Ciprofloxacin Adsorption onto a Smectite–Chitosan-Derived Nanocomposite Obtained by Hydrothermal Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Cobalt–Chitosan-Derived Carbon–Smectite Nanocomposite by a Hydrothermal Procedure
2.2. Characterization Methods
2.3. Adsorption Experiments
2.4. Interpretation of Adsorption Results
3. Results
3.1. Results of Characterization
3.2. Adsorption Results
3.2.1. The Preliminary Test of Adsorption Efficiency of Na-S and H_Co/C-S
3.2.2. The Effect of the Initial pH on CIP Adsorption
3.2.3. The Effect of the Initial CIP Concentration and Kinetic of Adsorption Process
3.2.4. Adsorption Isotherm Analysis for CIP Removal
3.2.5. The Effect of Temperature on CIP Adsorption onto H_Co/C-S and Thermodynamics of the Adsorption Process
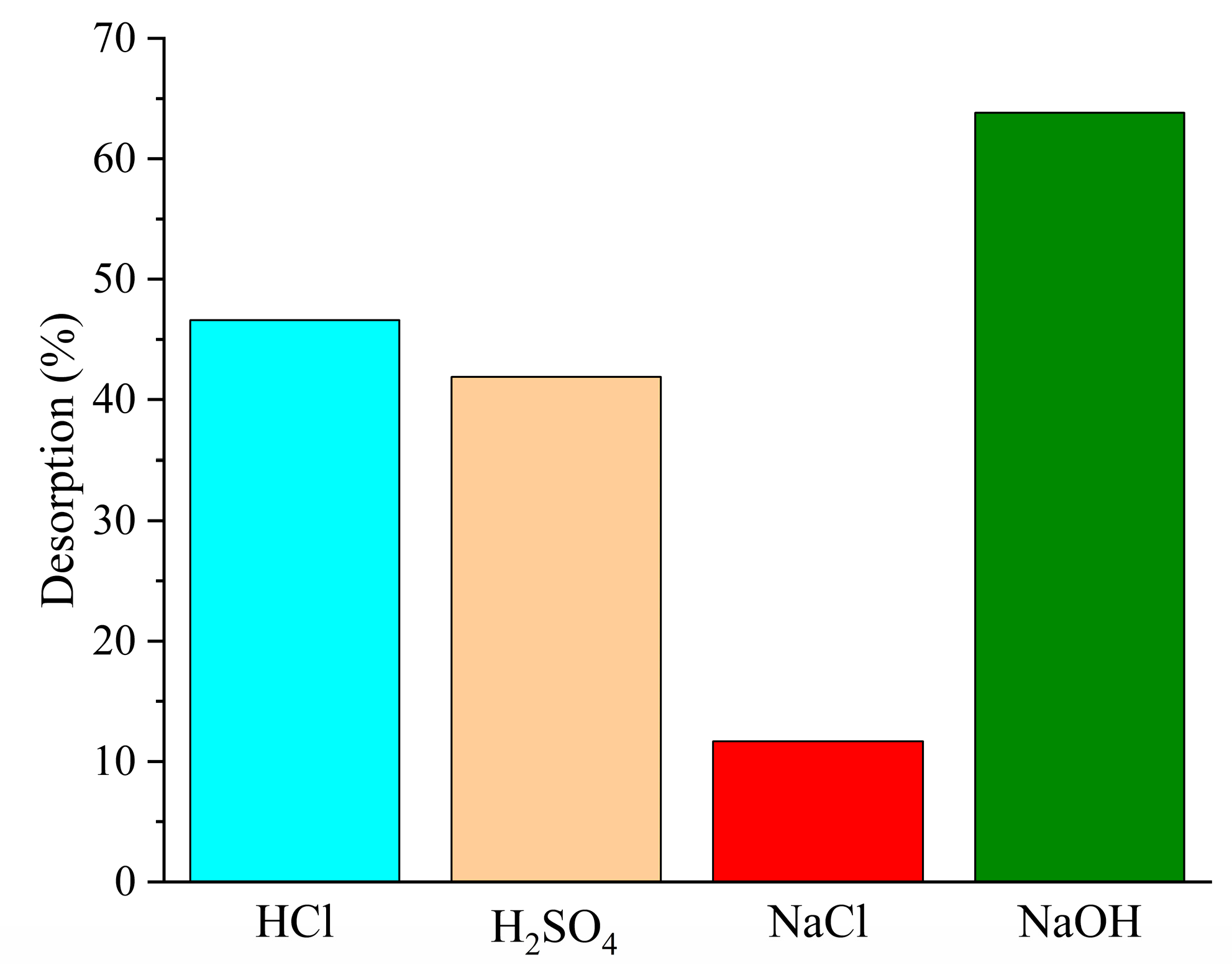
3.2.6. Results of Desorption Study
3.2.7. FTIR Analysis of H_Co/C-S after Adsorption of CIP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akhil, D.; Lakshmi, D.; Senthil Kumar, P. Occurrence and removal of antibiotics from industrial wastewater. Environ. Chem. Lett. 2021, 19, 1477–1507. [Google Scholar] [CrossRef]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Poll. 2009, 157, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Sagaseta de Ilurdoz, M.; Jaime Sadhwani, J.; Vaswani Reboso, J. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment—A review. J. Water Process Eng. 2022, 45, 102474. [Google Scholar]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Borghi, A.A.; Alves Palma, M.S. Tetracycline: Production, waste treatment and environmental impact assessment. Braz. J. Pharm. Sci. 2014, 50, 25–40. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, T.; Fang, H.H. Antibiotic resistance genes in water environment. Appl. Microbiol. Biotechnol. 2009, 82, 397–414. [Google Scholar] [CrossRef]
- Qalyoubi, L.; Al-Othman, A.; Al-Asheh, S. Removal of ciprofloxacin antibiotic pollutants from wastewater using nano-composite adsorptive membranes. Environ. Res. 2022, 215, 114182. [Google Scholar] [CrossRef]
- Al-Buriahia, A.K.; Al-Shaibania, M.M.; Radin Mohamed, R.M.S.; Al-Gheethi, A.A.; Sharmab, A.; Norli, I. Ciprofloxacin removal from non-clinical environment: A critical review of current methods and future trend prospects. J. Water Process Eng. 2022, 47, 102725. [Google Scholar] [CrossRef]
- Hacıosmanoglu, G.G.; Mejías, C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Antibiotic adsorption by natural and modified clay minerals as designer adsorbents for wastewater treatment: A comprehensive review. J. Environ. Manag. 2022, 317, 115397. [Google Scholar] [CrossRef]
