First Estimation of the Annual Biosynthetic Calorie Production by Phytoplankton in the Yellow Sea, South Sea of Korea, East China Sea, and East Sea
Abstract
1. Introduction
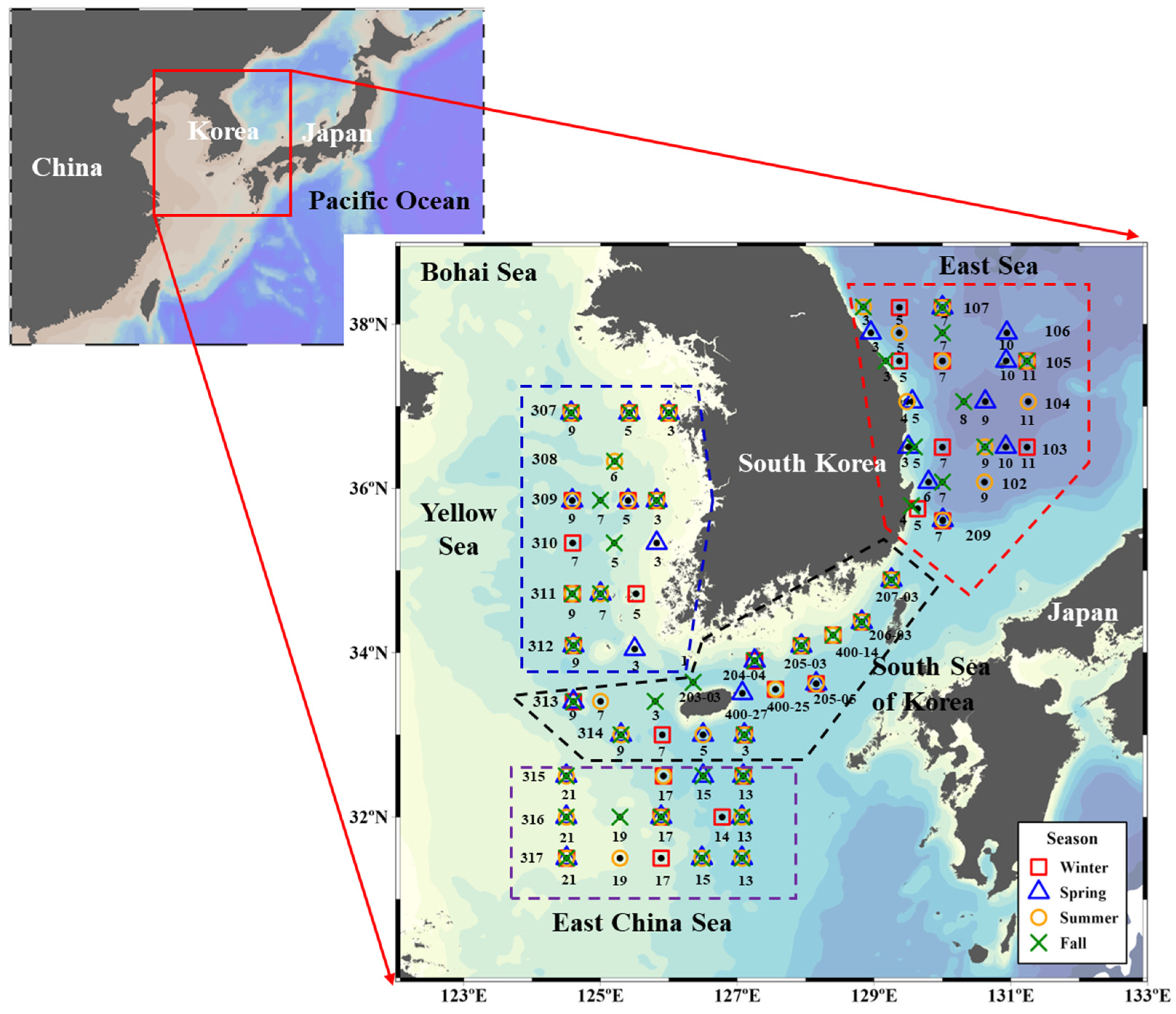
2. Materials and Methods
2.1. Water Sampling and Analysis for Concentrations of Chlorophyll a, Particulate Organic Carbon (POC), and Macromolecules
2.2. Calorie Content of POM
2.3. Calorie Production Rate of Phytoplankton
2.4. Statistical Analysis
3. Results and Discussion
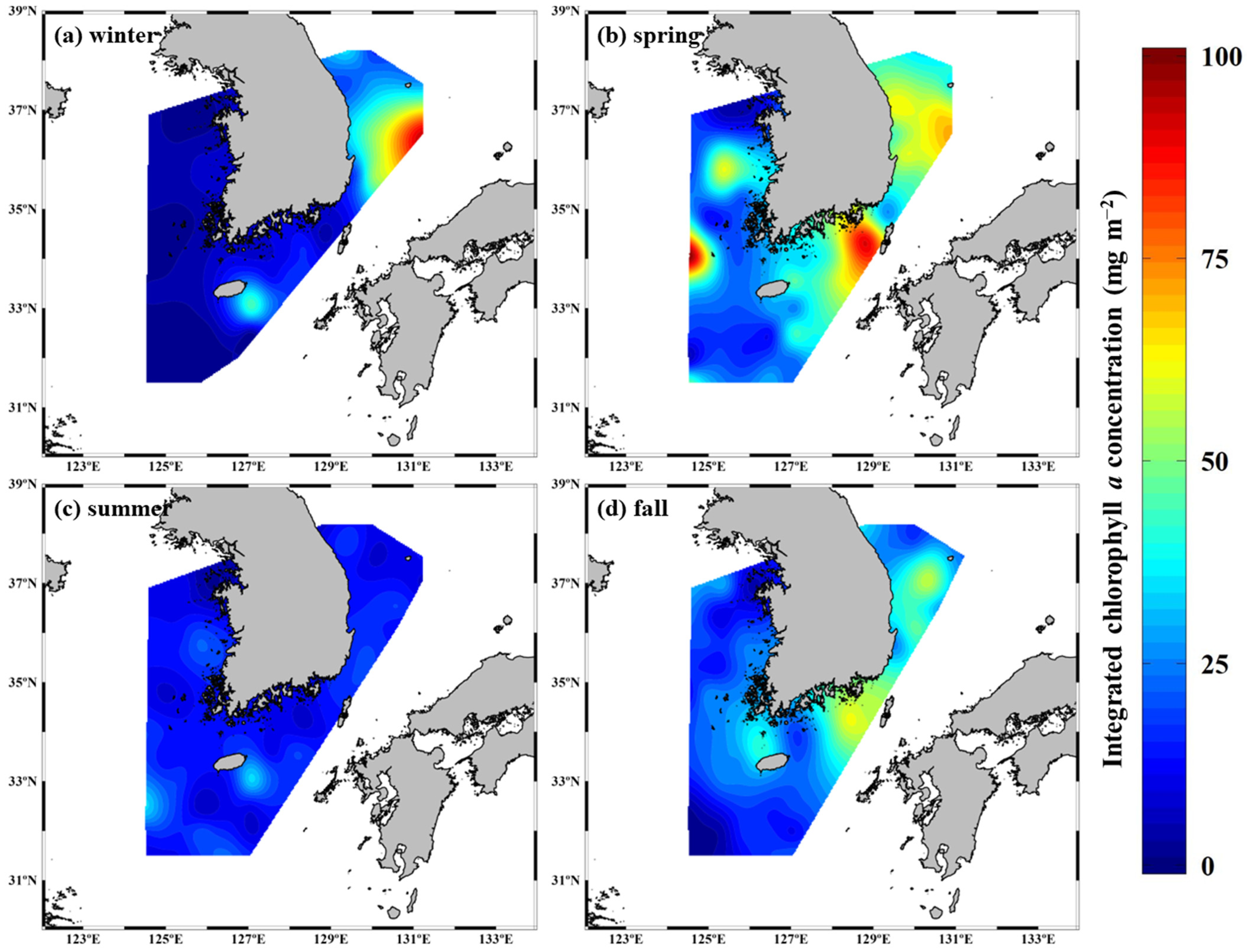
3.1. Seasonal Variation in Chlorophyll a Concentrations in the YS, SS, ECS, and ES
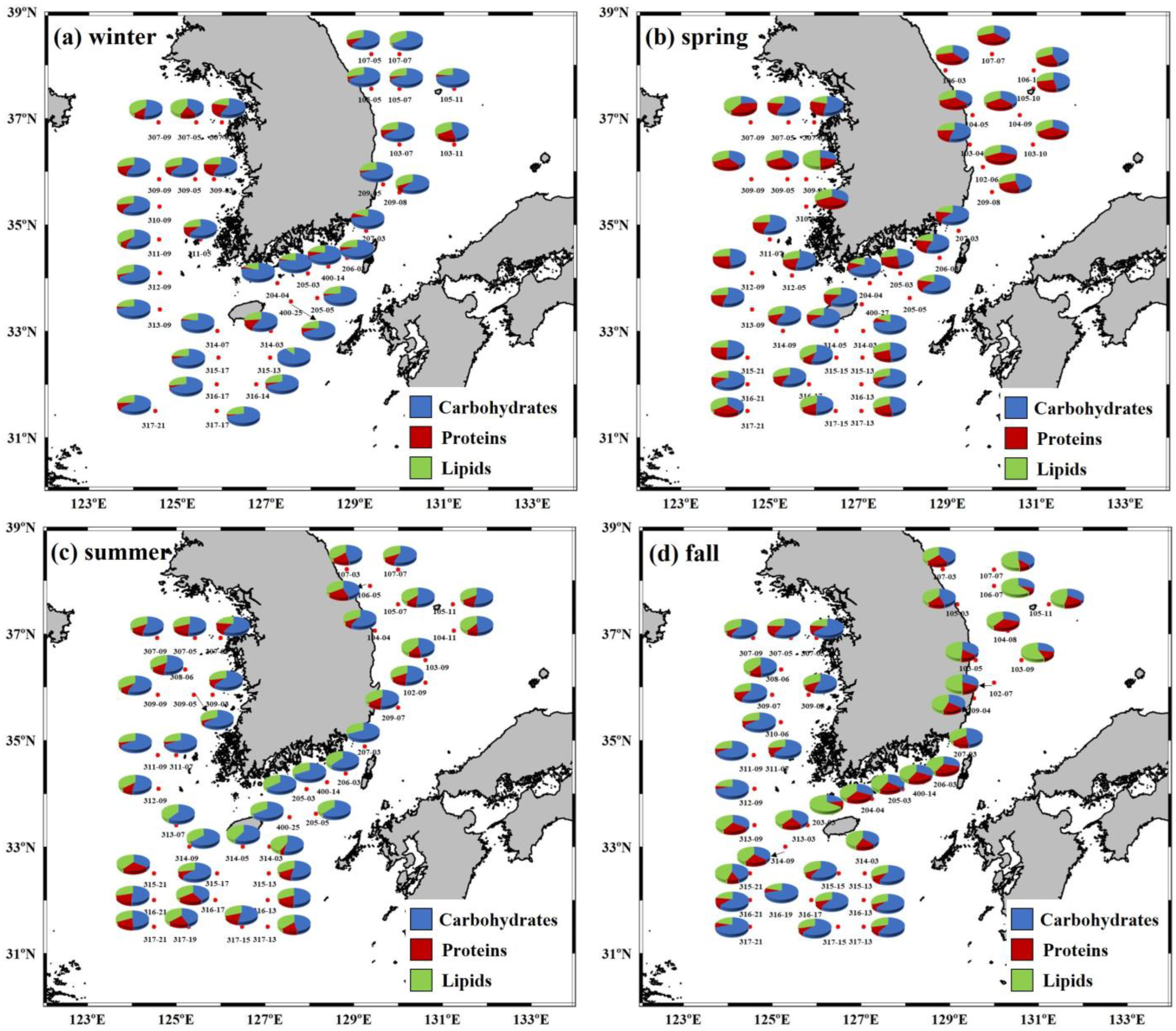
3.2. Seasonal Variations in the Macromolecular Compositions of POM in the YS, SS, ECS, and ES
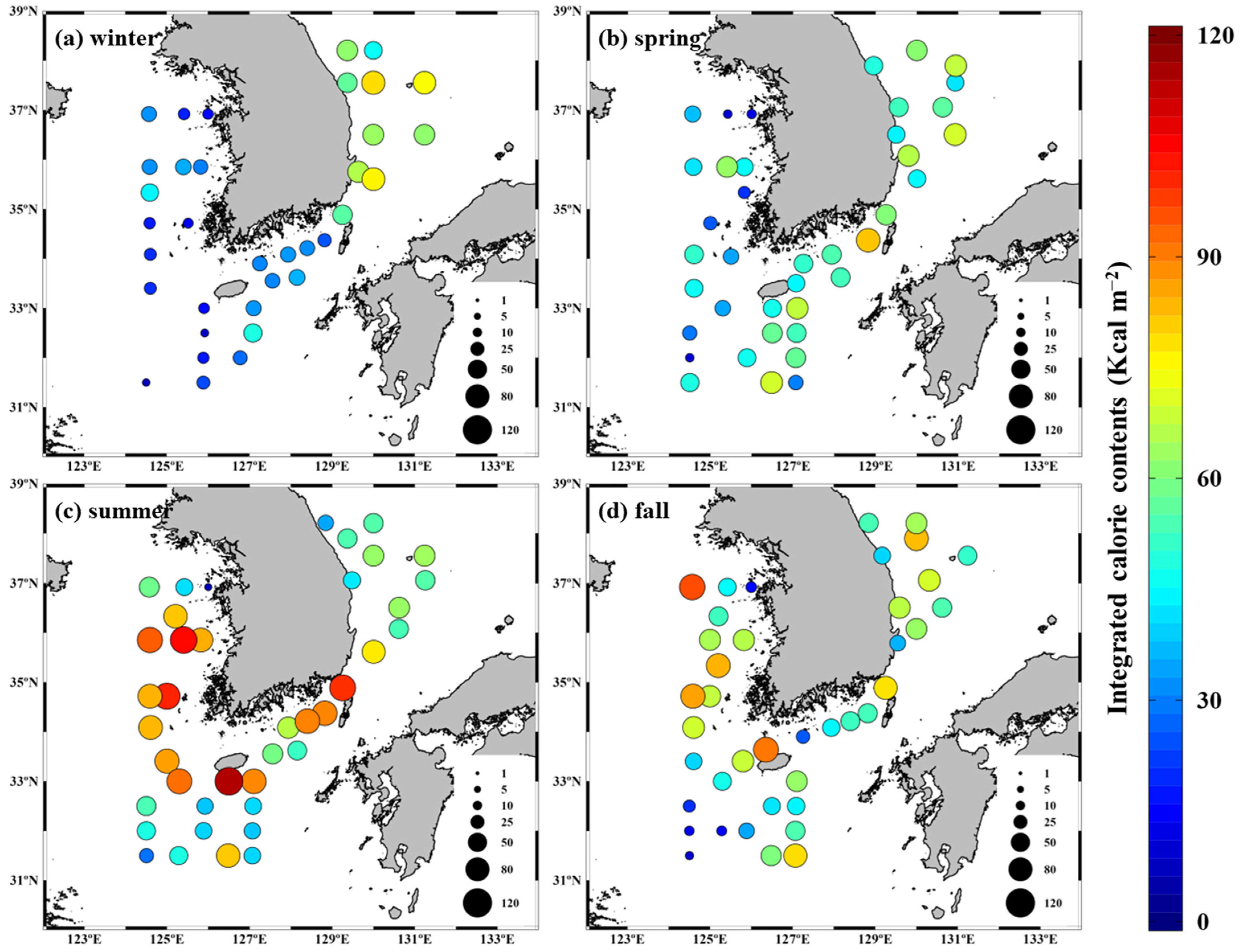
3.3. Seasonal Calorie Contents and Annual Calorie Productions of POM in the YS, SS, ECS, and ES
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liebezeit, G. Particulate carbohydrates in relation to phytoplankton in the euphotic zone of the Bransfield Strait. Polar Biol. 1984, 2, 225–228. [Google Scholar] [CrossRef]
- Moal, J.; Martin-Jezequel, V.; Harris, R.P.; Samain, J.F.; Poulet, S.A. Interspecific and intraspecific variability of the chemical composition of marine phytoplankton. Oceanol. Acta 1987, 10, 339–346. [Google Scholar]
- Fernández-Reiriz, M.J.; Perez-Camacho, A.; Ferreiro, M.J.; Blanco, J.; Planas, M.; Campos, M.J.; Labarta, U. Biomass production and variation in the biochemical profile (total protein, carbohydrates, RNA, lipids and fatty acids) of seven species of marine microalgae. Aquaculture 1989, 83, 17–37. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic diversity in the macromolecular composition of microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef]
- Morris, I. Photosynthetic products, physiological state, and phytoplankton growth. Can. Bull. Fish. Aquat. Sci. 1981, 210, 83–102. [Google Scholar]
- Parrish, C.C. Time series of particulate and dissolved lipid classes during spring phytoplankton blooms in Bedford Basin, a marine inlet. Mar. Ecol. Prog. Ser. 1987, 35, 129–139. [Google Scholar] [CrossRef]
- Van Oijen, T.; Van Leeuwe, M.A.; Gieskes, W.W.C.; De Baar, H.J.W. Effects of iron limitation on photosynthesis and carbohydrate metabolism in the Antarctic diatom Chaetoceros brevis (Bacillariophyceae). Eur. J. Phycol. 2004, 39, 161–171. [Google Scholar] [CrossRef]
- Duncan, R.J.; Petrou, K. Biomolecular composition of sea ice microalgae and its influence on marine biogeochemical cycling and carbon Transfer through polar marine food webs. Geoscience 2022, 12, 38. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, S.H.; Ha, S.Y.; Jung, J.; Kim, T.W.; Yang, E.J.; Jo, N.; Lim, Y.J.; Park, J.; Lee, S.H. Vertical distributions of macromolecular composition of particulate organic matter in the water column of the Amundsen Sea Polynya during the summer in 2014. J. Geophys. Res. Ocean 2018, 123, 1393–1405. [Google Scholar] [CrossRef]
