Using Multiscale Environmental and Spatial Analyses to Understand Natural and Anthropogenic Influence on Fish Communities in Four Canadian Rivers
Abstract
1. Introduction
2. Materials and Methods
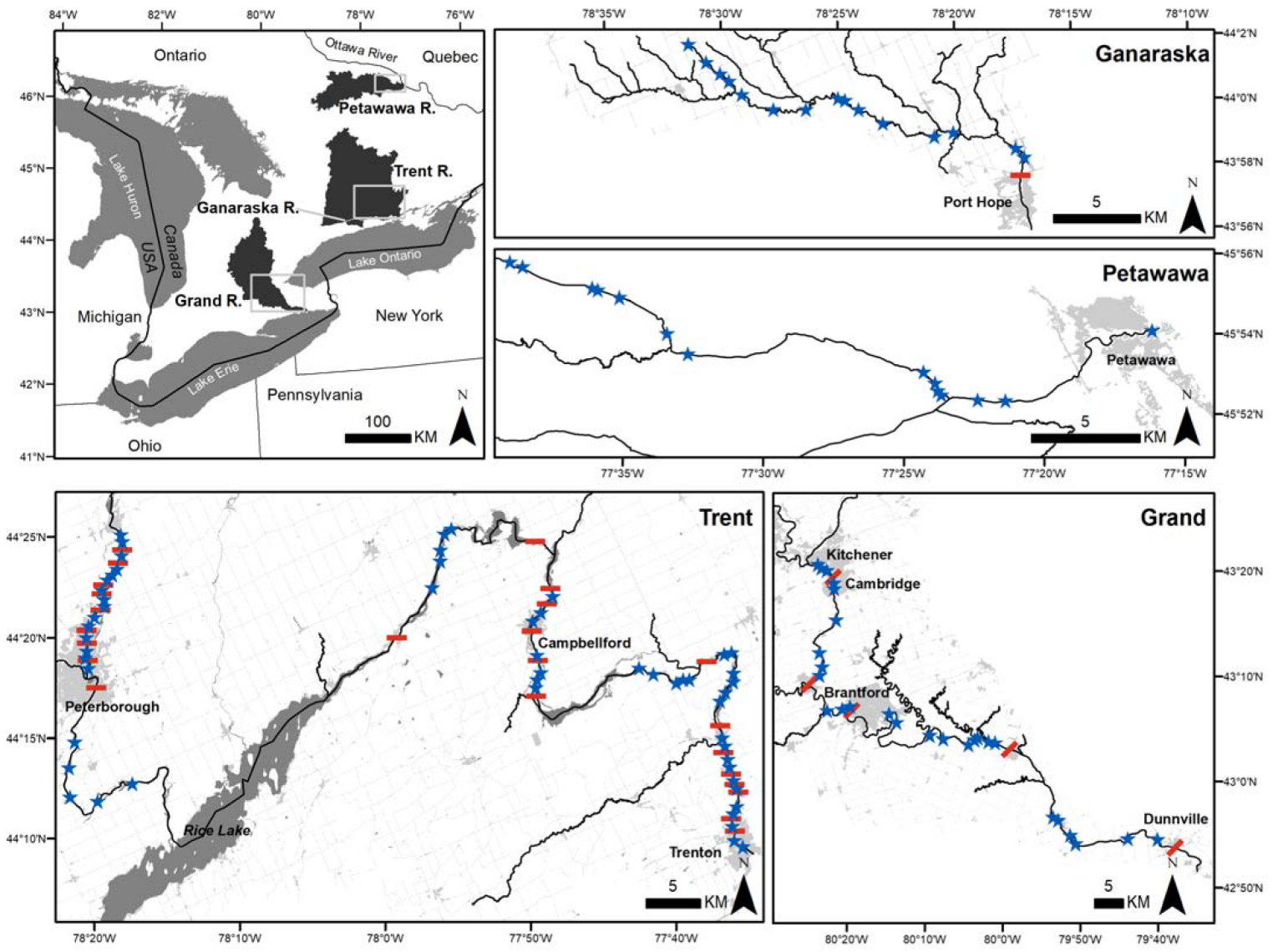
2.1. Study Area
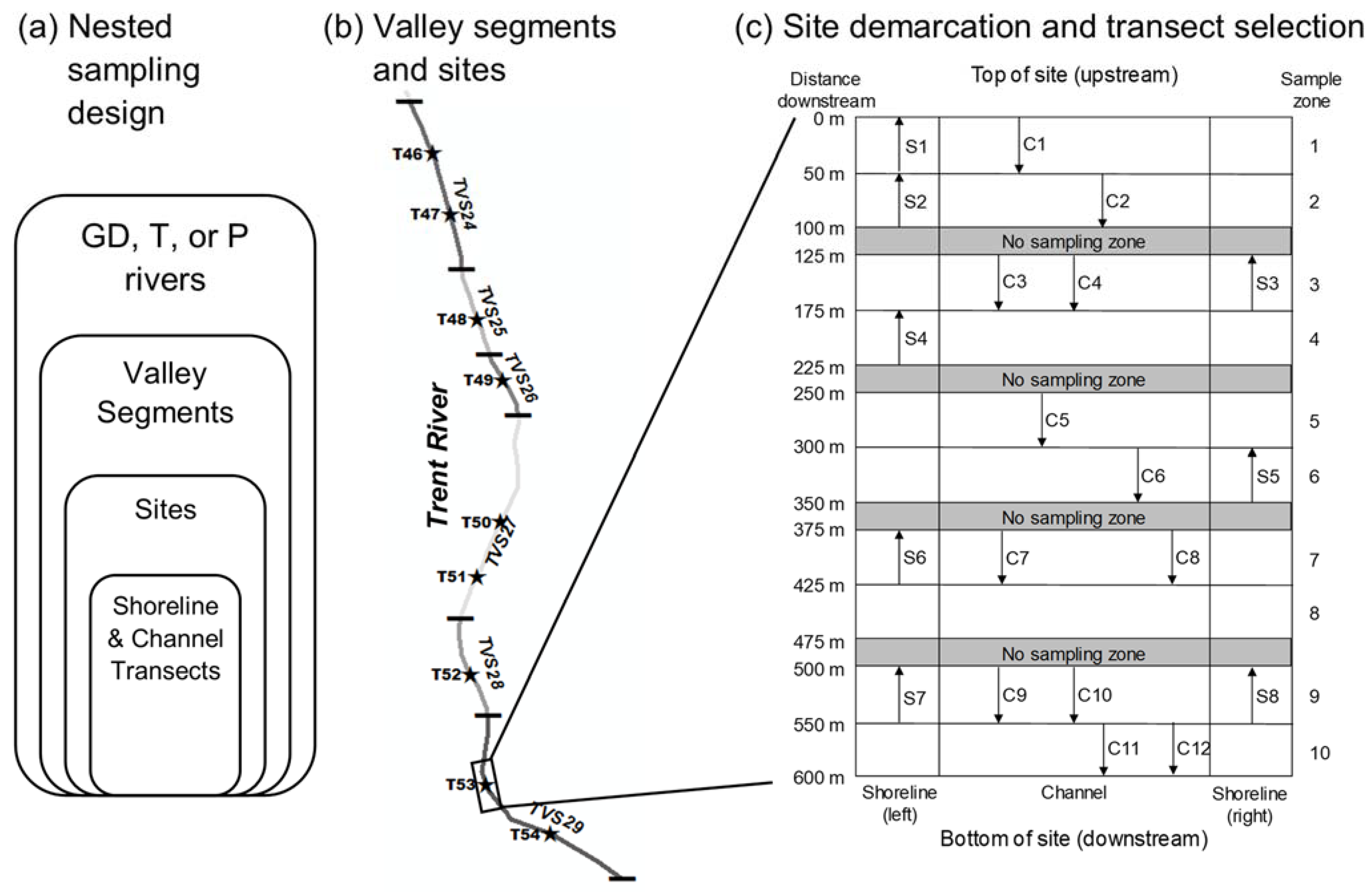
2.2. Study Sites
2.3. Instream Habit and Fish Sampling
2.4. Catchment and Valley Variables
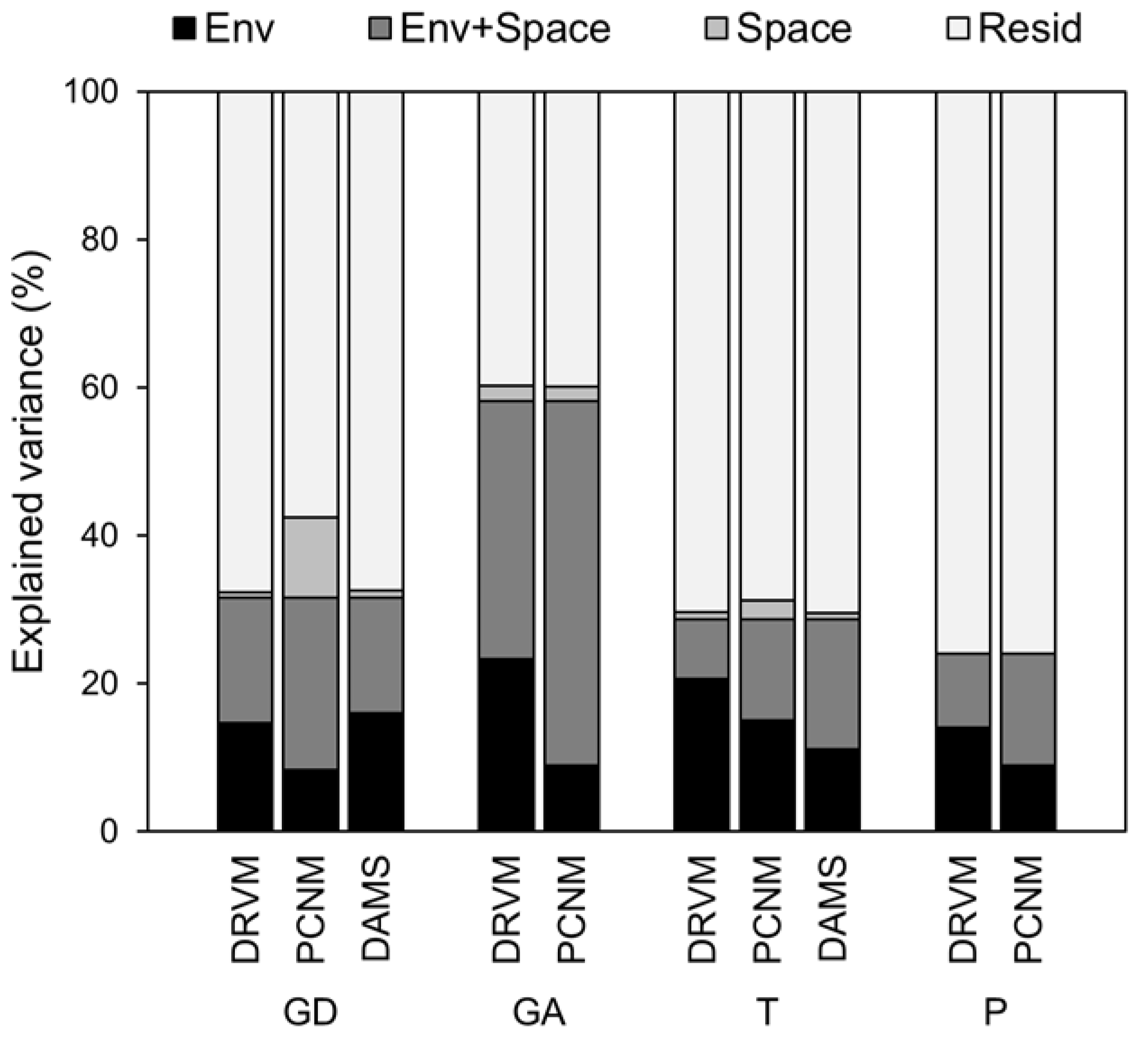
2.5. Spatial Variable Development
2.6. Data Analyses
3. Results
3.1. Data Summary
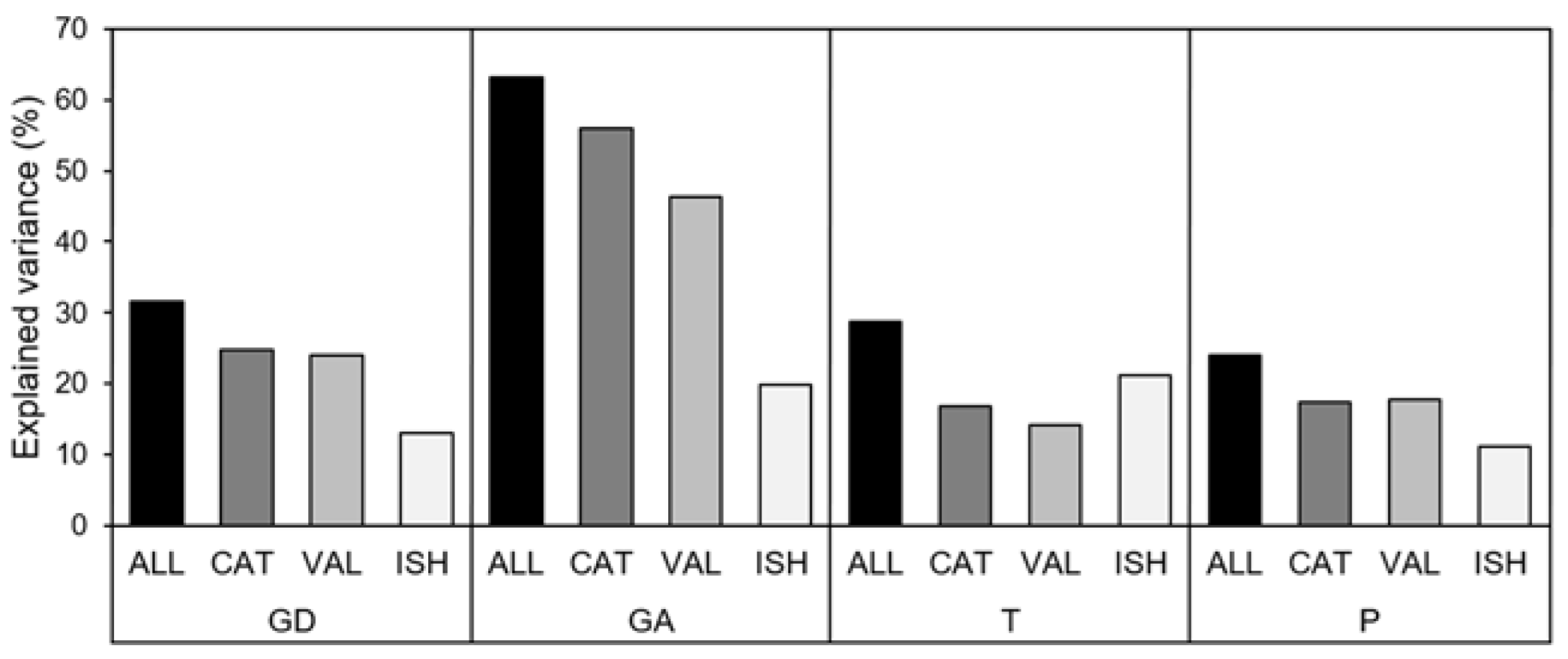
3.2. Environment and Fish Communities
3.3. Environment, Space, and Fish Communities
4. Discussion
4.1. Anthropogenic Influences Are Pervasive and Affect Fish Community Composition
4.2. Dams and Other Discontinuities Influence Fish Community Composition
4.3. Multiscale Control on Fish Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Sampling Design
Appendix B
| Family | Scientific Name | Common Name | Code | Tolerance | GD | GA | T | P |
|---|---|---|---|---|---|---|---|---|
| Atherinopsidae | Labidesthes sicculus | Brook Silverside | SILV | Intermediate | X | X | ||
| Catostomidae | Carpiodes cyprinus | Quillback | QUIL | Intermediate | X | X | X | X |
| Catostomus commersonii | White Sucker | WSUK | Tolerant | X | X | X | X | |
| Hypentelium nigricans | Northern Hog Sucker | HOGS | Intermediate | X | ||||
| Moxostoma anisurum | Silver Redhorse | SRDH | Intermediate | X | X | X | ||
| Moxostoma carinatum | River Redhorse | RVRH | Intolerant | X | ||||
| Moxostoma erythrurum | Golden Redhorse | GRED | Intermediate | X | ||||
| Moxostoma macrolepidotum | Shorthead Redhorse | SHRH | Intermediate | X | X | X | ||
| Moxostoma valenciennesi | Greater Redhorse | GRDH | Intolerant | X | X | |||
| Centrarchidae | Ambloplites rupestris | Rock Bass | ROCK | Intermediate | X | X | X | |
| Lepomis gibbosus | Pumpkinseed | PUNK | Intermediate | X | X | X | X | |
| Lepomis macrochirus | Bluegill | BLUE | Intermediate | X | X | |||
| Micropterus dolomieu | Smallmouth Bass | SBAS | Intermediate | X | X | X | ||
| Micropterus salmoides | Largemouth Bass | LBAS | Tolerant | X | X | X | ||
