‘Dual Purpose’ Surface Flow Constructed Treatment Wetlands Support Native Biodiversity in Intensified Agricultural Landscapes
Abstract
:1. Introduction
Study Aims
2. Methods
2.1. Study Region and FWS Description
2.2. Characterising the FWS and the Surrounding Landscape
2.3. Vegetation Survey
2.4. Fauna Survey
2.5. Statistical Analysis
2.5.1. Whole-Wetland Scale
2.5.2. Sub-Wetland/Plot Scale
3. Results
3.1. Landscape and Wetland Habitat
3.2. Vegetation Diversity
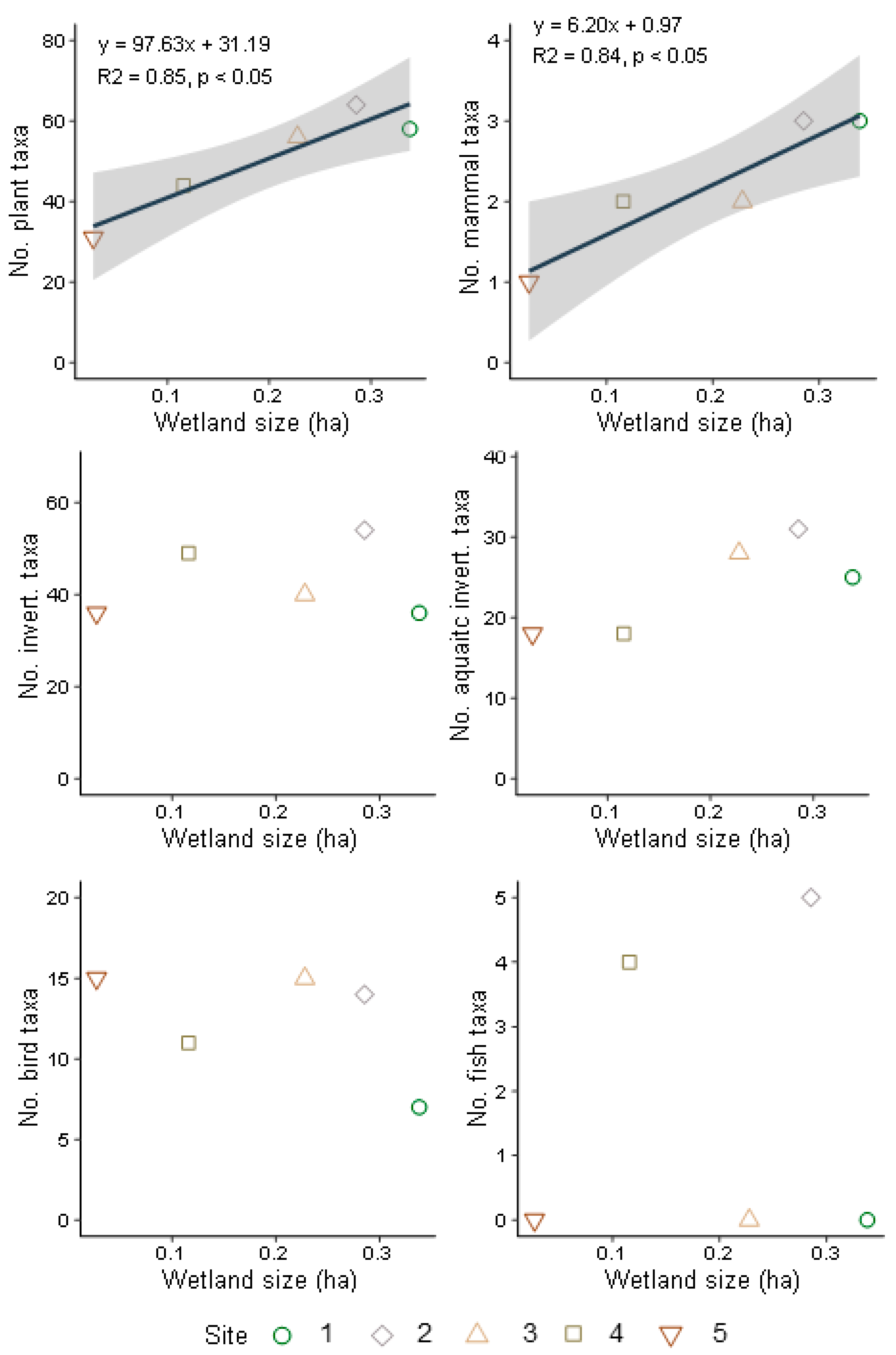
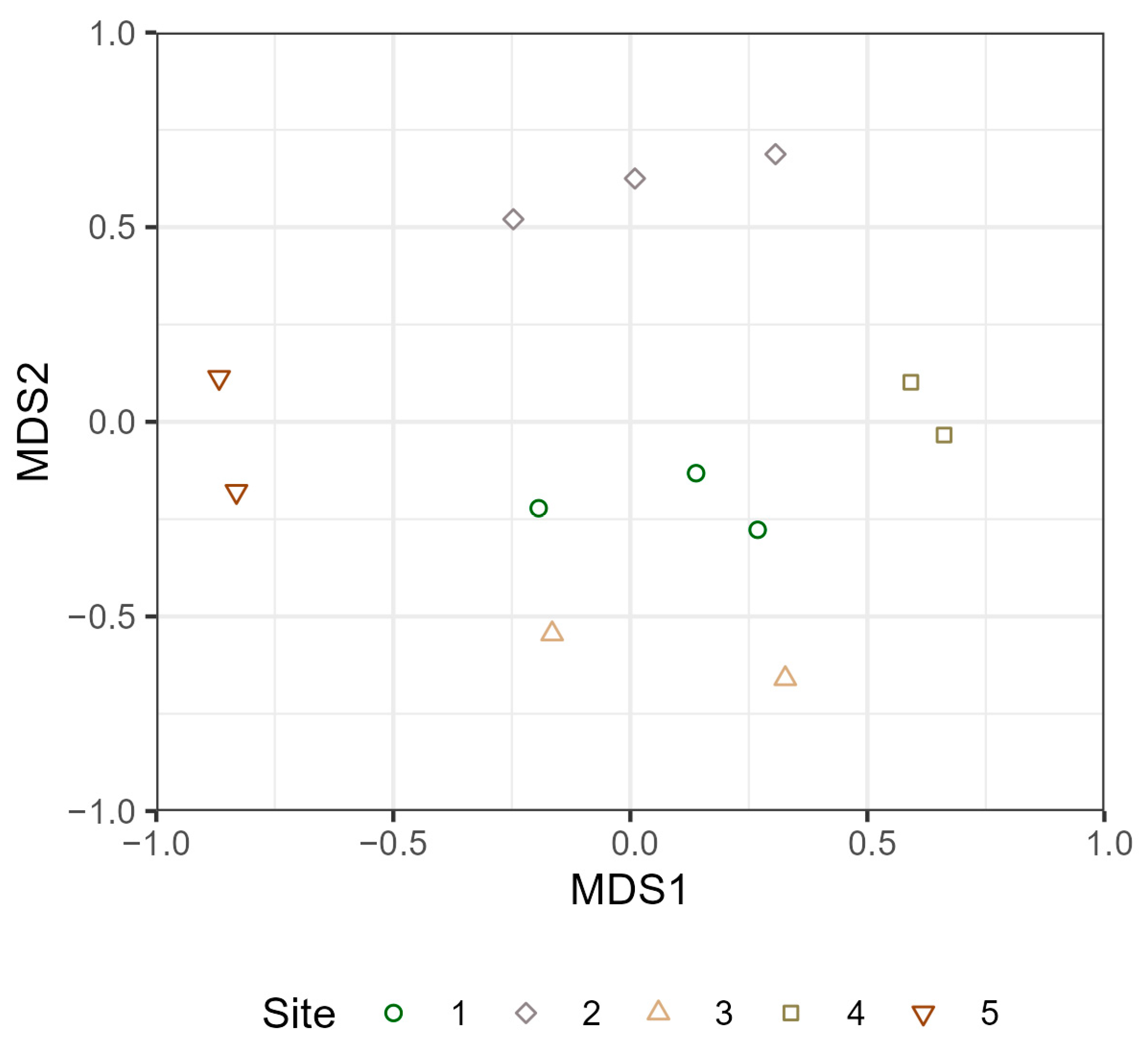
3.3. Terrestrial and Aquatic Invertebrate Diversity
3.4. Bird and Mammal Diversity
3.5. Fish and Amphibian Diversity
4. Discussion
4.1. Wetland Size, Habitat Connectivity, and Biodiversity
4.2. Supporting Native Biodiversity by Managing Exotic Species
4.3. Conclusions and Future Research
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitsch, W.J.; Bernal, B.; Hernandez, M.E. Ecosystem Services of Wetlands. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2015, 11, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Health Synthesis; Corvalán, C., Hales, S., McMichael, A.J., Millennium Ecosystem Assessment (Program), World Health Organization, Eds.; Millennium Ecosystem Assessment; World Health Organization: Geneva, Switzerland, 2005; ISBN 92-4-156309-5. [Google Scholar]
- Zedler, J.B.; Kercher, S. Wetland Resources: Status, Trends, Ecosystem Services, and Restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef] [Green Version]
- Mitsch, W.J.; Gosselink, J.G. (Eds.) Wetlands, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Myers, S.C.; Clarkson, B.R.; Reeves, P.N.; Clarkson, B.D. Wetland Management in New Zealand: Are Current Approaches and Policies Sustaining Wetland Ecosystems in Agricultural Landscapes? Ecol. Eng. 2013, 56, 107–120. [Google Scholar] [CrossRef]
- Gibbs, J.P. Wetland Loss and Biodiversity Conservation. Conserv. Biol. 2000, 14, 314–317. [Google Scholar] [CrossRef] [Green Version]
- Murillo-Pacheco, J.; López-Iborra, G.M.; Escobar, F.; Bonilla-Rojas, W.F.; Verdú, J.R. The Value of Small, Natural and Man-Made Wetlands for Bird Diversity in the East Colombian Piedmont. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 87–97. [Google Scholar] [CrossRef]
