1. Introduction
Wastewater reclamation and reuse offer a practical solution to enhance water supply resilience and alleviate local freshwater demand [
1,
2,
3]. Reverse osmosis (RO) is an advanced membrane desalination technology that effectively removes pathogens, organic, and inorganic substances in wastewater, providing high-quality water for beneficial reuse [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11]. However, membrane fouling and scaling are major operational problems that, if not controlled, can lead to a decreased water flux, deteriorated permeate water quality, elevated feed pressures, increased energy demand, and more frequent chemical cleaning. Operation and maintenance costs can increase considerably due to the need for extensive pretreatment and frequent cleaning; the latter may shorten membrane lifespans, increase downtime, and reduce water production [
5,
6,
9,
10,
12,
13,
14,
15,
16,
17,
18].
Membrane fouling and scaling often occur together during treatment of wastewater with RO because organics and sparingly soluble salts coexist in this matrix. Colloidal particles, microorganisms, organic matter, and sparingly soluble salts (e.g., CaCO
3, CaSO
4, SiO
2, and BaSO
4) present in feedwater adhere to, and precipitate, either within the polymer matrix of the membrane or on its surface. The mechanisms of membrane fouling and scaling are complicated during wastewater reclamation, primarily due to the high concentration of biological activity, suspended particles, colloids, and the presence of complex dissolved organic matter generated during biological wastewater treatment in secondary effluent [
10,
15,
19,
20,
21,
22,
23,
24].
Ensuring adequate pretreatment is crucial for the reliable operation and prolonged lifetime of RO membranes for wastewater reclamation. During wastewater desalination, RO trains mostly experience bio/organic fouling in the lead-position membrane elements, whereas the end-position elements are primally affected by inorganic scaling, such as calcium phosphate, due to higher ion concentrations in the water [
16]. Various technological approaches are available for pretreatment, including modification of membrane properties, pH adjustment, use of antiscalants and/or disinfectants, hydraulic flush, and chemical cleaning [
25].
Instead of relying on chemical pretreatment to prevent membrane fouling and scaling, an alternative approach is to use non-chemical pretreatment methods, such as an electromagnetic field (EMF) [
26,
27,
28]. EMFs can be generated and induced by ferrite magnets [
29], by wrapping metallic wire around a pipe carrying water, or wrapping wire directly around membrane pressure vessels [
30]. Water does not come into direct contact with the electrodes, and an EMF is induced due to alternating current (AC) or permanent magnets (e.g., neodymium magnets and ferrite magnets). Water is subject to a quick variation of coil voltage in the hertz (Hz) to megahertz frequency range [
17]. There are many types of commercial EMF water conditioning devices with differing EMF signal generation methods, directions, and intensities in terms of electric field strength (measured in volts per meter) and magnetic flux densities (measured in Tesla).
Several studies have demonstrated the effectiveness of using EMFs for treating wastewater, although there are no agreed-upon mechanisms. Zaidi et al. attributed the enhanced solid–liquid separation performance of the applied magnetic field to promote the aggregation of colloidal particles [
31]. Wang et al. in a paper review summarized improvements during the treatment, which included the removal of turbidity (efficiency not stated), suspended solids (efficiency not stated), chemical oxygen demand (COD) decreased by 25%, and total nitrogen and ammonia nitrogen decreased by 22.4% to 39.5% [
32]. Sibiya et al. observed similar results and demonstrated that the magnetic field (20 mT) increased the removal of turbidity, total suspended solids (TSS), and COD [
33]. An increase in COD removal efficiency of up to 82% using an EMF was also reported in other studies [
34,
35,
36].
Conversely, some studies have shown the use of an EMF to be ineffective for preventing or retarding fouling formation [
37,
38]. The conflicting outcomes reported may be attributed to various factors such as the utilization of different magnetic or electric fields, variations in their frequency and intensity, variations in water composition, and disparities in the treatment processes [
39]. Additionally, the efficacy of EMF treatment may be influenced by the characteristics of the pipe materials through which the EMF is transmitted [
40,
41].
