Dry and Wet Weather Survey for Human Fecal Sources in the San Diego River Watershed
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Analysis
2.2. Data Analysis
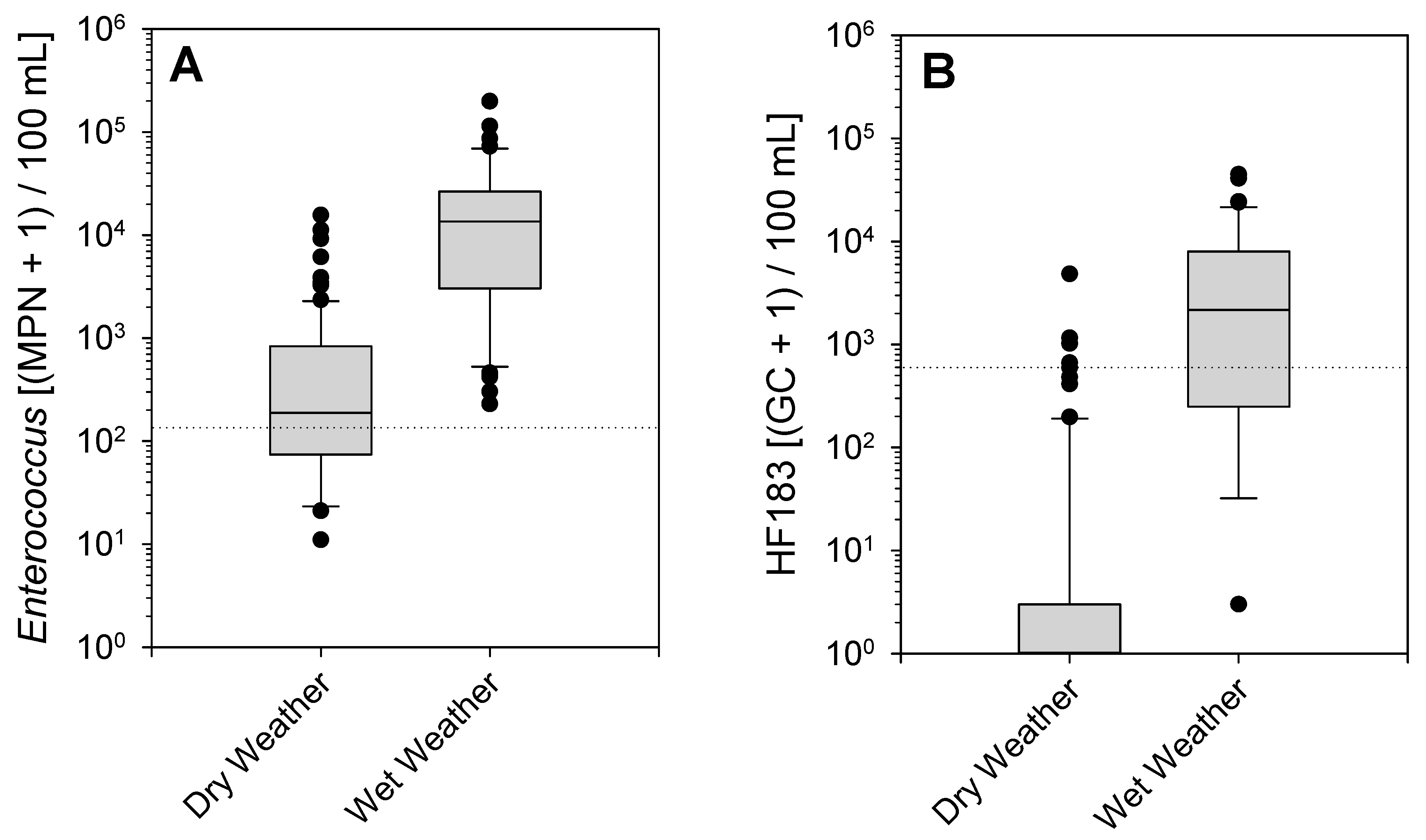
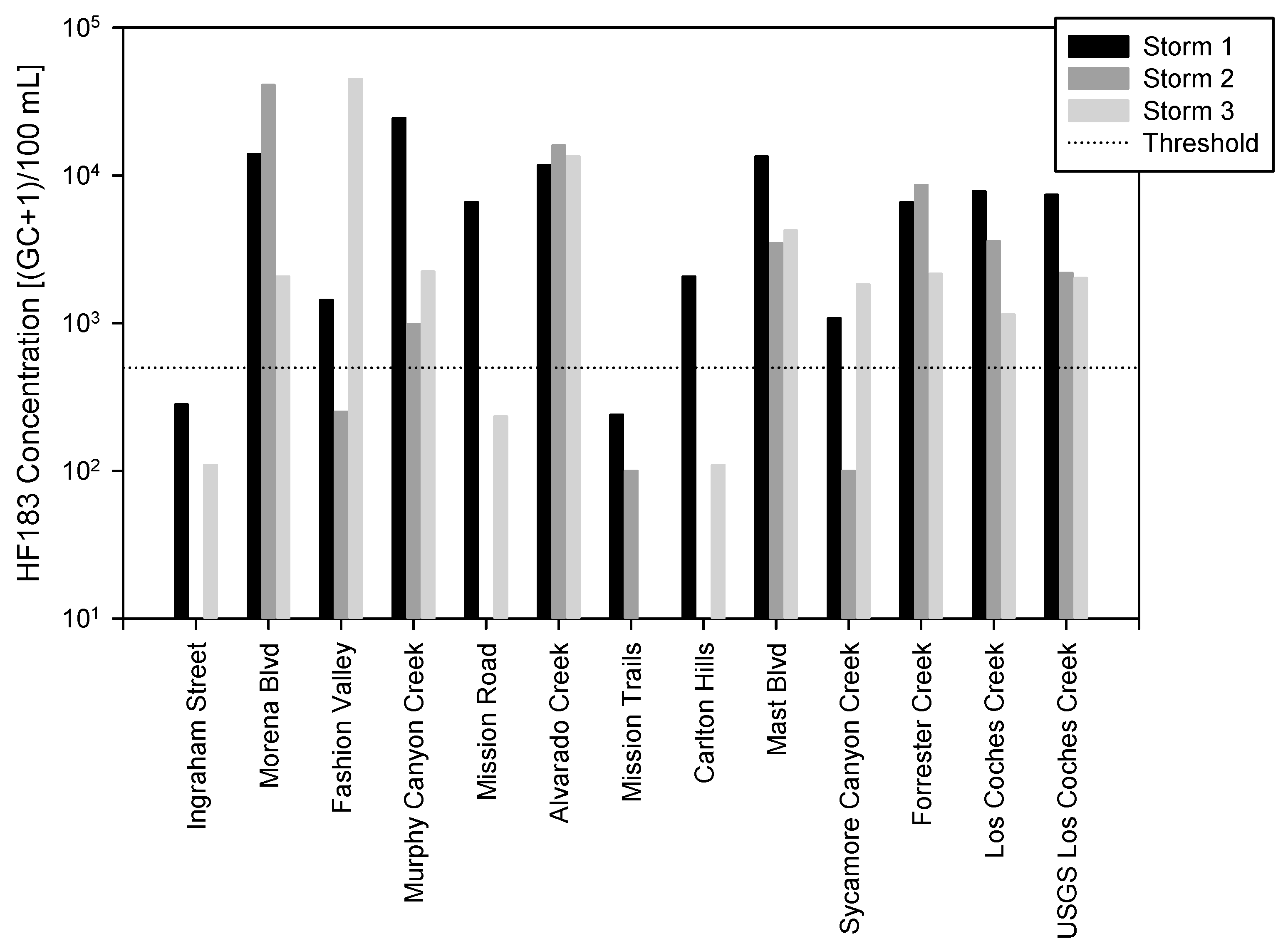
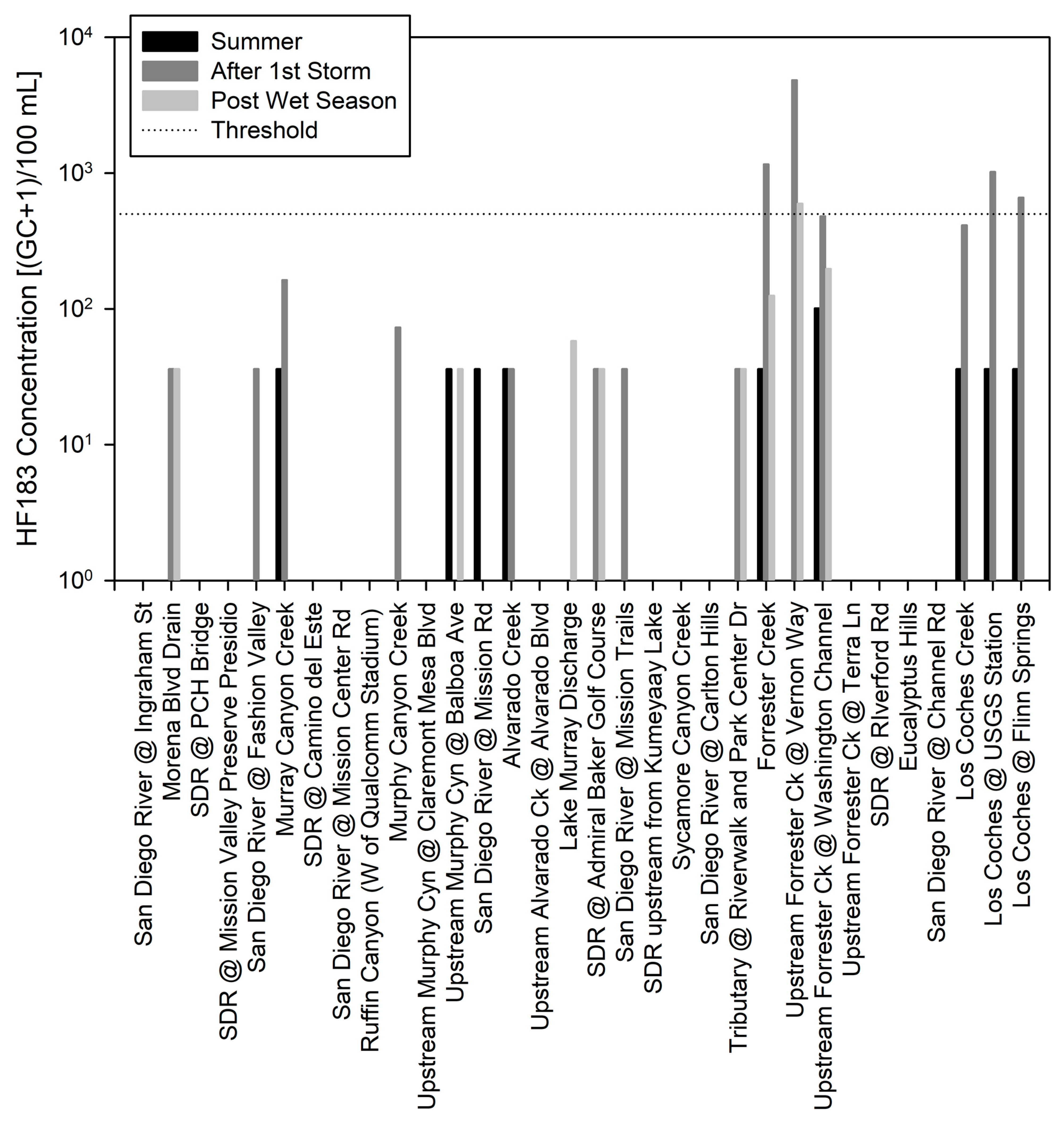
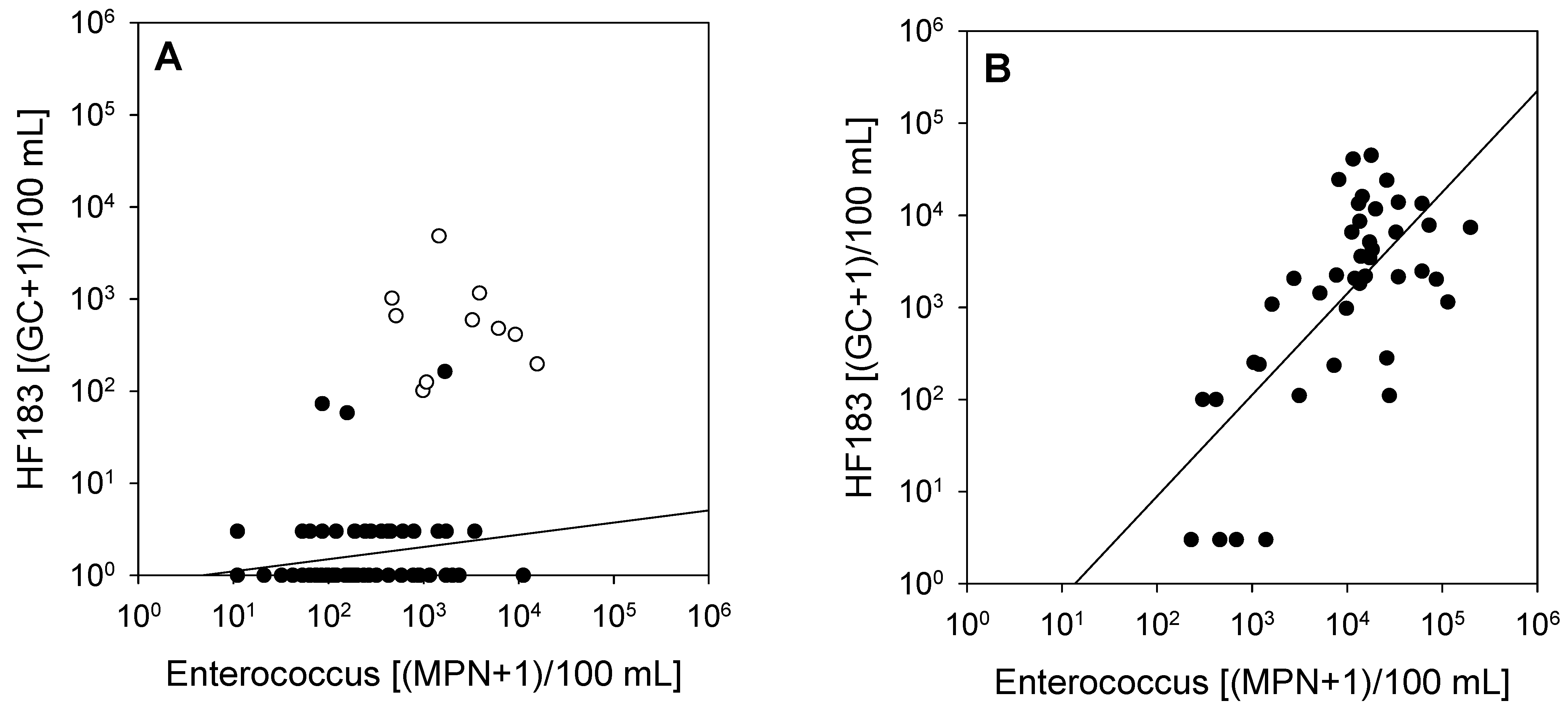
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPA. Recreational Water Quality Criteria; 20-F-12-058; United States Environmental Protection Agency, Office of Science and Technology, Health and Ecological Criteria Division, Office of Water: Washington, DC, USA, 1986; 69p.
- Cabelli, V.J.; Dufour, A.P.; Levin, M.A.; McCabe, L.J.; Haberman, P.W. Relationship of microbial indicators to health effects at marine bathing beaches. Am. J. Public Health 1979, 69, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Wade, T.J.; Sams, E.; Brenner, K.P.; Haugland, R.; Chern, E.; Beach, M.; Wymer, L.; Rankin, C.C.; Love, D.; Li, Q.; et al. Rapidly measured indicators of recreational water quality and swimming-associated illness at marine beaches: A prospective cohort study. Environ. Health 2010, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Arnold, B.F.; Wade, T.J.; Benjamin-Chung, J.; Schiff, K.C.; Griffith, J.F.; Dufour, A.P.; Weisberg, S.B.; Colford, J.M., Jr. Acute gastroenteritis and recreational water: Highest burden among young US children. Am. J. Public Health 2016, 106, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.F.C.; Garside, R.; Ukoumunne, O.C.; Gaze, W.H. A Cross-Sectional Study on the Prevalence of Illness in Coastal Bathers Compared to Non-Bathers in England and Wales: Findings from the Beach User Health Survey. Water Res. 2020, 176, 115700. [Google Scholar] [CrossRef]