- Hansson, L.-A.; Bronmark, C.; Anders Nilsson, P.; Abjornsson, K. Conflicting Demands on Wetland Ecosystem Services: Nutrient Retention, Biodiversity or Both? Freshw. Biol. 2005, 50, 705–714. [Google Scholar] [CrossRef]
- Blankenberg, A.-G.B.; Haarstad, K.; Paruch, A.M. Agricultural Runoff in Norway: The Problem, the Regulations, and the Role of Wetlands. In The Role of Natural and Constructed Wetlands in Nutrient Cycling and Retention on the Landscape; Vymazal, J., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 137–147. ISBN 978-3-319-08177-9. [Google Scholar]
- Mitchell, M.E.; Newcomer-Johnson, T.; Christensen, J.; Crumpton, W.; Richmond, S.; Dyson, B.; Canfield, T.J.; Helmers, M.; Lemke, D.; Lechtenberg, M.; et al. Potential of Water Quality Wetlands to Mitigate Habitat Losses from Agricultural Drainage Modernization. Sci. Total Environ. 2022, 838, 156358. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, M.V.; Hashemi, F.; Hoffmann, C.C.; Zak, D.; Audet, J.; Kronvang, B. Efficiency of Mitigation Measures Targeting Nutrient Losses from Agricultural Drainage Systems: A Review. Ambio 2020, 49, 1820–1837. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-1-56670-526-4. [Google Scholar]
- Tanner, C.C.; Depree, C.; Sukias, J.P.S.; Wright-Stow, A.E.; Burger, D.F.; Goeller, B.C. Constructed Wetland Practitioner Guide: Design and Performance Estimates; DaryNZ/NIWA: Hamilton, New Zealand, 2022; p. 40. [Google Scholar]
- Hefting, M.M.; van den Heuvel, R.N.; Verhoeven, J.T.A. Wetlands in Agricultural Landscapes for Nitrogen Attenuation and Biodiversity Enhancement: Opportunities and Limitations. Ecol. Eng. 2013, 56, 5–13. [Google Scholar] [CrossRef]
- Mander, Ü.; Tournebize, J.; Tonderski, K.; Verhoeven, J.T.A.; Mitsch, W.J. Planning and Establishment Principles for Constructed Wetlands and Riparian Buffer Zones in Agricultural Catchments. Ecol. Eng. 2017, 103, 296–300. [Google Scholar] [CrossRef]
