Advances in the Efficient Enrichment of Anammox Bacteria
Abstract
:1. Introduction
2. Physiological Properties of Anammox Bacteria
2.1. Types of Anammox Bacteria
2.2. Environmental Factors Affecting the Growth and Adaptation of Anammox Bacteria
2.3. Short-Term Starvation Tolerance of Bacteria
3. Strategies for Accelerating the Enrichment of Anammox Bacterial Activity
3.1. Process Regulation
3.1.1. Addition of Carbon/Charcoal-Containing Material
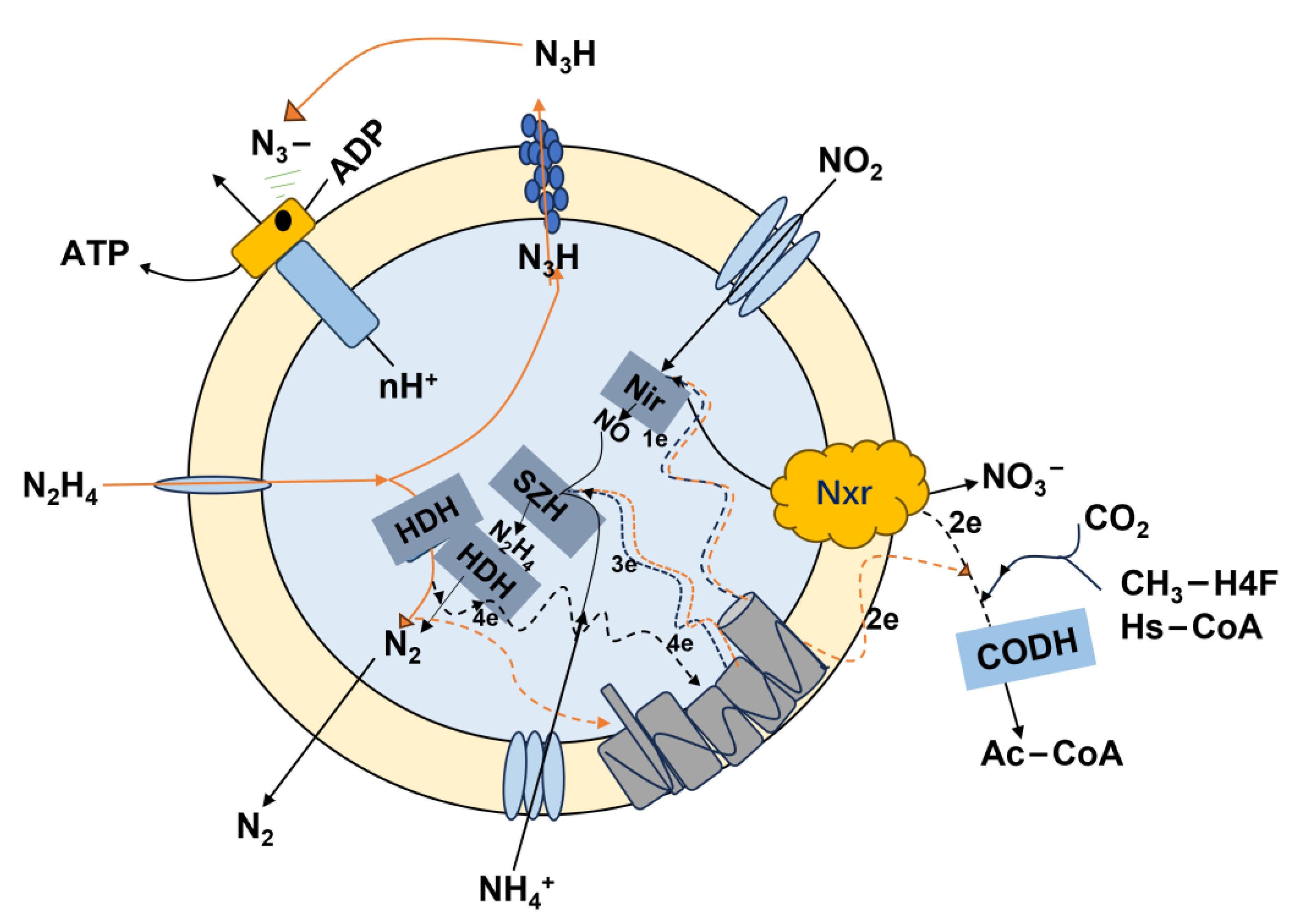
3.1.2. Addition of Hydrazine N2H4
3.1.3. Add Tourmaline
3.1.4. New Reactor Granules Circulating EGSB (EGSBGC)
3.1.5. Addition of Biological Carriers
3.2. Building Granulation Models
3.3. Biomass Management Strategies for Suspended Sludge
3.4. Strain Preservation
3.4.1. Bacterial Dormancy
3.4.2. Activation Recovery
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full form |
| Anammox | anaerobic ammonia oxidation |
| SNAP | single-stage short-course nitrification-anammox |
| PDA | partial nitrification-anammox |
| OLAND | restricted autotrophic nitrification-denitrification |
| CANON | total autotrophic denitrification |
| SNAD-DPR | short-course nitrification/anammox/denitrification coupled-denitrification phosphorus removal |
| OUT | operational taxonomic units |
| DNA | deoxyribonucleic acid |
| RNA | ribonucleic acid |
| rRNA | ribosomal RNA |
| pH | pondus hydrogenii |
| FNA | free nitrous acid |
| DS-EPSCN | denitrification sludge EPS enhanced |
| HRT | hydraulic retention time |
| NLR | N loading rate |
| DO | dissolved oxygen |
| NZVI | nano zero-valent iron |
| Nir | nitrite reductase |
| HZS | hydrazine synthetase |
| HDH | hydrazine dehydrogenase |
| Nxr | nitrite oxidoreductase |
| EPS | extracellular polymeric substances |
| ATP | adenosine triphosphate |
| RGO | reduced graphene oxide |
| GAC | granular activated carbons |
| NRR | a nitrogen removal rate |
| AOB | ammonia-oxidizing bacteria |
| NOB | nitrite-oxidizing bacteria |
| EGSBGC | granules circulating EGSB |
| UASB | up-flow anaerobic sludge blanket |
| EGSB | expanded granular sludge bed reactor |
| MBR | membrane bioreactor |
| ABR | anaerobic baffled reactor |
| UBF | up-flow blanket filter |
| SBBR | sequencing biofilm batch reactor |
| RBC | rotating biological contactor |
| PN/PS | protein/polysaccharide |
| AHLs | adding high serine lactones |
| PVA | polyvinyl alcohol |
| SA | sodium alginate |
| PVA/CS/Fe | polyvinyl alcohol/chitosan/iron |
| DB | denitrifying bacteria |
| A2O-BCO | anaerobic-anoxic-aerobic combined biological contact oxidation |
| NTR | nitrate-nitrite conversion ratio |
| SAD | sulfur-driven autotrophic denitrification |
| PSAD | partial S (0)-driven autotrophic denitrification |
| TNRE | total nitrogen removal efficiency |
| CODH | CO dehydrogenase/acetyl coenzyme A (Ac-CoA) synthase |
| CH3-H4 | methyltet-rahydrofolate |
| FDH | formic acid dehydrogenase |
| Ac-CoA | dehydrogenase/acetyl coenzyme A |
References
- Chen, S.; Liu, T.; Zheng, Z.; Ishaq, M.; Liang, G.; Fan, P.; Chen, T.; Tang, J. Recent progress and perspectives on Sb2Se3-based photocathodes for solar hydrogen production via photoelectrochemical water splitting. J. Energy Chem. 2022, 67, 508–523. [Google Scholar] [CrossRef]
- Padervand, M.; Asgarpour, F.; Akbari, A.; Eftekhari Sis, B.; Lammel, G. Hexagonal Core–Shell SiO2[–MOYI]Cl–]Ag Nanoframeworks for Efficient Photodegradation of the Environmental Pollutants and Pathogenic Bacteria. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1314–1323. [Google Scholar] [CrossRef]
- Karimidastenaei, Z.; Klöve, B.; Sadegh, M.; Haghighi, A.T. Polar Ice as an Unconventional Water Resource: Opportunities and Challenges. Water 2021, 13, 3220. [Google Scholar] [CrossRef]
- Yokota, N.; Mineshima, R.; Watanabe, Y.; Tokutomi, T.; Kiyokawa, T.; Nishiyama, T.; Fujii, T.; Furukawa, K. Startup of pilot-scale single-stage nitrogen removal using anammox and partial nitritation (SNAP) reactor for waste brine treatment using marine anammox bacteria. J. Biosci. Bioeng. 2021, 132, 505–512. [Google Scholar] [CrossRef]
- Qiu, J.; Li, X.; Peng, Y.; Jiang, H. Advanced nitrogen removal from landfill leachate via a two-stage combined process of partial nitrification-Anammox (PNA) and partial denitrification-Anammox (PDA). Sci. Total Environ. 2022, 810, 151186. [Google Scholar] [CrossRef]
- Valverde-Pérez, B.; Mauricio-Iglesias, M.; Sin, G. Systematic design of an optimal control system for the SHARON-Anammox process. J. Process Control 2016, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Nhu Hien, N.; Van Tuan, D.; Nhat, P.T.; Thi Thanh Van, T.; Van Tam, N.; Xuan Que, V.O.N.; Phuoc Dan, N. Application of Oxygen Limited Autotrophic Nitritation/Denitrification (OLAND) for anaerobic latex processing wastewater treatment. Int. Biodeterior. Biodegrad. 2017, 124, 45–55. [Google Scholar] [CrossRef]
- Gong, S.; Qin, Y.; Zheng, S.; Lu, T.; Yang, X.; Zeng, M.; Zhou, H.; Chen, J.; Huang, W. The rapid start-up of CANON process through adding partial nitration sludge to ANAMMOX system. J. Environ. Manag. 2023, 338, 117821. [Google Scholar] [CrossRef]
- Wen, X.; Zhou, J.; Li, Y.; Qing, X.; He, Q. A novel process combining simultaneous partial nitrification, anammox and denitrification (SNAD) with denitrifying phosphorus removal (DPR) to treat sewage. Bioresour. Technol. 2016, 222, 309–316. [Google Scholar] [CrossRef]
- Yang, X.-R.; Weng, B.-S.; Li, H.; Marshall, C.W.; Li, H.; Chen, Y.-S.; Yu, S.; Zhu, G.-B.; Zhu, Y.-G. An overlooked nitrogen loss linked to anaerobic ammonium oxidation in estuarine sediments in China. J. Soils Sediments 2017, 17, 2537–2546. [Google Scholar] [CrossRef] [Green Version]