- Lee, S.H.; Whitledge, T.E.; Kang, S.-H. Carbon uptake rates of sea ice algae and phytoplankton under different light intensities in a landfast sea ice zone, Barrow, Alaska. Arctic 2008, 61, 281–291. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.; Kang, J.J.; Joo, H.T.; Lee, J.H.; Lee, H.W.; Ahn, S.H.; Kang, C.K.; Lee, S.H. The effects of different environmental factors on the biochemical composition of particulate organic matter in Gwangyang Bay, South Korea. Biogeosciences 2017, 14, 1903–1917. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, J.J.; Jang, H.K.; Jo, N.; Lee, D.; Yun, M.S.; Lee, S.H. Major controlling factors for spatio-temporal variations in the macromolecular composition and primary production by phytoplankton in Garolim and Asan Bays in the Yellow Sea. Reg. Stud. Mar. Sci. 2020, 36, 101269. [Google Scholar] [CrossRef]
- Tanoue, E.; Handa, N. Monosaccharide composition of marine particles and sediments from the Bering Sea and northern North Pacific. Oceanol. Acta 1987, 10, 91–99. [Google Scholar]
- Bhosle, N.B.; Wagh, A.B. Particulate carbohydrates in the Arabian Sea. Oceanol. Acta. 1989, 12, 57–63. [Google Scholar]
- Myklestad, S.; Holm-Hansen, O.; Vårum, K.M.; Volcani, B.E. Rate of release of extracellular amino acids and carbohydrates from the marine diatom Chaetoceros affinis. J. Plankton Res. 1989, 11, 763–773. [Google Scholar] [CrossRef]
- Kang, J.J.; Joo, H.T.; Lee, J.H.; Lee, J.H.; Lee, H.W.; Lee, D.; Kang, C.K.; Yun, M.S.; Lee, S.H. Comparison of biochemical compositions of phytoplankton during spring and fall seasons in the northern East/Japan Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2017, 143, 73–81. [Google Scholar] [CrossRef]
- Jo, N.; Kang, J.J.; Park, W.G.; Lee, B.R.; Yun, M.S.; Lee, J.H.; Kim, S.M.; Lee, D.; Joo, H.T.; Lee, J.H.; et al. Seasonal variation in the biochemical compositions of phytoplankton and zooplankton communities in the southwestern East/Japan Sea. Deep Res. Part II Top. Stud. Oceanogr. 2017, 143, 82–90. [Google Scholar] [CrossRef]
- Ríos, A.F.; Fraga, F.; Pérez, F.F.; Figueiras, F.G. Chemical composition of phytoplankton and particulate organic matter in the Ría de Vigo (NW Spain). Sci. Mar. 1998, 62, 257–271. [Google Scholar] [CrossRef]
- Mortensen, S.H.; Børsheim, K.Y.; Rainuzzo, J.; Knutsen, G. Fatty acid and elemental composition of the marine diatom Chaetoceros gracilis Schütt. Effects of silicate deprivation, temperature and light intensity. J. Exp. Mar. Biol. Ecol. 1988, 122, 173–185. [Google Scholar] [CrossRef]