Despite numerous laboratory and pilot studies exploring the use of EMFs for scaling control in brackish water desalination [
42,
43], there is a lack of systematic research on the impact of EMFs on RO membrane fouling and scaling during wastewater treatment [
41]. This research is the first study that quantitatively assessed the effect of an AC-induced EMF on foulant formation and accumulation on RO membrane surfaces, as well as its influence on permeate water quality during secondary effluent treatment. This study filled the knowledge gap in comparing the effectiveness of the EMF to antiscalants, a commonly employed pretreatment method, and explored the synergistic effects of EMF in combination with antiscalants on RO membrane performance. Additional experiments were conducted to determine if membrane flux restoration was possible through periodic hydraulic flushing, both with and without the presence of the EMF. The findings of this study enhance our understanding of how EMFs influence the performance of RO systems in wastewater treatment and offer insights into the reduction or elimination of chemical usage during advanced wastewater treatment and reuse.
2. Materials and Methods
2.1. Feedwater Quality
The effects of EMF on RO membrane fouling and scaling control were studied using the secondary effluent from the Roberto Bustamante Wastewater Treatment Plant (RBWWTP), El Paso, TX, USA (
Table 1).
Electrical conductivity (EC) and pH and temperature were measured using a hand-held conductivity meter (Hach SensION 5 conductivity meter, Cole-Palmer, Vernon Hills, IL, USA) and a pH meter (Oakton PC 300 m, Cole-Palmer, Vernon Hills, IL, USA), respectively. Alkalinity was measured using the acid titration method (Hach Digital Titrator, Model 16900, Cole-Palmer, Vernon Hills, IL, USA). Elements and metals were analyzed with inductively coupled plasma–optical emission spectrometry (ICP-OES; Optima 4300 DV, PerkinElmer, MA, USA), and common cations and anions were measured using an ion chromatograph (IC; ICS-2100, Dionex, Sunnyvale, CA, USA). Organics in water were measured as dissolved organic carbon (DOC; Shimadzu TOC-L, Kyoto, Japan) using fluorescence excitation–emission matrix spectroscopy (F-EEM, Aqualog-UV-800-C, Horiba Instruments Inc., Piscataway, NJ, USA). All the samples for IC, ICP-OES, DOC, and F-EEM measurements were filtered using Cole-Parmer Nylon Chromatography Syringe filters (0.45 μm pore size) before the instrumental analysis.
