Analysis of Water Quality and Habitat Suitability for Benthic Macro-Invertebrates in the Majiagou Urban River, China
Abstract
1. Introduction
2. Materials and Methods
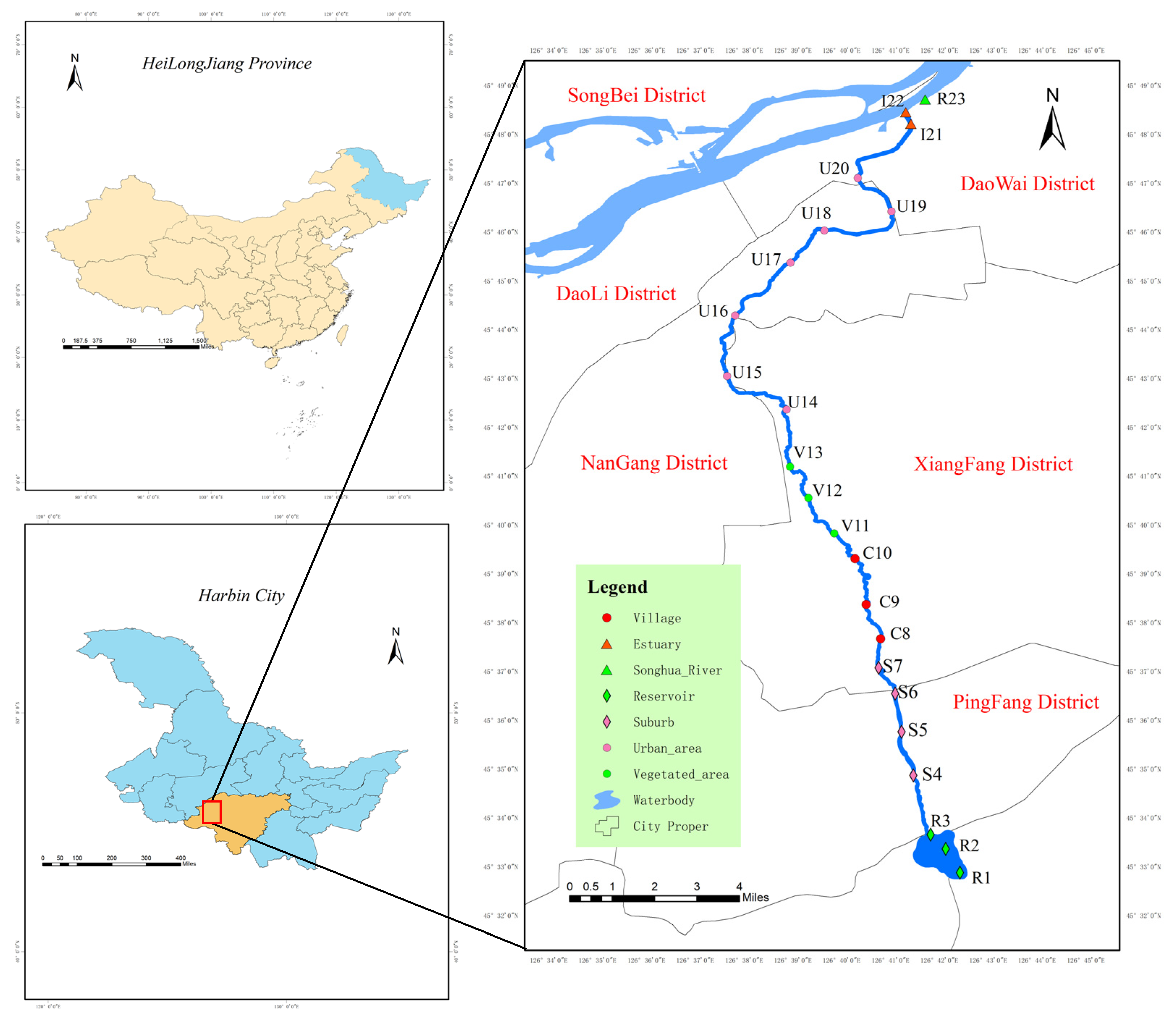
2.1. Study Area
2.2. Sampling and Measurements
2.3. Data Analysis Methods
2.3.1. One-Way Analysis of Variance
2.3.2. Factors Analysis Method
2.3.3. Generalized Additive Models (GAM)
3. Results
3.1. Macro-Invertebrate Diversity and Water Quality Factors
3.1.1. Distribution Characteristics of Macro-Invertebrates
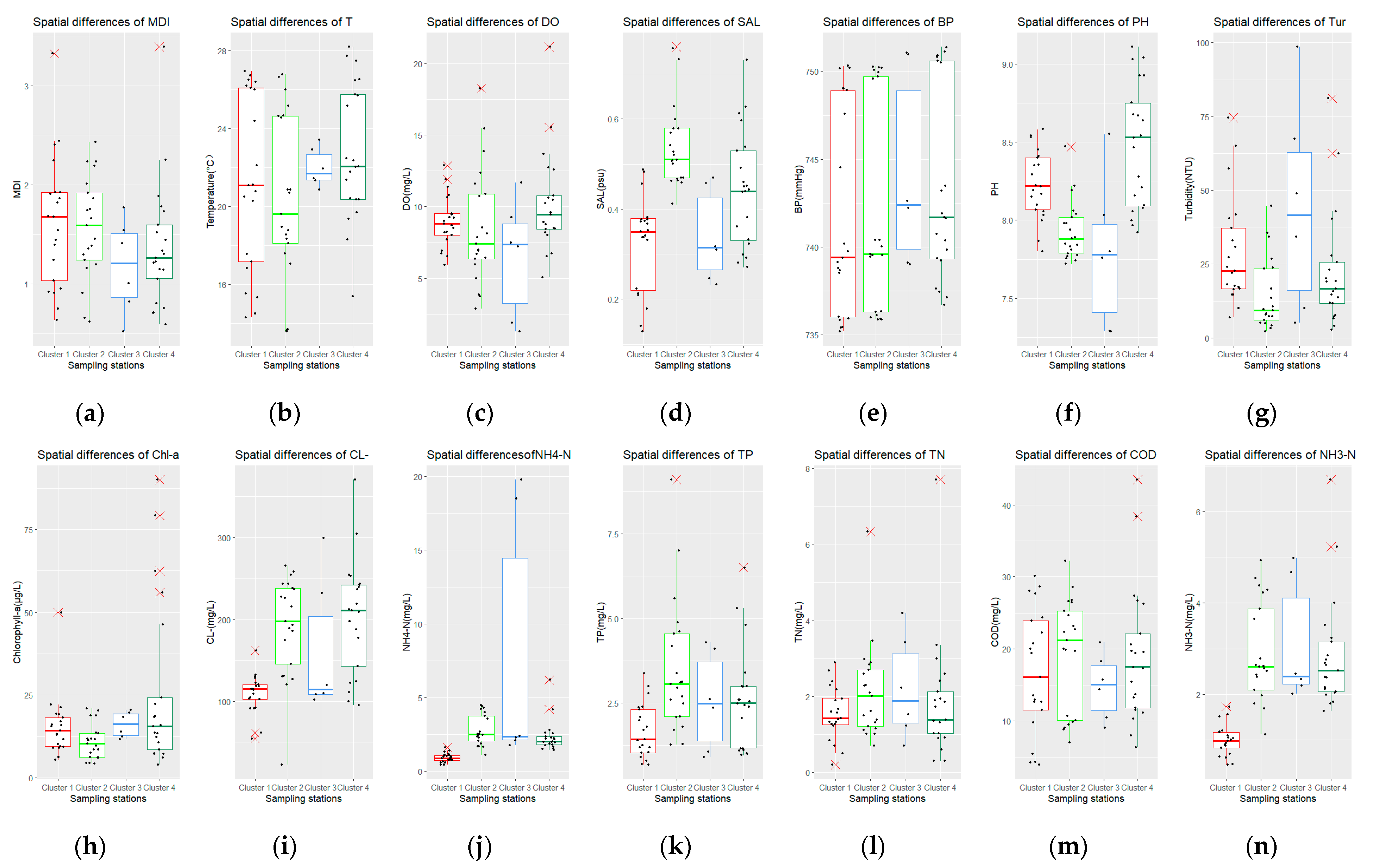
3.1.2. Spatial and Temporal Differences of Water Quality