- Land, M.; Granéli, W.; Grimvall, A.; Hoffmann, C.C.; Mitsch, W.J.; Tonderski, K.S.; Verhoeven, J.T.A. How Effective Are Created or Restored Freshwater Wetlands for Nitrogen and Phosphorus Removal? A Systematic Review. Environ. Evid. 2016, 5, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Thiere, G.; Milenkovski, S.; Lindgren, P.-E.; Sahlén, G.; Berglund, O.; Weisner, S.E.B. Wetland Creation in Agricultural Landscapes: Biodiversity Benefits on Local and Regional Scales. Biol. Conserv. 2009, 142, 964–973. [Google Scholar] [CrossRef]
- Spieles, D.J. Wetland Construction, Restoration, and Integration: A Comparative Review. Land 2022, 11, 554. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, L.; Wang, Y.; Liu, C.; Zhou, Y.; Lei, G. Can Constructed Wetlands Be Wildlife Refuges? A Review of Their Potential Biodiversity Conservation Value. Sustainability 2020, 12, 1442. [Google Scholar] [CrossRef] [Green Version]
- Wiegleb, G.; Dahms, H.-U.; Byeon, W.I.; Choi, G. To What Extent Can Constructed Wetlands Enhance Biodiversity? Int. J. Environ. Sci. Technol. 2017, 8, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Becerra-Jurado, G.; Harrington, R.; Kelly-Quinn, M. A Review of the Potential of Surface Flow Constructed Wetlands to Enhance Macroinvertebrate Diversity in Agricultural Landscapes with Particular Reference to Integrated Constructed Wetlands (ICWs). Hydrobiologia 2012, 692, 121–130. [Google Scholar] [CrossRef]
- Boets, P.; Michels, E.; Meers, E.; Lock, K.; Tack, F.M.G.; Goethals, P.L.M. Integrated Constructed Wetlands (ICW): Ecological Cevelopment in Constructed Wetlands for Manure Treatment. Wetlands 2011, 31, 763–771. [Google Scholar] [CrossRef]
- Eivers, R.S.; Duggan, I.C.; Hamilton, D.P.; Quinn, J.M. Constructed Treatment Wetlands Provide Habitat for Zooplankton Communities in Agricultural Peat Lake Catchments. Wetlands 2018, 38, 95–108. [Google Scholar] [CrossRef]
- Alsfeld, A.J.; Bowman, J.L.; Deller-Jacobs, A. The Influence of Landscape Composition on the Biotic Community of Constructed Depressional Wetlands. Restor. Ecol. 2010, 18, 370–378. [Google Scholar] [CrossRef]
- Strand, J.A.; Weisner, S.E.B. Effects of Wetland Construction on Nitrogen Transport and Species Richness in the Agricultural Landscape—Experiences from Sweden. Ecol. Eng. 2013, 56, 14–25. [Google Scholar] [CrossRef]