| Pomoxis annularis | White Crappie | WCRP | Tolerant | X | ||||
| Pomoxis nigromaculatus | Black Crappie | CRAP | Tolerant | X | X | |||
| Clupeidae | Alosa pseudoharengus | Alewife | ALEW | Intermediate | X | |||
| Dorosoma cepedianum | Gizzard Shad | GIZZ | Tolerant | X | ||||
| Cottidae | Cottus bairdii | Mottled Sculpin | MOTT | Intermediate | X | |||
| Cottus cognatus | Slimy Sculpin | SLIM | Intolerant | X | X | |||
| Cyprinidae | Carassius auratus | Goldfish | GLDF | Tolerant | X | |||
| Cyprinella spiloptera | Spotfin Shiner | SFSH | Intermediate | X | ||||
| Cyprinus carpio | Common Carp | CARP | Tolerant | X | X | |||
| Hybognathus hankinsoni | Brassy Minnow | BRSS | Intermediate | X | ||||
| Luxilus chrysocephalus | Striped Shiner | STSH | Intermediate | X | ||||
| Luxilus cornutus | Common Shiner | COMM | Intermediate | X | X | X | ||
| Margariscus margarita | Pearl Dace | PERL | Intermediate | X | ||||
| Nocomis biguttatus | Hornyhead Chub | HRNY | Intermediate | X | X | |||
| Notemigonus crysoleucas | Golden Shiner | GOLD | Intermediate | X | X | X | ||
| Notropis atherinoides | Emerald Shiner | EMRL | Intermediate | X | X | X | X | |
| Notropis heterodon | Blackchin Shiner | CHIN | Intolerant | X | X | |||
| Notropis heterolepis | Blacknose Shiner | BLAK | Intolerant | X | ||||
| Notropis hudsonius | Spottail Shiner | SPOT | Intermediate | X | X | X | X | |
| Notropis photogenis | Silver Shiner | SILS | Intolerant | X | ||||
| Notropis rubellus | Rosyface Shiner | ROSE | Intermediate | X | X | X | ||
| Notropis volucellus | Mimic Shiner | MIMC | Intermediate | X | X | X | ||
| Phoxinus eos | Northern Redbelly Dace | NRBD | Intermediate | X | ||||
| Pimephales notatus | Bluntnose Minnow | BNOS | Intermediate | X | X | X | X | |
| Pimephales promelas | Fathead Minnow | FATH | Tolerant | X | X | X | ||
| Rhinichthys atratulus | Blacknose Dace | BNDC | Intermediate | X | ||||
| Rhinichthys cataractae | Longnose Dace | LONG | Intermediate | X | X | X | ||
| Semotilus atromaculatus | Creek Chub | CHUB | Intermediate | X | X | X | ||
| Semotilus corporalis | Fallfish | FALL | Intermediate | X | X | |||