- Morris, I.; Glover, H.E.; Yentsch, C.S. Products of photosynthesis by marine phytoplankton: The effect of environmental factors on the relative rates of protein synthesis. Mar. Biol. 1974, 27, 1–9. [Google Scholar] [CrossRef]
- Kowallik, W. Blue light effects on carbohydrate and protein metabolism. In Blue Light Responses: Phenomena and Occurrence in Plants; Senger, H., Ed.; CRC Press: Boca Raton, FL, USA, 1978; Volume 1, pp. 8–13. [Google Scholar]
- Suárez, I.; Marañón, E. Photosynthate allocation in a temperate sea over an annual cycle: The relationship between protein synthesis and phytoplankton physiological state. J. Sea Res. 2003, 50, 285–299. [Google Scholar] [CrossRef]
- Kilham, S.S.; Kreeger, D.A.; Goulden, C.E.; Lynn, S.G. Effects of nutrient limitation on biochemical constituents of An-kistrodesmus falcatus. Freshw. Biol. 1997, 38, 591–596. [Google Scholar] [CrossRef]
- Harrison, P.J.; Thompson, P.A.; Calderwood, G.S. Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J. Appl. Phycol. 1990, 2, 45–56. [Google Scholar] [CrossRef]
- Berdalet, E.; Latasa, M.; Estrada, M. Effects of nitrogen and phosphorus starvation on nucleic acid and protein content of Heterocapsa sp. J. Plankton Res. 1994, 16, 303–316. [Google Scholar] [CrossRef]
- Chu, W.L.; Phang, S.M.; Goh, S.H. Environmental effects on growth and biochemical composition of Nitzschia inconspicua Grunow. J. Appl. Phycol. 1996, 8, 389–396. [Google Scholar] [CrossRef]
- Biddanda, B.; Benner, R. Carbon, nitrogen, and carbohydrate fluxes during the production of particulate and dissolved organic matter by marine phytoplankton. Limnol. Oceanogr. 1997, 42, 506–518. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.J.; Whitledge, T.E. High incorporation of carbon into proteins by the phytoplankton of the Bering Strait and Chukchi Sea. Cont. Shelf Res. 2009, 29, 1689–1696. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, J.H.; Yun, M.S.; Joo, H.T.; Song, H.J.; Yang, E.J.; Chung, K.H.; Kang, S.H.; Lee, S.H. High lipid composition of particulate organic matter in the northern Chukchi Sea, 2011. Deep. Res. Part II Top. Stud. Oceanogr. 2015, 120, 72–81. [Google Scholar] [CrossRef]
- Yun, M.S.; Lee, D.B.; Kim, B.K.; Kang, J.J.; Lee, J.H.; Yang, E.J.; Park, W.G.; Chung, K.H.; Lee, S.H. Comparison of phytoplankton macromolecular compositions and zooplankton proximate compositions in the northern Chukchi Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2015, 120, 82–90. [Google Scholar] [CrossRef]
- Jo, N.; La, H.S.; Kim, J.H.; Kim, K.; Kim, B.K.; Kim, M.J.; Son, W.; Lee, S.H. Different biochemical compositions of particulate organic matter driven by major phytoplankton communities in the northwestern Ross Sea. Front. Microbiol. 2021, 12, 623600. [Google Scholar] [CrossRef]