- Peng, B.; Chen, L.; Que, C.; Yang, K.; Deng, F.; Deng, X.; Shi, G.; Xu, G.; Wu, M. Adsorption of Antibiotics on Graphene and Biochar in Aqueous Solutions Induced by π-π Interactions. Sci. Rep. 2016, 6, 31920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Ma, W.; Lu, G.; Meng, F.; Duan, S.; Zhang, Z.; Wei, L.; Pan, Y. Adsorption of levofloxacin onto mechanochemistry treated zeolite: Modeling and site energy distribution analysis. Sep. Purif. Technol. 2019, 222, 30–34. [Google Scholar] [CrossRef]
- Premarathna, K.; Rajapaksha, A.U.; Adassoriya, N.; Sarkar, B.; Sirimuthu, N.M.; Cooray, A.; Ok, Y.S.; Vithanage, M. Clay-biochar composites for sorptive removal of tetracycline antibiotic in aqueous media. J. Environ. Manag. 2019, 238, 315–322. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.L.; Feng, D.; Xie, L.H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.R.; Zhou, H.C. Highly stable Zr(IV)-Based metal-organic frameworks for the detection and removal of antibiotics and organic explosives in water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef]
- Antonelli, R.; Malpass, G.R.P.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of ciprofloxacin onto thermally modified bentonite clay: Experimental design, characterization, and adsorbent regeneration. J. Environ. Chem. Eng. 2020, 8, 10455. [Google Scholar] [CrossRef]
- Ahrouch, M.; Gatica, J.M.; Draoui, K.; Vidal, H. Adding value to natural clays as low-cost adsorbents of methylene blue in polluted water through honeycomb monoliths manufacture. SN Appl. Sci. 2019, 1, 1595. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.H.; Li, Z.; Jean, J.S.; Jiang, W.T.; Wang, C.J.; Lin, K.H. Adsorption of tetracycline on 2:1 layered non-swelling clay mineral illite. App. Clay Sci. 2012, 67–68, 158–163. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Q. Sodium Alginate/Modified Bentonite Composite Bead Adsorptive Removal of Norfloxacin: Static and Dynamic Adsorption. Polymers 2022, 14, 3984. [Google Scholar] [CrossRef]
- Zou, C.; Liang, J.; Jiang, W.; Guan, Y.; Zhang, Y. Adsorption behavior of magnetic bentonite for removing Hg(II) from aqueous solutions. RSC Adv. 2018, 8, 27587–27595. [Google Scholar] [CrossRef]