- Seasonal differences in water quality
- Hierarchical cluster analysis (HCA) of samples
- Spatial differences of water quality and MDI
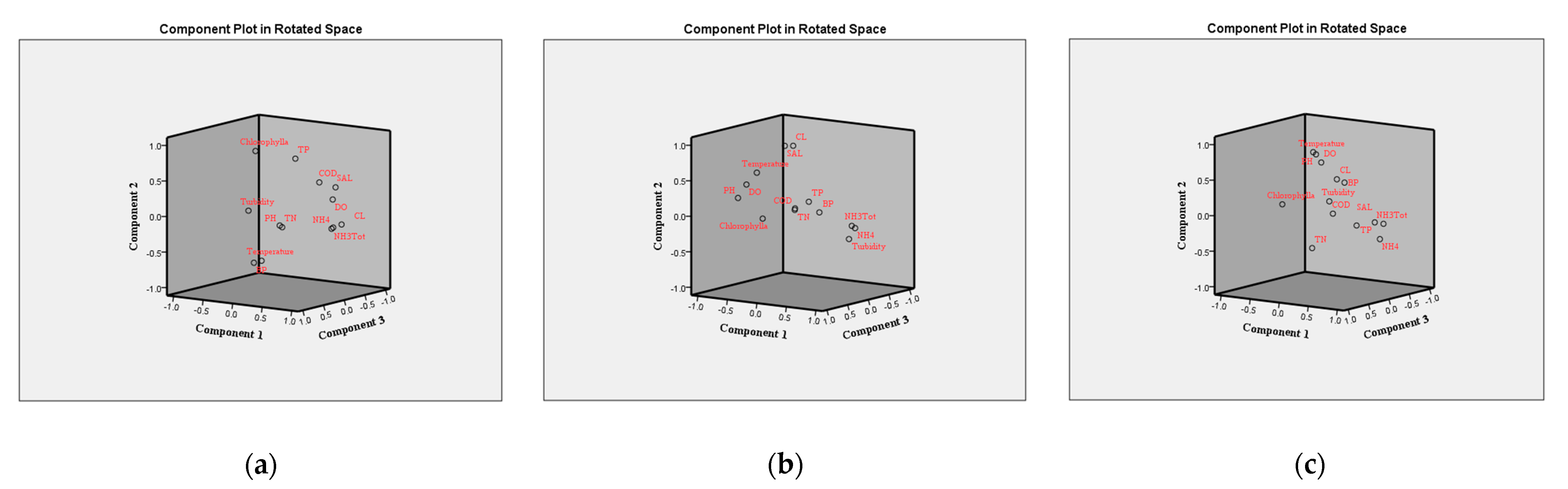
3.2. Identification of Critical Environmental Parameters
3.3. Response between Macro-Invertebrate MDI and Critical Quality Parameters Using GAM
3.3.1. Single-Factor Significance Test Used for Critical Water Quality Factors
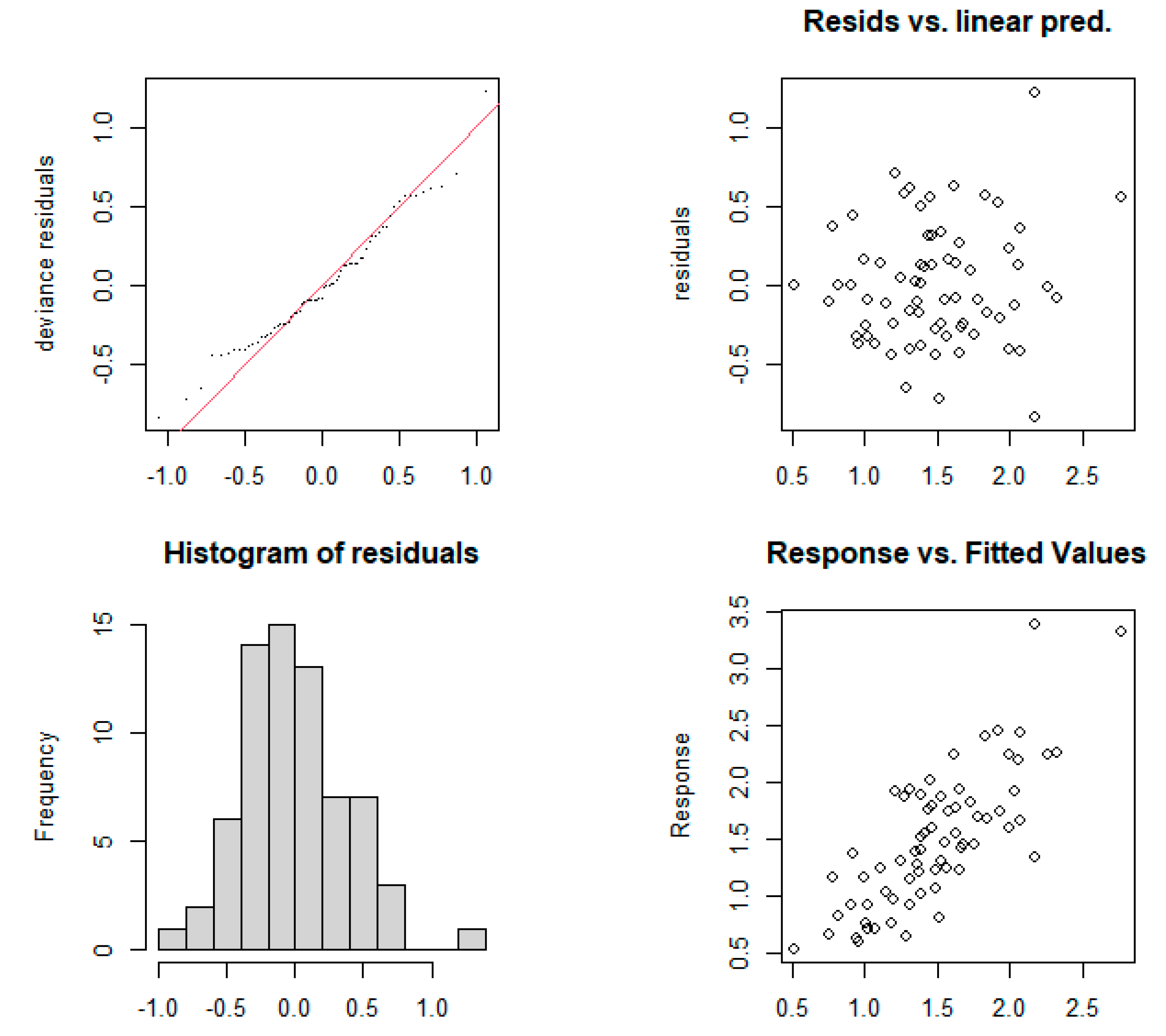
3.3.2. Multi-Factor GAM Optimization
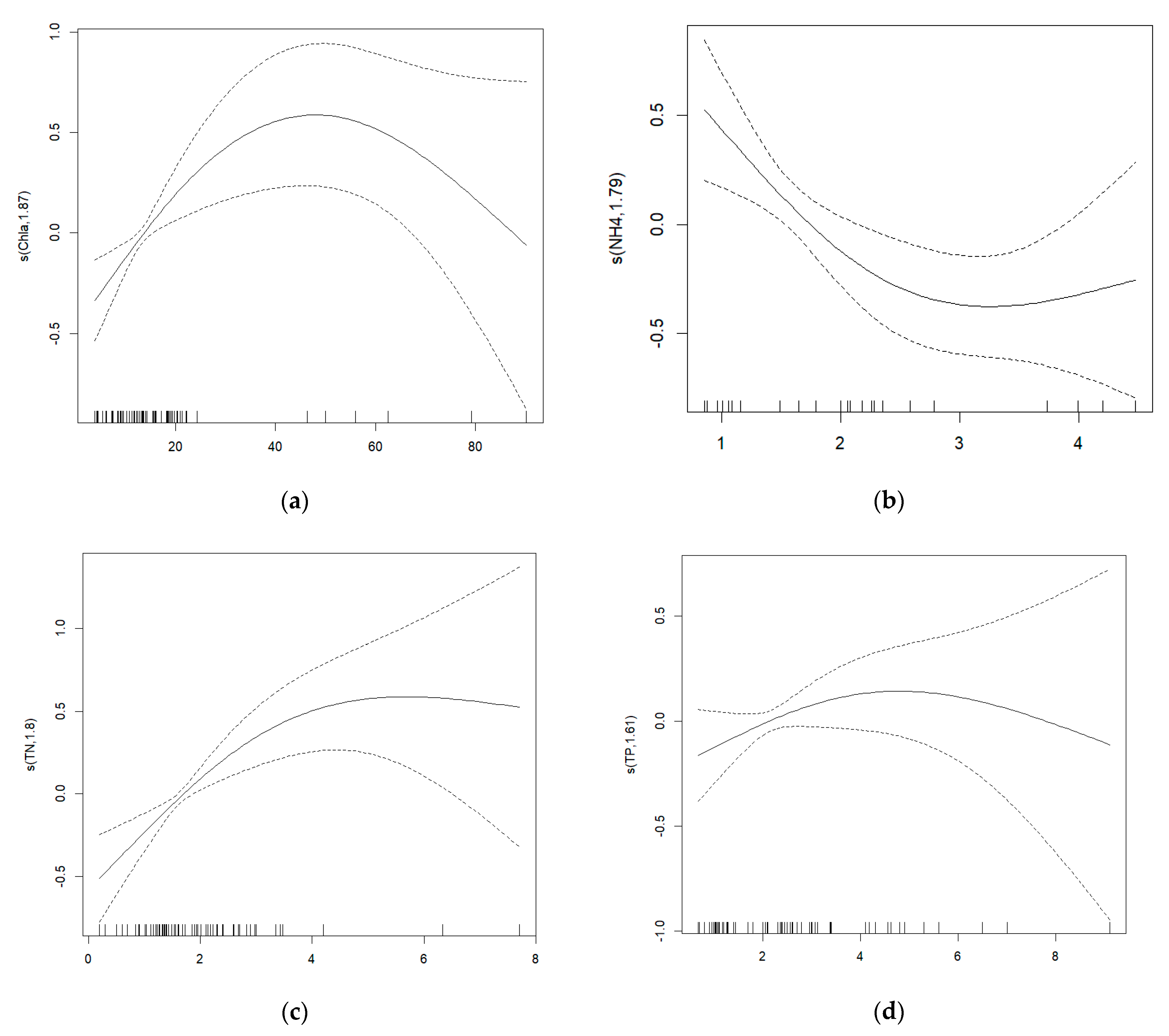
3.3.3. Response between MDI and Critical Quality Parameters
4. Discussion
4.1. Response of Benthic Macro-Invertebrates to Critical Water Quality Factors
4.2. Construction of GAM Model Based on HCA and PCA
4.3. The Limitations and Prospects of Our Study
5. Conclusions
- The macro-invertebrate community in the Majiagou River had differences in their spatiotemporal distribution. Due to human disturbance, the macro-invertebrate community tended to be homogeneous and resistant to pollution, with a decrease in the biodiversity index.
- In this study, one-way ANOVA and hierarchical cluster analysis (HCA) were used to analyze the temporal and spatial variations in water quality factors in the Majiagou Watershed. The results showed that COD in the Majiagou River had obvious seasonal differences, and the water quality in different reaches had spatial distribution differences; CL−, NH4+-N, TP, TN and COD in the downstream area were higher than they were in the upstream area.
- Principal component analysis was used to identify the key water environmental factors and three environmental factors with a significant impact on benthic animals were finally selected through a significance test, which reduced data redundancy.
