Diatom Voucher Flora and Comparison of Collection and Taxonomic Methods for Biodiversity Hotspot Upper Three Runs Creek
Abstract
:1. Introduction
Past Monitoring of Upper Three Runs Biodiversity
2. Materials and Methods
2.1. Study Site
2.2. Sampling
2.3. Laboratory Methods
2.3.1. Processing
2.3.2. Enumeration
2.3.3. Data and Statistical Analysis
3. Results
3.1. Physicochemical Results
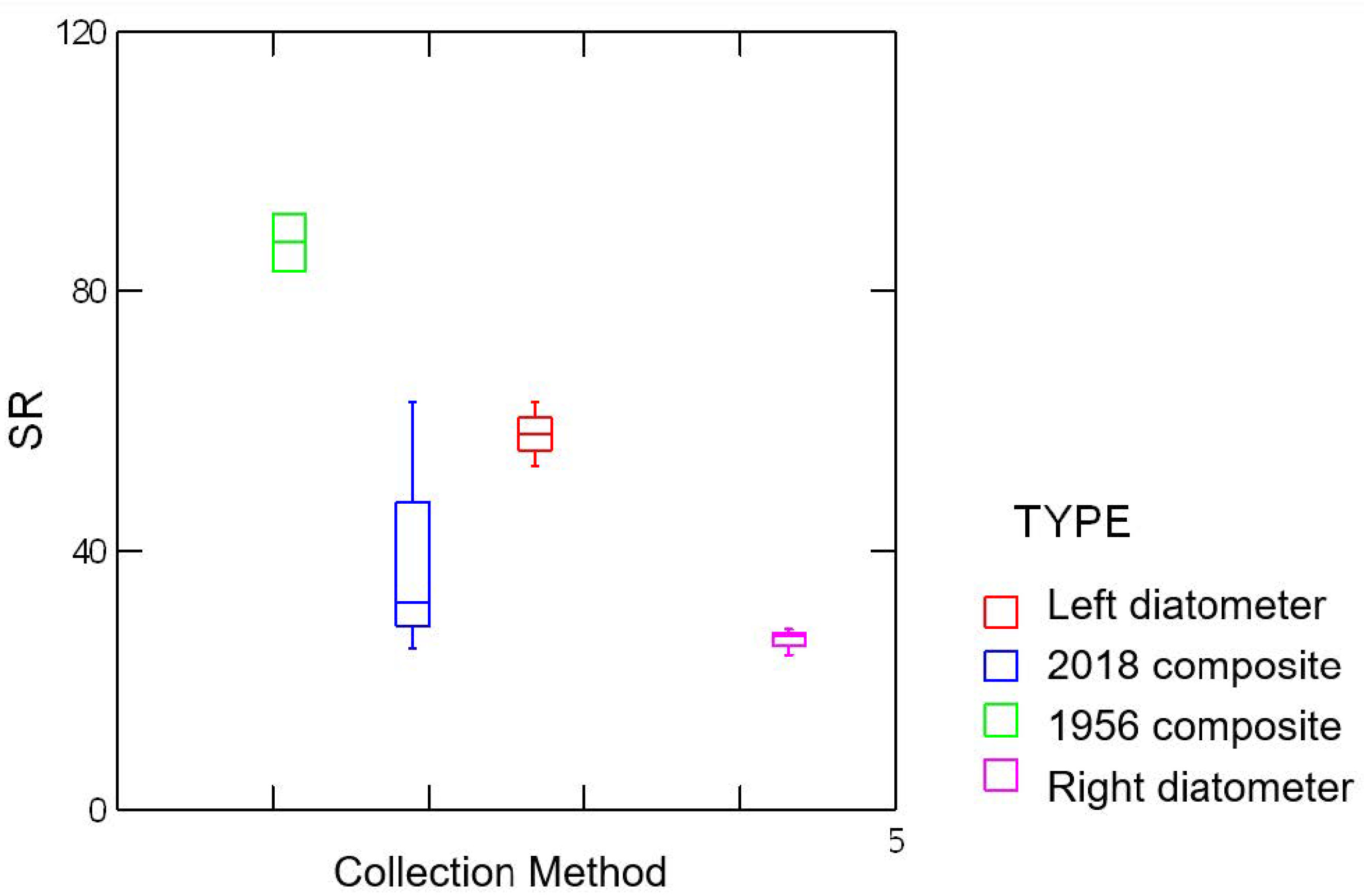
3.2. Biodiversity
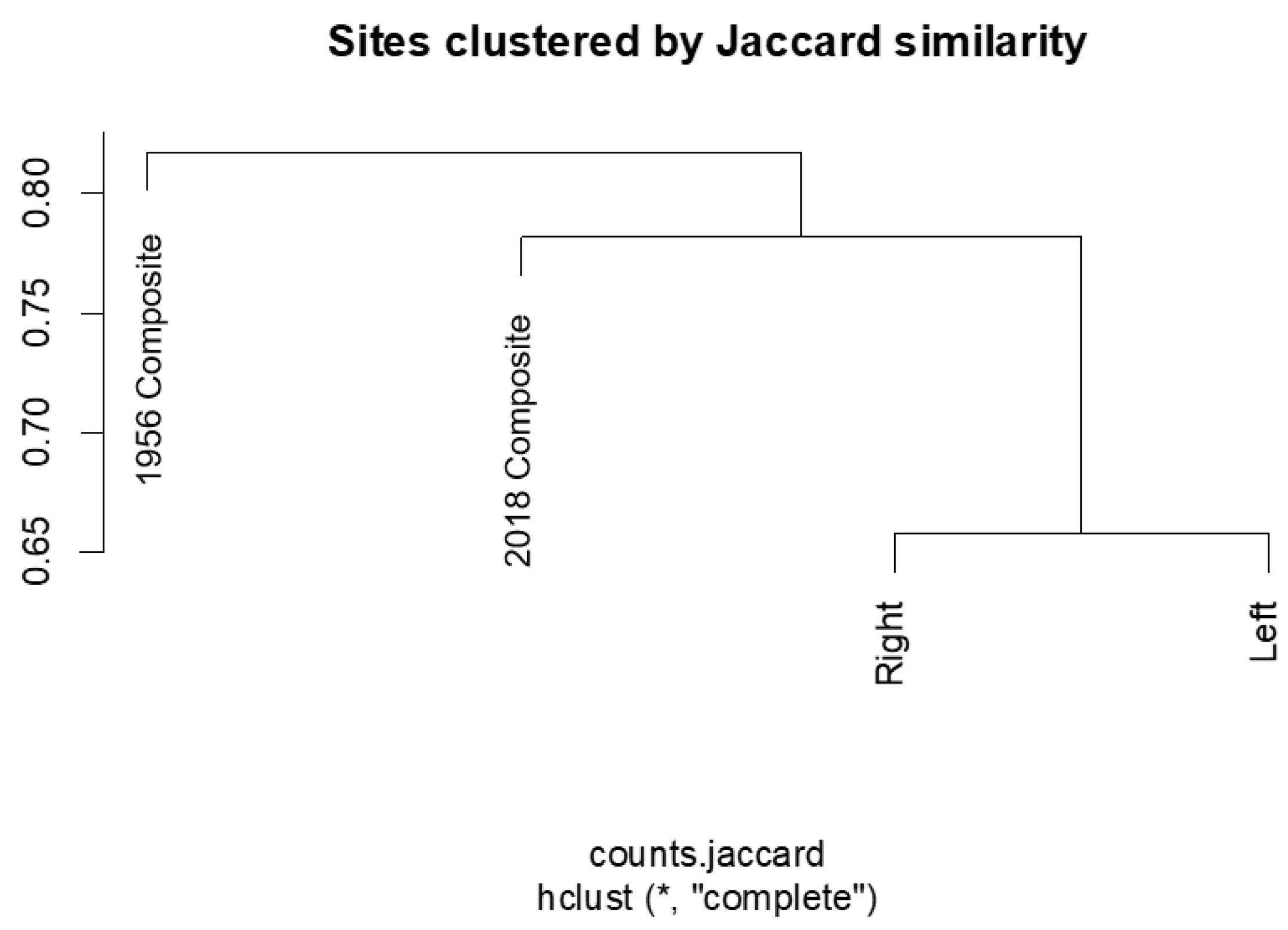
3.3. Similarity
3.4. Relative Abundance and Biological Indication
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butcher, R.W. Studies in the Ecology of Rivers: VII. The Algae of Organically Enriched Waters. J. Ecol. 1947, 35, 186–191. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Pan, Y.; Van Dam, H. Assessing Environmental Conditions in Rivers and Streams with Diatoms. The Diatoms: Applications for the Environmental and Earth Sciences; Cambridge University Press: Cambridge, UK, 1999; Volume 1. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Smol, J.P. Use of Algae in Environmental Assessments. In Freshwater Algae of North America: Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 921–962. ISBN 9780123858764. [Google Scholar]
- Spaulding, S.A.; Potapova, M.G.; Bishop, I.W.; Lee, S.S.; Gasperak, T.S.; Jovanoska, E.; Furey, P.C.; Edlund, M.B. Diatoms.org: Supporting taxonomists, connecting communities. Diatom Res. 2021, 36, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Alers-García, J.; Lee, S.S.; Spaulding, S.A. Resources and Practices to Improve Diatom Data Quality. Limnol. Oceanogr. Bull. 2021, 30, 48–53. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Pan, Y.; Manoylov, K.M.; Parker, C.A.; Larsen, D.P.; Herlihy, A.T. Development of diatom indicators of ecological conditions for streams of the western US. J. N. Am. Benthol. Soc. 2008, 27, 1000–1016. [Google Scholar] [CrossRef]