- Bhavya, P.S.; Kim, B.K.; Jo, N.; Kim, K.; Kang, J.J.; Lee, J.H.; Lee, D.; Lee, J.H.; Joo, H.T.; Ahn, S.H.; et al. A review on the macromolecular compositions of phytoplankton and the implications for aquatic biogeochemistry. Ocean Sci. J. 2019, 54, 1–14. [Google Scholar] [CrossRef]
- Platt, T.; Irwin, B. Caloric content of phytoplankton. Limnol. Oceanogr. 1973, 18, 306–310. [Google Scholar] [CrossRef]
- Fabiano, M.; Povero, P.; Danovaro, R. Distribution and composition of particulate organic matter in the Ross Sea (Antarctica). Polar Biol. 1993, 3, 525–533. [Google Scholar] [CrossRef]
- Danovaro, R.; Fabiano, M. Seasonal changes in quality and quantity of food available for benthic suspension-feeders in the Golfo Marconi (North-western Mediterranean). Estuar. Coast. Shelf Sci. 1997, 44, 723–736. [Google Scholar] [CrossRef]
- Kang, J.J.; Jang, H.K.; Lim, J.H.; Lee, D.; Lee, J.H.; Bae, H.; Lee, C.H.; Kang, C.K.; Lee, S.H. Characteristics of different size phytoplankton for primary production and biochemical compositions in the western East/Japan Sea. Front. Microbiol. 2020, 11, 560102. [Google Scholar] [CrossRef]
- Jang, H.K.; Youn, S.H.; Joo, H.; Kim, Y.; Kang, J.J.; Lee, D.; Jo, N.; Kim, K.; Kim, M.J.; Kim, S.; et al. First concurrent measurement of primary production in the Yellow Sea, the South Sea of Korea, and the East/Japan Sea, 2018. J. Mar. Sci. Eng. 2021, 9, 1237. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A manual of chemical & biological methods for seawater analysis. Mar. Pollut. Bull. 1984, 15, 419–420. [Google Scholar]
- Lee, J.H.; Lee, W.C.; Kim, H.C.; Jo, N.; Kim, K.; Lee, D.; Kang, J.J.; Sim, B.R.; Kwon, J., II; Lee, S.H. Temporal and spatial variations of the biochemical composition of phytoplankton and potential food material (FM) in Jaran Bay, South Korea. Water 2020, 12, 3093. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Roserough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Fiset, C.; Liefer, J.; Irwin, A.J.; Finkel, Z.V. Methodological biases in estimates of macroalgal macromolecular composition. Limnol. Oceanogr. Methods 2017, 15, 618–630. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar] [CrossRef]
- Winberg, G.G. Symbols, Units and Conversion Factors in Study of Fresh Waters Productivity; International Biological Programme: London, UK, 1971; p. 23. [Google Scholar]
- Yamada, K.; Ishizaka, J.; Yoo, S.; Kim, H.-C.; Chiba, S. Seasonal and interannual variability of sea surface chlorophyll a con-centration in the Japan/East Sea (JES). Prog. Oceanogr. 2004, 61, 193–211. [Google Scholar] [CrossRef]
- Lee, S.H.; Son, S.; Dahms, H.-U.; Park, J.W.; Lim, J.-H.; Noh, J.H.; Joo, H.T.; Jeong, J.Y.; Kang, C.K. Decadal changes of phytoplankton chlorophyll-a in the East Sea/Sea of Japan. Oceanology 2014, 54, 771–779. [Google Scholar] [CrossRef]