- A GAM model fitted the nonlinear relationship between MDI and critical water environmental factors and fitted the response curve for the critical environmental factors and macro-invertebrates. This could be used to further analyze ecological suitability. The purpose of our study was to provide a reference for the integrated management of urban river water ecosystems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beißler, H. A Combined Field and Remote-Sensing based Methodology to Assess the Ecosystem Service Potential of Urban Rivers in Developing Countries. Remote Sens. 2019, 11, 1697. [Google Scholar] [CrossRef]
- Begum, W.; Goswami, L.; Sharma, B.-B.; Kushwaha, A. Assessment of urban river pollution using the water quality index and macro-invertebrate community index. Environ. Dev. Sustain. 2022, 1–26. [Google Scholar] [CrossRef]
- Edegbene, A.O.; Odume, O.N.; Arimoro, F.O.; Keke, U.N. Identifying and classifying macroinvertebrate indicator signature traits and ecological preferences along urban pollution gradient in the Niger Delta. Environ. Pollut. 2021, 281, 117076. [Google Scholar] [CrossRef]
- Wei, C.; Gao, C.; Han, D.; Zhao, W.; Lin, Q.; Wang, G. Spatial and Temporal Variations of Water Quality in Songhua River from 2006 to 2015: Implication for Regional Ecological Health and Food Safety. Sustainability 2017, 9, 1502. [Google Scholar] [CrossRef]
- Sterling, J.-L.; Rosemond, A.-D.; Wenger, S.-J. Watershed urbanization affects macroinvertebrate community structure and reduces biomass through similar pathways in Piedmont streams, Georgia, USA. Freshw. Sci. 2016, 35, 676–688. [Google Scholar] [CrossRef]
- Gál, B.; András, W.; János, F.; Schmera, D. The effects of road crossings on stream macro-invertebrate diversity. Biodivers. Conserv. 2020, 29, 729–745. [Google Scholar] [CrossRef]
- Gallardo, L.-I.; Juan-Manuel, C.; Alicia-Susana-Guadalupe, P. Urban rain-fed lakes: Macro-invertebrate assemblages associated with Egeria najas as indicators of biological integrity in wetlands of Corrientes Province (Argentina). Biodivers. Conserv. 2019, 28, 1549–1568. [Google Scholar] [CrossRef]
- Andem, A.B.; Ibor, O.R.; Oku, E.E.; Ekanem, S.B.; Chukwuka, A.V.; Adeogun, A.O. Urbanisation gradients, riparian-loss and contaminant effects on macroinvertebrate distribution within a tropical river (Nigeria). Chem. Ecol. 2022, 38, 503–526. [Google Scholar] [CrossRef]
- Van Damme, P.-A.; Caroli, H.; Alfredo, A.; Bervoets, L. Macroinvertebrate community response to acid mine drainage in rivers of the High Andes (Bolivia). Environ. Pollut. 2008, 156, 1061–1068. [Google Scholar] [CrossRef]
- Arimoro, F.O.; Odume, O.N.; Uhunoma, S.I.; Edegbene, O.A. Anthropogenic impact on water chemistry and benthic macroinvertebrate associated changes in a southern Nigeria stream. Environ. Monit. Assess. 2015, 187, 1–14. [Google Scholar] [CrossRef]
- Belle, G.; Fossey, A.; Esterhuizen, L. Use of multiple indicators to assess the pollution condition of urban streams: A case study of Bloemspruit, Free State Province, South Africa. Water Environ. J. 2020, 34, 93–105. [Google Scholar] [CrossRef]
- Aazami, J.; Maghsodlo, H.; Mira, S.S.; Valikhani, H. Health evaluation of riverine ecosystems using aquatic macroinvertebrates: A case study of the Mohammad-Abad River, Iran. Int. J. Environ. Sci. Technol. 2020, 17, 2637–2644. [Google Scholar] [CrossRef]
- Blocksom, K.A.; Kurtenbach, J.P.; Klemm, D.J.; Fulk, F.A.; Cormier, S.M. Development and evaluation of the lake Macroinvertebrate Integrity Index (lmii) for New Jersey Lakes and Reservoirs. Environ. Monit. Assess. 2001, 77, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Pecher, C.; Fritz, S.A.; Marini, L.; Fontaneto, D.; Pautasso, M. Scale-dependence of the correlation between human population and the species richness of stream macro-invertebrates. Basic Appl. Ecol. 2010, 11, 272–280. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Kou, Z.; Teng, X.; Cai, W.; Hu, J. Shift of Sediments Bacterial Community in the Black-Odor Urban River during In Situ Remediation by Comprehensive Measures. Water 2019, 11, 2129. [Google Scholar] [CrossRef]
