Potential of Macrophytes for Wastewater Remediation with Constructed Floating Wetlands in Cold Climates
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field and Laboratory Measurements
2.3. Data Analyses
3. Results
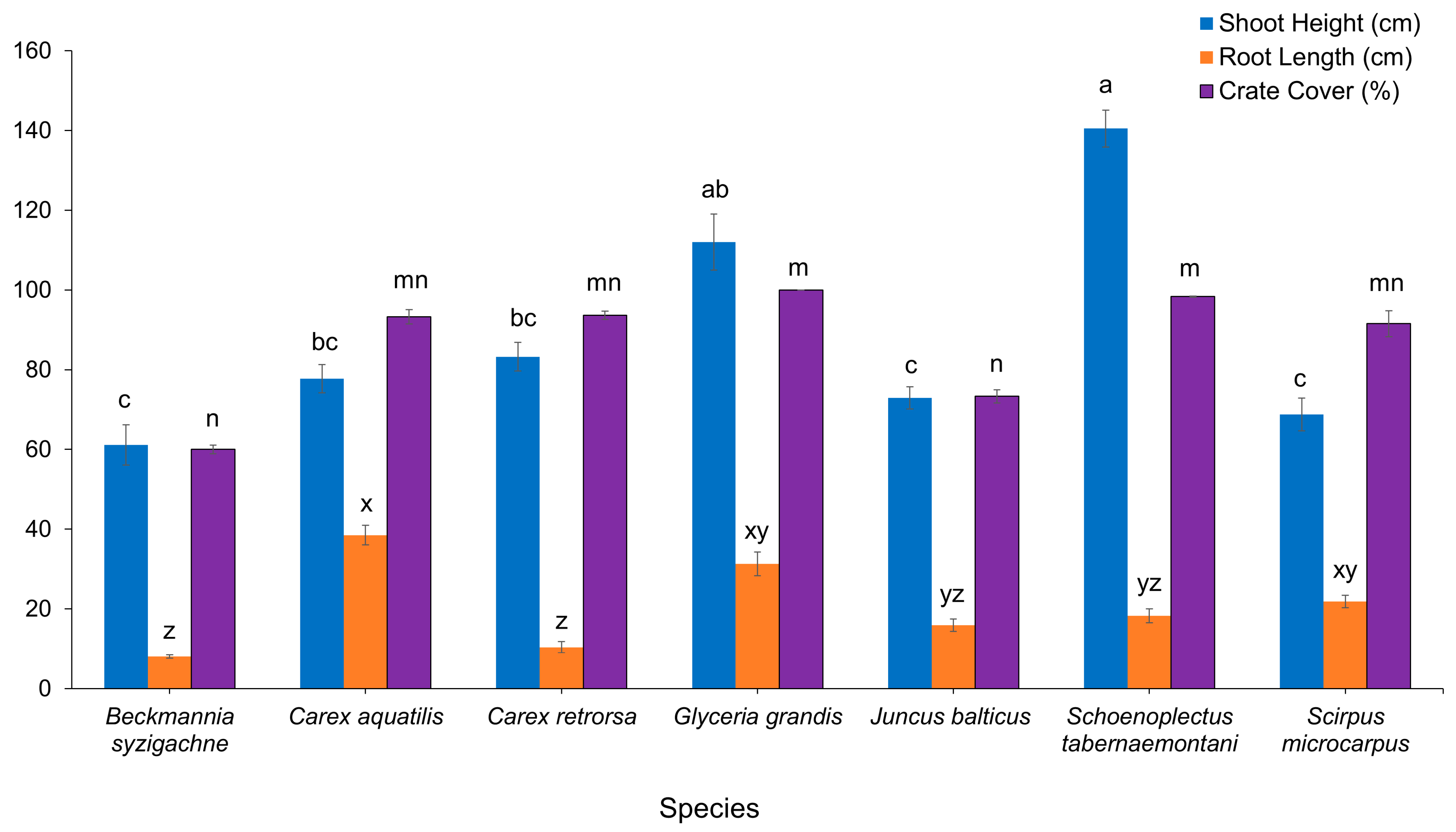
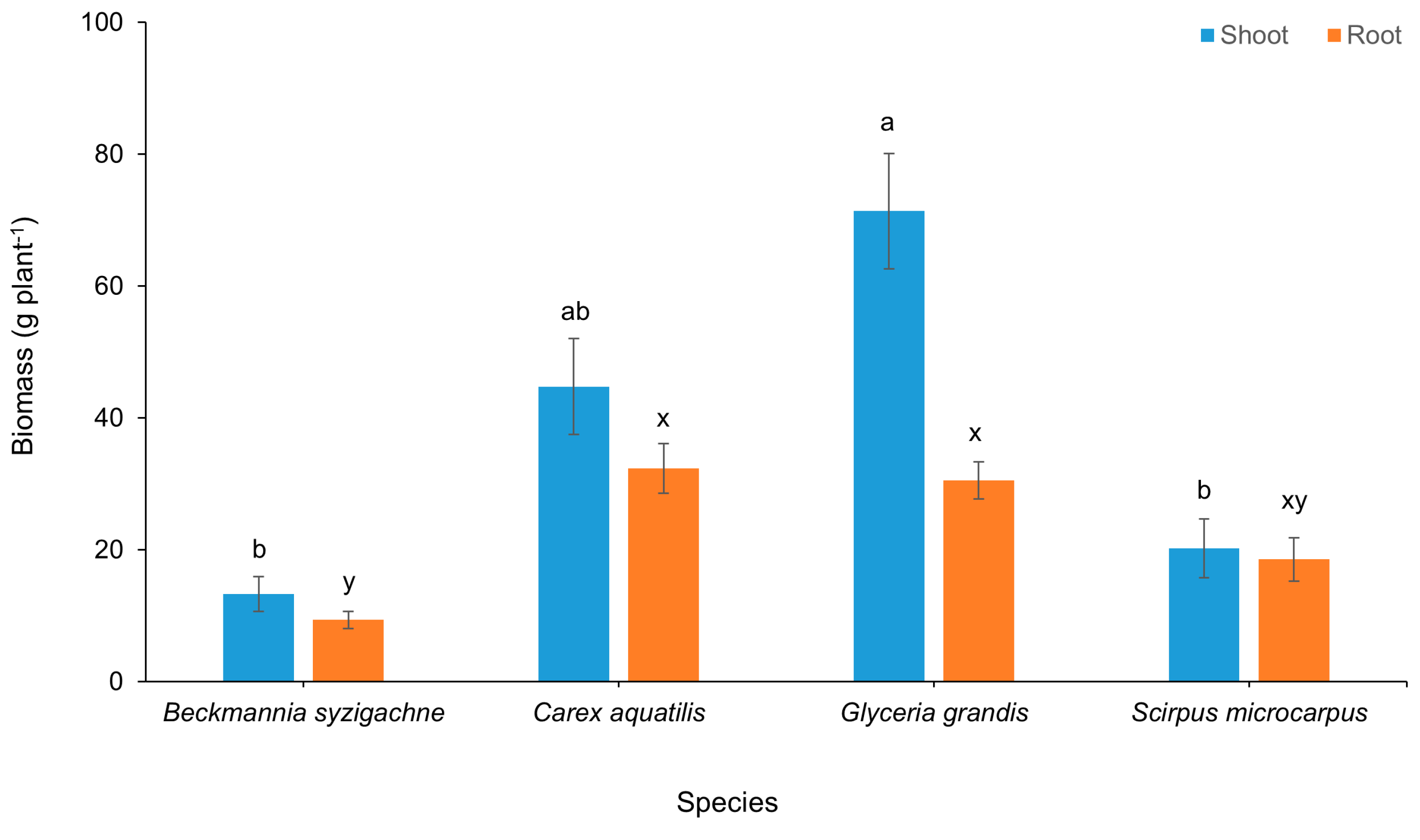
3.1. Plant Growth
3.2. Plant Uptake of Nutrients
3.3. Plant Uptake of Metals and Trace Elements
3.4. Total Plant Uptake per Unit Area of CFW
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahapatra, S.; Samal, K.; Dash, R.R. Waste stabilization pond (WSP) for wastewater treatment: A review on factors, modelling and cost analysis. J. Environ. Manag. 2022, 308, 114668. [Google Scholar] [CrossRef]
- Al-Hashimi, M.A.I.; Hussain, H.T. Stabilization pond for wastewater treatment. Eur. Sci. J. 2013, 9, 279–294. [Google Scholar]
- Cantinho, P.; Matos, M.; Transcoso, M.A.; Correia dos Santos, M.M. Behaviour and fate of metals in urban wastewater treatment plants: A review. Int. J. Environ. Sci. Technol. 2015, 13, 359–386. [Google Scholar] [CrossRef]
- Hargraves, A.J.; Constantino, C.; Dotro, G.; Cartmell, E.; Campo, P. Fate and removal f metals in municipal wastewater treatment: A review. Environ. Technol. Rev. 2018, 7, 1–18. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, P. Wastewater stabilization ponds: Removal of emerging contaminants. J. Sustain. Dev. Energy Water Environ. Syst. 2020, 8, 344–359. [Google Scholar] [CrossRef]
- Borne, K.E.; Fassman, E.A.; Tanner, C.C. Floating treatment wetland retrofit to improve stormwater pond performance for suspended solids, copper and zinc. Ecol. Eng. 2013, 54, 173–182. [Google Scholar] [CrossRef]
- Pavlineri, N.; Skoulikidis, N.T.; Tsihrintzis, V.A. Constructed floating wetlands: A review of research, design, operation and management aspects, and data meta-analysis. Chem. Eng. J. 2017, 308, 1120–1132. [Google Scholar] [CrossRef]
- Lucke, T.; Walker, C.; Beecham, S. Experimental designs of field-based constructed floating wetland studies: A review. Sci. Total Environ. 2019, 660, 199–208. [Google Scholar] [CrossRef]