- Dongmin, Y.; Jialiang, Z.; Cancan, J.; Danhua, W.; Likun, G.; Shujun, Z.; Huijie, L.; Dongsheng, W.; Shengjun, X.; Zhihui, B.; et al. Fast start-up of anammox process: Effects of extracellular polymeric substances addition on performance, granule properties, and bacterial community structure. J. Environ. Manag. 2023, 338, 117836. [Google Scholar] [CrossRef]
- Zongbao, Y. New System of Anammox: Effect of Added N2H4, NO Removal and Fe(III) Oxidation of NH4+. Ph.D. Thesis, Chongqing University, Chongqing, China, 2015. [Google Scholar]
- Weigang, W.; Tong, W.; Yufei, F.; Yayi, W. Research progress on the aggregation mechanism of anaerobic ammonia oxidation granular sludge. Microbiol. Bull. 2022, 49, 1927–1940. [Google Scholar] [CrossRef]
- Xiaoli, H. Enrichment Culture of Anaerobic Ammonia-Oxidizing Bacteria and Organic Nutrient Characterization of the Dominant Bacterium Jettenia Asiatica. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2015. [Google Scholar]
- Padhy, S.R.; Bhattacharyya, P.; Dash, P.K.; Nayak, S.K.; Baig, M.J.; Swain, P.; Mohapatra, T. Soil Metagenome Revealed Contrasting Anammox Bacterial Diversity in Coastal Mangrove and Rice Ecology. Geomicrobiol. J. 2022, 39, 659–668. [Google Scholar] [CrossRef]
- Strous, M.; Fuerst, J.A.; Kramer, E.H.; Logemann, S.; Muyzer, G.; van de Pas-Schoonen, K.T.; Webb, R.; Kuenen, J.G.; Jetten, M.S. Missing lithotroph identified as new planctomycete. Nature 1999, 400, 446–449. [Google Scholar] [CrossRef] [Green Version]
- Kartal, B.; van Niftrik, L.; Rattray, J.; van de Vossenberg, J.L.; Schmid, M.C.; Sinninghe Damste, J.; Jetten, M.S.; Strous, M. Candidatus ‘Brocadia fulgida’: An autofluorescent anaerobic ammonium oxidizing bacterium. FEMS Microbiol. Ecol. 2008, 63, 46–55. [Google Scholar] [CrossRef]
- Hu, B.L.; Zheng, P.; Tang, C.J.; Chen, J.W.; van der Biezen, E.; Zhang, L.; Ni, B.J.; Jetten, M.S.; Yan, J.; Yu, H.Q.; et al. Identification and quantification of anammox bacteria in eight nitrogen removal reactors. Water Res. 2010, 44, 5014–5020. [Google Scholar] [CrossRef]
- Araujo, J.C.; Campos, A.C.; Correa, M.M.; Silva, E.C.; Matte, M.H.; Matte, G.R.; Von Sperling, M.; Chernicharo, C.A. Anammox bacteria enrichment and characterization from municipal activated sludge. Water Sci. Technol. 2011, 64, 1428–1434. [Google Scholar] [CrossRef] [Green Version]
- Vanotti, M.B.; Fujii, T.; Szogi, A.A.; Rothrock, M.J.; García-González, M.C.; Kunz, A.; Aloy, A.M.; Furukawa, K. Experiences with anammox in the USA: Isolation, preservation and treatment performance of Brocadia caroliniensis. In Proceedings of the First International Anammox Symposium 2011 (IANAS 2011), Kumamoto, Japan, 19–21 May 2011. [Google Scholar]
- Narita, Y.; Zhang, L.; Kimura, Z.I.; Ali, M.; Fujii, T.; Okabe, S. Enrichment and physiological characterization of an anaerobic ammonium-oxidizing bacterium ‘Candidatus Brocadia sapporoensis’. Syst. Appl. Microbiol. 2017, 40, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Quan, Z.X.; Rhee, S.K.; Zuo, J.E.; Yang, Y.; Bae, J.W.; Park, J.R.; Lee, S.T.; Park, Y.H. Diversity of ammonium-oxidizing bacteria in a granular sludge anaerobic ammonium-oxidizing (anammox) reactor. Environ. Microbiol. 2008, 10, 3130–3139. [Google Scholar] [CrossRef]
- Ali, M.; Oshiki, M.; Awata, T.; Isobe, K.; Kimura, Z.; Yoshikawa, H.; Hira, D.; Kindaichi, T.; Satoh, H.; Fujii, T.; et al. Physiological characterization of anaerobic ammonium oxidizing bacterium ‘Candidatus Jettenia caeni’. Environ. Microbiol. 2015, 17, 2172–2189. [Google Scholar] [CrossRef]
- Botchkova, E.A.; Litti, Y.V.; Novikov, A.A.; Grouzdev, D.S.; Bochkareva, E.S.; Beskorovayny, A.V.; Kuznetsov, B.B.; Nozhevnikova, A.N. Description of “Candidatus Jettenia ecosi” sp. nov., a New Species of Anammox Bacteria. Microbiology 2018, 87, 766–776. [Google Scholar] [CrossRef]
- Nikolaev, Y.A.; Kozlov, M.N.; Kevbrina, M.V.; Dorofeev, A.G.; Pimenov, N.V.; Kallistova, A.Y.; Grachev, V.A.; Kazakova, E.A.; Zharkov, A.V.; Kuznetsov, B.B.; et al. Candidatus “Jettenia moscovienalis” sp. nov., a new species of bacteria carrying out anaerobic ammonium oxidation. Microbiology 2015, 84, 256–262. [Google Scholar] [CrossRef]
- Kartal, B.; Rattray, J.; van Niftrik, L.A.; van de Vossenberg, J.; Schmid, M.C.; Webb, R.I.; Schouten, S.; Fuerst, J.A.; Damste, J.S.; Jetten, M.S.; et al. Candidatus “Anammoxoglobus propionicus” a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Syst. Appl. Microbiol. 2007, 30, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, F.; Gong, Z.; Meng, F.; Chen, H.; Xue, Y.; Furukawa, K. Application of anaerobic ammonium-oxidizing consortium to achieve completely autotrophic ammonium and sulfate removal. Bioresour. Technol. 2008, 99, 6817–6825. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Walsh, K.; Webb, R.; Rijpstra, W.I.; van de Pas-Schoonen, K.; Verbruggen, M.J.; Hill, T.; Moffett, B.; Fuerst, J.; Schouten, S.; et al. Candidatus “Scalindua brodae”, sp. nov., Candidatus “Scalindua wagneri”, sp. nov., two new species of anaerobic ammonium oxidizing bacteria. Syst. Appl. Microbiol. 2003, 26, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Kuypers, M.M.; Sliekers, A.O.; Lavik, G.; Schmid, M.; Jørgensen, B.B.; Kuenen, J.G.; Damsté, J.S.S.; Strous, M.; Jetten, M.S. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 2003, 422, 608–611. [Google Scholar] [CrossRef]
- Woebken, D.; Lam, P.; Kuypers, M.M.; Naqvi, S.W.; Kartal, B.; Strous, M.; Jetten, M.S.; Fuchs, B.M.; Amann, R. A microdiversity study of anammox bacteria reveals a novel Candidatus Scalindua phylotype in marine oxygen minimum zones. Environ. Microbiol. 2008, 10, 3106–3119. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Mu, B.Z.; Gu, J.D. Molecular detection of anaerobic ammonium-oxidizing (anammox) bacteria in high-temperature petroleum reservoirs. Microb. Ecol. 2010, 60, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Brandsma, J.; van de Vossenberg, J.; Risgaard-Petersen, N.; Schmid, M.C.; Engstrom, P.; Eurenius, K.; Hulth, S.; Jaeschke, A.; Abbas, B.; Hopmans, E.C.; et al. A multi-proxy study of anaerobic ammonium oxidation in marine sediments of the Gullmar Fjord, Sweden. Environ. Microbiol. Rep. 2011, 3, 360–366. [Google Scholar] [CrossRef]