- Mitchell, E.-G.; Whittle, R.-J.; Griffiths, H.-J. Benthic ecosystem cascade effects in Antarctica using Bayesian network inference. Commun. Biol. 2020, 3, 582. [Google Scholar] [CrossRef]
- Yang, L.; Peng, S.; Sun, J.; Zhao, X.; Li, X. A case study of an enhanced eutrophication model with stoichiometric zooplankton growth sub-model calibrated by Bayesian method. Environ. Sci. Pollut. Res. 2016, 23, 8398–8409. [Google Scholar] [CrossRef]
- Cao, Y.; Cummings, K.; Hinz, L.; Douglass, S.A.; Stodola, A.P.; Holtrop, A.M. Reconstructing the natural distribution of individual unionid mussel species and species diversity in wadeable streams of Illinois, USA, with reference to stream bioassessment. Freshw. Sci. 2017, 36, 669–682. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Wang, H.; Ma, C. Phytoplankton community structure in relation to environmental factors and ecological assessment of water quality in the upper reaches of the Genhe River in the Greater Hinggan Mountains. Environ. Sci. Pollut. Res. 2019, 26, 17512–17519. [Google Scholar] [CrossRef]
- Sun, X.; Mwagona, P.C.; Shabani, I.E.; Hou, W.; Li, X.; Zhao, F.; Chen, Q.; Zhao, Y.; Liu, D.; Li, X.; et al. Phytoplankton functional groups response to environmental parameters in Muling River basin of northeast China. Ann. Limnol.-Int. J. Limnol. 2019, 55, 17. [Google Scholar] [CrossRef]
- Arias, M.J.; Vaschetto, P.A.; Marchese, M.; Regaldo, L.; Gagneten, A.M. Benthic Macroinvertebrates and Zooplankton Communities as Ecological Indicators in Urban Wetlands of Argentina. Sustainability 2022, 14, 4045. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, F.; Hu, P.; Hough, R.; Fu, Q.; Zhang, Z.; An, L.; Li, Y.-F.; Li, K.; Liu, D.; et al. Heavy Metals in Sediment from the Urban and Rural Rivers in Harbin City, Northeast China. Int. J. Environ. Res. Public Health 2019, 16, 4313. [Google Scholar] [CrossRef] [PubMed]
- Razmkhah, H.; Abrishamchi, A.; Torkian, A. Evaluation of spatial and temporal variation in water quality by pattern recognition techniques: A case study on Jajrood River (Tehran, Iran). J. Environ. Manag. 2010, 91, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Passos JB DM, C.; Teixeira DB, D.S.; Campos, J.A.; Lima RP, C.; Fernandes-Filho, E.I.; da Silva, D.D. Multivariate statistics for spatial and seasonal quality assessment of water in the Doce River basin, Southeastern Brazil. Environ. Monit. Assess. 2021, 193, 125. [Google Scholar] [CrossRef] [PubMed]
- Bertossi AP, A.; de Menezes JP, C.; Cecílio, R.A.; Garcia GD, O.; Neves, M.A. Selection and grouping of indicators of water quality using Multivariate Statistics. Semin.-Cienc. Agrar. 2013, 34, 2025–2036. [Google Scholar]
- Konishi, T. Principal component analysis for designed experiments. BMC Bioinform. 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Sulong, R.S.; Ma, H.; Wu, L. Comprehensive Evaluation of Water Carrying Capacity in Hebei Province, China on Principal Component Analysis. Front. Environ. Sci. 2021, 9. [Google Scholar] [CrossRef]
- Khatri, N.; Raval, K.; Jha, A. Integrated water quality monitoring of Mahi river using benthic macroinvertebrates and comparison of its biodiversity among various stretches. Appl Water Sci. 2021, 11, 143. [Google Scholar] [CrossRef]
- Salmeron, R.; Garcia, C.-B.; Garcia, J. Variance Inflation Factor and Condition Number in multiple linear regression. J. Stat. Comput. Simul. 2018, 88, 2365–2384. [Google Scholar] [CrossRef]
- Thompson, C.G.; Kim, R.S.; Aloe, A.M.; Becker, B.J. Extracting the Variance In flation Factor and Other Multicollinearity Diagnostics from Typical Regression Results. Basic Appl. Soc. Psychol. 2017, 39, 81–90. [Google Scholar] [CrossRef]
- Chauhan, N.; Paliwal, R.; Kumar, V.; Kumar, S.; Kumar, R. Watershed Prioritization in Lower Shivaliks Region of India Using Integrated Principal Component and Hierarchical Cluster Analysis Techniques: A Case of Upper Ghaggar Watershed. J. Indian Soc. Remote Sens. 2022, 50, 1051–1070. [Google Scholar] [CrossRef]