- Vymazal, J.; Zhao, Y.; Mander, Ü. Recent research challenges in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Colares, G.S.; Dell-Osbel, N.; Wiesel, P.G.; Oliveria, G.A.; Lemos, P.H.Z.; da Silva, F.P.; Lutterbeck, C.A.; Kist, L.T.; Machado, E.L. Floating treatment wetlands: A review and bibliometric analysis. Sci. Total Environ. 2020, 714, 136776. [Google Scholar] [CrossRef]
- Kulshreshtha, N.M.; Verma, V.; Soti, A.; Brighu, U.; Gupta, A.B. Exploring the contribution of plant species in the performance of constructed wetlands for domestic wastewater treatment. Bioresour. Technol. Rep. 2022, 18, 101038. [Google Scholar] [CrossRef]
- Ragush, C.; Schmidt, J.J.; Krkosek, W.H.; Gagnon, G.A.; Truelstrup-Hansen, L.; Jamieson, R.C. Treatment performance of wastewater stabilization ponds in Canada’s far north. Ecol. Eng. 2015, 83, 413–421. [Google Scholar] [CrossRef]
- Susarla, S.; Medina, V.F.; McCutcheon, S.C. Phytoremediation: An ecological solution to organic chemical contamination. Ecol. Eng. 2002, 18, 647–658. [Google Scholar] [CrossRef]
- Solomou, A.D.; Germani, R.; Proutsos, N.; Petropoulou, M.; Koutroumpilas, P.; Galanis, C.; Maroulis, G.; Kolimenakis, A. Utilizing mediterranean plants to remove contaminants from the soil environment: A short review. Agriculture 2022, 12, 238. [Google Scholar] [CrossRef]
- Yeh, N.; Yeh, P.; Chang, Y.-H. Artificial floating islands for environmental improvement. Renew. Sustain. Energy Rev. 2015, 47, 616–622. [Google Scholar] [CrossRef]
- Berg, E.C.; Borges, A.C. Use of plants in the remediation of arsenic-contaminated waters. Water Environ. Res. 2020, 92, 1669–1676. [Google Scholar] [CrossRef]
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Total Environ. 2009, 407, 3923–3930. [Google Scholar] [CrossRef]
- Vymazal, J. Emergent plants used in free water surface constructed wetlands: A review. Ecol. Eng. 2013, 61, 582–592. [Google Scholar] [CrossRef]
- Bhatia, M.; Goyal, D. Analyzing remediation potential of wastewater through wetland plants: A review. Environ. Prog. Sustain. Energy 2014, 33, 9–27. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, Y.; Dong, Y. Phytoremediation of polluted waters potentials and prospects of wetland plant. Engineering 2022, 22, 199–208. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy metal phytoremediation from aquatic ecosystems with special reference to macrophytes. Crit. Rev. Environ. Sci. Technol. 2009, 39, 697–753. [Google Scholar] [CrossRef]