- Wade, T.J.; Arnold, B.F.; Schiff, K.; Colford, J.M.; Weisberg, S.B.; Griffith, J.F.; Dufour, A.P. Health Risks to Children from Exposure to Fecally-Contaminated Recreational Water. PLoS ONE 2022, 17, e0266749. [Google Scholar] [CrossRef] [PubMed]
- Zimmer-Faust, A.G.; Steele, J.A.; Griffith, J.F.; Schiff, K.C. The challenges of microbial source tracking at urban beaches for Quantitative Microbial Risk Assessment (QMRA). Mar. Pollut. Bull. 2020, 160, 111546. [Google Scholar] [CrossRef]
- Tiefenthaler, L.L.; Stein, E.D.; Schiff, K.C. Levels and patterns of fecal indicator bacteria in stormwater runoff from homogenous land use sites and urban watersheds. J. Water Health 2011, 9, 279–290. [Google Scholar] [CrossRef]
- Schiff, K.C.; Kinney, P. Tracking sources of bacterial contamination in stormwater discharges to Mission Bay, California. Water Environ. Res. 2001, 73, 534–542. [Google Scholar] [CrossRef]
- Soller, J.; Bartrand, T.; Molina, M.; Whelan, G.; Schoen, M.; Ashbolt, A. Estimated human health risks from recreational exposures to stormwater containing animal faecal material. Environ. Model. Softw. 2015, 72, 21–32. [Google Scholar] [CrossRef]
- Soller, J.A.; Schoen, M.; Steele, J.A.; Griffith, J.F.; Schiff, K.C. Incidence of gastrointestinal illness following wet weather recreational exposures: Harmonization of quantitative microbial risk assessment with an epidemiologic investigation of surfers. Water Res. 2017, 121, 280–289. [Google Scholar] [CrossRef]