- Weisner, S.E.B.; Thiere, G. Effects of Vegetation State on Biodiversity and Nitrogen Retention in Created Wetlands: A Test of the Biodiversity–Ecosystem Functioning Hypothesis. Freshw. Biol. 2010, 55, 387–396. [Google Scholar] [CrossRef]
- Zak, D.; Stutter, M.; Jensen, H.S.; Egemose, S.; Carstensen, M.V.; Audet, J.; Strand, J.A.; Feuerbach, P.; Hoffmann, C.C.; Christen, B.; et al. An Assessment of the Multifunctionality of Integrated Buffer Zones in Northwestern Europe. J. Environ. Qual. 2019, 48, 362–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett-Walker, J.; Collier, K.J.; Daniel, A.; Hicks, B.J.; Klee, D. Design Features of Constructed Floodplain Ponds Influence Waterbird and Fish Communities in Northern New Zealand. Freshw. Biol. 2020, 65, 2066–2080. [Google Scholar] [CrossRef]
- Moreno-Mateos, D.; Comin, F.A. Integrating Objectives and Scales for Planning and Implementing Wetland Restoration and Creation in Agricultural Landscapes. J. Environ. Manag. 2010, 91, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Maseyk, F.J.; Dominati, E.J.; Mackay, A.D. More than a’nice to Have’: Integrating Indigenous Biodiversity into Agroecosystems in New Zealand. N. Z. J. Ecol. 2019, 43, 1–12. [Google Scholar]
- Findlay, C.S.; Houlahan, J. Anthropogenic Correlates of Species Richness in Southeastern Ontario Wetlands. Conserv. Biol. 1997, 11, 1000–1009. [Google Scholar] [CrossRef]
- Croft, M.V.; Chow-Fraser, P. Non-Random Sampling and Its Role in Habitat Conservation: A Comparison of Three Wetland Macrophyte Sampling Protocols. Biodivers Conserv. 2009, 18, 2283–2306. [Google Scholar] [CrossRef]
- Hughes, J.M.R.; Clarkson, B.R.; Castro-Castellon, A.T.; Hess, L.L. Wetland Plants and Aquatic Macrophytes. In Freshwater Ecology and Conservation; Oxford University Press: Oxford, UK, 2019; pp. 173–207. ISBN 978-0-19-876638-4. [Google Scholar]
- Clarkson, B.R.; Sorrell, B.K.; Reeves, P.N.; Champion, P.D.; Partridge, T.R.; Clarkson, B.D. Handbook for Monitoring Wetland Condition; Coordinated Monitoring of New Zealand Wetlands: Ministry for the Environment Sustainable Management Fund Project 5105; Ministry for the Environment: Wellington, New Zealand, 2004; Volume 74, p. 35.
