Analysis of Polycyclic Aromatic Hydrocarbons in Water Samples Using Deep Eutectic Solvent as a Dispersant in Dispersive Liquid–Liquid Microextraction Based on the Solidification of Floating Organic Droplet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentations
2.3. Sample Preparation
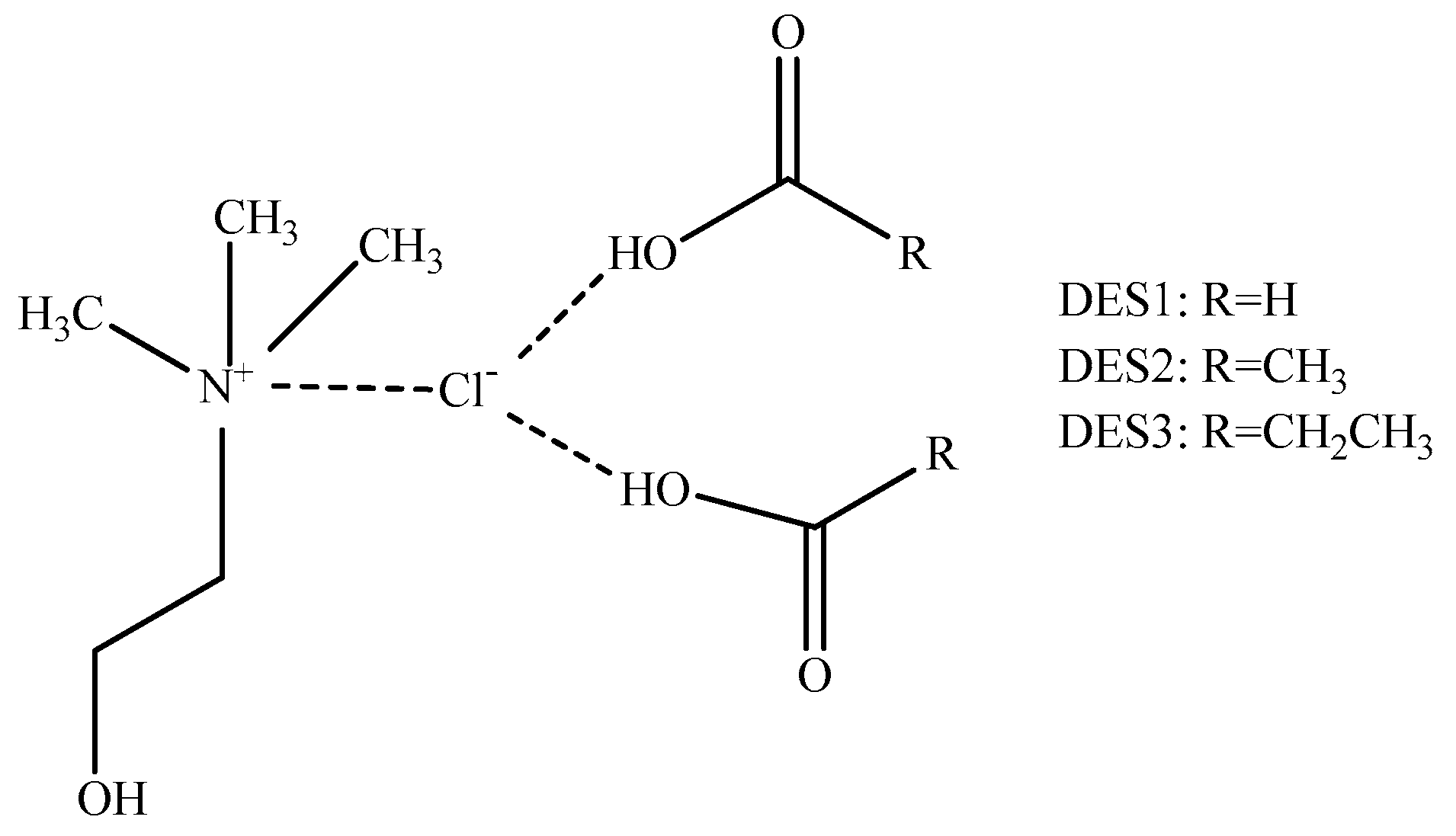
2.4. DES Preparation
2.5. DLLME-SFOD Procedure
2.6. Enrichment Factor Calculation
3. Results and Discussion
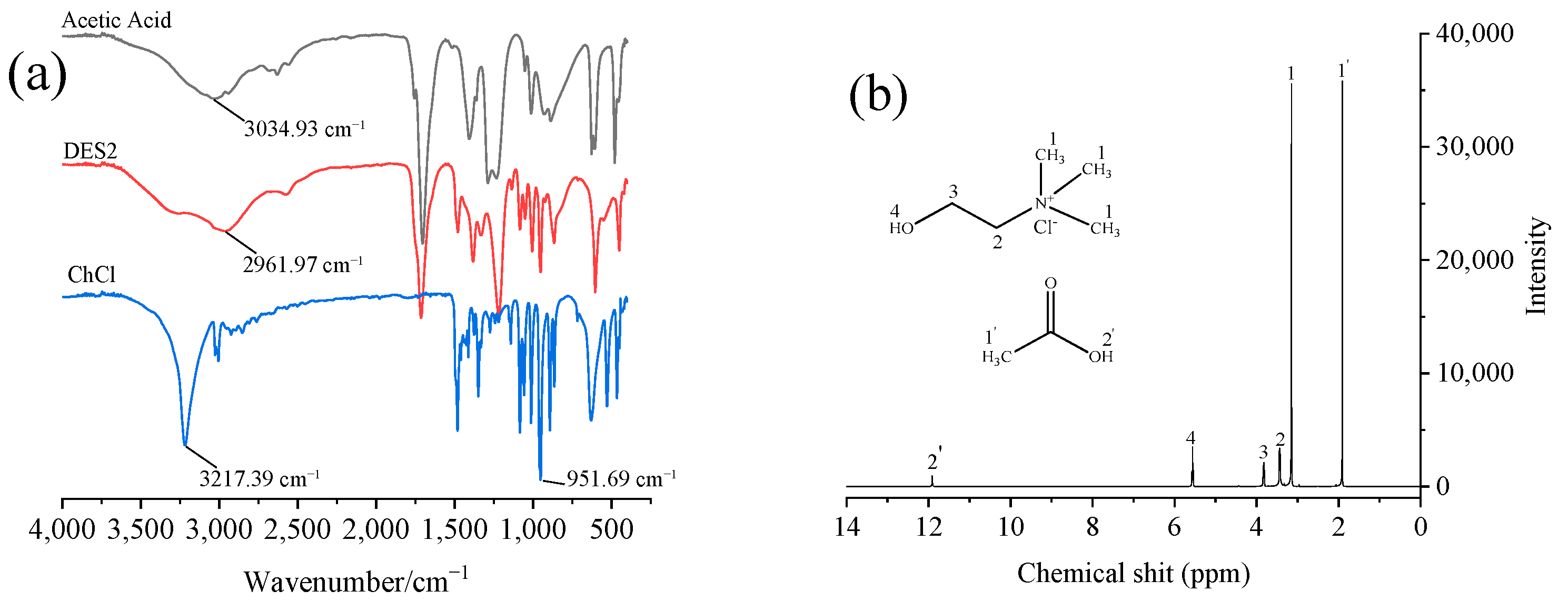
3.1. Characterization of DESs
3.2. Optimization of the Extraction Procedure
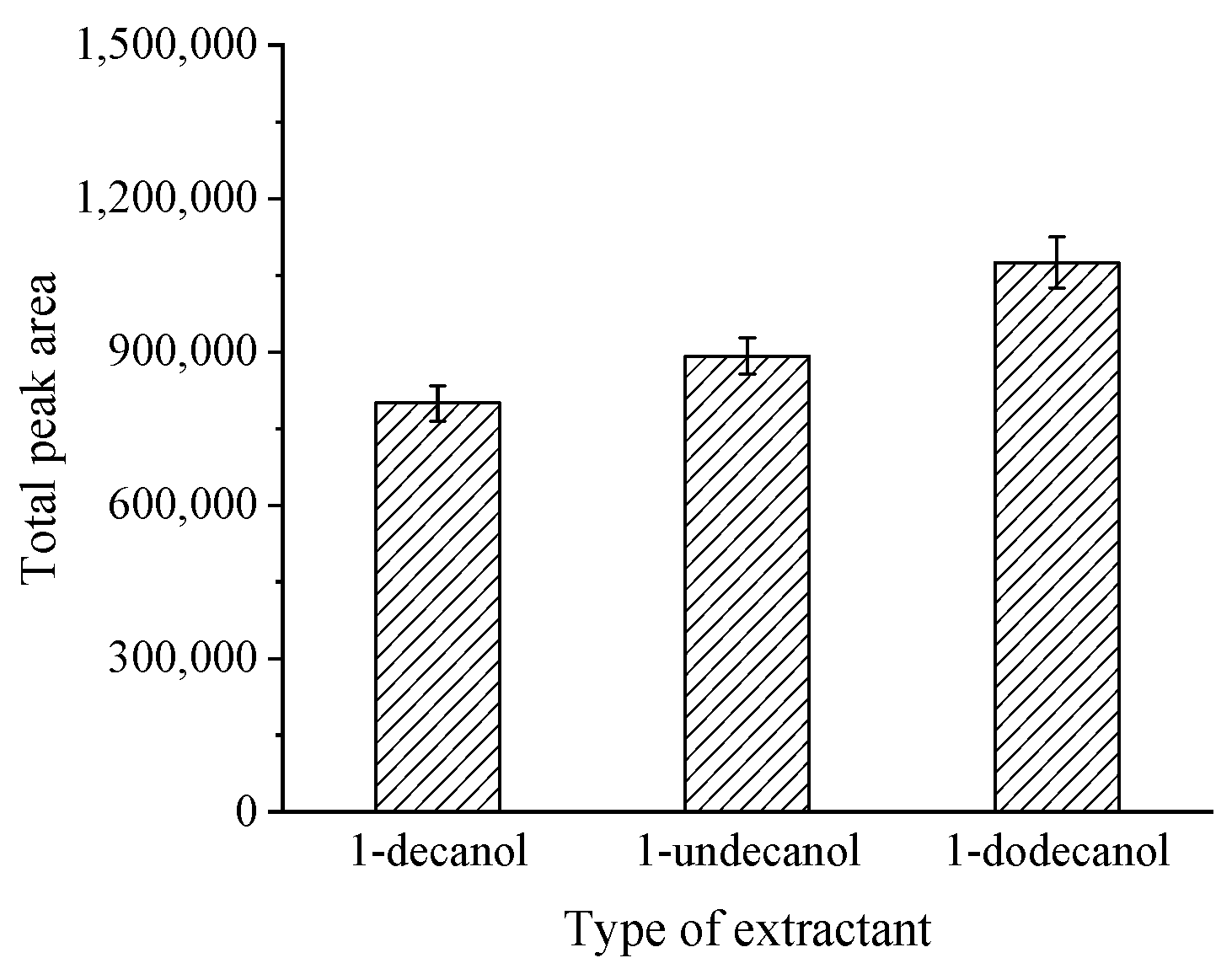
3.2.1. Selection of Extractant
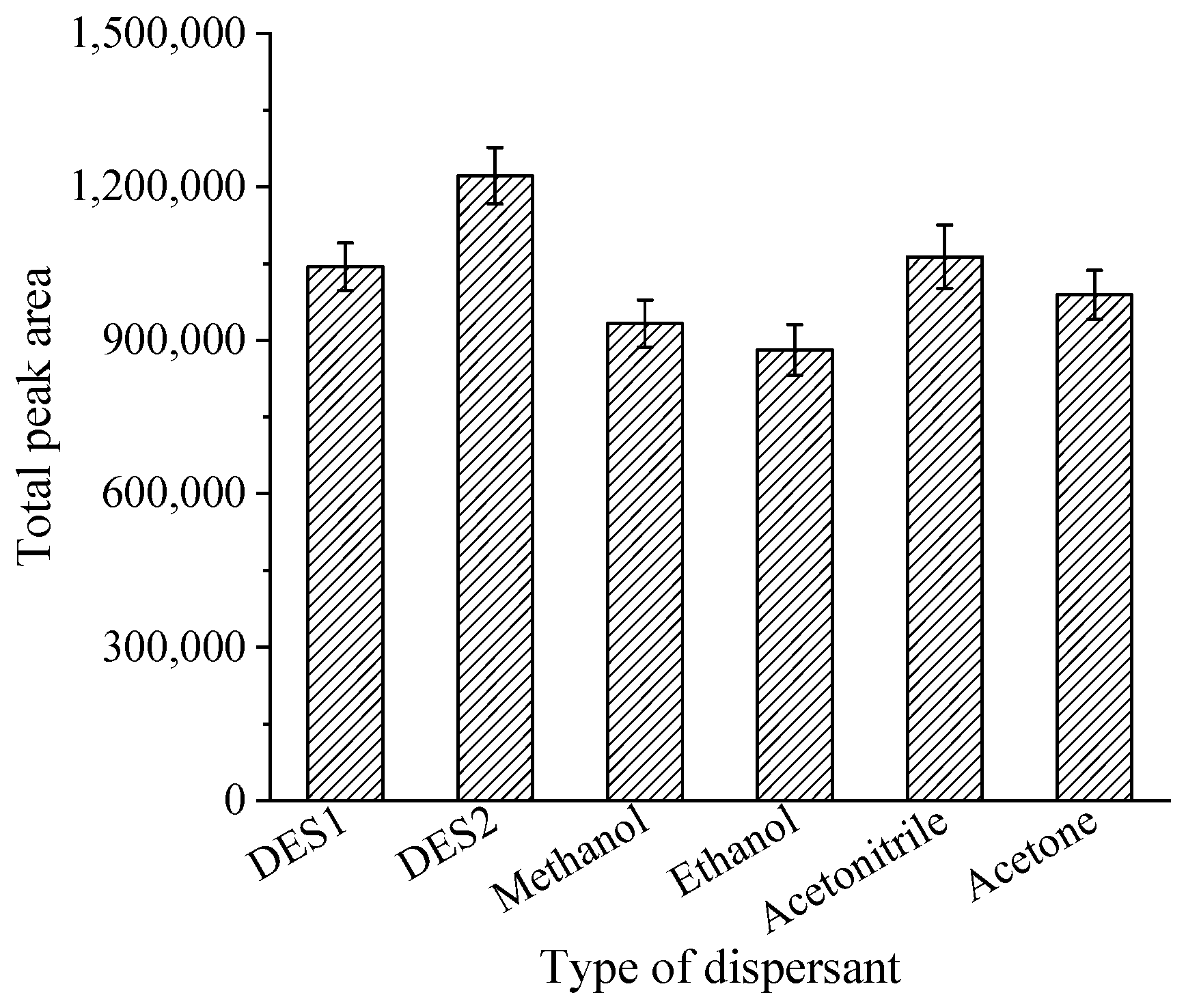
3.2.2. Selection of Dispersant
3.2.3. Box–Behnken Design
3.3. Method Validation
3.4. Analysis of Real Water Samples
3.5. Comparison of the Proposed Method with Other Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zainal, P.; Alang, A.S.; Abdul, A.S.; Rosly, N.Z. Polycyclic aromatic hydrocarbons: Occurrence, electroanalysis, challenges, and future outlooks. Crit. Rev. Anal. Chem. 2022, 52, 878–896. [Google Scholar] [CrossRef]
- Kozak, K.; Ruman, M.; Kosek, K.; Karasiński, G.; Stachnik, A.; Polkowska, Ż. Impact of volcanic eruptions on the occurrence of pahs compounds in the aquatic ecosystem of the southern part of west spitsbergen (hornsund fjord, svalbard). Water 2017, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic aromatic hydrocarbons: A critical review of environmental occurrence and bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef]
- Zhen, H.; Zhang, F.; Cheng, H.; Hu, F.; Jia, Y.; Hou, Y.; Shang, M.; Yu, H.; Jiang, M. Association of polycyclic aromatic hydrocarbons exposure with child neurodevelopment and adult emotional disorders: A meta-analysis study. Ecotox. Environ. Saf. 2023, 255, 114770. [Google Scholar] [CrossRef]
- Stading, R.; Gastelum, G.; Chu, C.; Jiang, W.; Moorthy, B. Molecular mechanisms of pulmonary carcinogenesis by polycyclic aromatic hydrocarbons (pahs): Implications for human lung cancer. Semin. Cancer Biol. 2021, 76, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (pahs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Han, C.; Fang, H.; Weng, J.; Shu, X.; Pan, Y.; Ma, L. Polycyclic aromatic hydrocarbons in surface waters from the seven main river basins of china: Spatial distribution, source apportionment, and potential risk assessment. Sci. Total Environ. 