- Chang, K.I.; Zhang, C.I.; Park, C.; Kang, D.J.; Ju, S.J.; Lee, S.H.; Wimbush, M. (Eds.) Oceanography of the East Sea (Japan Sea); Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-22719-1. [Google Scholar]
- Wang, Y.; Gao, Z. Contrasting chlorophyll-a seasonal patterns between nearshore and offshore waters in the Bohai and Yellow Seas, China: A new analysis using improved satellite data. Cont. Shelf Res. 2020, 203, 104173. [Google Scholar] [CrossRef]
- Kim, Y.; Youn, S.H.; Oh, H.J.; Joo, H.; Jang, H.K.; Kang, J.J.; Lee, D.; Jo, N.; Kim, K.; Park, S.; et al. Seasonal compositions of size-fractionated surface phytoplankton communities in the Yellow Sea. J. Mar. Sci. Eng. 2022, 10, 1087. [Google Scholar] [CrossRef]
- Wie, J.; Moon, B.-K.; Hyun, Y.-K.; Lee, J. Impact of local atmospheric circulation and sea surface temperature of the East Sea (Sea of Japan) on heat waves over the Korean Peninsula. Theor. Appl. Climatol. 2021, 144, 431–446. [Google Scholar] [CrossRef]
- Ahn, S.H.; Whitledge, T.E.; Stockwell, D.A.; Lee, J.H.; Lee, H.W.; Lee, S.H. The biochemical composition of phytoplankton in the Laptev and East Siberian seas during the summer of 2013. Polar Biol. 2019, 42, 133–148. [Google Scholar] [CrossRef]
- Fogg, G.E.; Thake, B. Algal Cultures and Phytoplankton Ecology; University of Wisconsin Press: Madison, WI, USA, 1987. [Google Scholar]
- DiTullio, G.R.; Laws, E.A. Diel periodicity of nitrogen and carbon assimilation in five species of marine phytoplankton: Accuracy of methodology for predicting N-assimilation rates and N/C composition ratios. Mar. Ecol. Prog. Ser. 1986, 32, 123–132. [Google Scholar] [CrossRef]
- Palmisano, A.C.; Lizotte, M.P.; Smith, G.A.; Nichols, P.D.; White, D.C.; Sullivan, C.W. Changes in photosynthetic carbon assimilation in Antarctic sea-ice diatoms during spring bloom: Variation in synthesis of lipid classes. J. Exp. Mar. Biol. Ecol. 1988, 116, 1–13. [Google Scholar] [CrossRef]
- Moon, C.-H.; Choi, H.-J. Studies on the environmental characteristics and phytoplankton community in the Nakdong River estuary. J. Oceanogr Soc. Korea 1991, 26, 144–154. [Google Scholar]
- Myklestad, S. Production of carbohydrates by marine planktonic diatoms. I. Comparison of nine different species in culture. J. Exp. Mar. Bio. Ecol. 1974, 15, 261–274. [Google Scholar] [CrossRef]
- Jo, N.; Youn, S.H.; Joo, H.T.; Jang, H.K.; Kim, Y.; Park, S.; Kim, J.; Kim, K.; Kang, J.J.; Lee, S.H. Seasonal variations in biochemical (biomolecular and amino Acid) compositions and protein quality of particulate organic matter in the southwestern East/Japan Sea. Front. Mar. Sci. 2022, 9, 979137. [Google Scholar] [CrossRef]