- Rytwo, G.G.; Kohavi, Y.; Botnick, I.; Gonen, Y. Use of CV- and TPP-montmorillonite for the removal of priority pollutants from water. Appl. Clay Sci. 2007, 36, 182–190. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M.; Rytwob, G. Hybrid materials based on clays for environmental and biomedical applications. J. Mater. Chem. 2010, 20, 9306–9321. [Google Scholar] [CrossRef]
- Beall, G.W. The use of organo-clays in water treatment. Appl. Clay Sci. 2003, 24, 11–20. [Google Scholar] [CrossRef]
- Chang, M.Y.; Juang, R.S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. J. Colloid Interface Sci. 2004, 278, 18–25. [Google Scholar] [CrossRef]
- Shen, Y.H. Phenol sorption by organoclays having different charge characteristics. Coll. Surf. A 2004, 232, 143–149. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Iakovleva, E.; Sillanpää, M. Efficient carbon interlayed magnetic chitosan adsorbent for anionic dye removal: Synthesis, characterization and adsorption study. Int. J. Biol. Macromol. 2020, 164, 3621–3631. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Bikiaris, D.N.; Mitropoulos, A.C. Chitosan adsorbents for dye removal: A review. Polym. Int. 2017, 66, 1800–1811. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Francis, A.O.; Zaini, M.A.A.; Muhammad, I.M.; Abdulsalam, S.; El-Nafaty, U.A. Physicochemical modification of chitosan adsorbent: A perspective. Biomass Conv. Bioref. 2023, 13, 5557–5575. [Google Scholar] [CrossRef]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.d.A.; Cadaval, T.R.S., Jr.; Breslin, C.B. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Mamadiev, M.; Zhao, C.; Wang, Z.; Xu, H. Synthesis of interstratified graphene/montmorillonite composite material through organics-pillared, delamination and co-stacking and its application in hexavalent chromium removal from aqueous solution. Adv. Pow. Technol. 2017, 28, 521–533. [Google Scholar] [CrossRef]
- Hani Ababneh, H.; Hameed, B.H. Chitosan-derived hydrothermally carbonized materials and its applications: A review of recent literature. Int. J. Biol. Macromol. 2021, 186, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Senmache, J.C.; Fernández-Reyes, B.; Hernández-Maldonado, A.J. Progress in the design of nanoporous adsorbent materials containing transition metals for the removal of contaminants of emerging concern. Env. Pollut. Bioavail. 2021, 33, 41–54. [Google Scholar] [CrossRef]
- King, C.J. Separation Processes Based on Reversible Chemical Complexation; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Stevanović, G.; Jović-Jovičić, N.; Krstić, J.; Milutinović-Nikolić, A.; Banković, P.; Popović, A.; Ajduković, M. Nanocomposite Co-catalysts, based on smectite and biowaste-derived carbon, as peroxymonosulfate activators in degradation of tartrazine. Appl. Clay Sci. 2022, 230, 106718. [Google Scholar] [CrossRef]