2021, 752, 141764. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; Li, M.; Ren, L.; Zheng, B.; Zou, X. Binary mixed solvent-based solvent demulsification-dispersive liquid-liquid microextraction coupled with gas chromatography-tandem mass spectrometry in determination of polycyclic aromatic hydrocarbons in water samples. Anal. Methods 2017, 9, 1855–1863. [Google Scholar] [CrossRef]
- Santana, J.L.; Massone, C.G.; Valdés, M.; Vazquez, R.; Lima, L.A.; Olivares-Rieumont, S. Occurrence and source appraisal of polycyclic aromatic hydrocarbons (pahs) in surface waters of the almendares river, cuba. Arch. Environ. Contam. Toxicol. 2015, 69, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Si, X.; Yuan, Y.; Chen, K.; Qin, K. Ultra-trace extraction of two bactericides via ultrasound-assisted dispersive liquid-liquid microextraction. J. Chromatogr. Sci. 2021, 59, 182–190. [Google Scholar] [CrossRef]
- Machado, I.; Tissot, F. Dispersive liquid-liquid microextraction as a preconcentration alternative to increase etaas sensitivity in the analysis of molybdenum in bovine meat and pasture samples. Talanta 2020, 212, 120783. [Google Scholar] [CrossRef]
- Mansour, F.R.; Danielson, N.D. Solvent-terminated dispersive liquid-liquid microextraction: A tutorial. Anal. Chim. Acta 2018, 1016, 1–11. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Leong, M.; Huang, S. Dispersive liquid–liquid microextraction method based on solidification of floating organic drop combined with gas chromatography with electron-capture or mass spectrometry detection. J. Chromatogr. A 2008, 1211, 8–12. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry. A review. Microchem J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Grau, J.; Azorín, C.; Benedé, J.L.; Chisvert, A.; Salvador, A. Use of green alternative solvents in dispersive liquid–liquid microextraction: A review. J. Sep. Sci. 2022, 45, 210–222. [Google Scholar] [CrossRef]