| Esocidae | Esox lucius | Northern Pike | PIKE | Intermediate | X | X | ||
| Esox masquinongy | Muskellunge | MUSK | Intermediate | X | X | |||
| Fundulidae | Fundulus diaphanus | Banded Killifish | KILL | Tolerant | X | |||
| Gasterosteidae | Culaea inconstans | Brook Stickleback | STIK | Intermediate | X | |||
| Hiodontidae | Hiodon tergisus | Mooneye | MOON | Intolerant | X | |||
| Ictaluridae | Ameiurus nebulosus | Brown Bullhead | BBUL | Intermediate | X | X | X | X |
| Ictalurus punctatus | Channel Catfish | CCAT | Tolerant | X | X | |||
| Noturus flavus | Stonecat | STON | Tolerant | X | ||||
| Lepisosteidae | Lepisosteus osseus | Longnose Gar | LGAR | Tolerant | X | X | X | |
| Percidae | Etheostoma blennioides | Greenside Darter | GSID | Intermediate | X | |||
| Etheostoma caeruleum | Rainbow Darter | RDRT | Intolerant | X | ||||
| Etheostoma exile | Iowa Darter | IOWA | Intermediate | X | X | |||
| Etheostoma flabellare | Fantail Darter | FANT | Intolerant | X | ||||
| Etheostoma nigrum | Johnny Darter | JOHN | Tolerant | X | X | X | X | |
| Perca flavescens | Yellow Perch | PRCH | Intermediate | X | X | |||
| Percina caprodes | Logperch | LOGP | Intolerant | X | X | X | ||
| Percina maculata | Blackside Darter | BDRT | Intermediate | X | ||||
| Sander vitreus | Walleye | WALL | Intermediate | X | X | X | ||
| Percopsidae | Percopsis omiscomaycus | Trout-Perch | TRPR | Intermediate | X | |||
| Salmonidae | Oncorhynchus kisutch | Coho Salmon | COHO | Intolerant | X | |||
| Oncorhynchus mykiss | Rainbow Trout | RAIN | Intolerant | X | X | |||
| Oncorhynchus tshawytscha | Chinook Salmon | KING | Intolerant | X | ||||
| Salmo trutta | Brown Trout | BTRT | Intolerant | X | ||||
| Salvelinus fontinalis | Brook Trout | BROK | Intolerant | X | ||||
| Sciaenidae | Aplodinotus grunniens | Freshwater Drum | DRUM | Tolerant | X | X | ||
| Umbridae | Umbra limi | Central Mudminnow | MUDM | Tolerant | X | X |
References
- Allen, T.F.H.; Starr, T.B. Hierarchy: Perspectives for Ecological Complexity, 2nd ed.; University Chicago Press: Chicago, IL, USA, 1982; ISBN 978-0-226-01431-9. [Google Scholar]