- Prochaska, C.; Gallios, G. Nano-Adsorbents for Cobalt Removal from Wastewater: A Bibliometric Analysis of Research Articles Indexed in the Scopus Database. Processes 2021, 9, 1177. [Google Scholar] [CrossRef]
- Jović-Jovičić, N.; Milutinović-Nikolić, A.; Banković, P.; Mojović, Z.; Žunić, M.; Gržetić, I.; Jovanović, D. Organo-inorganic bentonite for simultaneous adsorption of Acid Orange 10 and lead ions. Appl. Clay Sci. 2010, 47, 452–456. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Ganteaume, M. Thermal decomposition of gibbsite under low pressures: I. Formation of the boehmitic phase. J. Catal. 1975, 36, 99–110. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lippens, B.C.; de Boer, J.H. Studies on pore systems in catalysts: V. The t method. J. Catal. 1965, 4, 319–323. [Google Scholar] [CrossRef]
- Marinović, S.; Milutinović-Nikolić, A.; Žunić, M.; Vuković, Z.; Maksin, D.; Nastasović, A.; Jovanović, D. Porous glycidyl methacrylate-bentonite composite. Russ. J. Phys. Chem. A 2011, 85, 2386–2391. [Google Scholar]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakad. Handl. 1898, 24, 1–39. [Google Scholar]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H.; Heller, W. The Adsorption of Cis- and Trans-Azobenzene. J. Am. Chem. Soc. 1939, 61, 2228–2230. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, Q.; Wang, W.; Hu, Y. Adsorptive removal of ciprofloxacin by a chitosan modified Fe pretreatment biochar composite from aqueous solution. New J. Chem. 2023, 47, 7910–7921. [Google Scholar] [CrossRef]
- Khankhasaeva, S.T.; Badmaeva, S.V.; Ukhinova, M.V. Adsorption of diclofenac onto Fe2O3-pillared montmorillonite: Equilibrium, kinetics and thermodynamic studies. J. Molec. Liq. 2023, 380, 121725. [Google Scholar] [CrossRef]
- JCPDS. International Center for Diffraction Data; Joint Committee on Powder Diffraction Standards (JCPDS): Swarthmore, PA, USA, 1990; p. 1990. [Google Scholar]
- Subotić, B.; Tonejc, A.M.; Bagović, D.; Čižmek, A.; Antonić, T. Zeolites and Related Microporous Materials: State of the Art; Weitkamp, J., Karge, H.G., Pfeifer, H., Eds.; Elsevier Science: Amsterdam, The Netherland, 1994; Volume 3. [Google Scholar]
- Hartati, H.; Purwaningsih, A.; Tjahjandarie, T.S.; Saputri, N.H.; Puspitasari, I.S.; Lamanele, C.N.; Saadah, A.A.; Haque, A.S.; Mardho, D.Z. Synthesis of amorphous aluminosilicate from impure Indonesian kaolin. Open Chem. 2020, 18, 295–302. [Google Scholar] [CrossRef]