- Navarro, J.M.; Clasing, E.; Urrutia, G.; Asencio, G.; Stead, R.; Herrera, C. Biochemical composition and nutritive value of suspended particulate matter over a tidal flat of southern Chile. Estuar. Coast. Shelf Sci. 1993, 37, 59–73. [Google Scholar] [CrossRef]
- Danovaro, R.; Dell’anno, A.; Pusceddu, A.; Marrale, D.; Croce, N.D.; Fabiano, M.; Tselepides, A. Biochemical composition of pico-, nano- and micro-particulate organic matter and bacterioplankton biomass in the oligotrophic Cretan Sea (NE Mediterranean). Prog. Oceanogr. 2000, 46, 279–310. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, M.S.; Kim, B.K.; Joo, H.; Kang, S.H.; Kang, C.K.; Whitledge, T.E. Contribution of small phytoplankton to total primary production in the Chukchi Sea. Cont. Shelf Res. 2013, 68, 43–50. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, T.W.; Lee, S.; Lee, D.; Park, J.; Kim, B.K.; Kim, K.; Jang, H.K.; Bhavya, P.S.; Lee, S.H. Seasonal variations in the small phytoplankton contribution to the total primary production in the Amundsen Sea, Antarctica. J. Geophys. Res. Oceans 2019, 124, 8324–8341. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, M.S.; Jang, H.K.; Kang, J.J.; Kim, K.; Lee, D.; Jo, N.; Park, S.H.; Lee, J.H.; Ahn, S.H.; et al. Size-differential photosynthetic traits of phytoplankton in the Chukchi Sea. Cont. Shelf Res. 2023, 255, 104933. [Google Scholar] [CrossRef]
- Joo, H.; Park, J.W.; Son, S.; Noh, J.H.; Jeong, J.Y.; Kwak, J.H.; Saux-Picart, S.; Choi, J.H.; Kang, C.-K.; Lee, S.H. Long-term annual primary production in the Ulleung Basin as a biological hot spot in the East/Japan Sea. J. Geophys. Res. Oceans. 2014, 119, 3002–3011. [Google Scholar] [CrossRef]
- Hyun, J.H.; Kim, S.H.; Mok, J.S.; Cho, H.; Lee, T.; Vandieken, V.; Thamdrup, B. Manganese and iron reduction dominate organic carbon oxidation in surface sediments of the deep Ulleung Basin, East Sea. Biogeoscience 2017, 14, 941–958. [Google Scholar] [CrossRef]
- Lee, S.H.; Joo, H.T.; Lee, J.H.; Lee, J.H.; Kang, J.J.; Lee, H.W.; Lee, D.; Kang, C.K. Seasonal carbon uptake rates of phytoplankton in the northern East/Japan Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2017, 143, 45–53. [Google Scholar] [CrossRef]
- Morán, X.A.G.; López-Urrutia, Á.; Calvo-Díaz, A.; LI, W.K.W. Increasing importance of small phytoplankton in a warmer ocean. Glob. Chang. Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Mousing, E.A.; Ellegaard, M.; Richardson, K. Global patterns in phytoplankton community size structure-evidence for a direct temperature effect. Mar. Ecol. Prog. Ser. 2014, 497, 25–38. [Google Scholar] [CrossRef]
