHCe | Average WHC | WHCe Std. Dev. | WHC Std. Dev. | Origin | Habitat | |
|---|---|---|---|---|---|---|---|
| 1 | Aulacomnium palustre (Hedw.) Schwägr. | 32.72 | 7.87 | 20.68 | 3.56 | Kha | Wooded bog |
| 2 | Aulacomnium palustre (Hedw.) Schwägr. | 17.19 | 3.95 | 2.15 | 0.97 | G | Herb-dwarf shrub-moss tundra |
| 3 | Dicranum angustifolium Kindb. | 32.52 | 6.15 | 7.58 | 1 | Taz | Dryas tundra |
| 4 | Dicranum spadiceum J.E.Zetterst. | 16.06 | 4.54 | 6.19 | 0.96 | G | Herb-dwarf shrub-moss tundra |
| 5 | Dicranum spadiceum J.E.Zetterst. | 31.67 | 5.19 | 11.21 | 1.79 | Kha | Wooded bog |
| 6 | Dicranum elongatum Schleich. ex Schwägr. | 17 | 3.21 | 4.04 | 4.14 | G | Dryas tundra |
| 7 | Pleurozium schreberi (Brid.) Mitt. | 24.67 | 7.6 | 2.43 | 0.87 | Taz | Shrub-dwarf shrub-moss tundra |
| 8 | Sphagnum angustifolium (C.E.O.Jensen ex Russow) C.E.O.Jensen | 26.75 | 9.63 | 13.9 | 5.06 | Kha | Wooded bog |
| 9 | Sphagnum balticum (Russow) C.E.O.Jensen | 32.62 | 9.07 | 7.3 | 0.91 | Taz | Shrub-dwarf shrub-moss tundra |
| 10 | Sphagnum capillifolium (Ehrh.) Hedw. | 32.51 | 8.57 | 4.15 | 1.28 | N | Dwarf shrub-moss-lichen community |
| 11 | Sphagnum fuscum (Schimp.) H.Klinggr. | 30.94 | 11.78 | 2.8 | 2.5 | Kha | Bog near its border |
| 12 | Sphagnum fuscum (Schimp.) H.Klinggr. | 27.53 | 11.11 | 2.19 | 2.11 | N | Dwarf shrub-moss-lichen community |
| 13 | Sphagnum fuscum (Schimp.) H.Klinggr. | 31.05 | 11.24 | 2.24 | 2.42 | Taz | Shrub-dwarf shrub-moss tundra |
| 14 | Sphagnum jensenii H. Lindb. | 35.41 | 13.85 | 11.79 | 2.9 | Kha | Pine forest, dwarf shrub moss-lichen community |
| 15 | Sphagnum lenense H.Lindb. ex L.I.Savicz | 8.51 | 3.62 | 8.88 | 3.59 | N | Dwarf shrub-moss-lichen community |
| 16 | Sphagnum lindbergii Schimp. | 30.34 | 12.55 | 4.62 | 1.98 | Kha | Wooded bog |
| 17 | Sphagnum lindbergii Schimp. | 28.73 | 10.97 | 3.17 | 2.26 | Taz | Shrub-dwarf shrub-moss tundra |
| 18 | Sphagnum magellanicum Brid. s. l. * | 38.87 | 21.24 | 5.59 | 4.94 | Kha | Palsa mire, mossy frozen mound |
| 19 | Sphagnum warnstorfii Russow | 24.41 | 13.82 | 1.22 | 0.82 | Kha | Palsa mire, mossy frozen mound |
| Origin | Habitat | Average WHCe | Average WHC | |
|---|---|---|---|---|
| 1 | Khanymey | Wooded bog | 32.72 | 7.87 |
| 2 | Gyda | Herb-dwarf shrub-moss tundra | 17.19 | 3.95 |
| Origin | Habitat | Average WHCe | Average WHC | |
|---|---|---|---|---|
| 1 | Khanymey | Wooded bog | 31.67 | 5.19 |
| 2 | Gyda | Herb-dwarf shrub-moss tundra | 16.06 | 4.54 |
| Origin | Habitat | Average WHCe | Average WHC | |
|---|---|---|---|---|
| 1 | Taz | Shrub-dwarf shrub-moss tundra | 31.05 | 11.24 |
| 2 | Khanymey | Bog near its border | 30.94 | 11.78 |
| 3 | Noyabrsk | Dwarf shrub-moss-lichen community | 27.53 | 11.11 |
| Origin | Habitat | Average WHCe | Average WHC | |
|---|---|---|---|---|
| 1 | Khanymey | Wooded bog | 30.34 | 12.55 |
| 2 | Taz | Shrub-dwarf shrub-moss tundra | 28.73 | 10.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkova, I.I.; Volkov, I.V.; Morozova, Y.A.; Nikitkin, V.A.; Vishnyakova, E.K.; Mironycheva-Tokareva, N.P. Water Holding Capacity of Some Bryophyta Species from Tundra and North Taiga of the West Siberia. Water 2023, 15, 2626. https://doi.org/10.3390/w15142626
Volkova II, Volkov IV, Morozova YA, Nikitkin VA, Vishnyakova EK, Mironycheva-Tokareva NP. Water Holding Capacity of Some Bryophyta Species from Tundra and North Taiga of the West Siberia. Water. 2023; 15(14):2626. https://doi.org/10.3390/w15142626
Chicago/Turabian StyleVolkova, Irina I., Igor V. Volkov, Yana A. Morozova, Viktor A. Nikitkin, Evgenia K. Vishnyakova, and Nina P. Mironycheva-Tokareva. 2023. "Water Holding Capacity of Some Bryophyta Species from Tundra and North Taiga of the West Siberia" Water 15, no. 14: 2626. https://doi.org/10.3390/w15142626