- Syranidou, E.; Christofilopoulos, S.; Kalogerakis, N. Juncus spp.—The helophyte for all (phyto)remediation purposes? New Biotechnol. 2017, 38, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, S.; Molofsky, J. Reed canary grass (Phalaris arundinacea) as a biological model in the study of plant invasions. Crit. Rev. Plant Sci. 2004, 23, 415–429. [Google Scholar] [CrossRef]
- Rezania, S.; Park, J.; Rupani, P.F.; Darajeh, N.; Xu, X.; Shahrokhishahraki, R. Phytoremediation potential and control of Phragmites australis as a green phytomass: An overview. Environ. Sci. Pollut. Res. 2019, 26, 7428–7441. [Google Scholar] [CrossRef]
- Yates, C.N.; Wootton, B.C.; Murphy, S.D. Performance assessment of arctic tundra municipal wastewater treatment wetlands through an arctic summer. Ecol. Eng. 2012, 44, 160–173. [Google Scholar] [CrossRef]
- MacDonald, R.L.; Burke, J.M.; Chen, H.Y.H.; Prepas, E.E. Relationship between aboveground biomass and percent cover of ground vegetation in Canadian boreal plain riparian forests. For. Sci. 2012, 58, 47–53. [Google Scholar] [CrossRef]
- Porte, A.J.; Samalens, J.-C.; Dulhoste, R.; Teissier Du Cros, R.; Bosc, A.; Meredieu, C. Using cover measurements to estimate above ground understory biomass in maritime pine stands. Annu. For. Sci. 2009, 66, 307. [Google Scholar] [CrossRef]
- Röttgermann, M.; Steinlein, T.; Beyschlag, W.; Dietz, H. Linear relationships between aboveground biomass and plant cover in low open herbaceous vegetation. J. Veg. Sci. 2020, 11, 145–148. [Google Scholar] [CrossRef]
- Cooke, J.A.; Johnson, M.S.; Davison, A.W. Determination of fluoride in vegetation: A review of modern techniques. Environ. Pollut. 1976, 11, 257–268. [Google Scholar] [CrossRef]
- Skinner, K.; Wright, N.; Porter-Goff, E. Mercury uptake and accumulation by four species of aquatic plants. Environ. Pollut. 2007, 145, 234–237. [Google Scholar] [CrossRef]
- McQueen, A.D.; Maas, H.; Gasparib, D.P.; Kinleya, C.M.; Rodgers, J.H., Jr.; Castle, J.W. Performance of a hybrid pilot-scale constructed wetland system for treating oil sands process-affected water from the Athabasca oil sands. Ecol. Eng. 2017, 101, 152–165. [Google Scholar] [CrossRef]