- Fuchsman, C.A.; Staley, J.T.; Oakley, B.B.; Kirkpatrick, J.B.; Murray, J.W. Free-living and aggregate-associated Planctomycetes in the Black Sea. FEMS Microbiol. Ecol. 2012, 80, 402–416. [Google Scholar] [CrossRef] [Green Version]
- van de Vossenberg, J.; Rattray, J.E.; Geerts, W.; Kartal, B.; van Niftrik, L.; van Donselaar, E.G.; Sinninghe Damste, J.S.; Strous, M.; Jetten, M.S. Enrichment and characterization of marine anammox bacteria associated with global nitrogen gas production. Environ. Microbiol. 2008, 10, 3120–3129. [Google Scholar] [CrossRef]
- Speth, D.R.; Lagkouvardos, I.; Wang, Y.; Qian, P.Y.; Dutilh, B.E.; Jetten, M.S.M. Draft Genome of Scalindua rubra, Obtained from the Interface Above the Discovery Deep Brine in the Red Sea, Sheds Light on Potential Salt Adaptation Strategies in Anammox Bacteria. Microb. Ecol. 2017, 74, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Oshiki, M.; Mizuto, K.; Kimura, Z.I.; Kindaichi, T.; Satoh, H.; Okabe, S. Genetic diversity of marine anaerobic ammonium-oxidizing bacteria as revealed by genomic and proteomic analyses of ‘Candidatus Scalindua japonica’. Environ. Microbiol. Rep. 2017, 9, 550–561. [Google Scholar] [CrossRef]
- Hong, Y.G.; Li, M.; Cao, H.; Gu, J.D. Residence of habitat-specific anammox bacteria in the deep-sea subsurface sediments of the South China Sea: Analyses of marker gene abundance with physical chemical parameters. Microb. Ecol. 2011, 62, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Twachtmann, U.; Klein, M.; Strous, M.; Juretschko, S.; Jetten, M.; Metzger, J.W.; Schleifer, K.H.; Wagner, M. Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst. Appl. Microbiol. 2000, 23, 93–106. [Google Scholar] [CrossRef]
- Viancelli, A.; Kunz, A.; Esteves, P.A.; Bauermann, F.V.; Furukawa, K.; Fujii, T.; Antônio, R.V.; Vanotti, M. Bacterial biodiversity from an anaerobic up flow bioreactor with ANAMMOX activity inoculated with swine sludge. Braz. Arch. Biol. Technol. 2011, 54, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Khramenkov, S.V.; Kozlov, M.N.; Kevbrina, M.V.; Dorofeev, A.G.; Kazakova, E.A.; Grachev, V.A.; Kuznetsov, B.B.; Polyakov, D.Y.; Nikolaev, Y.A. A novel bacterium carrying out anaerobic ammonium oxidation in a reactor for biological treatment of the filtrate of wastewater fermented sludge. Microbiology 2013, 82, 628–636. [Google Scholar] [CrossRef]
- Weralupitiya, C.; Wanigatunge, R.; Joseph, S.; Athapattu, B.C.L.; Lee, T.H.; Kumar Biswas, J.; Ginige, M.P.; Shiung Lam, S.; Senthil Kumar, P.; Vithanage, M. Anammox bacteria in treating ammonium rich wastewater: Recent perspective and appraisal. Bioresour. Technol. 2021, 334, 125240. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Cheng, Y.-F.; Zhou, Y.-H.; Buayi, X.; Jin, R.-C. A novel strategy for accelerating the recovery of an anammox reactor inhibited by copper(II): EDTA washing combined with biostimulation via low-intensity ultrasound. Chem. Eng. J. 2015, 279, 912–920. [Google Scholar] [CrossRef]
- De Cocker, P.; Bessiere, Y.; Hernandez-Raquet, G.; Dubos, S.; Mozo, I.; Gaval, G.; Caligaris, M.; Barillon, B.; Vlaeminck, S.E.; Sperandio, M. Enrichment and adaptation yield high anammox conversion rates under low temperatures. Bioresour. Technol. 2018, 250, 505–512. [Google Scholar] [CrossRef]
- Pereira, A.D.; Cabezas, A.; Etchebehere, C.; de Lemos Chernicharo, C.A.; de Araújo, J.C. Microbial communities in anammox reactors: A review. Environ. Technol. Rev. 2017, 6, 74–93. [Google Scholar] [CrossRef]
- Isanta, E.; Bezerra, T.; Fernández, I.; Suárez-Ojeda, M.E.; Pérez, J.; Carrera, J. Microbial community shifts on an anammox reactor after a temperature shock using 454-pyrosequencing analysis. Bioresour. Technol. 2015, 181, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; van Loosdrecht, M.C.; Daigger, G.T. Mainstream partial nitritation-anammox in municipal wastewater treatment: Status, bottlenecks, and further studies. Appl. Microbiol. Biotechnol. 2017, 101, 1365–1383. [Google Scholar] [CrossRef]
- Yu, Z.; He, X.; Li, Z.; Zhou, S.; Guo, D.; Pu, H.; Luo, H. Anammox bacterial abundance and diversity in different temperatures of purple paddy soils by (13)C-DNA stable-isotope probing combined with high-throughput sequencing. Front. Microbiol. 2023, 14, 1098681. [Google Scholar] [CrossRef] [PubMed]
- Kai, W. Study on the Preservation of Anaerobic Ammonia Oxidation Sludge and Recovery of Denitrification Performance. Master’s Thesis, Xiangtan University, Xiangtan, China, 2016. [Google Scholar]
- Yang, J.; Zhang, L.; Hira, D.; Fukuzaki, Y.; Furukawa, K. Anammox treatment of high-salinity wastewater at ambient temperature. Bioresour. Technol. 2011, 102, 2367–2372. [Google Scholar] [CrossRef]
- Blum, J.M.; Su, Q.; Ma, Y.; Valverde-Perez, B.; Domingo-Felez, C.; Jensen, M.M.; Smets, B.F. The pH dependency of N-converting enzymatic processes, pathways and microbes: Effect on net N2O production. Environ. Microbiol. 2018, 20, 1623–1640. [Google Scholar] [CrossRef] [Green Version]
- Jetten, M.S.M.; Wagner, M.; Fuerst, J.; van Loosdrecht, M.; Kuenen, G.; Strous, M. Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Curr. Opin. Biotechnol. 2001, 12, 283–288. [Google Scholar] [CrossRef]
- Waki, M.; Yasuda, T.; Suzuki, K.; Sakai, T.; Suzuki, N.; Suzuki, R.; Matsuba, K.; Yokoyama, H.; Ogino, A.; Tanaka, Y.; et al. Rate determination and distribution of anammox activity in activated sludge treating swine wastewater. Bioresour. Technol. 2010, 101, 2685–2690. [Google Scholar] [CrossRef]