- O’Neill, R.V.; DeAngelis, D.L.; Waide, J.B.; Allen, T.F.H. A Hierarchical Concept of Ecosystems; Princeton University Press: Princeton, NJ, USA, 1986; ISBN 0-691-08436-X. [Google Scholar]
- Parsons, M.; Thoms, M.C.; Norris, R.H. Using Hierarchy to Select Scales of Measurement in Multiscale Studies of Stream Macroinvertebrate Assemblages. J. N. Am. Benthol. Soc. 2004, 23, 157–170. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The Ecology of Running Waters; University of Toronto Press: Toronto, ON, Canada, 1970; ISBN 0-8020-1689-8. [Google Scholar]
- Frissell, C.A.; Liss, W.J.; Warren, C.E.; Hurley, M.D. A Hierarchical Framework for Stream Habitat Classification—Viewing Streams in a Watershed Context. Environ. Manag. 1986, 10, 199–214. [Google Scholar] [CrossRef]
- Johnson, L.B.; Richards, C.; Host, G.E.; Arthur, J.W. Landscape Influences on Water Chemistry in Midwestern Stream Ecosystems. Freshwat. Biol. 1997, 37, 193–208. [Google Scholar] [CrossRef]
- Wang, L.; Lyons, J.; Rasmussen, P.; Seelbach, P.; Simon, T.; Wiley, M.; Kanehl, P.; Baker, E.; Niemela, S.; Stewart, P.M. Watershed, Reach, and Riparian Influences on Stream Fish Assemblages in the Northern Lakes and Forest Ecoregion, USA. Can. Fish. Aquat. Sci. 2003, 60, 491–505. [Google Scholar] [CrossRef]
- Gordon, N.D.; McMahon, T.A.; Finlayson, B.L.; Gippel, C.J.; Nathan, R.J. Stream Hydrology. An Introduction for Ecologists, 2nd ed.; John Wiley & Sons: Chichester, UK, 2004; ISBN 978-0-470-84358-1. [Google Scholar]
- Huet, M. Profiles and Biology of Western European Streams as Related to Fish Management. Trans. Am. Fish. Soc. 1959, 88, 155–163. [Google Scholar] [CrossRef]
- Schlosser, I.J. Environmental Variation, Life History Attributes, and Community Structure in Stream Fishes: Implications for Environmental Management and Assessment. Environ. Manag. 1990, 14, 621–628. [Google Scholar] [CrossRef]
- Fischer, J.R.; Paukert, C.P. Habitat Relationships with Fish Assemblages in Minimally Disturbed Great Plains Regions. Ecol. Freshw. Fish 2008, 17, 597–609. [Google Scholar] [CrossRef]