- Schiff, K.C.; Weisberg, S.B.; Dorsey, J.H. Microbiological monitoring of marine recreational waters in southern California. Environ. Manag. 2001, 27, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Environmental Incentives and Econorthwest. Cost-Benefit Analysis: San Diego Region Bacteria Total Maximum Daily Loads. 2017; p. 649. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiewfyUpcD8AhUdJkQIHXhlAukQFnoECBgQAQ&url=https%3A%2F%2Fwww.waterboards.ca.gov%2Fsandiego%2Fwater_issues%2Fprograms%2Fbasin_plan%2Fdocs%2Fissue3%2FFinal_CBA.pdf&usg=AOvVaw3dBfW53P_EjA-3dZLSJNtw (accessed on 1 May 2023).
- Boehm, A.B.; Van De Werfhorst, L.C.; Griffith, J.F.; Holden, P.A.; Jay, J.A.; Shanks, O.C.; Wanga, D.; Weisberg, S.B. Performance of forty-one microbial source tracking methods: A twenty-seven lab evaluation study. Water Res. 2013, 47, 6812–6828. [Google Scholar] [CrossRef] [PubMed]
- Harwood, V.; Shanks, O.; Koraijkic, A.; Verbyla, M.; Ahmed, W.; Iriate, M. General and host-associated bacterial indicators of faecal pollution. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management; Global Water Pathogen Project, Part 2: Indicators and Microbial Source Tracking Markers; Rose, J.B., Jiménez-Cisneros, B., Eds.; Michigan State University: East Lansing, MI, USA, 2017. [Google Scholar] [CrossRef]