- Christidis, G.E.; Scott, P.W.; Dunham, A.C. Acid activation and bleaching capacity of bentonites from the islands of Milos and Chios, Aegean, Greece. Appl. Clay Sci. 1997, 12, 329–347. [Google Scholar] [CrossRef]
- Jović-Jovičić, N.; Bajuk Bogdanović, D.; Novaković, T.; Banković, P.; Milutinović-Nikolić, A.; Mojović, Z. Electrochemical properties of carbonized bentonite. J. Serb. Chem. Soc. 2023, 88, 41–54. [Google Scholar] [CrossRef]
- Ruiz-García, C.; Perez-Carvajal, J.; Berenguer-Murci, A.; Darder, M.; Aranda, P.; Cazorla-Amoros, D.; Ruiz-Hitzky, E. Clay-supported graphene materials: Application to hydrogen storage. Phys. Chem. Chem. Phys. 2013, 15, 18635. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pieroti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Slides; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Vuković, Z.; Milutinović-Nikolić, A.; Krstić, J.; Abu-Rabi, S.; Novaković, T.; Jovanović, D. The Influence of Acid Treatment on the Nanostructure and Textural Properties of Bentonite Clays. Mater. Sci. Forum 2005, 494, 339–344. [Google Scholar] [CrossRef]
- Jović-Jovičić, N.; Milutinović-Nikolić, A.; Žunić, M.; Mojović, Z.; Banković, P.; Gržetić, I.; Jovanović, D. Synergic adsorption of Pb2+ and reactive dye—RB5 on two series of organomodified bentonites. J. Contam. Hydrol. 2013, 150, 1–11. [Google Scholar] [CrossRef]
- Ashiq, A.; Sarkar, B.; Adassooriya, N.; Walpita, J.; Rajapaksha, A.U.; Ok, Y.S.; Vithanage, M. Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 2019, 236, 124384. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline Studies of the Clay Minerals Society Source Clays. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Madejová, J.; Pentrák, M.; Pálková, H.; Komadel, P. Near-infrared spectroscopy: A powerful tool in studies of acid-treated clay minerals. Vib. Spectrosc. 2009, 49, 211–218. [Google Scholar] [CrossRef]
- Vasilev, A.; Efimov, M.; Bondarenko, G.; Kozlov, V.; Dzidziguri, E.; Karpacheva, G. Thermal behavior of chitosan as a carbon material precursor under IR radiation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 693, 012002. [Google Scholar] [CrossRef]