- Pedrouso, A.; Val del Rio, A.; Morales, N.; Vazquez-Padin, J.R.; Campos, J.L.; Mosquera-Corral, A. Mainstream anammox reactor performance treating municipal wastewater and batch study of temperature, pH and organic matter concentration cross-effects. Process Saf. Environ. Prot. 2021, 145, 195–202. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, Y.Z.; Zhang, L.H.; Li, J.; Wei, J.; Zheng, Z.M.; Zhang, K. Improving the resistance of Anammox granules to extreme pH shock: The effects of denitrification sludge EPS enhanced by a fluctuating C/N ratio cultivation on granules. Sci. Total Environ. 2021, 763, 144610. [Google Scholar] [CrossRef]
- Karasuta, C.; Wang, X.; Zheng, X.; Chen, Y.; Chen, Z. Effect of hydraulic retention time on effluent pH in anammox bioreactors: Characteristics of effluent pH and pH as an indicator of reactor performance. J. Environ. Manag. 2021, 280, 111716. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wang, X.; Liang, H.; Wei, Q.; Dou, Y.; Li, L. Anaerobic ammonia oxidizing bacteria: Ecological distribution, metabolism, and microbial interactions. Front. Environ. Sci. Eng. 2018, 12, 10. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Y.; Wang, W.; Zhou, S.; Wang, J.; Guo, J. Comparison of short-term dosing ferrous ion and nanoscale zero-valent iron for rapid recovery of anammox activity from dissolved oxygen inhibition. Water Res. 2019, 153, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Morales, N.; Val del Rio, A.; Vazquez-Padin, J.R.; Gutierrez, R.; Fernandez-Gonzalez, R.; Icaran, P.; Rogalla, F.; Campos, J.L.; Mendez, R.; Mosquera-Corral, A. Influence of dissolved oxygen concentration on the start-up of the anammox-based process: ELAN(R). Water Sci. Technol. 2015, 72, 520–527. [Google Scholar] [CrossRef]
- Liu, S.; Yang, F.; Xue, Y.; Gong, Z.; Chen, H.; Wang, T.; Su, Z. Evaluation of oxygen adaptation and identification of functional bacteria composition for anammox consortium in non-woven biological rotating contactor. Bioresour. Technol. 2008, 99, 8273–8279. [Google Scholar] [CrossRef]
- Weixing, M. Study on the Performance, N2O Production and Microbial Community of CANON Process for Treating Low Ammonia Nitrogen Wastewater. Master’s Thesis, Chang’an University, Xi’an, China, 2018. [Google Scholar]
- Wang, X.; Huang, J.; Gao, D. Effects of three storage conditions on the long-term storage and short-term reactivation performances of anammox granular sludge. Int. Biodeterior. Biodegrad. 2021, 164, 105310. [Google Scholar] [CrossRef]
- Reeve, P.J.; Mouilleron, I.; Chuang, H.P.; Thwaites, B.; Hyde, K.; Dinesh, N.; Krampe, J.; Lin, T.F.; van den Akker, B. Effect of feed starvation on side-stream anammox activity and key microbial populations. J. Environ. Manag. 2016, 171, 121–127. [Google Scholar] [CrossRef]
- Ganesan, S.; Vadivelu, V.M. Effect of external hydrazine addition on anammox reactor start-up time. Chemosphere 2019, 223, 668–674. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, L.; Zhang, F.; Ge, C.; Yang, F. Vacuum lyophilization preservation and rejuvenation performance of anammox bacteria. J. Biosci. Bioeng. 2020, 129, 519–527. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, J.; Peng, Y.; Wang, C.; Guo, G.; Liu, S. A comparison of endogenous processes during anaerobic starvation in anaerobic end sludge and aerobic end sludge from an anaerobic/anoxic/oxic sequencing batch reactor performing denitrifying phosphorus removal. Bioresour. Technol. 2012, 104, 19–27. [Google Scholar] [CrossRef]
- Hao, X.-D.; Wang, Q.-L.; Zhu, J.-Y.; Van Loosdrecht, M.C.M. Microbiological Endogenous Processes in Biological Wastewater Treatment Systems. Crit. Rev. Environ. Sci. Technol. 2010, 40, 239–265. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Zhou, S.; Yan, Y.; Lin, X.; Wu, M. Endogenous metabolism of anaerobic ammonium oxidizing bacteria in response to short-term anaerobic and anoxic starvation stress. Chem. Eng. J. 2017, 313, 1233–1241. [Google Scholar] [CrossRef]
- Ye, L.; Li, D.; Zhang, J.; Zeng, H. Resuscitation of starved anaerobic ammonium oxidation sludge system: Impacts of repeated short-term starvation. Bioresour. Technol. 2018, 263, 458–466. [Google Scholar] [CrossRef]
- Phanwilai, S.; Wantawin, C.; Terada, A.; Noophan, P.L.; Munakata-Marr, J. Resuscitation of starved suspended- and attached-growth anaerobic ammonium oxidizing bacteria with and without acetate. Water Sci. Technol. 2017, 75, 115–127. [Google Scholar] [CrossRef]
- Xu, D.; Kang, D.; Ding, A.; Li, Y.; Yu, T.; Li, W.; Zeng, Z.; Guo, L.; Zheng, P. Response of FANIR system to starvation stress: “Dormancy”. Water Res. 2020, 171, 115380. [Google Scholar] [CrossRef]
- Ji, Y.-X.; Jin, R.-C. Effect of different preservation conditions on the reactivation performance of anammox sludge. Sep. Purif. Technol. 2014, 133, 32–39. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Buayi, X.; Cheng, Y.-F.; Zhou, Y.-H.; Wang, H.-Z.; Jin, R.-C. Anammox endogenous metabolism during long-term starvation: Impacts of intermittent and persistent modes and phosphates. Sep. Purif. Technol. 2015, 151, 309–317. [Google Scholar] [CrossRef]
- Jin, R.C.; Yu, J.J.; Ma, C.; Yang, G.F.; Zhang, J.; Chen, H.; Zhang, Q.Q.; Ji, Y.X.; Hu, B.L. Transient and long-term effects of bicarbonate on the ANAMMOX process. Appl. Microbiol. Biotechnol. 2014, 98, 1377–1388. [Google Scholar] [CrossRef]
- Xu, J.; Wu, X.; Zhu, N.; Shen, Y.; Yuan, H. Anammox process dosed with biochars for enhanced nitrogen removal: Role of surface functional groups. Sci. Total Environ. 2020, 748, 141367. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, D.; Jin, Y. Effects of inorganic carbon on the nitrous oxide emissions and microbial diversity of an anaerobic ammonia oxidation reactor. Bioresour. Technol. 2018, 250, 124–130. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Fukuzaki, Y.; Hira, D.; Furukawa, K. High-rate nitrogen removal by the Anammox process with a sufficient inorganic carbon source. Bioresour. Technol. 2010, 101, 9471–9478. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, G.; Zhang, G.; Xu, X.; Yang, F. Using graphene oxide to enhance the activity of anammox bacteria for nitrogen removal. Bioresour. Technol. 2013, 131, 527–530. [Google Scholar] [CrossRef]