- Joo, H.T.; Son, S.H.; Park, J.-W.; Kang, J.J.; Jeong, J.-Y.; Kwon, J.-I.; Kang, C.-K.; Lee, S.H. Small phytoplankton contribution to the total primary production in the highly productive Ulleung Basin in the East/Japan Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2017, 143, 54–61. [Google Scholar] [CrossRef]
- Jang, H.K.; Kang, J.J.; Lee, J.H.; Kim, M.; Ahn, S.H.; Jeong, J.-Y.; Yun, M.S.; Han, I.-S.; Lee, S.H. Recent primary production and small phytoplankton contribution in the Yellow Sea during the summer in 2016. Ocean Sci. 2018, 53, 509–519. [Google Scholar] [CrossRef]
- Lin, C.; Ning, X.; Su, J.; Lin, Y.; Xu, B. Environmental changes and the responses of the ecosystems of the Yellow Sea during 1976–2000. J. Mar. Syst. 2005, 55, 223–234. [Google Scholar] [CrossRef]
- Fang, T.; Li, D.; Yu, L.; Gao, L.; Zhang, L. Effects of irradiance and phosphate on growth of nanophytoplankton and picophytoplankton. Acta Ecol. Sin. 2006, 26, 2783–2789. [Google Scholar] [CrossRef]
- Jin, J.; Liu, S.M.; Ren, J.L.; Liu, C.G.; Zhang, J.; Zhang, G.L. Nutrient dynamics and coupling with phytoplankton species composition during the spring blooms in the Yellow Sea. Deep. Res. Part II Top. Stud. Oceanogr. 2013, 97, 16–32. [Google Scholar] [CrossRef]
- Taniguchi, A. Geographical variation of primary production in the Western Pacific Ocean and adjacent seas with reference to the inter-relations between various parameters of primary production. Mem. Fac. Fish. Hokkaido Univ. 1972, 19, 1–33. [Google Scholar]
- Kwak, J.H.; Hwang, J.; Choy, E.J.; Park, H.J.; Kang, D.J.; Lee, T.; Chang, K.-I.; Kim, K.-R.; Kang, C.-K. High primary productivity and f-ratio in summer in the Ulleung Basin of the East/Japan Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2013, 79, 74–85. [Google Scholar] [CrossRef]
- Lee, S.H.; Yun, M.S.; Kim, B.K.; Saitoh, S.I.; Kang, C.K.; Kang, S.-H.; Whitledge, T. Latitudinal carbon productivity in the Bering and Chukchi Seas during the summer in 2007. Cont. Shelf Res. 2013, 59, 28–36. [Google Scholar] [CrossRef]
- Kim, B.K.; Joo, H.; Song, H.J.; Yang, E.J.; Lee, S.H.; Hahm, D.; Rhee, T.S.; Lee, S.H. Large seasonal variation in phytoplankton production in the Amundsen Sea. Polar Biol. 2014, 38, 319–331. [Google Scholar] [CrossRef]
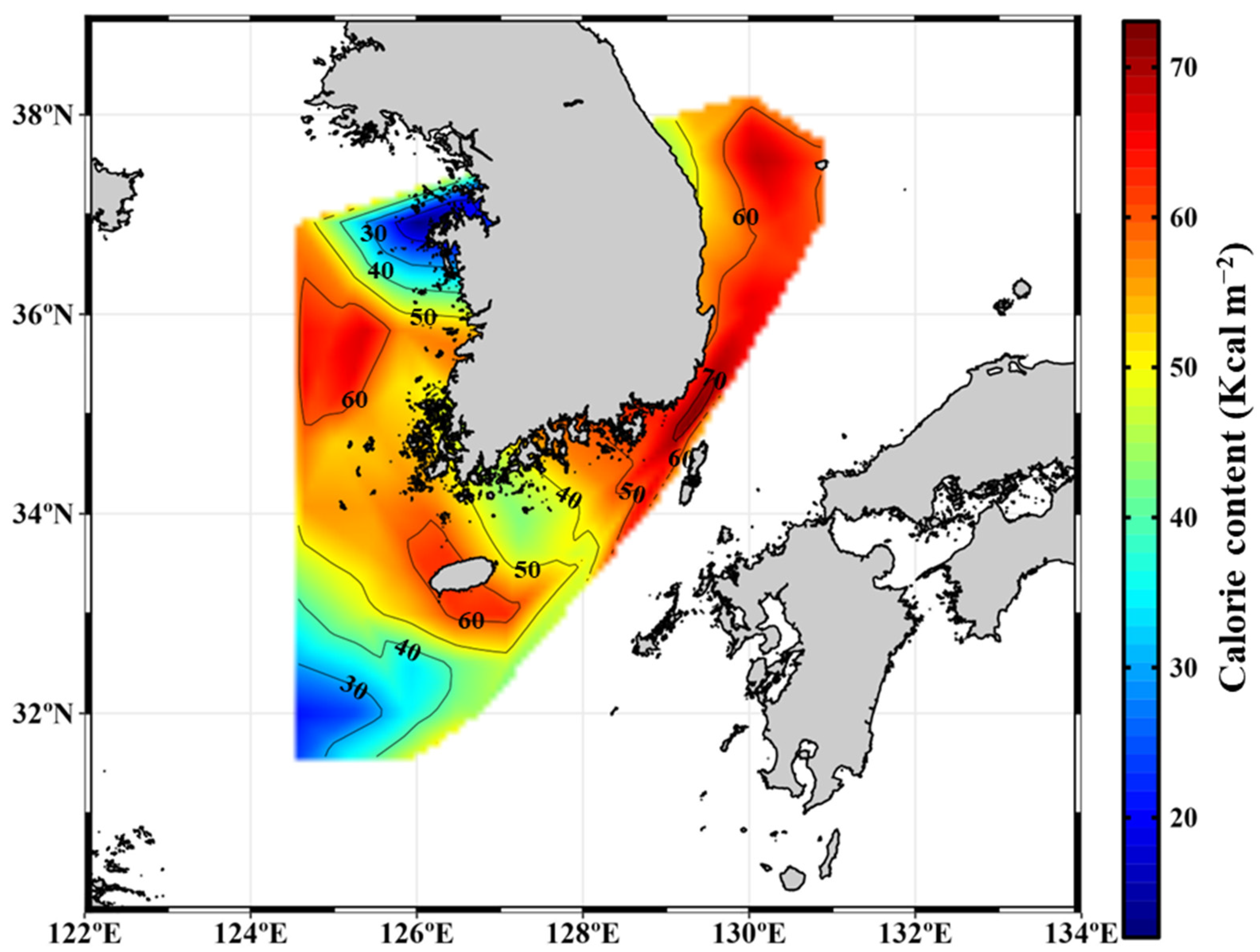
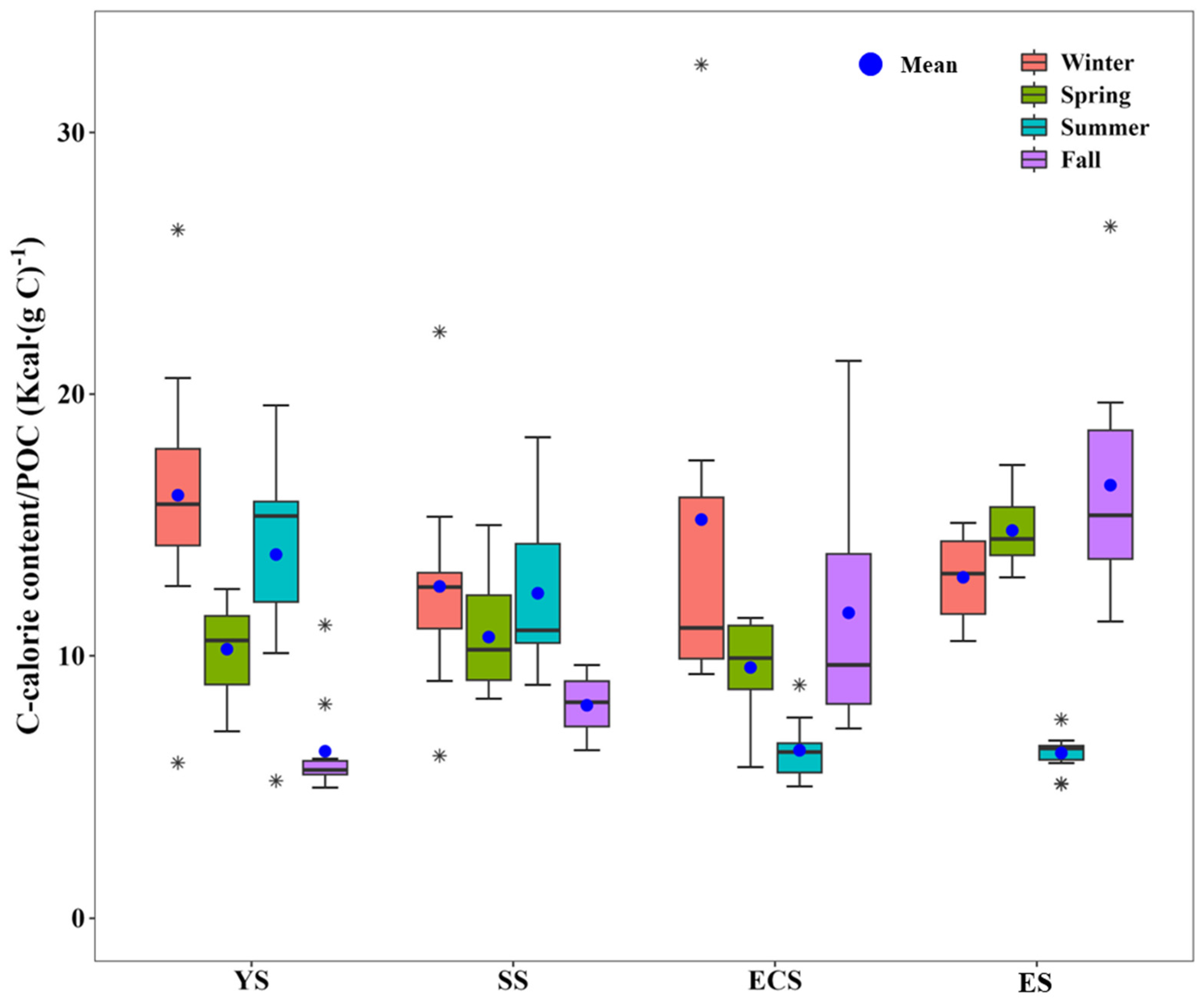
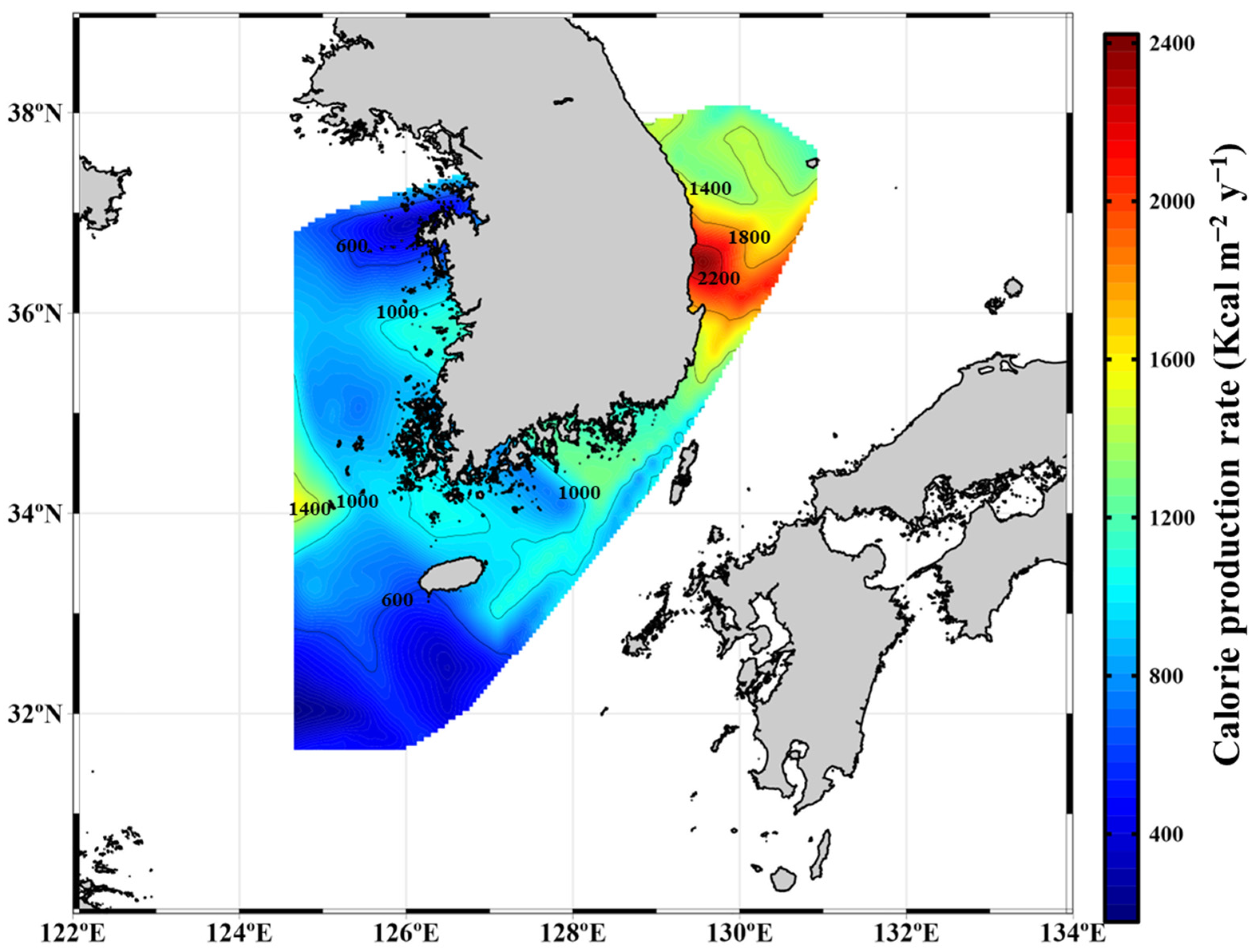
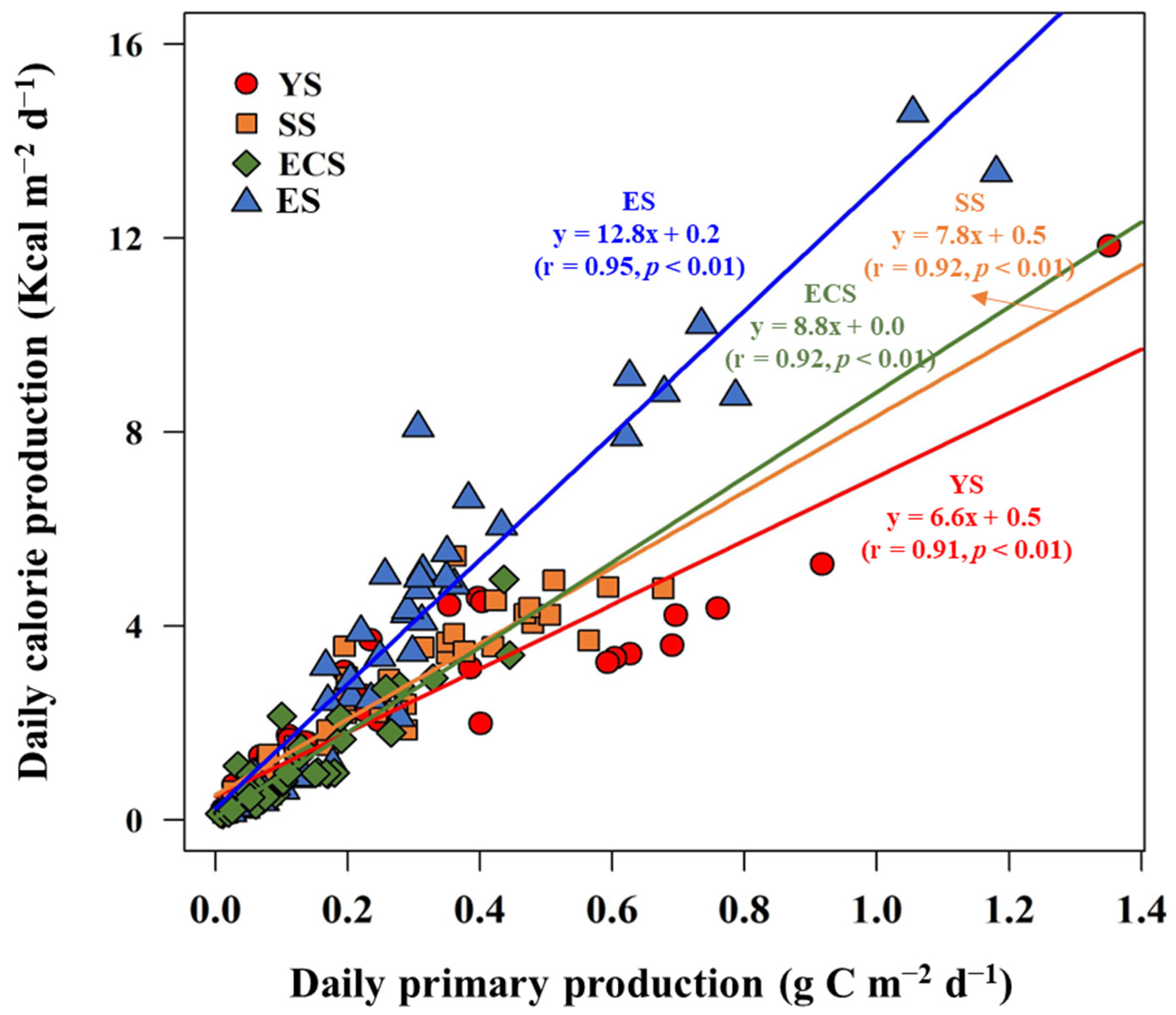
| Location | Period | Carbohydrates (mg m−3) | Proteins (mg m−3) | Lipids (mg m−3) | FM (mg m−3) | Calorie Content (Kcal m−3) | References | |
|---|---|---|---|---|---|---|---|---|
| East Sea | Southwestern part | 2014 (Apr.–Nov.) | 199 ± 93 | 85 ± 59 | 90 ± 36 | [17] | ||
| Northern part | 2012 (Oct.) | 41–86 (67 ± 16) | 40–122 (66 ± 23) | 80–136 (95 ± 17) | 170–340 (230 ± 50) | 1–2 (2 ± 0.3) | [16] | |