- Troia, M.J.; Gido, K.B. Predicting Community-Environment Relationships of Stream Fishes across Multiple Drainage Basins: Insights into Model Generality and the Effect of Spatial Extent. J. Environ. Manag. 2013, 128, 313–323. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The Stream and Its Valley. Verb. Internat. Verein Limnol. 1975, 19, 1–15. [Google Scholar] [CrossRef]
- Seelbach, P.W.; Wiley, M.J.; Baker, M.E.; Wehrly, K.E. Initial Classification of River Valley Segments across Michigan’s Lower Peninsula. In American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 2006; Volume 48, pp. 25–48. [Google Scholar]
- Jones, N.E.; Schmidt, B.J. Tributary Effects in Rivers: Interactions of Spatial Scale, Network Structure, and Landscape Characteristics. Can. J. Fish. Aquat. Sci. 2016, 74, 503–510. [Google Scholar] [CrossRef]
- Statzner, B.; Gore, J.A.; Resh, V.H. Hydraulic Stream Ecology: Observed Patterns and Potential Applications. J. N. Am. Benthol. Soc. 1988, 7, 307–360. [Google Scholar] [CrossRef]
- Zorn, T.G.; Seelbach, P.W.; Wiley, M.J. Distributions of Stream Fishes and Their Relationship to Stream Size and Hydrology in Michigan’s Lower Peninsula. Trans. Am. Fish. Soc. 2002, 131, 70–85. [Google Scholar] [CrossRef]
- Esselman, P.C.; Allan, J.D. Relative Influences of Catchment- and Reach-Scale Abiotic Factors on Freshwater Fish Communities in Rivers of Northeastern Mesoamerica. Ecol. Freshw. Fish 2010, 19, 439–454. [Google Scholar] [CrossRef]
- Pease, A.A.; Taylor, J.M.; Winemiller, K.O.; King, R.S. Multiscale Environmental Influences on Fish Assemblage Structure in Central Texas Streams. T. Am. Fish. Soc. 2011, 140, 1409–1427. [Google Scholar] [CrossRef]
- Johnson, R.K.; Furse, M.T.; Hering, D.; Sandin, L. Ecological Relationships between Stream Communities and Spatial Scale: Implications for Designing Catchment-Level Monitoring Programmes. Freshwat. Biol. 2007, 52, 939–958. [Google Scholar] [CrossRef]
- Terra, B.d.F.; Hughes, R.M.; Araújo, F.G. Fish Assemblages in Atlantic Forest Streams: The Relative Influence of Local and Catchment Environments on Taxonomic and Functional Species. Ecol. Freshw. Fish 2016, 25, 527–544. [Google Scholar] [CrossRef]