- Xu, J.; Li, C.; Zhu, N.; Shen, Y.; Yuan, H. Particle size-dependent behavior of redox-active biochar to promote anaerobic ammonium oxidation (anammox). Chem. Eng. J. 2021, 410, 127925. [Google Scholar] [CrossRef]
- Izadi, P.; Izadi, P.; Eldyasti, A. Holistic insights into extracellular polymeric substance (EPS) in anammosx bacterial matrix and the potential sustainable biopolymer recovery: A review. Chemosphere 2021, 274, 129703. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Qiao, S.; Zhou, J.; Tang, X. Fast start-up of the anammox process with addition of reduced graphene oxides. Chem. Eng. J. 2016, 283, 160–166. [Google Scholar] [CrossRef]
- Chen, H.; Cao, S.; Chen, L.; Zhang, Z.; Tian, J.; Jin, R.; Yao, J. Biochar accelerates the start-up of the anammox process: Phenomenon and potential mechanisms. J. Water Process Eng. 2023, 53, 103662. [Google Scholar] [CrossRef]
- Junxiang, X. Biochar-Mediated Anaerobic Ammonia Oxidation Granular Sludge Formation and Mechanism of Tolerance to Organic Carbon Source Stress. Master’s Thesis, Suzhou University of Science and Technology, Suzhou, China, 2022. [Google Scholar]
- Adams, M.; Xie, J.; Xie, J.; Chang, Y.; Guo, M.; Chen, C.; Zhang, T.C. The effect of carrier addition on Anammox start-up and microbial community: A review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 355–368. [Google Scholar] [CrossRef]
- Liang, L.; Luo, J.; Xiao, X.; Wang, J.; Hong, M.; Deng, C.; Li, Y.Y.; Liu, J. Granular activated carbon promoting re-granulation of anammox-hydroxyapatite granules for stable nitrogen removal at low phosphate concentration. Sci. Total Environ. 2022, 805, 150359. [Google Scholar] [CrossRef]
- Yao, Z.B.; Cai, Q.; Zhang, D.J.; Xiao, P.Y.; Lu, P.L. The enhancement of completely autotrophic nitrogen removal over nitrite (CANON) by N2H4 addition. Bioresour. Technol. 2013, 146, 591–596. [Google Scholar] [CrossRef]
- Jinyuan, M. Research on the Performance and Pathway of ANAMMOX Bacteria Degrade NO and N2H4. Master’s Thesis, Beijing Jiaotong University, Beijing, China, 2017. [Google Scholar]
- Qing, C.; Jiajia, D. Mechanism and kinetic properties of N2H4-enhanced anaerobic ammonia oxidation. Water Treat. Technol. 2015, 41, 73–77. [Google Scholar] [CrossRef]
- Li, W.; Tan, C.; Cui, D.; Liu, Y.; Jiang, L.; Yao, L.; Chen, Y. Influence of Tourmaline on the Activity of ANAMMOX Bacteria and ANAMMOX Reaction. J. Environ. Eng. 2018, 144, 04018059. [Google Scholar] [CrossRef]
- Qiu, S.; Ma, F.; Wo, Y.; Xu, S. Study on the biological effect of Tourmaline on the cell membrane of E. coli. Surf. Interface Anal. 2011, 43, 1069–1073. [Google Scholar] [CrossRef]
- Irisa, T.; Hira, D.; Furukawa, K.; Fujii, T. Reduction of nitric oxide catalyzed by hydroxylamine oxidoreductase from an anammox bacterium. J. Biosci. Bioeng. 2014, 118, 616–621. [Google Scholar] [CrossRef]
- Chong, T.; Yingjie, L.; Wei, W.; Shan, Q. Effect of tourmaline on anaerobic ammonia oxidizing bacteria and anaerobic ammonia oxidation reaction. China Water Supply Drain. 2014, 30, 11–15. [Google Scholar] [CrossRef]
- Tan, C.; Cui, D.; Liu, Y.; Ji, Y. Influence of Tourmaline on the Anaerobic Ammonium Oxidation Process in Sequencing Batch Reactors. J. Environ. Eng. 2017, 143, 04017053. [Google Scholar] [CrossRef]
- Ali, M.; Okabe, S. Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues. Chemosphere 2015, 141, 144–153. [Google Scholar] [CrossRef]
- Ni, L.; Lin, X.; Yan, H.; Wang, Y. A novel anammox granules-circulating expanded granular sludge bed reactor for the efficient circulation and retention of floating anammox granules. Chemosphere 2019, 235, 316–326. [Google Scholar] [CrossRef]
- Yin, X.; Qiao, S.; Zhou, J.; Quan, X. Using three-bio-electrode reactor to enhance the activity of anammox biomass. Bioresour. Technol. 2015, 196, 376–382. [Google Scholar] [CrossRef]
- Noophan, P.; Sripiboon, S.; Damrongsri, M.; Munakata-Marr, J. Anaerobic ammonium oxidation by Nitrosomonas spp. and anammox bacteria in a sequencing batch reactor. J. Environ. Manag. 2008, 90, 967–972. [Google Scholar] [CrossRef]
- Huang, X.; Urata, K.; Wei, Q.; Yamashita, Y.; Hama, T.; Kawagoshi, Y. Fast start-up of partial nitritation as pre-treatment for anammox in membrane bioreactor. Biochem. Eng. J. 2016, 105, 371–378. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Niu, Q.; Ma, H.; Li, Y.-Y. Operation stability and recovery performance in an Anammox EGSB reactor after pH shock. Ecol. Eng. 2016, 90, 50–56. [Google Scholar] [CrossRef]
- Tang, C.-J.; Zheng, P.; Wang, C.-H.; Mahmood, Q.; Zhang, J.-Q.; Chen, X.-G.; Zhang, L.; Chen, J.-W. Performance of high-loaded ANAMMOX UASB reactors containing granular sludge. Water Res. 2010, 45, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Luzi, Y.; Tao, W.; Fanghua, X.; Xian, W.; Hongying, Y. Enhancement of Anammox performances in an ABR at normal temperature by the low-intensity ultrasonic irradiation. Ultrason. Sonochemistry 2021, 73, 105468. [Google Scholar] [CrossRef]
- Ying, Z.Q.; Chang, W.X. Study on ANAMMOX Treatment of Ammonium-Rich Wastewater. Adv. Mater. Res. 2011, 396–398, 2044–2047. [Google Scholar] [CrossRef]
- Jiatong, D.; Yongzhen, P.; Jianhua, Z.; Liang, Z. Anammox bacteria enrichment in fixed biofilm successfully enhanced nitrogen removal of domestic wastewater in a sequencing biofilm batch reactor (SBBR). J. Water Process Eng. 2021, 42, 102154. [Google Scholar] [CrossRef]