- Jiang, B.; Xing, Y.; Zhang, B.; Cai, R.; Zhang, D.; Sun, G. Effective phytoremediation of low-level heavy metals by native macrophytes in a vanadium mining area, China. Environ. Sci. Pollut. Res. 2018, 25, 31272–31282. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.N.; Varickanickal, J.; Cousins, S.; Wootton, B.C. Testing the ability to enhance nitrogen removal at cold temperatures with C. aquatilis in a horizontal subsurface flow wetland system. Ecol. Eng. 2016, 94, 344–351. [Google Scholar] [CrossRef]
- Picard, C.R.; Fraser, L.H.; Steer, D. The interacting effects of temperature and plant community type on nutrient removal in wetland microcosms. Bioresour. Technol. 2004, 96, 1039–1047. [Google Scholar] [CrossRef]
- Zhang, C.-B.; Liu, W.-L.; Pan, X.-C.; Guan, M.; Liu, S.-L.; Ge, Y.; Chang, J. Comparison of effects of plant and biofilm bacterial community parameters on removal performances of pollutants in floating island systems. Ecol. Eng. 2014, 73, 58–63. [Google Scholar] [CrossRef]
- Fraser, L.H.; Carty, S.M.; Steer, D. A test of four plant species to reduce total nitrogen and total phosphorus from soil leachate in subsurface wetland microcosms. Bioresour. Technol. 2004, 94, 185–192. [Google Scholar] [CrossRef]
- He, N.; Sun, Z.; Zhang, Y.; Liu, M. Nitrogen and phosphorus removal from simulated wastewater with aquatic macrophytes. Adv. Mater. Res. 2012, 518–523, 2597–2603. [Google Scholar] [CrossRef]
- Gourand, C.; Giroux, J.-F.; Mesleard, F.; Desnouhes, L. Non-destructive sampling of Schoenoplectus maritimus in southern France. Wetlands 2008, 28, 532–537. [Google Scholar] [CrossRef]
- Gamal El-Din, M.; Naeth, M.A. Evaluating the performance of constructed floating wetlands in treating wastewater in cold climates. In Final Report Prepared for Brazeau County, Alberta and Covey and Associates; 2020; pp. 1–40. [Google Scholar]
- Weiss, J.; Hondzo, M.; Biesboer, D.; Semmens, M. Laboratory study of heavy metal phytoremediation by three wetland macrophytes. Int. J. Phytoremed. 2006, 8, 245–259. [Google Scholar] [CrossRef]
- Deng, H.; Ye, Z.N.; Wong, M.H. Lead and zinc accumulation and tolerance in populations of six wetland plants. Environ. Pollut. 2006, 141, 69–80. [Google Scholar] [CrossRef]