- An, J.; Trujillo-Rodríguez, M.J.; Pino, V.; Anderson, J.L. Non-conventional solvents in liquid phase microextraction and aqueous biphasic systems. J. Chromatogr. A 2017, 1500, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.; Moral, R.; Thakuria, S.; Mitra, A.; Paul, S. Hydrophobic deep eutectic solvents as greener substitutes for conventional extraction media: Examples and techniques. ACS Omega 2023, 8, 9702–9728. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Liu, S.; Wu, Z. Recent application of deep eutectic solvents as green solvent in dispersive liquid-liquid microextraction of trace level chemical contaminants in food and water. Crit. Rev. Anal. Chem. 2022, 52, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Makoś, P.; Przyjazny, A.; Boczkaj, G. Hydrophobic deep eutectic solvents as “green“ extraction media for polycyclic aromatic hydrocarbons in aqueous samples. J. Chromatogr. A 2018, 1570, 28–37. [Google Scholar] [CrossRef]
- Bian, Y.; Wang, Y.; Yu, J.; Zheng, S.; Qin, F.; Zhao, L. Analysis of six preservatives in beverages using hydrophilic deep eutectic solvent as disperser in dispersive liquid-liquid microextraction based on the solidification of floating organic droplet. J. Pharm. Biomed. Anal. 2021, 195, 113889. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Shimizu, K. Deep eutectic solvent as a novel disperser in dispersive liquid-liquid microextraction based on solidification of floating organic droplet (dllme-sfod) for preconcentration of steroids in water samples: Assessment of the method deleterious impact on the environment using analytical eco-scale and green analytical procedure index. Microchem J. 2019, 149, 103988. [Google Scholar]
- Shishov, A.; Volodina, N.; Nechaeva, D.; Gagarinova, S.; Bulatov, A. Deep eutectic solvents as a new kind of dispersive solvent for dispersive liquid–liquid microextraction. RSC Adv. 2018, 8, 38146–38149. [Google Scholar] [CrossRef]
- Khezeli, T.; Daneshfar, A.; Sahraei, R. A green ultrasonic-assisted liquid–liquid microextraction based on deep eutectic solvent for the hplc-uv determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta 2016, 150, 577–585. [Google Scholar] [CrossRef]