- Dou, W.; Yulin, W.; Lei, L.; Yiqiang, C.; Chunxiao, W.; Xiaoqing, X.; Yu, Y.; Yubo, W.; Tong, Z. Niche differentiation and symbiotic association among ammonia/nitrite oxidizers in a full-scale rotating biological contactor. Water Res. 2022, 225, 119137. [Google Scholar] [CrossRef]
- Arrojo, B.; Figueroa, M.; Mosquera-Corral, A.; Campos, J.L.; Méndez, R. Influence of gas flow-induced shear stress on the operation of the Anammox process in a SBR. Chemosphere 2008, 72, 1687–1693. [Google Scholar] [CrossRef]
- Arwa, E.; Hamza, A.; Mahjoub, J.; Adel, G. Development of Abrasives from Non-woven Based on Used Textiles. J. Nat. Fibers 2022, 19, 2189–2203. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Hira, D.; Fujii, T.; Zhang, W.; Furukawa, K. High-rate nitrogen removal from anaerobic digester liquor using an up-flow anammox reactor with polyethylene sponge as a biomass carrier. J. Biosci. Bioeng. 2010, 111, 306–311. [Google Scholar] [CrossRef]
- Taraning, R.N.W.S.; Jinyoung, P.; Minjune, Y.; Sookyun, W.; Minhee, L. Cesium removal from a water system using a polysulfone carrier containing nitric acid-treated bamboo charcoal. J. Environ. Radioact. 2020, 225, 106374. [Google Scholar] [CrossRef]
- Hang, L.J.; Jisun, S.; Jongsoo, K. Comparison of the Rate of Demineralization of Enamel using Synthetic Polymer Gel. J. Korean Acad. Pedtatric Dent. 2019, 46, 190–199. [Google Scholar] [CrossRef]
- Qiongpeng, D.; Jialin, L.; Rui, D.; Tiantian, S.; Xiyao, L.; Qiong, Z.; Yongzhen, P. Highly Enriched Anammox Bacteria with a Novel Granulation Model Regulated by Epistylis spp. in Domestic Wastewater Treatment. Environ. Sci. Technol. 2023, 57, 3571–3580. [Google Scholar] [CrossRef]
- Pérez-Uz, B.; Arregui, L.; Calvo, P.; Salvadó, H.; Fernández, N.; Rodríguez, E.; Zornoza, A.; Serrano, S. Assessment of plausible bioindicators for plant performance in advanced wastewater treatment systems. Water Res. 2010, 44, 5059–5069. [Google Scholar] [CrossRef]
- Xiaojing, W.; Donghui, L.; Yongyou, H.; Xiaoqiang, Z.; Guobin, W.; Jieyun, X. Performance and mechanism of simultaneous nitrification and denitrification in zeolite spheres internal loop airlift reactor. Bioresour. Technol. 2023, 380, 129073. [Google Scholar] [CrossRef]
- Isaka, K.; Kimura, Y.; Yamamoto, T.; Osaka, T.; Tsuneda, S. Complete autotrophic denitrification in a single reactor using nitritation and anammox gel carriers. Bioresour. Technol. 2013, 147, 96–101. [Google Scholar] [CrossRef]
- Hao, J.; Zhong, W.; Shang, R.; Jingang, Q.; Qiong, Z.; Xiyao, L.; Yongzhen, P. Enrichment and retention of key functional bacteria of partial denitrification-Anammox (PD/A) process via cell immobilization: A novel strategy for fast PD/A application. Bioresour. Technol. 2021, 326, 124744. [Google Scholar] [CrossRef]
- Kiprotich, K.; Kartik, C.; Jashan, G.; Lewis, K.S.; Faizal, B.; Sheena, K. Critical Analysis of Biomass Retention Strategies in Mainstream and Sidestream ANAMMOX-Mediated Nitrogen Removal Systems. Environ. Sci. Technol. 2020, 55, 9–24. [Google Scholar] [CrossRef]
- Yuqing, M.; Bo, W.; Xiaodi, L.; Shuo, W.; Wen, W.; Yongzhen, P. Enrichment of anammox biomass during mainstream wastewater treatment driven by achievement of partial denitrification through the addition of bio-carriers. J. Environ. Sci. 2024, 137, 181–194. [Google Scholar] [CrossRef]
- Yan, F.; Bo, W.; Yongzhen, P.; Xiyao, L.; Qiong, Z. Enhanced nitrogen removal from low COD/TIN mainstream wastewater in a continuous plug-flow reactor via partial nitrification, simultaneous anammox and endogenous denitrification (PN-SAED) process. Bioresour. Technol. 2021, 345, 126539. [Google Scholar] [CrossRef]
- Suneethi, S.; Joseph, K. ANAMMOX process start up and stabilization with an anaerobic seed in Anaerobic Membrane Bioreactor (AnMBR). Bioresour. Technol. 2011, 102, 8860–8867. [Google Scholar] [CrossRef]
- Abma, W.R.; Schultz, C.E.; Mulder, J.W.; van der Star, W.R.; Strous, M.; Tokutomi, T.; van Loosdrecht, M.C. Full-scale granular sludge Anammox process. Water Sci. Technol. 2007, 55, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Ma, H.; Kong, Z.; Guo, Y.; Li, Y.Y. Bulking and floatation of the anammox-HAP granule caused by low phosphate concentration in the anammox reactor of expanded granular sludge bed (EGSB). Bioresour. Technol. 2020, 310, 123421. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, H.; Jiang, Z.; Wang, Y. Visual evidence for anammox granules expanding their size by aggregation of anammox micro-granules. Sci. Total Environ. 2020, 745, 141052. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wang, Y.; Tang, X.; Li, L.; Feng, F.; Mahmood, Q.; Wu, D.; Tang, C.J. Quantitative determination of cavitation formation and sludge flotation in Anammox granules by using a new diffusion-reaction integrated mathematical model. Water Res. 2020, 174, 115632. [Google Scholar] [CrossRef]
- Yachao, Z.; Jing, Z.; Aiyue, H.; Rongxuan, Z.; Dongbo, L.; Kai, Z.; Yang, L.; Jun, L. Effect of extracellular polymers and signaling molecules on the activity of anaerobic ammonia-oxidizing sludge. China Environ. Sci. 2019, 39, 4133–4140. [Google Scholar] [CrossRef]
- Bae, H.; Choi, M.; Chung, Y.-C.; Lee, S.; Yoo, Y.J. Core-shell structured poly(vinyl alcohol)/sodium alginate bead for single-stage autotrophic nitrogen removal. Chem. Eng. J. 2017, 322, 408–416. [Google Scholar] [CrossRef]
- Qiaochong, H.; Yunpeng, S.; Rui, L.; Tong, P.; Nan, C.; Zhenjun, W.; Chuanping, F. Rice washing drainage (RWD) embedded in poly(vinyl alcohol)/sodium alginate as denitrification inoculum for high nitrate removal rate with low biodiversity. Bioresour. Technol. 2022, 355, 127288. [Google Scholar] [CrossRef]