- Grenouillet, G.; Pont, D.; Harisse, C. Within-Basin Fish Assemblage Structure: The Relative Influence of Habitat versus Stream Spatial Position on Local Species Richness. Can. J. Fish. Aquat. Sci. 2004, 61, 93–102. [Google Scholar] [CrossRef]
- Falke, J.A.; Gido, K.B. Spatial Effects of Reservoirs on Fish Assemblages in Great Plains Streams in Kansas, USA. River Res. Appl. 2006, 22, 55–68. [Google Scholar] [CrossRef]
- Zhao, K.; Song, K.; Pan, Y.; Wang, L.; Da, L.; Wang, Q. Metacommunity Structure of Zooplankton in River Networks: Roles of Environmental and Spatial Factors. Ecol. Indic. 2017, 73, 96–104. [Google Scholar] [CrossRef]
- Karr, J.R. Biological Integrity—A Long-Neglected Aspect of Water- Resource Management. Ecol. Appl. 1991, 1, 66–84. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Ward, J.V.; Stanford, J.A. The Serial Discontinuity Concept of Lotic Ecosystems. In Dynamics of Lotic Ecosystems; Fontaine, T.D., Bartell, S.M., Eds.; Ann Arbor Science: Ann Arbor, MI, USA, 1983; pp. 29–42. [Google Scholar]
- Stanford, J.A.; Ward, J.V. Revisiting the Serial Discontinuity Concept. Regul. Rivers 2001, 17, 303–310. [Google Scholar] [CrossRef]
- Rolls, R.J.; Stewart-Koster, B.; Ellison, T.; Faggotter, S.; Roberts, D.T. Multiple Factors Determine the Effect of Anthropogenic Barriers to Connectivity on Riverine Fish. Biodivers. Conserv. 2014, 23, 2201–2220. [Google Scholar] [CrossRef]
- Cooper, A.R.; Infante, D.M.; Wehrly, K.E.; Wang, L.; Brenden, T.O. Identifying Indicators and Quantifying Large-Scale Effects of Dams on Fishes. Ecol. Indic. 2016, 61, 646–657. [Google Scholar] [CrossRef]
- Fagan, W.F. Connectivity, Fragmentation, and Extinction Risk in Dendritic Metapopulations. Ecology 2002, 83, 3243–3249. [Google Scholar] [CrossRef]
- Rodeles, A.A.; Galicia, D.; Miranda, R. A Simple Method to Assess the Fragmentation of Freshwater Fish Meta-Populations: Implications for River Management and Conservation. Ecol. Indic. 2021, 125, 107557. [Google Scholar] [CrossRef]