- Hua, A.; Kusin, F.; Praveena, S. Spatial Variation Assessment of River Water Quality Using Environmetric Techniques. Pol. J. Environ. Stud. 2016, 25, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Pärn, J.; Verhoeven, J.T.; Butterbach-Bahl, K.; Dise, N.B.; Ullah, S.; Aasa, A.; Egorov, S.; Espenberg, M.; Järveoja, J.; Jauhiainen, J.; et al. Nitrogen-rich organic soils under warm well-drained conditions are global nitrous oxide emission hotspots. Nat. Commun. 2018, 9, 1135. [Google Scholar] [CrossRef]
- Vázquez, R.; Vimos-Lojano, D.; Hampel, H. Habitat Suitability Curves for Freshwater Macroinvertebrates of Tropical Andean Rivers. Water 2020, 12, 2703. [Google Scholar] [CrossRef]
- Su, Y.; Li, W.; Liu, L.; Li, J.; Sun, X.; Hu, W. Assessment of medium and small river health based on macroinvertebrates habitat suitability curves: A case study in a tributary of Yangtze River, China. Water Policy 2020, 22, 602–621. [Google Scholar] [CrossRef]
- Gutiérrez-Estrada, J.C.; Bilton, D.T. A heuristic approach to predicting water beetle diversity in temporary and fluctuating waters. Ecol. Model. 2010, 221, 1451–1462. [Google Scholar] [CrossRef]
- Zhang, H.; Huo, S.; Yeager, K.M.; Li, C.; Xi, B.; Zhang, J.; He, Z.; Ma, C. Apparent relationships between anthropogenic factors and climate change indicators and POPs deposition in a lacustrine system. J. Environ. Sci. 2019, 83, 83174–83182. [Google Scholar] [CrossRef]
- Jiang, Y.; He, K.; Li, Y.; Qin, M.; Cui, Z.; Zhang, Y.; Yao, Y.; Chen, X.; Deng, M.; Gray, A.; et al. Driving Factors of Total Organic Carbon in Danjiangkou Reservoir Using Generalized Additive Model. Water 2022, 14, 891. [Google Scholar] [CrossRef]
- Downie, A.-L.; von Numers, M.; Boström, C. Influence of model selection on the predicted distribution of the seagrass Zostera marina. Estuar. Coast. Shelf Sci. 2013, 121, 8–19. [Google Scholar] [CrossRef]
- Yang, Y.; Yi, Y.; Zhou, Y.; Wang, X.; Zhang, S.; Yang, Z. Spatio-temporal variations of benthic macroinvertebrates and the driving environmental variables in a shallow lake. Ecol. Indic. 2020, 110, 105948. [Google Scholar] [CrossRef]
- Bednarek, A.-T.; Hart, D.-D. Modifying dam operations to restore rivers: Ecological responses to Tennessee river dam mitigation. Ecol. Appl. 2005, 15, 997–1008. [Google Scholar] [CrossRef]
- Shen, Z.; Yang, Y.; Ai, L.; Yu, C.; Su, M. A hybrid CART-GAMs model to evaluate benthic macroinvertebrate habitat suitability in the Pearl River Estuary, China. Ecol. Indic. 2022, 143, 143109368. [Google Scholar] [CrossRef]
- Muvengwi, J.; Chikorowondo, G.; Mbiba, M.; Gandiwa, E. Diversity and abundance of macro-invertebrates on abandoned cattle kraals in a semi-arid savanna. Ecol. Evol. 2018, 8, 8477–8489. [Google Scholar] [CrossRef] [PubMed]
- Aspin, T.W.H.; Khamis, K.; Matthews, T.; Milner, A.M.; O’callaghan, M.J.; Trimmer, M.; Woodward, G.; Ledger, M.E. Extreme drought pushes stream invertebrate communities over functional thresholds. Glob. Chang. Biol. 2018, 25, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.A.; Hwang, S.J.; Park, S.R.; Lee, S.W. Examining the Relationships between Watershed Urban Land Use and Stream Water Quality Using Linear and Generalized Additive Models. Water 2016, 8, 155. [Google Scholar] [CrossRef]
- Torres, S.H.; Martin, J.P.; Gárgano, L.; Armendáriz, L.C. Análisis de la calidad ambiental en un sector del Río Chico (Santa Cruz, Argentina) basado en bioindicadores bentónicos. Inf. Científicos Técnicos-UNPA 2019, 11, 36–49. [Google Scholar] [CrossRef]
- Colla, M.-F.; Cesar, I.-I.; Salas, L.-B. Benthic insects of the El Tala River (Catamarca, Argentina): Longitudinal variation of their structure and the use of insects to assess water quality. Braz. J. Biol. 2013, 73, 357–366. [Google Scholar] [CrossRef] [PubMed]

| Season | Phylum | Class | Species |
|---|---|---|---|
| Spring | Arthropoda | Insecta | Chinonomus pallidivittatus |
| Dicrotendipes tritomus | |||
| Chironomus riparius | |||
| Malacostraca | Exopalaemon modestus | ||
| Clitellata | Glossiphonia lata | ||
| Annelida | Oligochaeta | Limnodrilus hoffmeisteri | |
| Tubifex tubifex | |||
| Mollusca | Gastropoda | Bithynia fuchsiana | |
| Lamellibranchia | Sphaerium lacustre | ||