- Ngā Tipa o Aotearoa New Zealand Plants Database. Available online: https://nzflora.landcareresearch.co.nz/ (accessed on 24 November 2020).
- Clarkson, B.R.; Fitzgerald, N.B.; Champion, P.D.; Forester, L.; Rance, B.D. New Zealand Wetland Plant List 2021; Manaaki Whenua-Landcare Research: Hamilton, New Zealand, 2021; p. 66. [Google Scholar]
- Clarkson, B.; Sorrell, B.K. New Zealand Wetland Condition Monitoring. In Wetland and Stream Rapid Assessment: Development, Validation, and Application; Dorney, J., Savage, R., Tiner, R., Adamus, P., Eds.; Elesvier: Cambridge, MA, USA, 2018; ISBN 978-0-12-805091-0. [Google Scholar]
- Sherley, G.; Stringer, I. DOCCM-248862 Invertebrates: Pitfall Trapping v1. 0. Dep. Conserv. Te Papa Atawhai Wellingt. 2016, 1, 1–30. [Google Scholar]
- Hartley, L.; Greene, T. Birds: Incomplete Counts—Five-Minute Bird Counts v1.0; Inventory and Monitoring Toolbox: Birds; Department of Conservation, Science & Capability Group: Hamilton, New Zealand, 2012; p. 22.
- Gillies, C. Interim DOC Trail Camera Guide v1.0.2: Using Camera Traps to Monitor Feral Cats and Mustelids; Department of Conservation, Science & Capability Group: Hamilton, New Zealand, 2018; p. 9.
- Gillies, C.; Williams, D. DOC Tracking Tunnel Guide v2.5.2: Using Tracking Tunnels to Monitor Rodents and Mustelids; Department of Conservation, Science & Capability Group: Hamilton, New Zealand, 2013; p. 14.
- Joy, M.; David, B.; Lake, M.D.; Massey University; Institute of Natural Resources; Ecology Group. New Zealand Freshwater Fish Sampling Protocols; Massey University: Palmerston North, New Zealand, 2013; ISBN 978-0-473-23437-9. [Google Scholar]
- Oertli, B.; Biggs, J.; Céréghino, R.; Grillas, P.; Joly, P.; Lachavanne, J.-B. Conservation and Monitoring of Pond Biodiversity: Introduction. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 535–540. [Google Scholar] [CrossRef]
- Winterbourn, M.J.; Gregson, K.L.; Dolphin, C.H. Guide to the Aquatic Insects of New Zealand; Entomological Society of New Zealand: Auckland, New Zealand, 1989; Volume 9. [Google Scholar]
- Grainger, N.; Harding, J.S.; Drinan, T.; Collier, K.J.; Smith, B.J.; Death, R.; Makan, T.; Rolfe, J.R. Conservation Status of New Zealand Freshwater Invertebrates, 2018; Publishing Team, Department of Conservation: Wellington, New Zealand, 2018. [Google Scholar]
- Phillips, N.; Smith, B. New Zealand Freshwater Macroinvertebrate Trait Database; NIWA National Institute of Water & Atmospheric Research: Hamilton, New Zealand, 2018; p. 25. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). Statistician 2017, 56, e24. [Google Scholar]
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships. R News 2002, 2, 7–10. [Google Scholar]
- Belbin, L. PATN: Pattern Analysis Package: Technical Reference; Division of Wildlife and Ecology, CSIRO: Canberra, Australia, 1995. [Google Scholar]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional Dissimilarity as a Robust Measure of Ecological Distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package 2022. Computer Software Manual. Available online: https://www.researchgate.net/publication/360782912_vegan_community_ecology_package_version_26-2_April_2022. (accessed on 6 July 2023).