- Shanks, O.C.; Korajkic, A. Microbial source tracking: Characterization of human fecal pollution in environmental waters with HF183 quantitative real-time PCR. In Microbial Forensics; Academic Press: Cambridge, MA, USA, 2020; pp. 71–87. [Google Scholar]
- Cao, Y.; Raith, M.R.; Griffith, J.F. Droplet digital PCR for simultaneous quantification of general and human-associated fecal indicators for water quality assessment. Water Res. 2015, 70, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.A.; Blackwood, A.D.; Griffith, J.F.; Noble, R.T.; Schiff, K.C. Quantification of pathogens and markers of fecal contamination during storm events along popular surfing beaches in San Diego, California. Water Res. 2018, 136, 137–149. [Google Scholar] [CrossRef]
- Boehm, A.; Soller, J. Refined ambient water quality thresholds for human-associated fecal indicator HF183 for recreational waters with and without co-occurring gull fecal contamination. Microb. Risk Anal. 2020, 16, 100139. [Google Scholar] [CrossRef]
- DeFlorio-Barker, S.; Wing, C.; Jones, R.M.; Dorevitch, S. Estimate of incidence and cost of recreational waterborne illness on United States surface waters. Environ. Health 2018, 17, 3. [Google Scholar] [CrossRef]
- Sauer, E.P.; VandeWalle, J.L.; Bootsma, M.J.; McLellan, S.L. Detection of the human specific Bacteroides genetic marker provides evidence of widespread sewage contamination of stormwater in the urban environment. Water Res. 2011, 45, 4081–4091. [Google Scholar] [CrossRef]
- Sidhu, J.P.S.; Ahmed, W.; Gernjak, W.; Aryal, R.; McCarthy, D.; Palmer, A.; Kolotelo, P.; Toze, S. Sewage Pollution in Urban Stormwater Runoff as Evident from the Widespread Presence of Multiple Microbial and Chemical Source Tracking Markers. Sci. Total Environ. 2013, 463–464, 488–496. [Google Scholar] [CrossRef]