- Khan, S.; Ahmad, I.; Shah, M.T.; Rehman, S.; Khaliq, A. Use of constructed wetland for the removal of heavy metals from industrial wastewater. J. Environ. Manag. 2009, 90, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.S.; Weiss, P. Metal uptake, transport, and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Z.; Gao, P.; Liu, P. Selection of aquatic plants for phytoremediation of heavy metal in electroplate wastewater. Acta Physiol. Plant. 2013, 35, 355–364. [Google Scholar] [CrossRef]
- Gupta, V.; Courtemanche, J.; Gunn, J.; Mykytczuk, N. Shallow floating treatment wetland capable of sulfate reduction in acid mine drainage impacted waters in a northern climate. J. Environ. Manag. 2020, 262, 110351. [Google Scholar] [CrossRef]
- Taylor, C.R.; Hook, P.B.; Stein, O.R.; Zabinski, C.A. Seasonal effects of 19 plant species on COD removal in subsurface treatment wetland microcosms. Ecol. Eng. 2011, 37, 703–710. [Google Scholar] [CrossRef]
- Arslan, M.; Wilkinson, S.; Naeth, M.A.; Gamal El-Din, M.; Khokhar, K.; Walker, C.; Lucke, T. Performance of constructed floating wetlands in a cold climate waste stabilization pond. Sci. Total Environ. 2023, 880, 163115. [Google Scholar] [CrossRef]
- Vymazal, J. Concentration is not enough to evaluate accumulation of heavy metals and nutrients in plants. Sci. Total Environ. 2016, 544, 495–498. [Google Scholar] [CrossRef]
- Sharma, R.; Vymazal, J.; Malaviya, P. Application of floating treatment wetlands for stormwater runoff: A critical review of the recent developments with emphasis on heavy metals and nutrient removal. Sci. Total Environ. 2021, 777, 146044. [Google Scholar] [CrossRef]
| Element | Beckmannia syzigachne | Carex aquatilis | Glyceria grandis | Scirpus microcarpus | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shoot (mg kg−1) | Root (mg kg−1) | TF | Shoot (mg kg−1) | Root (mg kg−1) | TF | Shoot (mg kg−1) | Root (mg kg−1) | TF | Shoot (mg kg−1) | Root (mg kg−1) | TF | |
| Nutrients | ||||||||||||
| Nitrogen | 23,060(1454) y | 22,320(753) b | 1.04 | 17,580(1821) z | 24,100(2020) b | 0.73 | 17,600(509) z | 19,260(644) c | 0.92 | 29,080(722) x | 38,640(1075) a | 0.75 |
| Phosphorus | 3344(265) xy | 3486(196) ab | 0.97 | 3460(587) xy | 3000(342) bc | 1.13 | 2202(142) y | 2524(118) c | 0.88 | 3746(266) x | 4078(106) a | 0.92 |