2.2. Properties of EMF
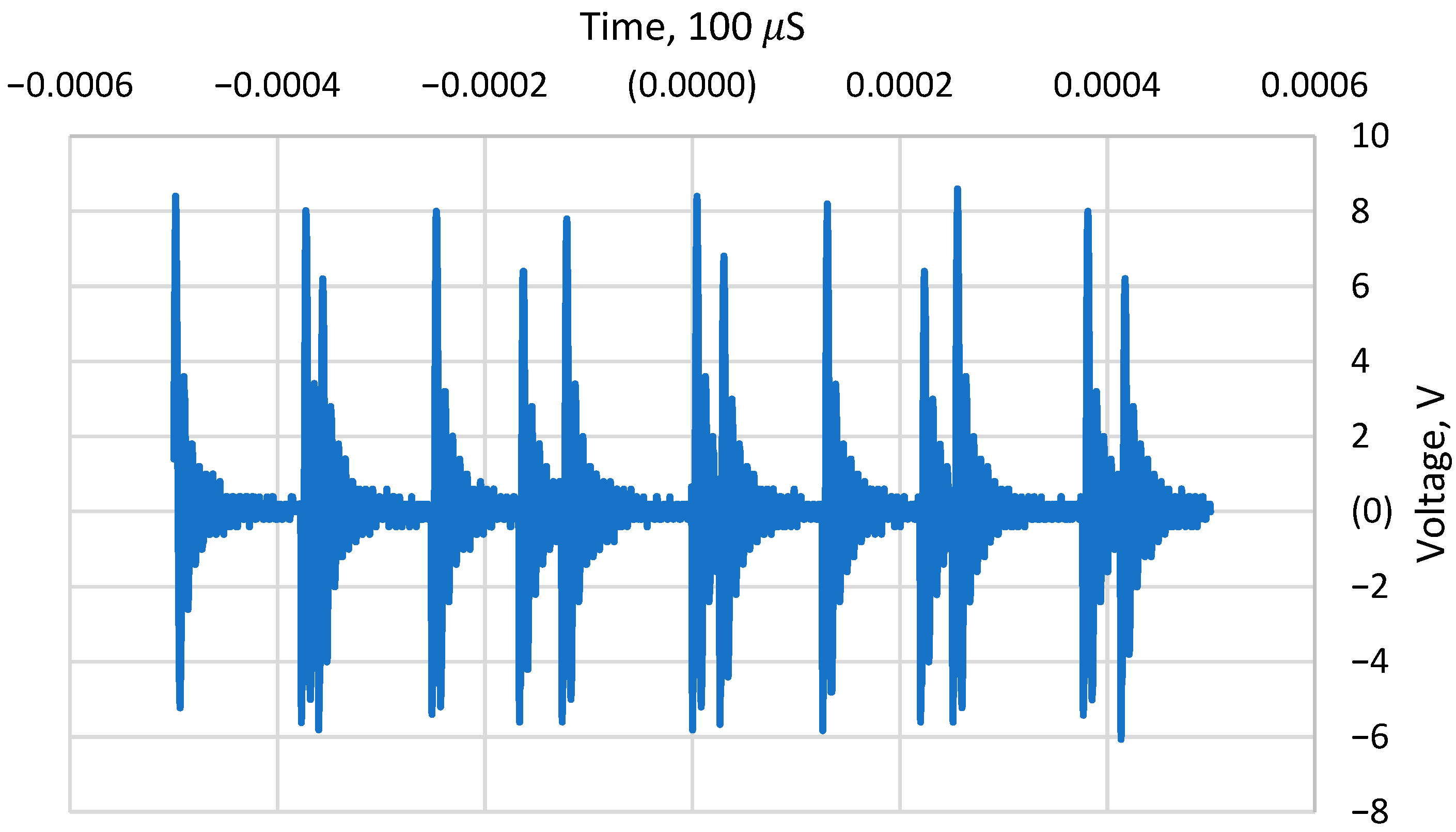
The EMF device utilized in this study is designed for scale control with a specialized transducer connected to a ferrite ring that generates pulsed, decaying sinusoidal electric signals at a frequency of ±150 kHz. The device operates at a maximum power of 1.2 W and produces a peak-to-peak voltage of 14.4 V (
Figure 1). The characterization of the EMF device was performed using an oscilloscope Tektronix TDS 1002B (Tektronix, Beaverton, OR, USA) and a 3DHALL magnetic sensor (Model SENM3DX, Senis, Switzerland) that allows the acquisition of all three magnetic-field components (Bx, By, and Bz) at the same time. The magnetic field and electric field generated by the device were measured as 0.51 mT and 811 V/m, respectively.
2.3. Bench-Scale Fouling and Scaling Experiments
The objective of this research is to assess the effectiveness of EMFs in reducing fouling and scaling on RO membranes during the treatment of municipal secondary effluent. In a typical potable reuse project, RO filtration is preceded by microfiltration (MF) or ultrafiltration (UF). However, in this study, the wastewater was intentionally not filtered by MF or UF to accelerate scaling and particulate fouling on the RO membranes.
A flat-sheet, bench-scale filtration system was employed to investigate the effect of EMF on low-pressure, brackish water RO membranes (Hydranautics’ ESPA2-LD flat sheet membranes) that included two standard cross-flow SEPA cells. The SEPA cells were installed in series and are regarded as duplicates in each test, with a slight increase in feedwater concentration to the second cell. The rectangular plate-and-frame cell used had the dimensions of a channel length of 14.6 cm, a width of 9.5 cm, and a height of 0.86 mm (34 mil). This configuration resulted in an effective membrane area of 139 cm2 per unit and a cross-sectional flow area of 0.82 cm2. The cells and tubing were made of stainless steel. To manage membrane fouling and scaling, the EMF device was placed at the inlet of the RO units through an SA 213 grade TP 316 stainless steel pipe with an outer diameter of 3/8 inches and an inner diameter of 0.255 inches.