- Khezeli, T.; Daneshfar, A.; Sahraei, R. Emulsification liquid–liquid microextraction based on deep eutectic solvent: An extraction method for the determination of benzene, toluene, ethylbenzene and seven polycyclic aromatic hydrocarbons from water samples. J. Chromatogr. A 2015, 1425, 25–33. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Shimizu, K. Deep eutectic solvents as promising green solvents in dispersive liquid–liquid microextraction based on solidification of floating organic droplet: Recent applications, challenges and future perspectives. Molecules 2021, 26, 7406. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, Y.; Zhao, Q.; Chen, A.; Jiao, B. Ultrasound–assisted dispersive liquid–phase microextraction by solidifying l–menthol–decanoic acid hydrophobic deep eutectic solvents for detection of five fungicides in fruit juices and tea drinks. J. Sep. Sci. 2021, 44, 3870–3882. [Google Scholar] [CrossRef]
- Albero, B.; Tadeo, J.L.; Pérez, R.A. Ultrasound-assisted extraction of organic contaminants. TrAC Trends Anal. Chem. 2019, 118, 739–750. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Effective method of treatment of industrial effluents under basic ph conditions using acoustic cavitation—A comprehensive comparison with hydrodynamic cavitation processes. Chem. Eng. Process. Process Intensif. 2018, 128, 103–113. [Google Scholar] [CrossRef]
- Manariotis, I.D.; Karapanagioti, H.K.; Chrysikopoulos, C.V. Degradation of pahs by high frequency ultrasound. Water Res. 2011, 45, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ding, Z.; Lv, L.; Song, D.; Feng, Y. A novel dispersive liquid–liquid microextraction based on solidification of floating organic droplet method for determination of polycyclic aromatic hydrocarbons in aqueous samples. Anal. Chim. Acta 2009, 636, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Vera, C.; Lasarte-Aragonés, G.; Bravo, M.A.; Muñoz-Lira, D.; Salazar, R.; Toledo-Neira, C. Ionic liquids-based dispersive liquid-liquid microextraction for determination of carcinogenic polycyclic aromatic hydrocarbons in tea beverages: Evaluation of infusion preparation on pollutants release. Food Control. 2019, 106, 106685. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Li, S.; Zhu, G. Air-assisted liquid-liquid microextraction based on the solidification of floating deep eutectic solvents for the simultaneous determination of bisphenols and polycyclic aromatic hydrocarbons in tea infusions via hplc. Food Chem. 2021, 348, 129106. [Google Scholar] [CrossRef]