| Potassium | 4490(781) y | 4134(532) c | 1.09 | 9208(1602) x | 6664(385) ab | 1.36 | 8978(494) x | 6964(210) a | 1.29 | 6250(1053) xy | 5238(362) bc | 1.22 |
| Sulfur | 2752(167) y | 3468(128) c | 0.80 | 3236(586) y | 3,554(453) c | 0.90 | 4228(201) xy | 5210(94) b | 0.81 | 5794(776) x | 6320(138) a | 0.92 |
| Calcium | 5290(436) y | 5826(294) | 0.92 | 4612(129) y | 4774(1318) | 1.14 | 5648(305) y | 3576(359) | 1.63 | 11526(910) x | 6268(1003) | 1.95 |
| Magnesium | 1566(152) y | 3916(222) a | 0.41 | 2106(85) xy | 1838(319) b | 1.23 | 3194(174) x | 2150(186) b | 1.52 | 2650(206) x | 1898(216) b | 1.45 |
| Metals and Trace Elements | ||||||||||||
| Aluminum | 86(8) | 1362(94) a | 0.06 | 89(29) | 283(68) b | 0.45 | 101(68) | 450(66) b | 0.19 | 188(62) | 434(102) b | 0.57 |
| Arsenic | 0.25(0.04) xy | 2.28(0.4) ab | 0.13 | 0.15(0.01) y | 1.70(0.35) b | 0.10 | 0.20(0.03) y | 3.26(0.39) a | 0.06 | 0.45(0.09) x | 2.33(0.23) ab | 0.19 |
| Barium | 135.20(9.0) x | 176.2(15.82) a | 0.81 | 78.54(9.84) y | 44.74(8.46) b | 2.00 | 84.56(5.55) y | 51.72(5.2) b | 1.70 | 104.46(19) xy | 77.26(9.6) b | 1.34 |
| Boron | 9.42(1.5) y | 19.72(2.86) a | 0.51 | 9.92(0.75) y | 10.92(3.52) ab | 1.19 | 11.98(0.59) y | 6.98(0.3) b | 1.74 | 23.68(2.47) x | 13.34(0.94) ab | 1.76 |
| Cadmium | 0.03(0.0) | 0.10(0.01) b | 0.26 | 0.01(0) | 0.04(0.01) c | 0.23 | 0.01(0.0) | 0.16(0.02) a | 0.07 | 0.02(0.0) | 0.06(0.01) c | 0.41 |
| Chromium | 0.49(0.12) | 11.86(1.06) a | 0.04 | 0.20(0.04) | 1.43(0.35) b | 0.20 | 0.23(0.09) | 3.04(0.4)b | 0.08 | 0.41(0.12) | 1.73(0.31) b | 0.28 |
| Cobalt | 0.32(0.04) xy | 3.64(0.21) a | 0.09 | 0.18(0.02) y | 1.87(0.3) b | 0.11 | 0.13(0.04) y | 3.45(0.27) a | 0.04 | 0.40(0.1) x | 1.62(0.14) b | 0.26 |
| Copper | 2.79(0.46) | 5.32(0.72) | 0.53 | 1.50(0.23) | 3.68(0.2) | 0.42 | 1.71(0.30) | 5.68(0.83) | 0.30 | 3.31(0.91) | 4.26(0.21) | 0.75 |
| Iron | 642(94) xy | 4,204(601) | 0.17 | 238(35) y | 2,206(394) | 0.13 | 248(89) xy | 4466(1058) | 0.06 | 783(230) x | 3854(297) | 0.20 |
| Lead | 0.15(0.02) | 0.57(0.1) | 0.30 | 0.10(0.02) | 0.36(0.1) | 0.32 | 0.12(0.04) | 0.68(0.09) | 0.18 | 0.26(0.06) | 0.58(0.07) | 0.47 |