- Daniel, W.M.; Infante, D.M.; Hughes, R.M.; Tsang, Y.; Esselman, P.C.; Wieferich, D.J.; Herreman, K.; Cooper, A.R.; Wang, L.; Taylor, W.W. Characterizing Coal and Mineral Mines as a Regional Source of Stress to Stream Fish Assemblages. Ecol. Indic. 2015, 50, 50–61. [Google Scholar] [CrossRef]
- Mandrak, N.E.; Crossman, E.J. Postglacial Dispersal of Freshwater Fishes into Ontario. Can. J. Zool. 1992, 70, 2247–2259. [Google Scholar] [CrossRef]
- Chu, C.; Jones, N.E.; Mandrak, N.E.; Piggott, A.R.; Minns, C.K. The Influence of Air Temperature, Groundwater Discharge, and Climate Change on the Thermal Diversity of Stream Fishes in Southern Ontario Watersheds. Can. J. Fish. Aquat. Sci. 2008, 65, 297–308. [Google Scholar] [CrossRef]
- Raab, D.; Mandrak, N.E.; Ricciardi, A. Low-Head Dams Facilitate Round Goby Neogobius Melanostomus Invasion. Biol. Invasions 2018, 20, 757–776. [Google Scholar] [CrossRef]
- Bunt, C.M.; Cooke, S.J.; Scott McKinley, R. Assessment of the Dunnville Fishway for Passage of Walleyes from Lake Erie to the Grand River, Ontario. J. Great Lakes Res. 2000, 26, 482–488. [Google Scholar] [CrossRef]
- Reid, S.M.; Wilson, C.C.; Mandrak, N.E.; Carl, L.M. Population Structure and Genetic Diversity of Black Redhorse (Moxostoma Duquesnei) in a Highly Fragmented Watershed. Conserv. Genet. 2008, 9, 531–546. [Google Scholar] [CrossRef]
- Carl, L.M.; Esselman, P.C.; Sparks-Jackson, B.L.; Wilson, C.C. The Species–Area Relationship for a Highly Fragmented Temperate River System. Ecosphere 2021, 12, e03411. [Google Scholar] [CrossRef]
- Carl, L.M.; Sparks-Jackson, B.L.; Munn, S.T.; Esselman, P.C.; Wilson, C.C. Habitat and Fish Assemblages along Four River Mainstems in Ontario, Canada, 1997 to 2001, with Supporting Spatial Data. U.S. Geological Survey Data Release, 2021 [Data Set]. Available online: https://www.sciencebase.gov/catalog/item/5afc91e1e4b0da30c1bc20ca (accessed on 13 February 2023).
- Ontario Ministry of Natural Resources. Ontario Ministry of Natural Resources Ontario Integrated Hydrology Data (OIHD), Version 2.0 [Data Set]. 2015. Available online: https://geohub.lio.gov.on.ca/maps/mnrf::ontario-integrated-hydrology-oih-data/about (accessed on 13 February 2023).