| PAH | Retention Time (Min) | Characteristic Ions (m/z) |
|---|---|---|
| Naphthalene | 5.804 | 127, 128 *, 129 |
| Acenaphthylene | 7.994 | 151, 152 *, 153 |
| Acenaphthene | 8.236 | 152, 153 *, 154 |
| Fluorene | 9.303 | 165, 166 *, 167 |
| Phenanthrene | 12.546 | 176, 178 *, 179 |
| Anthracene | 12.752 | 152, 176, 178 *, 179 |
| Fluoranthene | 16.057 | 101, 200, 202 *, 203 |
| Pyrene | 16.551 | 101, 200, 202 *, 203 |
| Benz[a]anthracene | 18.971 | 114, 226, 228 *, 229 |
| Chrysene | 19.030 | 113, 226, 228 *, 229 |
| Benzo[b]fluoranthene | 20.794 | 126, 250, 252 *, 253 |
| Benzo[k]fluoranthene | 20.845 | 126, 250, 252 *, 253 |
| Benzo[a]pyrene | 21.391 | 126, 250, 252 *, 253 |
| Indeno[1,2,3-c,d]pyrene | 23.950 | 138, 276 *, 274, 277 |
| Dibenz[a,h]anthracene | 24.040 | 139, 276, 278 *, 279 |
| Benzo[g,h,i]perylene | 24.664 | 138, 274, 276 *, 277 |
| Factors | Levels | |||
|---|---|---|---|---|
| Low (−1) | Central (0) | High (1) | ||
| X1-Volume of 1-dodecanol | 60 | 90 | 120 | |
| X2-Volume of DES2 | 150 | 350 | 550 | |
| X3-Ultrasound time | 1 | 3 | 5 | |
| Runs | X1 | X2 | X3 | Total peak area |
| 1 | 120 | 550 | 3 | 987,898 |
| 2 | 90 | 350 | 3 | 1,147,172 |
| 3 | 60 | 550 | 3 | 1,229,678 |
| 4 | 60 | 150 | 3 | 1,226,731 |
| 5 | 90 | 150 | 5 | 1,074,630 |
| 6 | 120 | 150 | 3 | 624,997 |
| 7 | 90 | 350 | 3 | 1,179,585 |
| 8 | 90 | 350 | 3 | 1,228,592 |
| 9 | 120 | 350 | 1 | 834,673 |
| 10 | 90 | 550 | 5 | 1,086,868 |
| 11 | 90 | 150 | 1 | 823,972 |
| 12 | 90 | 550 | 1 | 1,026,248 |
| 13 | 60 | 350 | 1 | 1,307,096 |
| 14 | 120 | 350 | 5 | 985,991 |
| 15 | 90 | 350 | 3 | 1,199,126 |
| 16 | 90 | 350 | 3 | 1,159,579 |
| 17 | 60 | 350 | 5 | 1,348,729 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 568,267,029,114 | 9 | 63,140,781,013 | 50.20 | <0.0001 ** |
| X1-Volume of 1-dodecanol | 352,243,719,453 | 1 | 352,243,719,453 | 280.07 | <0.0001 ** |
| X2-Volume of DES2 | 42,102,506,380 | 1 | 42,102,506,381 | 33.48 | 0.0007 ** |
| X3-Ultrasound time | 31,780,860,555 | 1 | 31,780,860,555 | 25.27 | 0.0015 ** |
| X1X2 | 32,391,720,529 | 1 | 32,391,720,529 | 25.757 | 0.0014 ** |