- Villemur, R.; Imbeau, M.; Vuong, M.N.; Masson, L.; Payment, P. An Environmental Survey of Surface Waters Using Mitochondrial DNA from Human, Bovine and Porcine Origin as Fecal Source Tracking Markers. Water Res. 2015, 69, 143–153. [Google Scholar] [CrossRef]
- Cao, Y.; Raith, M.; Smith, P.; Griffith, J.; Weisberg, S.; Schriewer, A.; Sheldon, A.; Crompton, C.; Amenu, G.; Gregory, J.; et al. Regional Assessment of Human Fecal Contamination in Southern California Coastal Drainages. Int. J. Environ. Res. Public Health 2017, 14, 874. [Google Scholar] [CrossRef]
- Nshimyimana, J.P.; Cruz, M.C.; Thompson, R.J.; Wuertz, S. Bacteroidales Markers for Microbial Source Tracking in Southeast Asia. Water Res. 2017, 118, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Jennings, W.C.; Gálvez-Arango, E.; Prieto, A.L.; Boehm, A.B. CrAssphage for Fecal Source Tracking in Chile: Covariation with Norovirus, HF183, and Bacterial Indicators. Water Res. X 2020, 9, 100071. [Google Scholar] [CrossRef] [PubMed]
- McKee, B.A.; Molina, M.; Cyterski, M.; Couch, A. Microbial Source Tracking (MST) in Chattahoochee River National Recreation Area: Seasonal and Precipitation Trends in MST Marker Concentrations, and Associations with E. Coli Levels, Pathogenic Marker Presence, and Land Use. Water Res. 2020, 171, 115435. [Google Scholar] [CrossRef] [PubMed]