- Isaka, K.; Date, Y.; Sumino, T.; Tsuneda, S. Ammonium removal performance of anaerobic ammonium-oxidizing bacteria immobilized in polyethylene glycol gel carrier: Anammox bacteria immobilized in gel carrier. Appl. Microbiol. Biotechnol. 2007, 76, 1457–1465. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.C.; Chen, Y.P. Nitrogen removal performance and characteristics of gel beads immobilized anammox bacteria under different PVA:SA ratios. Water Environ. Res. 2021, 93, 1627–1639. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Gao, S. PVA/CS and PVA/CS/Fe gel beads’ synthesis mechanism and their performance in cultivating anaerobic granular sludge. Chemosphere 2019, 219, 130–139. [Google Scholar] [CrossRef]
- Zhang, L.; Narita, Y.; Gao, L.; Ali, M.; Oshiki, M.; Okabe, S. Maximum specific growth rate of anammox bacteria revisited. Water Res. 2017, 116, 296–303. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Li, G.; Temmink, H.; Rijnaarts, H.H.M. Granule-based immobilization and activity enhancement of anammox biomass via PVA/CS and PVA/CS/Fe gel beads. Bioresour. Technol. 2020, 309, 123448. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, H.; Wang, D.; Wang, W. Insight into the response of anammox granule rheological intensity and size evolution to decreasing temperature and influent substrate concentration. Water Res. 2019, 162, 258–268. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Shen, J.; He, Z. Enhancing anammox resistance to low operating temperatures with the use of PVA gel beads. Sci. Total Environ. 2021, 774, 144826. [Google Scholar] [CrossRef]
- Cong, L.; Qingtao, L.; Jiarui, F.; Yongzhen, P.; Rui, D. Metagenomics-based interpretation of selective bioaugmentation promoting partial-denitrification coupling with anammox process reactivation in suspended sludge system. Chem. Eng. J. 2023, 454, 139977. [Google Scholar] [CrossRef]
- Ma, B.; Xu, X.; Wei, Y.; Ge, C.; Peng, Y. Recent advances in controlling denitritation for achieving denitratation/anammox in mainstream wastewater treatment plants. Bioresour. Technol. 2020, 299, 122697. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Yu, D.; Du, S.; Yuan, M.; Zhen, J. Evaluating the potential for sustaining mainstream anammox by endogenous partial denitrification and phosphorus removal for energy-efficient wastewater treatment. Bioresour. Technol. 2019, 284, 302–314. [Google Scholar] [CrossRef]
- Qi, Z.; Jianwei, L.; Liyan, D.; Tipei, J.; Yang, Z.; Xiyao, L.; Yongzhen, P. From hybrid process to pure biofilm anammox process: Suspended sludge biomass management contributing to high-level anammox enrichment in biofilms. Water Res. 2023, 236, 119959. [Google Scholar] [CrossRef]
- Ruitao, G.; Yongzhen, P.; Jianwei, L.; Ying, L.; Liyan, D.; Wenyu, L.; Chengkun, K. Mainstream partial denitrification-anammox (PD/A) for municipal sewage treatment from moderate to low temperature: Reactor performance and bacterial structure. Sci. Total Environ. 2022, 806, 150267. [Google Scholar] [CrossRef]
- Yang-Fan, D.; Di, W.; Hao, H.; Yan-Xiang, C.; van Loosdrecht, M.C.M.; Guang-Hao, C. Exploration and verification of the feasibility of sulfide-driven partial denitrification coupled with anammox for wastewater treatment. Water Res. 2021, 193, 116905. [Google Scholar] [CrossRef]
- Yan, Y.; Xiang, L.; Wei, L.; Miao, S.; Mao, Z.; PeiLin, X.; BoLin, L.; Yong, H. Effects of different reduced sulfur forms as electron donors in the start-up process of short-cut sulfur autotrophic denitrification. Bioresour. Technol. 2022, 354, 127194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, N.; Chen, Z.; Ma, Y.; Wang, L.; Zhang, H.; Jia, J. Long-term impact of sulfate on an autotrophic nitrogen removal system integrated partial nitrification, anammox and endogenous denitrification (PAED). Chemosphere 2019, 235, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Zhiqi, W.; Jingfeng, G.; Huihui, D.; Yukun, Y.; Yifan, Z.; Dingchang, L.; Yingchao, C. Partial S(0)-driven autotrophic denitrification process facilitated the quick natural enrichment of anammox bacteria at room temperature. Sci. Total Environ. 2022, 855, 158916. [Google Scholar] [CrossRef]
- Hoehler, T.M.; Jorgensen, B.B. Microbial life under extreme energy limitation. Nat. Rev. Microbiol. 2013, 11, 83–94. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Ma, B.; Wang, X.; Zhou, J.; Zhao, S.; Liu, R. Anammox granular sludge in low-ammonium sewage treatment: Not bigger size driving better performance. Water Res. 2018, 142, 147–158. [Google Scholar] [CrossRef]
- Xiruo, L. Experimental Study on the Recovery of Nitrogen Removal Efficiency of Long-Term Dormant Anaerobic Ammonia Oxidation Sludge. Master’s Thesis, Shenyang University of Architecture, Shenyang, China, 2022. [Google Scholar]
- Xing, B.-S.; Guo, Q.; Jiang, X.-Y.; Chen, Q.-Q.; He, M.-M.; Wu, L.-M.; Jin, R.-C. Long-term starvation and subsequent reactivation of anaerobic ammonium oxidation (anammox) granules. Chem. Eng. J. 2016, 287, 575–584. [Google Scholar] [CrossRef]
- Viancelli, A.; Pra, M.C.; Scussiato, L.A.; Cantão, M.; Ibelli, A.M.G.; Kunz, A. Preservation and reactivation of Candidatus Jettenia asiatica and Anammoxoglobus propionicus using different preservative agents. Chemosphere 2017, 186, 453–458. [Google Scholar] [CrossRef]
- Tang, X.; Guo, Y.; Jiang, B.; Liu, S. Metagenomic approaches to understanding bacterial communication during the anammox reactor start-up. Water Res. 2018, 136, 95–103. [Google Scholar] [CrossRef]
- Dong, L.; Mingyang, L.; Jie, Z.; Huiping, Z. Long-term preservation and rapid activity recovery of anaerobic ammonia-oxidized granular sludge. Environ. Sci. 2021, 42, 2957–2965. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Yang, F. Long-term storage and subsequent reactivation of Anammox sludge at 35 °C. Desalination Water Treat. 2016, 57, 24716–24723. [Google Scholar] [CrossRef]