| X1X3 | 3,007,699,806 | 1 | 3,007,699,806 | 2.39 | 0.1659 |
| X2X3 | 9,028,610,361 | 1 | 9,028,610,361 | 7.18 | 0.0316 * |
| X12 | 2,557,585,467 | 1 | 2,557,585,467 | 2.038 | 0.1969 |
| X22 | 83,518,149,657 | 1 | 83,518,149,657 | 66.40 | <0.0001 ** |
| X32 | 6,418,184,246 | 1 | 6,418,184,246 | 5.10 | 0.0584 |
| Residual | 8,803,979,294 | 7 | 1,257,711,328 | ||
| Lack of Fit | 4,621,628,887 | 3 | 1,540,542,962 | 1.47 | 0.3486 |
| Pure Error | 4,182,350,407 | 4 | 1,045,587,602 | ||
| Core Total | 577,071,008,408 | 16 | |||
| R2 | 0.9847 | ||||
| Adjusted R2 | 0.9651 | ||||
| Predicted R2 | 0.8605 |
| PAH | LR (μg/L) | R2 | LOD (ng/L) | LOQ (ng/L) | %RSD (n = 5) | EF |
|---|---|---|---|---|---|---|
| Naphthalene | 0.03–5.0 | 0.9989 | 6.0 | 19.9 | 3.9 | 164 |
| Acenaphthylene | 0.03–5.0 | 0.9992 | 5.8 | 19.2 | 4.8 | 157 |
| Acenaphthene | 0.02–5.0 | 0.9991 | 3.5 | 11.8 | 4.3 | 160 |
| Fluorene | 0.03–5.0 | 0.9992 | 4.4 | 14.6 | 3.1 | 169 |
| Phenanthrene | 0.03–5.0 | 0.9973 | 6.3 | 21.1 | 4.2 | 165 |
| Anthracene | 0.05–5.0 | 0.9968 | 9.3 | 31.0 | 5.6 | 172 |
| Fluoranthene | 0.03–5.0 | 0.9997 | 4.5 | 14.9 | 2.8 | 152 |
| Pyrene | 0.03–5.0 | 0.9994 | 3.9 | 13.1 | 3.8 | 175 |
| Benz[a]anthracene | 0.03–5.0 | 0.9985 | 5.3 | 17.5 | 4.9 | 148 |
| Chrysene | 0.03–5.0 | 0.9984 | 4.8 | 16.1 | 5.4 | 154 |
| Benzo[b]fluoranthene | 0.05–5.0 | 0.9958 | 8.7 | 29.0 | 4.7 | 163 |
| Benzo[k]fluoranthene | 0.05–5.0 | 0.9965 | 9.1 | 30.5 | 5.9 | 146 |
| Benzo[a]pyrene | 0.05–5.0 | 0.9983 | 9.7 | 32.4 | 3.7 | 158 |
| Indeno[1,2,3-c,d]pyrene | 0.08–5.0 | 0.9936 | 14.1 | 46.9 | 5.5 | 149 |
| Dibenz[a,h]anthracene | 0.08–5.0 | 0.9943 | 12.8 | 42.7 | 6.1 | 142 |
| Benzo[g,h,i]perylene | 0.08–5.0 | 0.9977 | 11.7 | 38.9 | 5.2 | 151 |
| PAH | Spiked (μg/L) | Tap Water | River Water | ||||
|---|---|---|---|---|---|---|---|
| Found (μg/L) | %RR a | %RSD (n = 3) | Found (μg/L) | %RR | %RSD (n = 3) | ||
| Naphthalene | 0 | nd b | — | — | 0.23 | — | — |
| 2 | 2.07 | 103.50 | 3.8 | 2.27 | 102.00 | 4.2 | |
| 5 | 4.89 | 97.80 | 3.6 | 5.15 | 98.40 | 3.8 | |
| Acenaphthylene | 0 | nd | — | — | nd | — | — |
| 2 | 1.89 | 94.50 | 5.0 | 1.93 | 96.50 | 4.9 | |
| 5 | 4.69 | 93.80 | 4.6 | 4.96 | 99.20 | 4.8 | |
| Acenaphthene | 0 | nd | — | — | 0.19 | — | — |