- Wang, G.; Xu, J.; Sun, Z.; Zheng, S. Surface Functionalization of Montmorillonite with Chitosan and the Role of Surface Properties on Its Adsorptive Performance: A Comparative Study on Mycotoxins Adsorption. Langmuir 2020, 36, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.W.; Wang, C.B.; Chien, S.H. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.G. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- Bhagat, C.; Kumar, M.; Tyagi, V.K. Proclivities for prevalence and treatment of antibiotics in the ambient water: A review. Npj Clean Water 2020, 3, 42. [Google Scholar] [CrossRef]
- Pei, Z.G.; Shan, X.Q.; Kong, J.; Wen, B.; Owens, G. Coadsorption of ciprofloxacin and Cu (II) on montmorillonite and kaolinite as affected by solution pH. Environ. Sci. Technol. 2010, 44, 915–920. [Google Scholar] [CrossRef]
- Gulen, B.; Demircivi, P. Adsorption Properties of Flouroquinolone Type Antibiotic Ciprofloxacin into 2:1 Dioctahedral Clay Structure: Box-Behnken Experimental Design. J. Mol. Struct. 2020, 1206, 127659. [Google Scholar] [CrossRef]
- Wang, L.; Chen, G.; Ling, C.; Zhang, J.; Szerlag, K. Adsorption of Ciprofloxacin on to Bamboo Charcoal: Effects of PH, Salinity, Cations, and Phosphate. Environ. Prog. Sustain. Energy 2017, 36, 1108–1115. [Google Scholar] [CrossRef]
- Tran, Q.T.; Do, T.H.; Ha, X.L.; Nguyen, H.P.; Nguyen, A.T.; Ngo, T.C.Q.; Chau, H.D. Study of the Ciprofloxacin Adsorption of Activated Carbon Prepared from Mangosteen Peel. Appl. Sci. 2022, 12, 8770. [Google Scholar] [CrossRef]
- Balarak, D.; Baniasadi, M.; Lee, S.; Joon, M. Ciprofloxacin adsorption onto Azolla filiculoides activated carbon from aqueous solutions. Desalin. Water Treat. 2021, 218, 444–453. [Google Scholar] [CrossRef]
- Wakejo, W.K.; Meshasha, B.T.; Kang, J.W.; Chebude, Y. Enhanced Ciprofloxacin Removal from Aqueous Solution Using a Chemically Modified Biochar Derived from Bamboo Sawdust: Adsorption Process Optimization with Response Surface Methodology. Ads. Sci. Technol. 2023, 2022, 2699530. [Google Scholar] [CrossRef]
- Magesh, N.; Renita, A.A.; Siva, R.; Harirajan, N.; Santhosh, A. Adsorption behavior of fluoroquinolone(ciprofloxacin) using zinc oxide impregnated activated carbon prepared from jack fruit peel: Kinetics and isotherm studies. Chemosphere 2020, 290, 133227. [Google Scholar] [CrossRef]
- Ambrósio, N.; Vieira, M.; Silva, M. Cu(II) Adsorption on Modified Bentonitic Clays: Different Isotherm Behaviors in Static and Dynamic Systems. Mater. Res. 2012, 15, 114–124. [Google Scholar]
- Bizi, M.; El Bachra, F.E. Evaluation of the ciprofloxacin adsorption capacity of common industrial minerals and application to tap water treatment. Powder Technol. 2020, 362, 323–333. [Google Scholar] [CrossRef]