- Sherchan, S.; Shahin, S.; Alarcon, J.; Brosky, H.; Potter, C.; Dada, A.C. Microbial Source Tracking of Fecal Contamination in Stormwater Runoff. J. Water Health 2022, 20, 1271–1283. [Google Scholar] [CrossRef]
- Boehm, A.B.; Graham, K.E.; Jennings, W.C. Can We Swim Yet? Systematic Review, Meta-Analysis, and Risk Assessment of Aging Sewage in Surface Waters. Environ. Sci. Technol. 2017, 52, 9634–9645. [Google Scholar] [CrossRef]
- Arnold, B.F.; Schiff, K.C.; Ercumen, A.; Benjamin-Chung, J.; Steele, J.A.; Griffith, J.F.; Steinberg, S.J.; Smith, P.D.; McGee, C.D.; Wilson, C.; et al. Acute Illness Among Surfers After Exposure to Seawater in Dry- and Wet-Weather Conditions. Am. J. Epidemiol. 2017, 186, 866–875. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiff, K.; Griffith, J.; Steele, J.; Zimmer-Faust, A. Dry and Wet Weather Survey for Human Fecal Sources in the San Diego River Watershed. Water 2023, 15, 2239. https://doi.org/10.3390/w15122239
Schiff K, Griffith J, Steele J, Zimmer-Faust A. Dry and Wet Weather Survey for Human Fecal Sources in the San Diego River Watershed. Water. 2023; 15(12):2239. https://doi.org/10.3390/w15122239
Chicago/Turabian StyleSchiff, Kenneth, John Griffith, Joshua Steele, and Amity Zimmer-Faust. 2023. "Dry and Wet Weather Survey for Human Fecal Sources in the San Diego River Watershed" Water 15, no. 12: 2239. https://doi.org/10.3390/w15122239
APA StyleSchiff, K., Griffith, J., Steele, J., & Zimmer-Faust, A. (2023). Dry and Wet Weather Survey for Human Fecal Sources in the San Diego River Watershed. Water, 15(12), 2239. https://doi.org/10.3390/w15122239