- Voelz, N.J.; McArthur, J.V. An exploration of factors influencing lotic insect species richness. Biodivers. Conserv. 2000, 9, 1543–1570. [Google Scholar] [CrossRef]
- Army Corps of Engineers. Savannah River Basin Water Management Frequently Asked Questions (FAQs). Available online: https://www.sas.usace.army.mil/Portals/61/docs/WaterMgmt/WaterMgmt%20FAQ.pdf (accessed on 12 May 2023).
- Patrick Center for Environmental Research. 1996 Savannah River Biological Surveys for Westinghouse Savannah River Company; Report No. 97-14F; Academy of Natural Sciences of Philadelphia: Philadelphia, PA, USA, 1997; 141p. [Google Scholar]
- Bouchard, R.; Acker, F.; Grant, R.J.; Jackson, J.; Horwitz, R.; Davis, R.; Thomas, R.; McNair, J. 2000 Savannah River Biological Surveys for Westinghouse Savannah River Company; Report No. 01-16F; PCER, ANSP: Philadelphia, PA, USA, 2001. [Google Scholar]
- U.S. Department of Energy, Office of Environmental Management. Savannah River Site Strategic Vision: 2022–2032. Available online: https://www.energy.gov/em/articles/savannah-river-site-strategic-vision-2022-2032 (accessed on 12 May 2023).
- Patrick, R.; Reimer, C.W. The Diatoms of the United States, Exclusive of Alaska and Hawaii: Fragilariaceae, Eunotiaceae, Achnanthaceae, Naviculacae; Academy of Natural Sciences: Philadelphia, PA, USA, 1966; Volume 1, pp. 112–336. [Google Scholar]