- Atkinson, I.A.E. Derivation of Vegetation Mapping Units for an Ecological Survey of Tongariro National North Island, New Zealand. N. Z. J. Bot. 1985, 23, 361–378. [Google Scholar] [CrossRef]
- Tanner, C.C.; Tomer, M.D.; Goeller, B.C. A Framework for Selecting and Applying Interceptive Agricultural Diffuse Pollution Mitigations. N. Z. J. Agric. Res. 2023; in-prep. [Google Scholar]
- Scheffer, M.; Geest, G.J.V.; Zimmer, K.; Jeppesen, E.; Søndergaard, M.; Butler, M.G.; Hanson, M.A.; Declerck, S.; Meester, L.D. Small Habitat Size and Isolation Can Promote Species Richness: Second-Order Effects on Biodiversity in Shallow Lakes and Ponds. Oikos 2006, 112, 227–231. [Google Scholar] [CrossRef]
- Spieles, D.J.; Mitsch, W.J. Macroinvertebrate Community Structure in High-and Low-Nutrient Constructed Wetlands. Wetlands 2000, 20, 716–729. [Google Scholar] [CrossRef]
- Briers, R.A.; Biggs, J. Spatial Patterns in Pond Invertebrate Communities: Separating Environmental and Distance Effects. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 549–557. [Google Scholar] [CrossRef]
- Moreno-Mateos, D.; Meli, P.; Vara-Rodríguez, M.I.; Aronson, J. Ecosystem Response to Interventions: Lessons from Restored and Created Wetland Ecosystems. J. Appl. Ecol. 2015, 52, 1528–1537. [Google Scholar] [CrossRef]
- Innes, J.; King, C.; Bartlam, S.; Forrester, G.; Howitt, R. Predator Control Improves Nesting Success in Waikato Forest Fragments. N. Z. J. Ecol. 2015, 39, 245–253. [Google Scholar]
- Smith, B.J.; Reid, D.J. Silent Assassins: Predation of Native New Zealand Trichopteran Eggs by Non-Native Freshwater Gastropods. Aquat. Insects 2016, 37, 293–302. [Google Scholar] [CrossRef]
- Angeler, D.G.; Álvarez-Cobelas, M.; Sánchez-Carrillo, S.; Rodrigo, M.A. Assessment of Exotic Fish Impacts on Water Quality and Zooplankton in a Degraded Semi-Arid Floodplain Wetland. Aquat. Sci. 2002, 64, 76–86. [Google Scholar] [CrossRef]
- Collier, K.J.; Grainger, N. New Zealand Invasive Fish Species. In New Zealand Invasive Fish Handbook; Lake Ecosystem Restoration New Zealand (LERNZ): Hamilton, New Zealand, 2015; pp. 8–22. [Google Scholar]
- Chappel, P.R. The Climate and Weather of Waikato. NIWA Sci. Technol. ND 2011, 61, 1–40. [Google Scholar]
- Shearer, J.C. Natural and Anthropogenic Influences on Peat Development in Waikato/Hauraki Plains Restiad Bogs. J. R. Soc. N. Z. 1997, 27, 295–313. [Google Scholar] [CrossRef]
- Ministry for the Environment; Stats New Zealand. New Zealand’s Environmental Reporting Series: Environment Aotearoa; Ministry for the Environment and Stats New Zealand: Wellington, New Zealand, 2019; p. 128.