- García-Alonso, J.; Sulbaran, B.; Bandala, E.; Real, J.; Sulbarán-Rangel, B. Adsorption and kinetic studies of the removal of ciprofloxacin from aqueous solutions by diatomaceous earth. Desalin. Water Treat. 2019, 162, 331–340. [Google Scholar] [CrossRef]
- Abramian, L.; El-Rassy, H. Adsorption Kinetics and Thermodynamics of Azo-Dye Orange II onto Highly Porous Titania Aerogel. Chem. Eng. J. 2009, 150, 403–410. [Google Scholar] [CrossRef]
- Tran, H.N.; Lima, E.C.; Juang, R.-S.; Bollinger, J.-C.; Chao, H.-P. Thermodynamic parameters of liquid–phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: A comparison study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Wang, Z.; Muhammad, Y.; Tang, R.; Lu, C.; Yu, S.; Song, R.; Tong, Z.; Han, B.; Zhang, H. Dually organic modified bentonite with enhanced adsorption and desorption of tetracycline and ciprofloxacine. Sep. Purif. Technol. 2021, 274, 119059. [Google Scholar] [CrossRef]
- Al-Omar, M.A. Ciprofloxacin: Physical Profile. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2005; Volume 31, pp. 163–178. [Google Scholar]
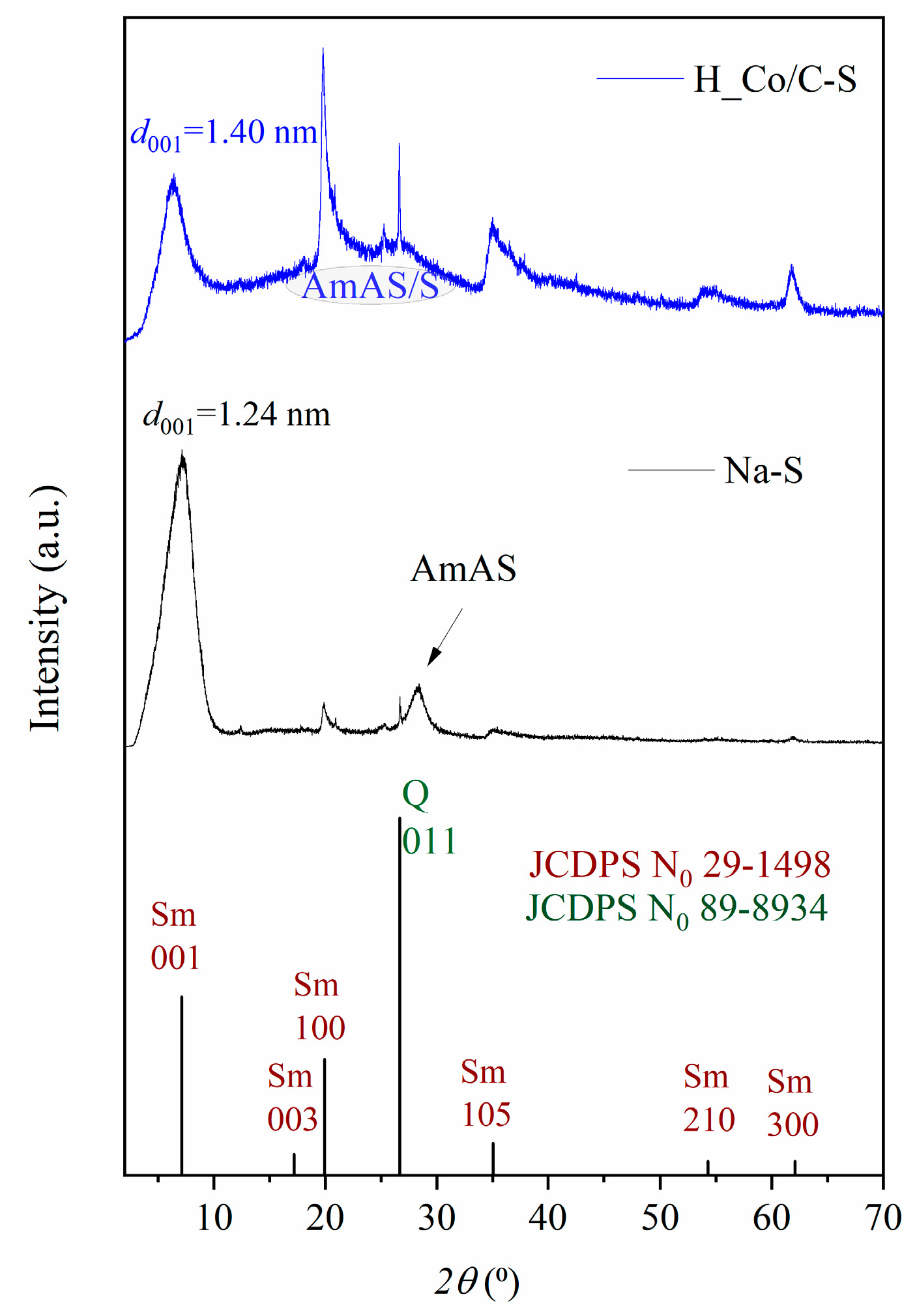


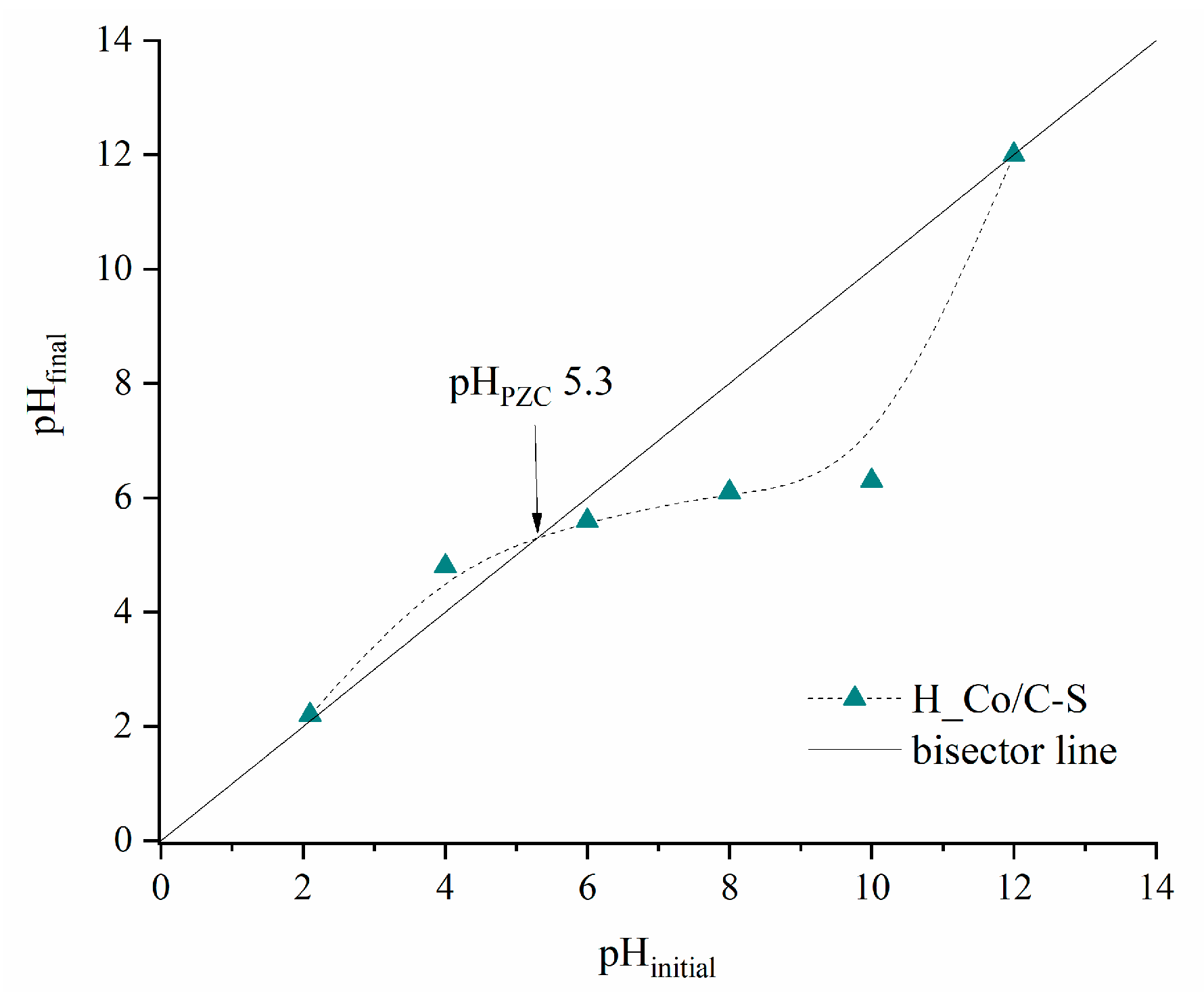
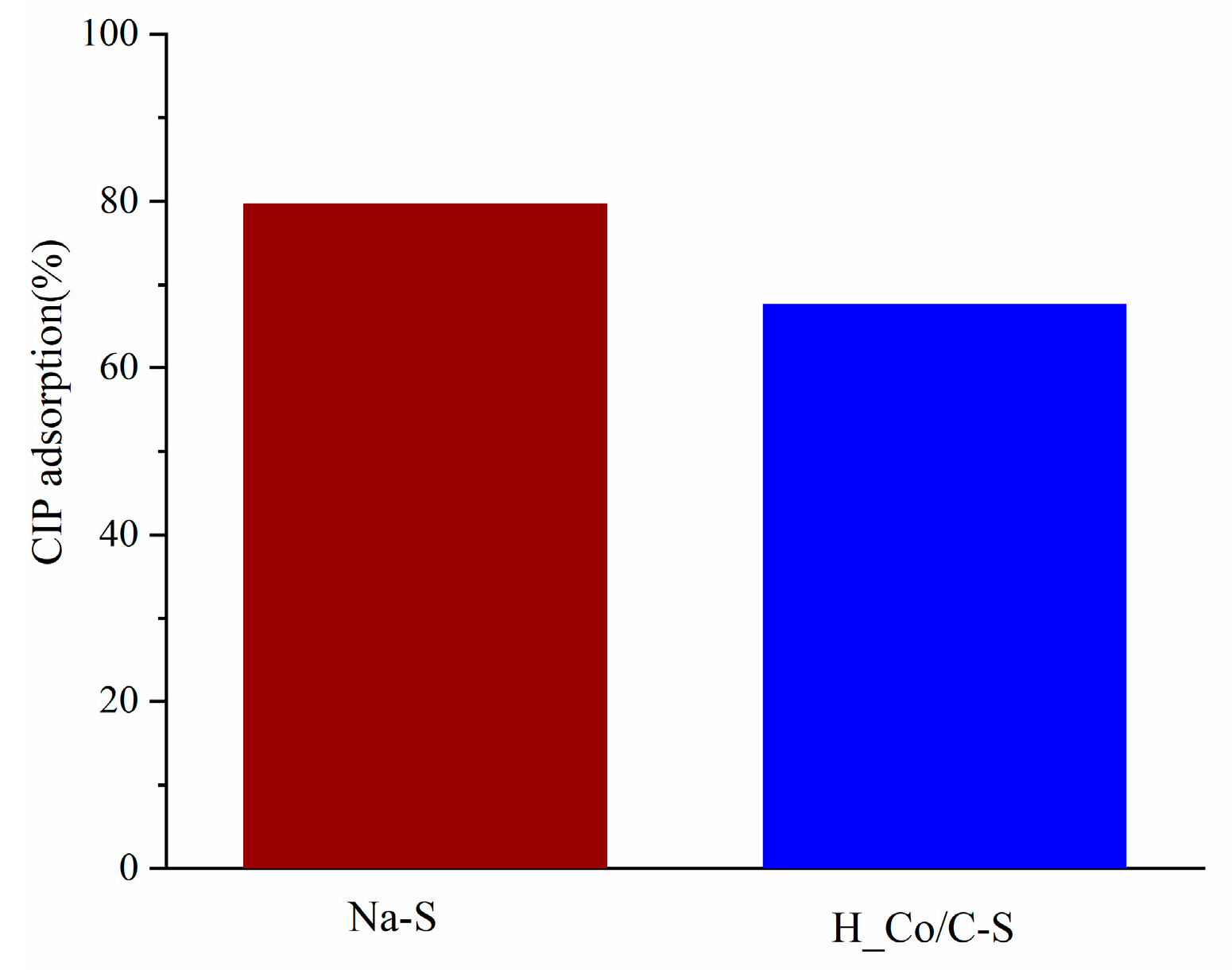
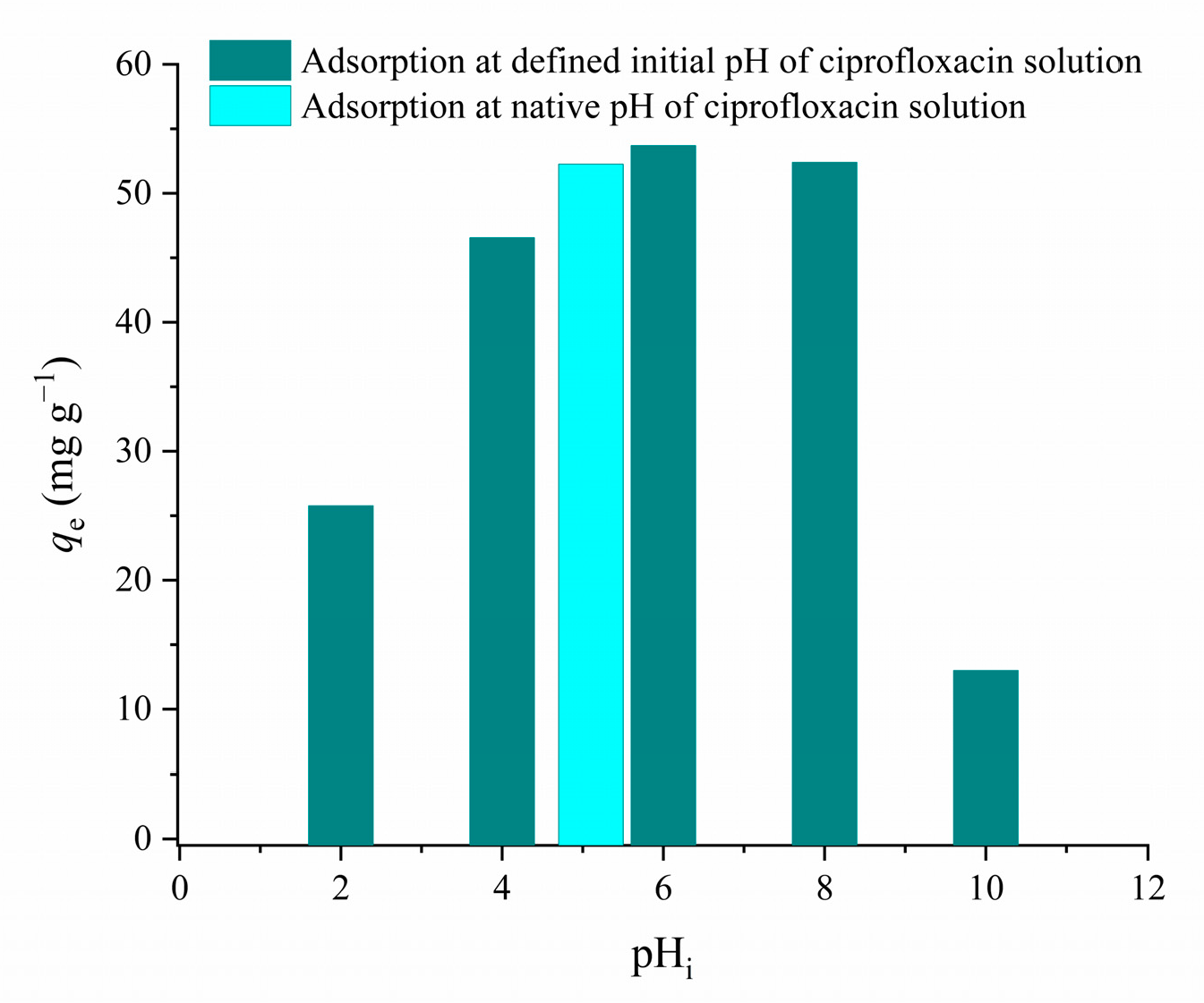
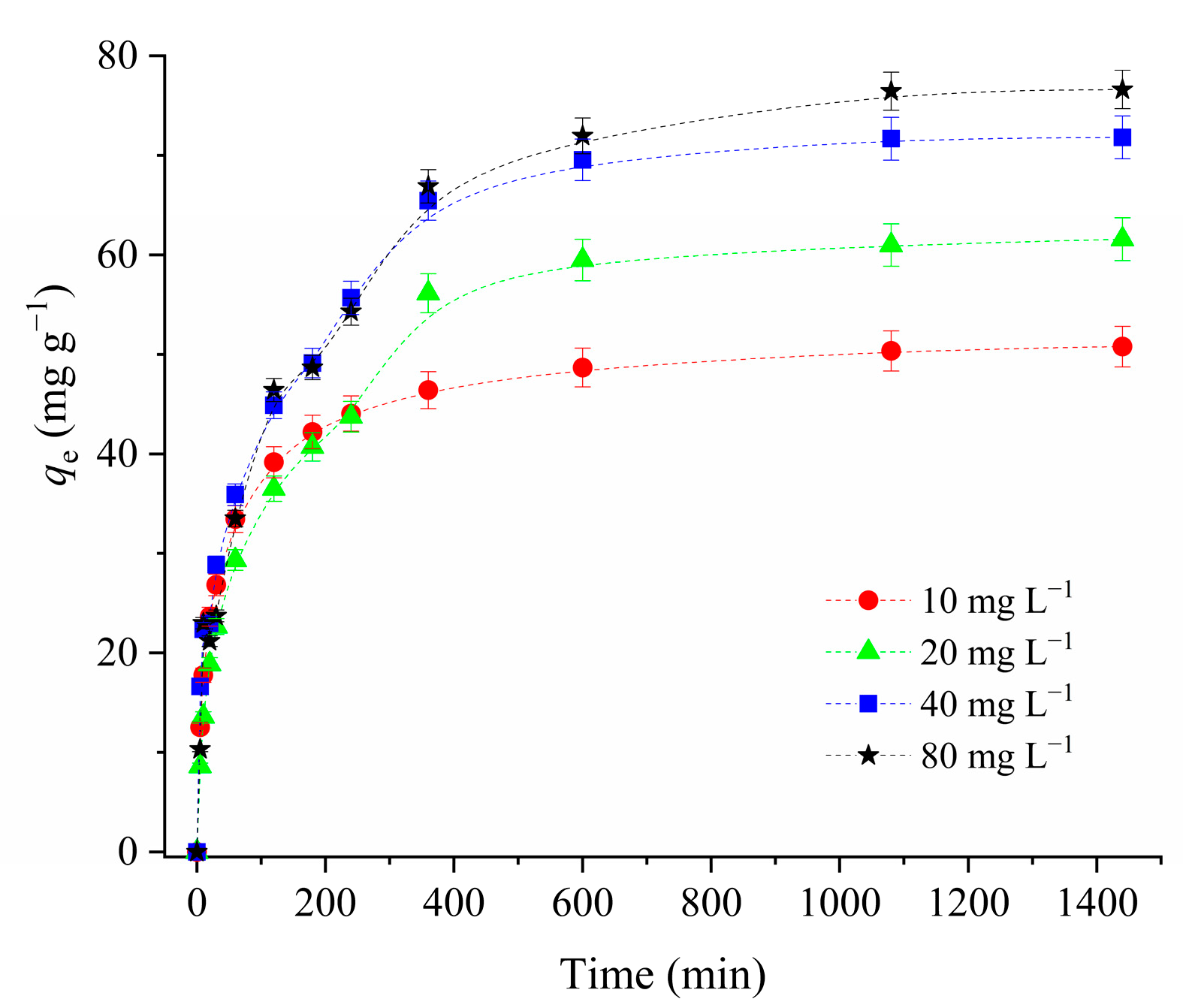
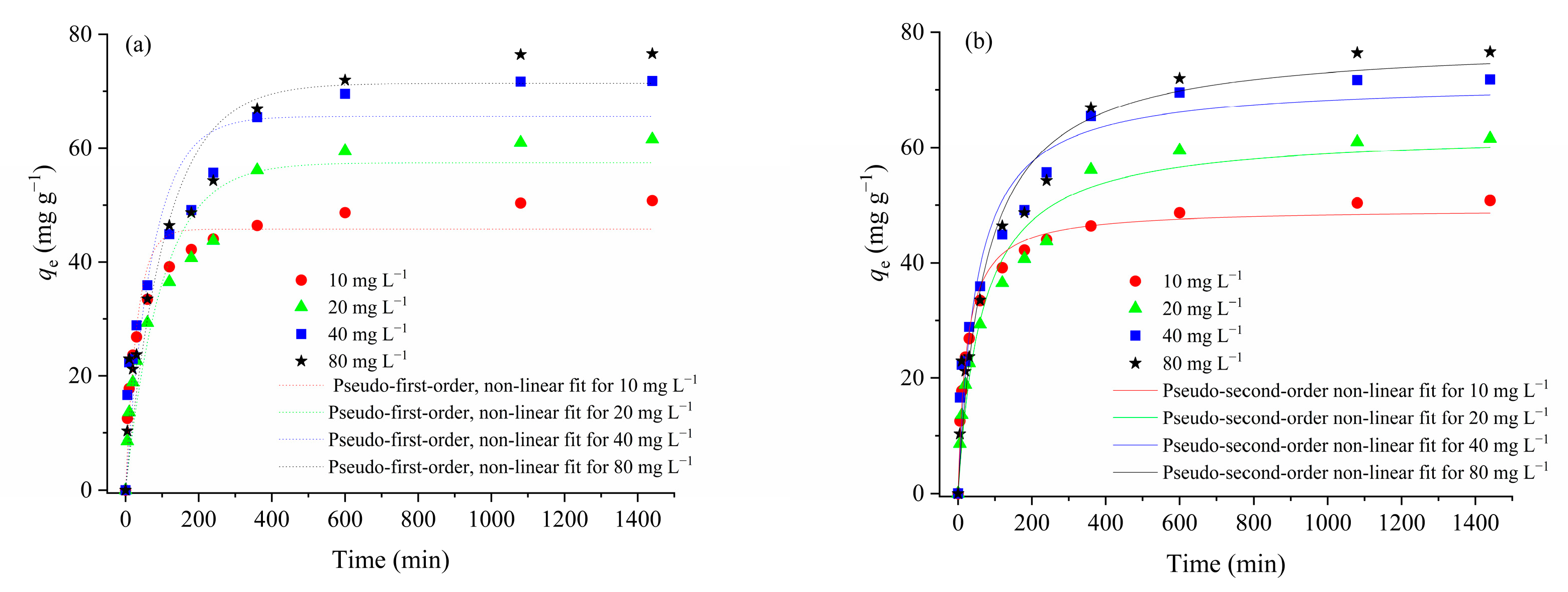
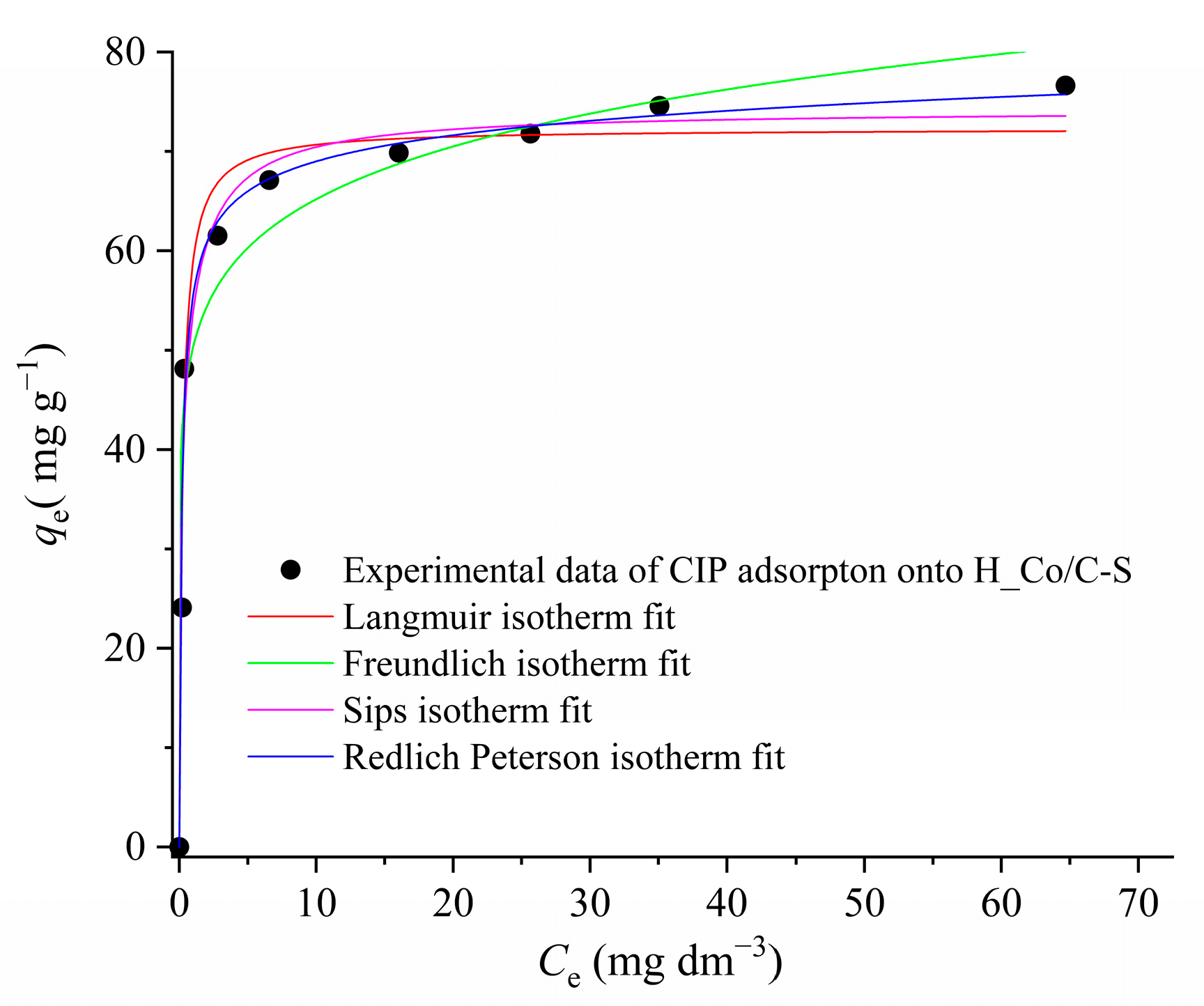
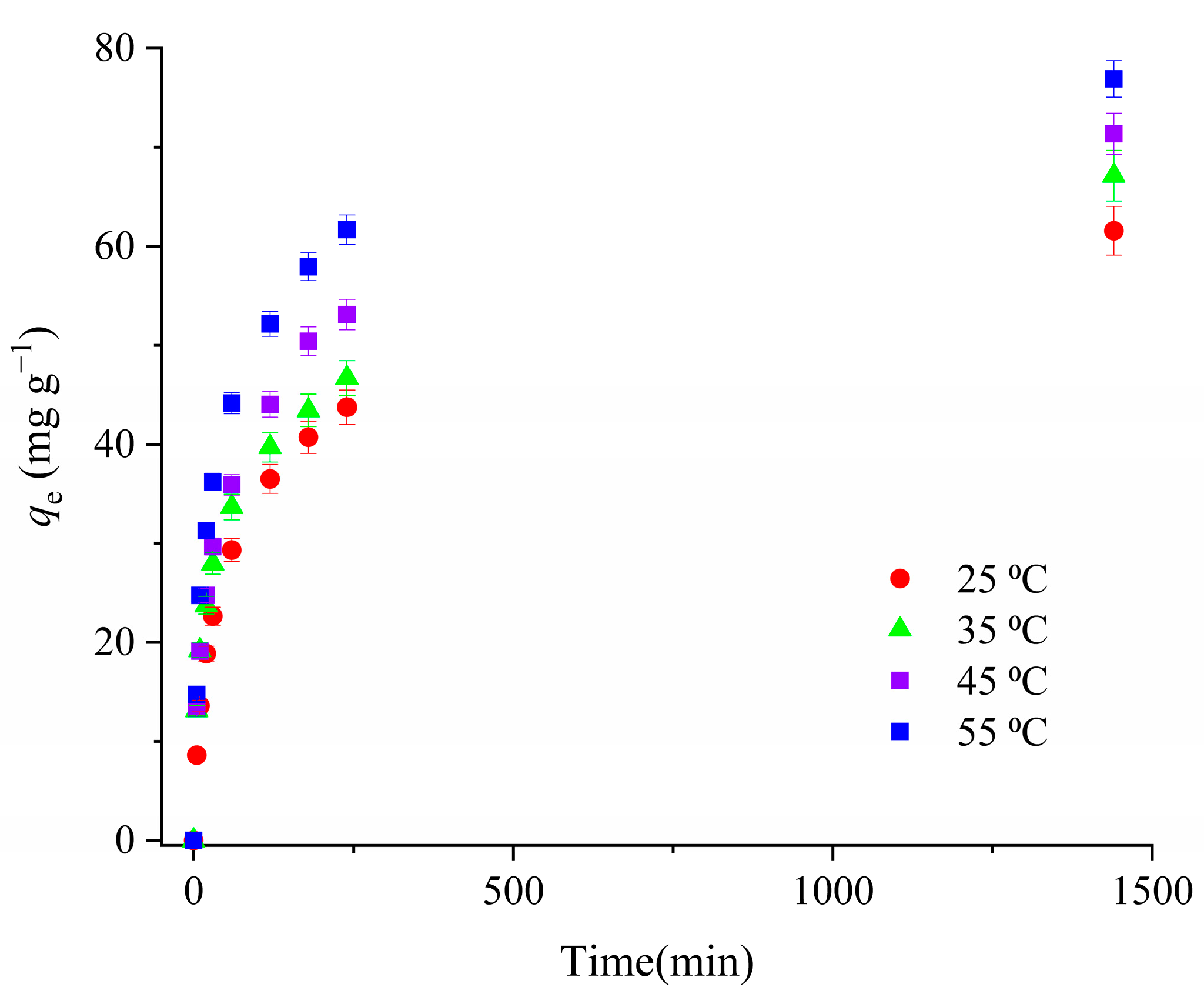


| Sample | SBET (m2 g−1) | Vtot (cm3 g−1) | Vmeso (cm3 g−1) | Vmicro (cm3 g−1) | DMED (nm) | Dmax (nm) |
|---|---|---|---|---|---|---|
| Na-S | 119 | 0.105 | 0.074 | 0.049 | 4.16 | 4.00 |
| H_Co/C-S | 0.73 | 0.012 | 0.019 | / | 13.8 | 13.0 |
| Sample | H_Co/C-S | |||
|---|---|---|---|---|
| C0 (mg g−1) | 10 | 20 | 40 | 80 |
| qeexp (mg g−1) | 50.8 | 61.6 | 71.8 | 76.6 |
| Pseudo-first-order kinetic model | ||||
| qecalc (mg g−1) | 45.8 | 57.4 | 65.6 | 71.4 |
| k1·10−2 (min−1) | 3.21 | 1.00 | 1.35 | 0.910 |
| R2 | 0.937 | 0.915 | 0.873 | 0.906 |
| Pseudo-second-order kinetic model | ||||
| qecalc (mg g−1) | 49.4 | 62.8 | 71.5 | 78.2 |
| k2·10−4 (g mg−1 min−1) | 8.92 | 2.43 | 2.88 | 1.75 |
| R2 | 0.984 | 0.965 | 0.941 | 0.954 |
| Adsorption Model | Isotherm Parameters | |||
|---|---|---|---|---|
| Freundlich equation | KF (mg g−1(dm3 mg−1)1/n) | nF | R2 | |
| 50.3 | 8.86 | 0.948 | ||
| Langmuir equation | KL (dm3 mg−1) | qmax (mg g−1) | RL | R2 |
| 4.38 | 72.3 | 0.022 | 0.977 | |
| Sips equation | K (dm3 mg−1)n | qsat (mg g−1) | bS | R2 |