| 2015 (May) | 97–313 (150 ± 64) | 52–106 (75 ± 18) | 38–125 (60 ± 27) | 194–542 (284 ± 98) | 1–3 (2 ± 0.5) | |||
| Ulleung Basin | 2016 (Apr.) | 72–215 (151 ± 54) | 41–92 (66 ± 18) | 91–189 (123 ± 33) | 232–401 (340 ± 61) | 1.6–2.4 (2 ± 0.3) | [36] | |
| Northwestern part | 89–191 (139 ± 34) | 42–143 (80 ± 31) | 92–284 (203 ± 56) | 309–594 (422 ± 81) | 2–4 (3 ± 1) | |||
| Korean Bay | Gwangyang bay | 2012 (Apr.)–2013 (Apr.) | 14–412 (130 ± 87) | 23–382 (155 ± 73) | 21–401 (155 ± 79) | 171–916 (435 ± 176) | 1–6 (3 ± 1) | [11] |
| Garolim and Asan bay | 2015 (Apr.)–2016 (Nov.) | 232–703 (455 ± 134) | 11–379 (150 ± 87) | 42–457 (177 ± 103) | 351–1270 (781 ± 238) | 2–8 (4 ± 2) | [12] | |
| Jaran bay | 2016 (Monthly) | 145–269 (210 ± 42) | 41–202 (90 ± 50) | 85–158 (111 ± 24) | 297–630 (111 ± 24) | 1.7–3.7 (2.4 ± 0.5) | [39] | |
| Korean Seas | Yellow Sea | 2018 (Feb., Apr., Aug., and Oct.) | 73–522 (201 ± 77) | 17–212 (59 ± 43) | 77–244 (111 ± 36) | 266–923 (372 ± 128) | 2–5(2 ± 0.7) | This study |
| South Sea | 67–401 (162 ± 76) | 0–98 (34 ± 29) | 36–230 (88 ± 33) | 173–549 (284 ± 96) | 1–3 (2 ± 0.5) | |||
| East Sea | 60–254 (112 ± 38) | 3–147 (44 ± 35) | 44–158 (88 ± 30) | 131–472 (244 ± 78) | 1–3 (2 ± 0.5) | |||
| East China Sea | 2018 (Feb., May, Aug., and Nov.) | 78–499 (164 ± 77) | 1–138 (40 ± 29) | 41–194 (85 ± 33) | 144–552 (288 ± 101) | 1–4 (2 ± 0.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.-K.; Youn, S.-H.; Joo, H.; Kang, J.-J.; Lee, J.-H.; Lee, D.; Jo, N.; Kim, Y.; Kim, K.; Kim, M.-J.; et al. First Estimation of the Annual Biosynthetic Calorie Production by Phytoplankton in the Yellow Sea, South Sea of Korea, East China Sea, and East Sea. Water 2023, 15, 2489. https://doi.org/10.3390/w15132489
Jang H-K, Youn S-H, Joo H, Kang J-J, Lee J-H, Lee D, Jo N, Kim Y, Kim K, Kim M-J, et al. First Estimation of the Annual Biosynthetic Calorie Production by Phytoplankton in the Yellow Sea, South Sea of Korea, East China Sea, and East Sea. Water. 2023; 15(13):2489. https://doi.org/10.3390/w15132489
Chicago/Turabian StyleJang, Hyo-Keun, Seok-Hyun Youn, Huitae Joo, Jae-Joong Kang, Jae-Hyung Lee, Dabin Lee, Naeun Jo, Yejin Kim, Kwanwoo Kim, Myung-Joon Kim, and et al. 2023. "First Estimation of the Annual Biosynthetic Calorie Production by Phytoplankton in the Yellow Sea, South Sea of Korea, East China Sea, and East Sea" Water 15, no. 13: 2489. https://doi.org/10.3390/w15132489
APA StyleJang, H.-K., Youn, S.-H., Joo, H., Kang, J.-J., Lee, J.-H., Lee, D., Jo, N., Kim, Y., Kim, K., Kim, M.-J., Park, S., Kim, J., Kim, J., Ahn, S.-H., & Lee, S.-H. (2023). First Estimation of the Annual Biosynthetic Calorie Production by Phytoplankton in the Yellow Sea, South Sea of Korea, East China Sea, and East Sea. Water, 15(13), 2489. https://doi.org/10.3390/w15132489