| 2 | 1.99 | 99.50 | 4.1 | 2.14 | 97.50 | 3.8 | |
| 5 | 4.76 | 95.20 | 3.7 | 5.03 | 96.80 | 4.2 | |
| Fluorene | 0 | nd | — | — | nd | — | — |
| 2 | 1.97 | 98.50 | 3.1 | 1.96 | 98.00 | 2.6 | |
| 5 | 5.08 | 101.60 | 2.7 | 5.16 | 103.20 | 2.9 | |
| Phenanthrene | 0 | nd | — | — | nd | — | — |
| 2 | 2.10 | 105.00 | 4.3 | 2.05 | 102.50 | 4.5 | |
| 5 | 4.89 | 97.80 | 4.0 | 5.03 | 100.60 | 4.1 | |
| Anthracene | 0 | nd | — | — | nd | — | — |
| 2 | 1.91 | 95.50 | 5.8 | 2.05 | 102.50 | 5.4 | |
| 5 | 4.81 | 96.20 | 5.2 | 4.96 | 99.20 | 5.6 | |
| Fluoranthene | 0 | nd | — | — | nd | — | — |
| 2 | 1.97 | 98.50 | 2.6 | 1.89 | 94.50 | 2.9 | |
| 5 | 5.08 | 101.60 | 2.9 | 4.99 | 99.80 | 3.1 | |
| Pyrene | 0 | nd | — | — | nd | — | — |
| 2 | 1.98 | 99.00 | 3.6 | 1.97 | 98.50 | 3.9 | |
| 5 | 4.79 | 95.80 | 3.7 | 5.08 | 101.60 | 4.1 | |
| Benz[a]anthracene | 0 | nd | — | — | nd | — | — |
| 2 | 1.94 | 97.00 | 4.9 | 1.91 | 95.50 | 4.8 | |
| 5 | 5.06 | 101.20 | 4.6 | 4.83 | 96.60 | 5.1 | |
| Chrysene | 0 | nd | — | — | nd | — | — |
| 2 | 1.93 | 96.50 | 4.7 | 2.03 | 101.50 | 4.9 | |
| 5 | 4.89 | 97.80 | 5.2 | 4.70 | 94.00 | 5.5 | |
| Benzo[b]fluoranthene | 0 | nd | — | — | nd | — | — |
| 2 | 2.02 | 101.00 | 4.9 | 2.07 | 103.50 | 4.6 | |
| 5 | 5.23 | 104.60 | 4.6 | 4.98 | 99.60 | 4.3 | |
| Benzo[k]fluoranthene | 0 | nd | — | — | nd | — | — |
| 2 | 1.98 | 99.00 | 5.6 | 1.96 | 98.00 | 5.8 | |
| 5 | 5.26 | 105.20 | 5.9 | 5.12 | 102.40 | 6.2 | |
| Benzo[a]pyrene | 0 | nd | — | — | nd | — | — |
| 2 | 2.06 | 103.00 | 3.6 | 1.99 | 99.50 | 4.2 | |
| 5 | 4.91 | 98.20 | 4.0 | 5.18 | 103.60 | 3.8 | |
| Indeno[1,2,3-c,d]pyrene | 0 | nd | — | — | nd | — | — |
| 2 | 1.95 | 97.50 | 5.6 | 2.09 | 104.50 | 5.7 | |
| 5 | 5.04 | 100.80 | 5.4 | 4.89 | 97.80 | 5.9 | |
| Dibenz[a,h]anthracene | 0 | nd | — | — | nd | — | — |
| 2 | 2.04 | 102.00 | 5.9 | 1.95 | 97.50 | 6.2 | |
| 5 | 4.92 | 98.40 | 6.0 | 5.16 | 103.20 | 6.4 | |
| Benzo[g,h,i]perylene | 0 | nd | — | — | nd | — | — |
| 2 | 1.89 | 94.50 | 4.9 | 2.05 | 102.50 | 4.9 | |
| 5 | 4.86 | 97.20 | 4.7 | 5.01 | 100.20 | 5.1 | |
| Extraction Method | Detection Method | Matrix | Extractant | Dispersant | LOD (μg/L) | %RSD | EF | Reference |
|---|---|---|---|---|---|---|---|---|