- Beard, C.M. Wetlands and Lowland Floodplains. In Waters Waikato: Ecology of New Zealand’s Longest River; Collier, K.J., Hamilt, D.P., Vant, W.N., Howard-Williams, C., Eds.; Environment Waikato and the Centre for Biodiversity and Ecology Research University of Waikato: Hamilton, New Zealand, 2010; pp. 265–279. [Google Scholar]
- McGlone, M.S. Postglacial History of New Zealand Wetlands and Implications for Their Conservation. N. Z. J. Ecol. 2009, 33, 23. [Google Scholar]

| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Mean | Median | SD | Min. | Max. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Vegetation (113 species) | ||||||||||
| No. plots | 13 | 18 | 14 | 13 | 6 | |||||
| No. species | 58 | 64 | 56 | 44 | 31 | 50.60 | 56.00 | 13.14 | 31.00 | 64.00 |
| Ratio native species | 0.28 | 0.39 | 0.35 | 0.34 | 0.26 | 0.32 | 0.34 | 0.06 | 0.26 | 0.39 |
| Ratio obligate hydrophyte species a | 0.10 | 0.22 | 0.25 | 0.09 | 0.10 | 0.15 | 0.10 | 0.08 | 0.09 | 0.25 |
| Ratio facultative hydrophyte species a | 0.43 | 0.34 | 0.38 | 0.52 | 0.52 | 0.44 | 0.43 | 0.08 | 0.34 | 0.52 |
| Ratio terrestrial species a | 0.45 | 0.38 | 0.38 | 0.36 | 0.39 | 0.39 | 0.38 | 0.03 | 0.36 | 0.45 |
| Terrestrial invertebrates (85 species) | ||||||||||
| No. 1 month traps | 10 | 12 | 9 | 9 | 5 | |||||
| No. species | 36 | 54 | 40 | 49 | 36 | 45.78 | 49.00 | 7.48 | 36.00 | 54.00 |
| Ratio native species b | 0.56 | 0.57 | 0.58 | 0.55 | 0.44 | 0.31 | 0.44 | 0.28 | 0.00 | 0.58 |
| Ratio native individuals b | 0.55 | 0.46 | 0.49 | 0.33 | 0.55 | 0.50 | 0.49 | 0.09 | 0.33 | 0.67 |
| Aquatic invertebrates (47 species) | ||||||||||
| No. pooled samples | 3 | 3 | 2 | 2 | 2 | |||||
| No. species | 25 | 31 | 28 | 18 | 18 | 25.50 | 25.00 | 5.00 | 18.00 | 31.00 |
| Ratio native species | 0.80 | 0.74 | 0.82 | 0.67 | 0.61 | 0.74 | 0.74 | 0.07 | 0.61 | 0.82 |
| Ratio native individuals c | 0.28 | 0.78 | 0.20 | 0.15 | 0.26 | 0.40 | 0.28 | 0.26 | 0.15 | 0.78 |
| Birds (20 species) | ||||||||||
| No. dawn point counts | 2 | 2 | 2 | 2 | 2 | |||||
| No. species | 7 | 14 | 15 | 11 | 15 | 13.16 | 14.00 | 2.65 | 7.00 | 15.00 |
| Ratio native species | 0.43 | 0.43 | 0.53 | 0.27 | 0.53 | 0.45 | 0.43 | 0.10 | 0.27 | 0.53 |
| Ratio waterbird species | 0.43 | 0.21 | 0.33 | 0.18 | 0.20 | 0.26 | 0.21 | 0.08 | 0.18 | 0.43 |
| Mammals (five species) | ||||||||||
| No. tracking tunnel nights | 20 | 28 | 16 | 18 | 10 | |||||
| No. camera trap nights | 4 | 6 | 4 | 4 | 2 | |||||
| No. species | 3 | 3 | 2 | 2 | 1 | 2.38 | 3.00 | 0.69 | 1.00 | 3.00 |
| Ratio native species | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fish (six species) | ||||||||||
| No. overnight net sets | 2 | 3 | 2 | 2 | 1 | |||||
| No. overnight trap sets | 10 | 14 | 7 | 10 | 4 | |||||
| No. species | 0 | 5 | 0 | 4 | 0 | NA | NA | NA | NA | NA |
| Ratio native species | 0.00 | 0.60 | 0.00 | 0.50 | 0.00 | NA | NA | NA | NA | NA |
| Ratio native individuals | 0.00 | 0.08 | 0.00 | 0.08 | 0.00 | NA | NA | NA | NA | NA |