- Yan, G.; Beihai, Z.; Chen, S. Initiation of idle anaerobic ammonia oxidation microorganisms and microbial analysis. Environ. Prot. Sci. 2017, 43, 43–50. [Google Scholar] [CrossRef]
| Bacillus spp. | Strain | Discover Time | Discover Country | Source | References |
|---|---|---|---|---|---|
| Candidatus Brocadia | B. anammoxidans | 1999 | The Netherlands | Wastewater | [16] |
| B. fulgida | 2008 | The Netherlands | Wastewater | [17] | |
| B. sinica | 2010 | China | Bioreactors | [18] | |
| B. brasiliensis | 2011 | Brazil | Wastewater | [19] | |
| B. caroliniensis | 2011 | USA | Livestock manure sludge | [20] | |
| B. sapporoensis | 2017 | Japan | Bioreactors | [21] | |
| Candidatus Jettenia | J. asiatica | 2008 | China | Bioreactors | [22] |
| J. caeni | 2012 | Japan | Wastewater | [23] | |
| J. ecosi | 2018 | Russia | Bioreactors | [24] | |
| J. moscovienalis | 2015 | Moscow | Bioreactors | [25] | |
| Candidatus Anammoxoglobus | A. propionicus | 2007 | The Netherlands | Bioreactors | [26] |
| A. sulfate | 2008 | China | Biological turntable | [27] | |
| Candidatus Scalindua | S. brodae | 2003 | Britain | Wastewater | [28] |
| S. wagneri | 2003 | Britain | Wastewater | [28] | |
| S. sorokinii | 2003 | Britain | Seawater | [29] | |
| S. arabica | 2008 | Arabian | Seawater | [30] | |
| S. sinooilfield | 2010 | China | Oil reservoirs | [31] | |
| S. marina | 2011 | Sweden | Submarine sediments | [32] | |
| S. richardsii | 2012 | Black Sea | Black Sea sub-box area | [33] | |
| S. profunda | 2013 | Sweden | Submarine sediments | [34] | |
| S. rubra | 2017 | Red Sea | Seawater | [35] | |
| S. japonica | 2017 | Japan | Bay sediments | [36] | |
| S. zhenghei | 2010 | South China Sea | Seawater | [37] | |
| Candidatus Kuenenia | K. stuttgartiensis | 2000 | Germany | Bioreactors Biofilm | [38] |
| Candidatus Brasilis | B. concordiensis | 2011 | Brazil | Bioreactors | [39] |
| Candidatus Anammoximicrobium | A. moscowii | 2012 | Moscow | River sediments | [40] |
| Reactor Type | Advantages | Disadvantages | References |
|---|---|---|---|
| SBR | Highly efficient mud and water separation with good biological cut-off capacity, full mixing of the substrate, high impact resistance, no backflow, stable operation, and simple operation. | Low operating load and high automatic control requirements, not suitable for coupling with other processes, still some sludge loss | [96] |
| Membrane Bioreactor (MBR) | Good separation of mud and water, membrane structure can effectively stop anammox loss, high strain activity, short doubling time, good effluent quality, simple process, and easy operation. | High resistance, high reactor price, and easy clogging of membranes | [97] |
| EGSB | Enrichment of anammox has certain advantages, high sludge retention capacity, good mass transfer conditions, and less clogging | High operating conditions and control requirements, frequent and violent particle sludge collisions, easy loss, and high energy consumption | [98] |
| UASB | High sludge concentration, high treatment load, high impact resistance, high bioretention, short HRT and low energy consumption, easy separation of gas–liquid–solid phases, high confinement, stable operation, good denitrification, accelerated granular sludge formation | Poor mass transfer, uneven mixing within the sludge, easy formation of dead zones, short flows, and trench flows, affect the start-up effect | [99] |
| Anaerobic Baffled Reactor (ABR) | Good bioretention capacity, easy solid–liquid separation, easy formation of granular sludge, advantages of cultivating anammox. | Difficult to achieve uniform water distribution, prone to gullying and dead spots | [100] |
| Up-flow Blanket Filter (UBF) | Good adaptation to changes in water quality and quantity, long sludge age, the high flow rate in the tank, and good mass transfer conditions. | Granular sludge is unstable and easily lost | [101] |
| Sequencing Biofilm Batch Reactor (SBBR) | Good bioretention capacity, high impact resistance, stable operation, and simple operation. | Anammox enrichment is influenced by the type and nature of the filler, and the control system is complex | [102] |
| Rotating Biological Contactor (RBC) | High biomass per unit of sludge and low sludge loss. | High rotational energy consumption | [103] |
| Serial Number | Possible Solutions |
|---|---|
| 1 | The current literature shows that no pure cultures of anammox bacteria have been found. Therefore, it is urgent to find a method that allows anammox bacteria to be isolated and cultured and remain active, contributing to the denitrification performance of the process. |
| 2 | A total of 4 mg/L of Fe3O4 is the most suitable input amount for the recovery of anammox bacterial activity, and NZVI has the advantages of a large specific surface area and strong reducing ability. Two substances, Fe3O4 and NZVI, were combined to explore the effect on the activity of anammox bacteria. |
| 3 | Seven genera of anammox microorganisms are known, and the exploration of other bacterial taxa with anammox functions will continue. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Wen, X.; Huang, J.; Sun, D.; Jin, L. Advances in the Efficient Enrichment of Anammox Bacteria. Water 2023, 15, 2556. https://doi.org/10.3390/w15142556
Fu Y, Wen X, Huang J, Sun D, Jin L. Advances in the Efficient Enrichment of Anammox Bacteria. Water. 2023; 15(14):2556. https://doi.org/10.3390/w15142556
Chicago/Turabian StyleFu, Yuting, Xin Wen, Jiansheng Huang, Da Sun, and Libo Jin. 2023. "Advances in the Efficient Enrichment of Anammox Bacteria" Water 15, no. 14: 2556. https://doi.org/10.3390/w15142556
APA StyleFu, Y., Wen, X., Huang, J., Sun, D., & Jin, L. (2023). Advances in the Efficient Enrichment of Anammox Bacteria. Water, 15(14), 2556. https://doi.org/10.3390/w15142556