| Lithium | 0.50(0.0) | 1.05(0.1) | 0.49 | 0.66(0.03) | 0.99(0.18) | 0.95 | 1.17(0.03) | 1.14(0.04) | 1.02 | 0.57(0.01) | 0.87(0.17) | 0.48 |
| Manganese | 405(60) | 497(67) a | 0.86 | 381(76.1) | 239(28) b | 1.62 | 316(15.2) | 197(20) b | 1.65 | 526(62) | 174(15) b | 3.00 |
| Molybdenum | 0.36(0.01) y | 0.62(0.06) b | 0.61 | 0.82(0.12) x | 0.49(0.04) b | 1.78 | 0.28(0.02) y | 0.55(0.09) b | 0.55 | 0.87(0.11) x | 1.01(0.09) a | 0.86 |
| Nickel | 1.16(0.17) x | 9.35(0.45) a | 0.13 | 0.34(0.04) y | 1.98(0.24) c | 0.19 | 0.43(0.12) y | 5.38(0.53) b | 0.07 | 1.41(0.35) x | 3.45(0.22) c | 0.42 |
| Rubidium | 4.78(0.79) y | 7.57(0.77) b | 0.64 | 3.15(0.52) y | 3.48(0.42) c | 0.89 | 11.56(0.81) x | 11.96(0.64) a | 0.97 | 3.04(0.52) y | 3.25(0.36) c | 0.95 |
| Sodium | 1706(114) y | 2510(521) ab | 0.76 | 1390(305) y | 1428(142) b | 1.01 | 5608(498) x | 7224(197) a | 0.78 | 666(104) y | 1924(160) b | 0.35 |
| Strontium | 40.62(3.89) y | 44.48(2.15) a | 0.93 | 42.10(2.32) y | 33.76(10.5) ab | 1.58 | 43.34(2.4) y | 22.60(1.19) b | 1.94 | 81.4(5.2) x | 37.18(1.4) ab | 2.20 |
| Uranium | 0.07(0.01) y | 0.23(0.02) ab | 0.33 | 0.03(0.0)y | 0.18(0.03) b | 0.16 | 0.03(0.01) y | 0.39(0.06) a | 0.08 | 0.15(0.05) x | 0.40(0.05) a | 0.37 |
| Vanadium | 0.28(0.04) y | 2.06(0.14) | 0.14 | 0.24(0.07)y | 1.09(0.24) | 0.28 | 0.57(0.25) xy | 2.17(0.58) | 0.22 | 0.81(0.23) x | 2.11(0.26) | 0.42 |
| Zinc | 11.97(1.07) xy | 17.50(0.75) a | 0.69 | 8.31(0.54) y | 13.46(0.92) bc | 0.62 | 7.52(0.68) y | 10.86(0.81) c | 0.70 | 15.18(1.87) x | 15.22(0.44) ab | 1.00 |
| Element | Beckmannia syzigachne | Carex aquatilis | Glyceria grandis | Scirpus microcarpus | ||||
|---|---|---|---|---|---|---|---|---|
| Shoots | Roots | Shoots | Roots | Shoots | Roots | Shoots | Roots | |
| Nutrients | ||||||||
| Nitrogen | 7287 | 4962 | 18,714 | 18,527 | 29,879 | 13,969 | 13,963 | 17,033 |
| Phosphorus | 1057 | 775 | 3683 | 2306 | 3738 | 1831 | 1799 | 1798 |
| Potassium | 1419 | 919 | 9802 | 5123 | 15,242 | 5051 | 3001 | 2309 |
| Sulfur | 870 | 771 | 3445 | 2732 | 7178 | 3779 | 2782 | 2786 |
| Calcium | 1672 | 1295 | 4910 | 3670 | 9588 | 2594 | 5534 | 2763 |
| Magnesium | 495 | 870 | 2242 | 1413 | 5422 | 1559 | 1272 | 837 |
| Metals and Trace Elements | ||||||||
| Aluminum | 27 | 303 | 95 | 218 | 171 | 326 | 90 | 191 |