| 0.552 | 74.2 | 0.313 | 0.976 | |
| Redlich–Peterson equation | KRP (dm3 g−1) | aRP (L mg−1)nRP | nRP | R2 |
| 431 | 6.78 | 0.957 | 0.985 | |
| Adsorbent | Adsorbent Properties | Adsorption Capacity (mg g−1) | References |
|---|---|---|---|
| Hydrothermally synthetized Co–carbon smectite (H_Co/C-S) | SBET = 0.73 m2 g−1; Vmeso = 0.019 cm3 g−1, DMED = 13.9 nm pHPZC 5.3 | 72.3 | This study |
| Activated carbon derived from mangosteen peel | SBET = 419.9 m2 g−1 Vcap = 0.280 cm3 g−1, Dmean = 2.7 nm pHPZC 5.34 | 29.8 | [72] |
| Calcined Verdelodo clay-packed fixed-bed | SBET = 62.1 m2 g−1, Vmeso = 0.019 cm3 g−1, | 12.6 | [15,76] |
| Smectite clay | SBET = 87.7 m2 g−1 Vmeso = 0.09 cm3 g−1, pHPZC 8.00 | 184 | [77] |
| Biochar derived from bamboo sawdust | SBET = 1158 m2 g−1 pHPZC 6.5 | 78.4 | [74] |
| Activated carbon derived from Azolla filiculoides | SBET = 716.4 m2 g−1 Dmean = 41.3 nm Vtot = 0.481 cm3 g−1 | 35.1 | [73] |
| Bamboo charcoal | SBET = 1228 m2 g−1 pHPZC 6.5 | 36.0 | [71] |
| Surface-modified tamarind shell | Dmean ≤ 10 µm | 21.7 | [75] |
| Diatomaceous earth | SBET = 29.14 m2 g−1, Dmean = 190 nm | 19.4 | [78] |
| T (K) | ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (J K−1 mol−1) |
|---|---|---|---|
| 298 | −5.16 | 19.7 | 83.5 |
| 308 | −5.99 | ||
| 318 | −6.83 | ||
| 328 | −7.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajduković, M.; Stevanović, G.; Marinović, S.; Mojović, Z.; Banković, P.; Radulović, K.; Jović-Jovičić, N. Ciprofloxacin Adsorption onto a Smectite–Chitosan-Derived Nanocomposite Obtained by Hydrothermal Synthesis. Water 2023, 15, 2608. https://doi.org/10.3390/w15142608
Ajduković M, Stevanović G, Marinović S, Mojović Z, Banković P, Radulović K, Jović-Jovičić N. Ciprofloxacin Adsorption onto a Smectite–Chitosan-Derived Nanocomposite Obtained by Hydrothermal Synthesis. Water. 2023; 15(14):2608. https://doi.org/10.3390/w15142608
Chicago/Turabian StyleAjduković, Marija, Gordana Stevanović, Sanja Marinović, Zorica Mojović, Predrag Banković, Katarina Radulović, and Nataša Jović-Jovičić. 2023. "Ciprofloxacin Adsorption onto a Smectite–Chitosan-Derived Nanocomposite Obtained by Hydrothermal Synthesis" Water 15, no. 14: 2608. https://doi.org/10.3390/w15142608
APA StyleAjduković, M., Stevanović, G., Marinović, S., Mojović, Z., Banković, P., Radulović, K., & Jović-Jovičić, N. (2023). Ciprofloxacin Adsorption onto a Smectite–Chitosan-Derived Nanocomposite Obtained by Hydrothermal Synthesis. Water, 15(14), 2608. https://doi.org/10.3390/w15142608