| SD- DLLME a | GC-MS | River water | Mixed solvent (Methylene chloride:n-hexane = 1:1, molar ratio) | Acetonitrile | 0.0021– 0.0136 | 5.8– 10.9 | 94.9–103 | [8] |
| USA- DLLME b | HPLC-UV | Effluent | DES (Thymol: ± Camphor = 1:1, molar ratio) | Acetonitrile | 0.0039– 0.0098 | 2.20– 6.09 | — | [20] |
| ELLME- DES c | HPLC-UV | Tap water, industrial wastewater | DES (Chcl:phenol = 1:2, molar ratio) | Tetrahydrofuran | 0.09–0.7 | — | 151–170 | [25] |
| DLLME- SFOD | HPLC-UV | Wastewater, lake water, tap water | 1-dodecanol | Methanol | 0.045–1.1 | 1.3–4.4 | 88–118 | [31] |
| IL- DLLME d | HPLC-FLD | Tea infusions | Ionic liquid ([MOEDEA][FAP]) f | Acetonitrile | 0.002–0.004 | 1.9–4.7 | 61–94 | [32] |
| AA-LLME-SFDES e | HPLC-UV | Tea infusions | DES (DL-methol:dedecanoic acid = 3:1, molar ratio) | — g | 0.16–0.75 | 0.9–2.3 | 15–18 | [33] |
| DLLME- SFOD | GC-MS | Tap water, river water | 1-dodecanol | DES (ChCl:acetic acid = 1:2, molar ratio) | 0.0035– 0.0141 | 2.8–6.1 | 142–175 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Hu, J.; Li, X. Analysis of Polycyclic Aromatic Hydrocarbons in Water Samples Using Deep Eutectic Solvent as a Dispersant in Dispersive Liquid–Liquid Microextraction Based on the Solidification of Floating Organic Droplet. Water 2023, 15, 2579. https://doi.org/10.3390/w15142579
Peng C, Hu J, Li X. Analysis of Polycyclic Aromatic Hydrocarbons in Water Samples Using Deep Eutectic Solvent as a Dispersant in Dispersive Liquid–Liquid Microextraction Based on the Solidification of Floating Organic Droplet. Water. 2023; 15(14):2579. https://doi.org/10.3390/w15142579
Chicago/Turabian StylePeng, Chunlong, Jinfeng Hu, and Xin Li. 2023. "Analysis of Polycyclic Aromatic Hydrocarbons in Water Samples Using Deep Eutectic Solvent as a Dispersant in Dispersive Liquid–Liquid Microextraction Based on the Solidification of Floating Organic Droplet" Water 15, no. 14: 2579. https://doi.org/10.3390/w15142579
APA StylePeng, C., Hu, J., & Li, X. (2023). Analysis of Polycyclic Aromatic Hydrocarbons in Water Samples Using Deep Eutectic Solvent as a Dispersant in Dispersive Liquid–Liquid Microextraction Based on the Solidification of Floating Organic Droplet. Water, 15(14), 2579. https://doi.org/10.3390/w15142579