| Arsenic | 0.079 | 0.507 | 0.160 | 1.307 | 0.340 | 2.364 | 0.216 | 1.027 |
| Barium | 42.73 | 39.17 | 83.61 | 34.39 | 143.55 | 37.51 | 50.16 | 34.06 |
| Boron | 2.98 | 4.38 | 10.56 | 8.39 | 20.34 | 5.06 | 11.37 | 5.88 |
| Cadmium | 0.009 | 0.022 | 0.011 | 0.031 | 0.017 | 0.116 | 0.010 | 0.026 |
| Chromium | 0.15 | 2.64 | 0.21 | 1.10 | 0.39 | 2.20 | 0.20 | 0.76 |
| Cobalt | 0.10 | 0.81 | 0.19 | 1.44 | 0.22 | 2.50 | 0.19 | 0.71 |
| Copper | 0.88 | 1.18 | 1.60 | 2.83 | 2.90 | 4.12 | 1.59 | 1.88 |
| Iron | 203 | 935 | 253 | 1,696 | 421 | 3239 | 376 | 1,699 |
| Lead | 0.05 | 0.13 | 0.11 | 0.28 | 0.20 | 0.49 | 0.12 | 0.26 |
| Lithium | 0.16 | 0.23 | 0.70 | 0.76 | 1.99 | 0.83 | 0.27 | 0.38 |
| Manganese | 128 | 110 | 405 | 183 | 536 | 143 | 252 | 77 |
| Molybdenum | 0.11 | 0.14 | 0.87 | 0.38 | 0.48 | 0.40 | 0.42 | 0.45 |
| Nickel | 0.37 | 2.08 | 0.36 | 1.52 | 0.73 | 3.90 | 0.68 | 1.52 |
| Rubidium | 1.51 | 1.68 | 3.35 | 2.68 | 19.62 | 8.67 | 1.46 | 1.43 |
| Sodium | 539 | 558 | 1480 | 1098 | 9520 | 5239 | 320 | 848 |
| Strontium | 12.84 | 9.89 | 44.82 | 25.95 | 73.58 | 16.39 | 39.09 | 16.39 |
| Uranium | 0.022 | 0.051 | 0.032 | 0.138 | 0.051 | 0.283 | 0.072 | 0.176 |
| Vanadium | 0.09 | 0.46 | 0.26 | 0.84 | 0.97 | 1.57 | 0.39 | 0.93 |
| Zinc | 3.78 | 3.89 | 8.85 | 10.35 | 12.77 | 7.88 | 7.29 | 6.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkinson, S.R.; Naeth, M.A.; Dhar, A. Potential of Macrophytes for Wastewater Remediation with Constructed Floating Wetlands in Cold Climates. Water 2023, 15, 2479. https://doi.org/10.3390/w15132479
Wilkinson SR, Naeth MA, Dhar A. Potential of Macrophytes for Wastewater Remediation with Constructed Floating Wetlands in Cold Climates. Water. 2023; 15(13):2479. https://doi.org/10.3390/w15132479
Chicago/Turabian StyleWilkinson, Sarah R., M. Anne Naeth, and Amalesh Dhar. 2023. "Potential of Macrophytes for Wastewater Remediation with Constructed Floating Wetlands in Cold Climates" Water 15, no. 13: 2479. https://doi.org/10.3390/w15132479
APA StyleWilkinson, S. R., Naeth, M. A., & Dhar, A. (2023). Potential of Macrophytes for Wastewater Remediation with Constructed Floating Wetlands in Cold Climates. Water, 15(13), 2479. https://doi.org/10.3390/w15132479