A LabVIEW data acquisition system was used for monitoring and controlling the RO system, which operated in either a continuous, recirculation mode (recirculating both concentrate and permeate to the feed tank) or feed-and-bleed mode (recirculating only concentrate to the feedwater tank while discharging permeate to enhance water recovery) (
Figure A1). The feedwater flow rate was controlled at 1 L/min (cross-flow or linear flow velocity of 0.20 m/s) using a Hydra-cell pump (M03EKSGSFSHA, Wanner Engineering, Inc., Minneapolis, MN, USA) with a Dayton motor (1F798, Grainger, IL, USA), and feed pressure was maintained at 150 psi (1034 kPa) through manual and automated pressure valves. Two different operation modes were tested with up to four different pretreatment conditions, including (a) no pretreatment, (b) only EMF, (c) only adding antiscalant, and (d) EMF combined with the addition of antiscalant. In feed-and-bleed operation, tests with 10 L of feedwater were conducted until the highest possible water recovery (with a target of 90%) was achieved. Hypersperse MDC714, manufactured by Suez Water, was used as the antiscalant in these experiments with a dosage of 1 mg/L. During the testing, pressure, flow rate, conductivity, pH, temperature, and turbidity were monitored for all the streams in the RO system, and water samples were collected for chemical analysis.
All the experiments were conducted in replicates to ensure experimental reproducibility, especially because this study used real wastewater that had variability in water quality, as shown in
Table 1. The variability of the membrane-fouling experiments was calculated as the standard deviation of permeate water flux/average water flux of duplicate testing. The experimental results demonstrate high reproducibility of the membrane fouling experiments with a variability of less than 10%. Four experiments had variability higher than 10%, which was caused by the high amounts of suspended solids in secondary effluent, resulting in different particulate fouling trends in duplicate experiments.
2.4. RO Membrane Fouling Characterization
After each experiment, membrane specimens were collected to characterize membrane foulants and scalants formed on the membrane surface. The specimens were stored in sterile polystyrene Petri dishes and kept in a refrigerator (~4 °C, unexposed to light) prior to analysis. The membrane morphology, surface structure, and elemental composition were characterized by scanning electron microscope (SEM, S-3400N II, Hitachi High-Technologies Corp., Pleasanton, CA, USA) and energy dispersive X-ray microanalysis (EDS, Noran System Six 300, Thermo Electron Corp., Madison, WI, USA). To quantify membrane fouling and scaling under different operating conditions, chemical extractions were conducted on membrane samples, using a virgin membrane as baseline control. The membranes were cut into pieces of 16 cm2 and further into smaller pieces, and then they were soaked separately in 0.8 M nitric acid (HNO3) solution and 0.1 M potassium hydroxide (KOH) solution to extract inorganic scalants and organic foulants, respectively. All membranes and solution samples were ultrasonicated for 120 min and then centrifuged for particle separation from the solution. DOC and F-EEM were used to determine organic fouling. The concentrations of inorganic scalants were measured using IC and ICP-OES.
4. Conclusions
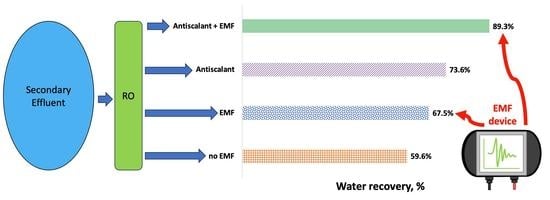
This study evaluated the membrane performance during desalination of municipal secondary effluent using an AC-induced EMF device as a standalone pretreatment option and in conjunction with antiscalants. Experiments demonstrated that the use of an EMF was effective in reducing fouling and scaling, leading to stable water production, higher water recovery rates, and longer operational times, compared to the no-EMF experimental setup. The bench-scale testing showed that the EMF could reduce membrane scaling by 2–8 times and organic fouling by 2 times when EMF was used compared to experiments without EMF. Interestingly, the highest water recovery (89.3%) was achieved when using a combination of EMF and antiscalants, which was higher than the recoveries achieved by the EMF-only (67.5%) and antiscalant-only (73.6%) experiments. This result was non-intuitive because the proposed mechanisms for EMFs and antiscalants are seemingly opposite, with the former acting to promote crystallization in the bulk solution and the latter acting to suppress crystallization. As such, additional research is needed to elucidate the fundamental mechanisms of EMF fouling and scaling control as a standalone process, and in conjunction with other pretreatment methods. Many factors need to be investigated individually and holistically, including water chemistry, applied pressure, magnetism generation source, shape and strength of the field, exposure time to EMF, RO membrane material, and feedwater velocity.