- Patrick, R.; Reimer, C.W. The Diatoms of the United States, Exclusive of Alaska and Hawaii: Entomoneidaceae, Cymbellaceae, Gomphonemaceae, Epithemiaceae; Monographs of the Academy of Natural Sciences of Philadelphia: Philadelphia, PA, USA, 1975; Volume 2, pp. 107–162. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Epithemiaceae, Bacillariaceae, Surirellaceae; Veb Gustav Fisher Verlag: Jena, Germany, 1988; Volume 2, pp. 394–425. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema; Veb Gustav Fisher Verlag: Jena, Germany, 1991; Volume 4, pp. 750–778. [Google Scholar]
- Levkov, Z.; Danijela, M.K.; Reichardt, E. Diatoms of Europe. Diatoms of the European Inland Waters and Comparable Habitats, Gomphonema; Koeltz Botanical Books: Hesse, Germany, 2016; Volume 8, pp. 2–546. [Google Scholar]
- Potapova, M.; Charles, D.F. Diatom metrics for monitoring eutrophication in rivers of the United States. Ecol. Indic. 2007, 7, 48–70. [Google Scholar] [CrossRef]
- Bishop, I.W.; Esposito, R.M.; Tyree, M.; Spaulding, S.A. A diatom voucher flora from selected southeast rivers (USA). Phytotaxa 2017, 332, 101–140. [Google Scholar] [CrossRef]
- Moseley, R.C.; Manoylov, K.M. Diatom community composition from low human impact areas in southeast U.S. Ga. J. Sci. 2012, 70, 123. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005; Volume 21, p. 1504. [Google Scholar]
- Stevenson, R.J.; Bahls, L.L. Periphyton Protocols. Chapter 6. In Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers; Mt Barbour, B., Gerritsen, J., Snyder, B.D., Stribling, J.B., Eds.; EPA/841-B-99-002; US Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999; p. 23. [Google Scholar]
- Johnson, K. Diatom Voucher Flora and Comparison of Collection Methods for Biodiversity Hotspot upper Three Runs Creek 2020. Biology Theses, Georgia College & State University. Available online: https://kb.gcsu.edu/biology/7 (accessed on 1 June 2023).