| Nemathelminthes | Gordiacea | ||
| Summer | Arthropoda | Insecta | Dicrotendipes lobifer |
| Glyptotendipes cauliginellus | |||
| Malacostraca | Exopalaemon modestus | ||
| Clitellata | Helobdella nuda | ||
| Annelida | Oligochaeta | Limnodrilus hoffmeisteri | |
| Tubifex tubifex | |||
| Mollusca | Gastropoda | Radix swinhonei | |
| Bithynia fuchsiana | |||
| Lamellibranchia | Sphaerium lacustre | ||
| Nemathelminthes | Gordiacea | ||
| Autumn | Arthropoda | Insecta | Cricotopus sylvestris |
| Chinonomus pallidivittatus | |||
| Dicrotendipes tritomus | |||
| Malacostraca | Exopalaemon modestus | ||
| Clitellata | Helobdella stagnalis | ||
| Annelida | Oligochaeta | Branchiura sowerbyi | |
| Limnodrilus hoffmeisteri | |||
| Tubifex tubifex | |||
| Mollusca | Gastropoda | Radix swinhonei | |
| Bithynia fuchsiana | |||
| Lamellibranchia | Sphaerium lacustre | ||
| Nemathelminthes | Gordiacea |
| Season | Effects of Season | ||||
|---|---|---|---|---|---|
| May | July | September | Statistics | p-Value | |
| COD | 22.73 ± 7.25 a | 20.13 ± 6.79 a | 11.3 ± 6.48 b | F = 17.59 | <0.001 |
| Salinity | 0.39 ± 0.12 ab | 0.48 ± 0.16 a | 0.41 ± 0.11 a | F = 2.896 | 0.062 |
| pH | 8.16 ± 0.28 a | 8.17 ± 0.48 a | 8.19 ± 0.39 a | F = 0.031 | 0.97 |
| PC | Initial Eigenvalues | ||
|---|---|---|---|
| Total | % of Variance | Cumulative % | |
| (a) | |||
| PC1 | 4.108 | 31.604 | 31.604 |
| PC2 | 3.127 | 24.053 | 55.657 |
| PC3 | 1.877 | 14.436 | 70.093 |
| PC4 | 1.303 | 10.025 | 80.118 |
| (b) | |||
| PC1 | 5.177 | 39.823 | 39.823 |
| PC2 | 2.164 | 16.648 | 56.471 |
| PC3 | 1.603 | 12.332 | 68.802 |
| PC4 | 1.222 | 9.401 | 78.203 |
| PC5 | 1.075 | 8.273 | 86.476 |
| (c) | |||
| PC1 | 4.517 | 34.743 | 34.743 |
| PC2 | 2.970 | 22.843 | 57.586 |
| PC3 | 1.754 | 13.491 | 71.077 |
| PC4 | 1.137 | 8.745 | 79.822 |
| GAM Model | edf | p-Value | GCV | Deviance Explained (DE) | Significance |
|---|---|---|---|---|---|
| MDI~s (NH4+-N, k = 3) | 1 | 0.0278 * | 0.34798 | 7.02% | √ |
| MDI~s (TN, k = 3) | 1.8 | 0.000843 *** | 0.30319 | 20.9% | √ |
| MDI~s (SAL, k = 3) | 1.753 | 0.118 | 0.35416 | 7.48% | × |
| MDI~s (Chl-a, k = 3) | 1.873 | 0.00632 ** | 0.32474 | 15.5% | √ |
| MDI~s (CL−, k = 3) | 1.52 | 0.445 | 0.36775 | 3.26% | × |
| MDI~s (pH, k = 3) | 1.214 | 0.901 | 0.37391 | 0.73% | × |
| MDI~te (NH4+-N, TN, k = 3) | 5.187 | 0.00219 ** | 0.30451 | 28.5% | √ |
| MDI~te (NH4+-N, Chl-a, k = 3) | 4.751 | 0.00187 ** | 0.30473 | 27.4% | √ |
| MDI~te (TN, Chl-a, k = 3) | 4.765 | 8.8 × 10−5 *** | 0.26659 | 36.5% | √ |
| GAM Model | GCV | AIC | Deviance Explained (DE) | DE Variation with Model 1 |
|---|---|---|---|---|
| Model 1: MDI~s (TN) + s (NH4+-N) + s (Chl-a) | 0.248 | 99.20836 | 52.2% | |
| Model 2: MDI~s (TN) + s (NH4+-N) + s (Chl-a) + te (NH4+-N, TN) | 0.24637 | 96.2688 | 60% | +7.8% |
| Model 3: MDI~s (TN) + s (NH4+-N) + s (Chl-a) + te (NH4+-N, Chl-a) | 0.24457 | 97.26073 | 56.2% | +4% |
| Model 4: MDI~s (TN) + s (NH4) + s (Chl-a) + te (TN, Chl-a) | 0.24847 | 96.93572 | 59.4% | +7.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yu, H.; Liu, M.; Liu, J.; Dong, W.; Xu, T.; Wang, Y.; Guo, Y. Analysis of Water Quality and Habitat Suitability for Benthic Macro-Invertebrates in the Majiagou Urban River, China. Water 2023, 15, 2269. https://doi.org/10.3390/w15122269
Zhang Y, Yu H, Liu M, Liu J, Dong W, Xu T, Wang Y, Guo Y. Analysis of Water Quality and Habitat Suitability for Benthic Macro-Invertebrates in the Majiagou Urban River, China. Water. 2023; 15(12):2269. https://doi.org/10.3390/w15122269
Chicago/Turabian StyleZhang, Yongxin, Hongxian Yu, Manhong Liu, Jiamin Liu, Wentao Dong, Tiantian Xu, Yunrui Wang, and Yao Guo. 2023. "Analysis of Water Quality and Habitat Suitability for Benthic Macro-Invertebrates in the Majiagou Urban River, China" Water 15, no. 12: 2269. https://doi.org/10.3390/w15122269
APA StyleZhang, Y., Yu, H., Liu, M., Liu, J., Dong, W., Xu, T., Wang, Y., & Guo, Y. (2023). Analysis of Water Quality and Habitat Suitability for Benthic Macro-Invertebrates in the Majiagou Urban River, China. Water, 15(12), 2269. https://doi.org/10.3390/w15122269