| Group | KW | Vegetation Classification Groups | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||
| Vegetation type | Typha orientalis reedland | Mixed sedgeland | Carex secta sedgeland | Phormium tenax flaxland | Mixed grassland | Ranunculus repens herbfield | |
| No. plots | 3 | 9 | 7 | 9 | 18 | 10 | |
| Species (cover %) | |||||||
| Ranunculus repens | 35.8 | 0 | 0.1 | 15.1 | 17.3 | 13.4 | 67.2 |
| Phormium tenax | 21.6 | 0 | 0.33 | 0 | 60 | 0.39 | 5 |
| Carex secta | 18.4 | 0 | 1.1 | 75.6 | 0.6 | 0 | 2 |
| Holcus lanatus | 11.6 | 0 | 0.2 | 1.3 | 6.1 | 13.2 | 5.1 |
| Typha orientalis | 8.4 | 86.7 | 0 | 0 | 0 | 0 | 0 |
| Plot variables | |||||||
| Prevalence Index | 35.1 | 1 | 1.39 | 1.51 | 2.34 | 2.62 | 2.91 |
| Canopy maximum height (cm) | 18.7 | 253 | 62 | 160 | 231 | 137 | 86 |
| Canopy mean height (cm) | 18.4 | 193 | 58 | 75 | 125 | 72 | 37 |
| Total species richness (n) | 15.7 | 2.3 | 2.7 | 4.6 | 7.1 | 5.4 | 5.8 |
| Total species cover (%) | 11 | 112 | 50 | 108 | 111 | 88 | 96 |
| Native species richness (n) | 9.4 | 1.7 | 1 | 1.7 | 1.8 | 0.9 | 0.7 |
| Group | KW | Invertebrate Classification Groups | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| No. samples | 21 | 5 | 2 | 5 | 5 | |
| Taxa (mean count) | ||||||
| Coleoptera Latridiidae Cartodere sp. | 4.0 | 1.0 | 7.0 | 5.6 | 27.8 | |
| Coleoptera Corylophidae Sericoderus sp. | 2.6 | 0.6 | 3.0 | 2.2 | 2.0 | |
| Amphipoda Talitdirdae Genus sp. | 1.8 | 22.0 | 5.0 | 0.6 | 0.6 | |
| Diptera Sphaeroceridae Genus sp. | 2.1 | 11.6 | 0.5 | 0 | 0.8 | |
| Diptera Phoridae Metopina sp. | 1.7 | 3.8 | 56.5 | 0.6 | 0.2 | |
| Orthoptera Gryllidae Genus sp. | 0.4 | 2.2 | 0.5 | 10.4 | 0 | |
| Hymenoptera Formicidae Chelaner sp. | 0.1 | 0 | 0 | 6.2 | 0 | |
| Sample variables | ||||||
| Canopy mean height (cm) | 13.3 | 68 | 41 | 165 | 84 | 150 |
| Total individuals (n) | 10.8 | 29.5 | 66.2 | 88 | 44.4 | 42.2 |
| Vegetation structure a | 10.2 | sedgeland-herbfield | herbfield | flaxland | flaxland-shrubland | shrubland |
| Canopy maximum height (cm) | 7.7 | 143.4 | 73.8 | 290 | 167.4 | 219 |
| Litter depth mean (cm) | 7.6 | 2.8 | 5.4 | 6.5 | 5.2 | 5.6 |
| Litter depth maximum (cm) | 7.3 | 5.7 | 11.6 | 14.5 | 8.6 | 11.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goeller, B.C.; Sukias, J.P.S.; Woodward, S.J.R.; Clarkson, B.R. ‘Dual Purpose’ Surface Flow Constructed Treatment Wetlands Support Native Biodiversity in Intensified Agricultural Landscapes. Water 2023, 15, 2526. https://doi.org/10.3390/w15142526
Goeller BC, Sukias JPS, Woodward SJR, Clarkson BR. ‘Dual Purpose’ Surface Flow Constructed Treatment Wetlands Support Native Biodiversity in Intensified Agricultural Landscapes. Water. 2023; 15(14):2526. https://doi.org/10.3390/w15142526
Chicago/Turabian StyleGoeller, Brandon C., James P. S. Sukias, Simon J. R. Woodward, and Beverley R. Clarkson. 2023. "‘Dual Purpose’ Surface Flow Constructed Treatment Wetlands Support Native Biodiversity in Intensified Agricultural Landscapes" Water 15, no. 14: 2526. https://doi.org/10.3390/w15142526