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Jaccard, P. Étude comparative de la distribution florale dans une portion des Alpes et du Jura. Bull. Soc. Vaud. Sci. Nat. 1901, 37, 547–579. [Google Scholar]
- Sörensen, T. Method of establishing groups of equal amplitude in plant sociology based on similarity of species content. K. Dan. Vidensk. Selskab. 1948, 4, 1–34. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 12 May 2023).
- Oksanen, J. Vegan: Ecological Diversity. R Proj. 2013, 368, 1–11. [Google Scholar]
- Wilkinson, L. Systat, the System for Statistics; SYSTAT, Inc.: Evanston, IL, USA, 1989. [Google Scholar] [CrossRef]
- Academy of Natural Sciences Report. Savannah River Biological Surveys, South Carolina and Georgia, August–September 1955, May 1956. Progress Report for E.I. du Pont de Nemours and Company, Savannah River Plant; ANSP: Philadelphia, PA, USA, 1957. [Google Scholar]
- Abarca, N.; Jahn, R.; Zimmermann, J.; Enke, N. Does the Cosmopolitan Diatom Gomphonema parvulum (Kützing) Kützing Have a Biogeography? PLoS ONE 2014, 9, e86885. [Google Scholar] [CrossRef]
- Abarca, N.; Zimmermann, J.; Kusber, W.-H.; Mora, D.; Van, A.T.; Skibbe, O.; Jahn, R. Defining the core group of the genus Gomphonema Ehrenberg with molecular and morphological methods. Bot. Lett. 2020, 167, 114–159. [Google Scholar] [CrossRef]
- Andrejić, J.Z.; Spaulding, S.A.; Manoylov, K.M.; Edlund, M.B. Phenotypic plasticity in diatoms: Janus cells in four Gomphonemataxa. Diatom Res. 2018, 33, 453–470. [Google Scholar] [CrossRef]
- Kermarrec, L.; Bouchez, A.; Rimet, F.; Humbert, J.-F. First Evidence of the Existence of Semi-Cryptic Species and of a Phylogeographic Structure in the Gomphonema parvulum (Kützing) Kützing Complex (Bacillariophyta). Protist 2013, 164, 686–705. [Google Scholar] [CrossRef]
- Mcbride, S.A.; Edgar, R.K. Janus cells unveiled: Frustular morphometric variability in Gomphonema angustatum. Diatom Res. 1998, 13, 293–310. [Google Scholar] [CrossRef]
- Gaiser, E.E.; Johansen, J. Freshwater diatoms from Carolina Bays and other isolated wetlands on the Atlantic coastal plain of South Carolina, USA, with descriptions of seven taxa new to science. Diatom Res. 2000, 15, 75–130. [Google Scholar] [CrossRef]
- Johnson, K. Gomphonema lagenula. In Diatoms of North America. Available online: https://diatoms.org/species/gomphonema-lagenula (accessed on 12 May 2023).
- Rose, D.T.; Cox, E.J. What constitutes Gomphonema parvulum? Long-term culture studies show that some varieties of G. parvulum belong with other Gomphonema species. Plant Ecol. Evol. 2014, 147, 366–373. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Bothwell, M.L.; Lowe, R.L. Algal Ecology: Freshwater Benthic Ecosystem; Academic Press: Cambridge, MA, USA, 1996; pp. 3–30. [Google Scholar] [CrossRef]
- Furey, P.C.; Lowe, R.L.; Johansen, J.R. Eunotia Ehrenberg (Bacillariophyta) of the Great Smoky Mountains National Park, USA. Bibl. Diatomol. 2011, 56, 1–134. [Google Scholar]
- Barinova, S.S.; Medvedeva, L.A.; Anissimova, O.V. Diversity of Algal Indicators in Environmental Assessment; Pilies Studio: Tel Aviv, Israel, 2006; p. 498. [Google Scholar]
- Lange-Bertalot, H.; Bak, M.; Witkowski, A.; Tagliaventi, N. Diatoms of Europe: Eunotia and Some Related Genera; Gantner Publishing: Nueziders, Austria, 2011; Volume 6, pp. 2–747. [Google Scholar]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar]
- Siver, P.A. Diatoms of North America. The freshwater flora of Cape Cod. Iconogr. Diatomol. 2005, 14, 463. [Google Scholar]
- Lowe, R.L. Environmental Requirements and Pollution Tolerance of Freshwater Diatoms; National Environmental Research Center, Office of Research and Development, US Environmental Protection Agency: Cincinnati, OH, USA, 1974; Volume 1, pp. 1–334. [Google Scholar]
- Cantonati, M.; Kelly, M.G.; Lange-Bertalot, H. Freshwater Benthic Diatoms of Central Europe. Over 800 Common Species Used in Ecological Assessment. English Edition with Updated Taxonomy and Added Species; Koeltz Botanical Books: Hesse, Germany, 2017; pp. 2–546. ISBN 978-3-946583-06-6. [Google Scholar]
- Goldsborough, L.G.; Robinson, G.G.C. Seasonal succession of diatom epiphyton on dense mats of Lemna minor L. Can. J. Bot. 1985, 63, 2332–2339. [Google Scholar] [CrossRef]
- Jüttner, I.; Ector, L.; Reichardt, E.; Van de Vijver, B.; Jarlman, A.; Krokowski, J.; Cox, E.J. Gomphonema varioreduncum sp. nov., a new species from northern and western Europe and a re-examination of Gomphonema exilissimum. Diatom Res. 2013, 28, 303–316. [Google Scholar] [CrossRef]
- Cox, E.J. Morphogenetic information and the selection of taxonomic characters for raphid diatom systematics. Plant Ecol. Evol. 2010, 143, 271–277. [Google Scholar] [CrossRef]

| Sampling Date | DO (%) | DO (mg/L) | pH | Conductivity (mS/cm) | Depth (cm) | Temperature (°C) |
|---|---|---|---|---|---|---|
| 23 March deployment | 106.4 * | 11.09 | 6.46 | 13 | 140 | 13.5 |
| 9 April retrieval | 99.1 | 10.12 | 5.28 | 0.019 * | 177 | 14.45 |
| 15 August deployment | 88.7 | 7.85 | 4.17 | 15 | 177 | 21.33 |
| 30 August retrieval | 53.1 | 4.69 | 4.55 | 15 | 177 | 21.43 |
| Sample | Species Richness | SWDI (H’) | Pielou’s Evenness (J’) |
|---|---|---|---|
| 2018 c | 111 | 3.242 | 0.688 |
| Right d | 67 | 3.263 | 0.776 |
| Left d | 129 | 3.661 | 0.753 |
| 1956 c | 153 | 4.342 | 0.863 |
| Sample | Species Richness | SWDI (H’) | Pielou’s Evenness (J’) |
|---|---|---|---|
| 2018 c | 89 | 2.320 | 0.517 |
| Right d | 42 | 2.217 | 0.593 |
| Left d | 103 | 3.138 | 0.677 |
| 1956 c | 136 | 4.09 | 0.833 |
| Sample Pair | Jaccard Similarity | Jaccard Distance | Sorenson |
|---|---|---|---|
| 2018 c & Right d | 0.219 | 0.781 | 0.360 |
| 2018 c & Left d | 0.218 | 0.782 | 0.358 |
| Right d & Left d | 0.347 | 0.658 | 0.510 |
| 2018 c & 1956 c | 0.211 | 0.789 | 0.348 |
| 1956 c & Left d | 0.236 | 0.763 | 0.383 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, K.M.; Gaiser, E.; Manoylov, K.M. Diatom Voucher Flora and Comparison of Collection and Taxonomic Methods for Biodiversity Hotspot Upper Three Runs Creek. Water 2023, 15, 2578. https://doi.org/10.3390/w15142578
Johnson KM, Gaiser E, Manoylov KM. Diatom Voucher Flora and Comparison of Collection and Taxonomic Methods for Biodiversity Hotspot Upper Three Runs Creek. Water. 2023; 15(14):2578. https://doi.org/10.3390/w15142578
Chicago/Turabian StyleJohnson, Katherine M., Evelyn Gaiser, and Kalina M. Manoylov. 2023. "Diatom Voucher Flora and Comparison of Collection and Taxonomic Methods for Biodiversity Hotspot Upper Three Runs Creek" Water 15, no. 14: 2578. https://doi.org/10.3390/w15142578