- Ontario Ministry of Natural Resources. Ontario Dam Inventory, Version 1 [Data Set]. 2014. Available online: https://geohub.lio.gov.on.ca/datasets/mnrf::ontario-dam-inventory/explore (accessed on 13 February 2023).
- Statistics Canada. Census Road Network File, 2001 [Data Set]. 2001. Available online: https://www12.statcan.gc.ca/census-recensement/2011/geo/RNF-FRR/index-2011-eng.cfm?year=01 (accessed on 13 February 2023).
- Wang, L.; Riseng, C.M.; Mason, L.A.; Wehrly, K.E.; Rutherford, E.S.; McKenna, J.E.; Castiglione, C.; Johnson, L.B.; Infante, D.M.; Sowa, S.; et al. A Spatial Classification and Database for Management, Research, and Policy Making: The Great Lakes Aquatic Habitat Framework. J. Great Lakes Res. 2015, 41, 584–596. [Google Scholar] [CrossRef]
- Ontario Geological Survey. Quaternary Geology, Seamless Coverage of the Province of Ontario: Ontario Geological Survey, Data Set 14 [Data Set]. 1997. Available online: https://data.ontario.ca/dataset/quaternary-geology-of-ontario (accessed on 13 February 2023).
- Ontario Geological Survey. 1:250 000 Scale Bedrock Geology of Ontario Ontario Geological Survey. Miscellaneous Release–Data 126—Revision 1 [Data Set]. 2011. Available online: https://data.ontario.ca/dataset/1250-000-scale-bedrock-geology-of-ontario/resource/8ef6fca2-9fa4-4958-8c6c-a190f639d6a6 (accessed on 13 February 2023).
- Soil Landscapes of Canada Working Group. Soil Landscapes of Canada Version 3.2 [Data Set]. 2011. Available online: https://sis.agr.gc.ca/cansis/nsdb/slc/v3.2/index.html (accessed on 13 February 2023).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Ontario Ministry of Natural Resources and Forestry. Provincial Digital Elevation Model-South [Data set]. 2016. Available online: https://geohub.lio.gov.on.ca/maps/mnrf::provincial-digital-elevation-model-pdem/about (accessed on 13 February 2023).
- Barnucz, J. A Comparative Study of Sampling Efficiency between Boat Electrofishing and Non-Lethal Gillnet/Minnow Trap Sampling Used in Non-Wadeable River Sampling Methodology, Petawawa River, Ontario; Peterborough, ON, Canada, 2002; Unpublished internal Report. [Google Scholar]
- ESRI ArcGIS Desktop: Release 10.4.1; Environmental Systems Research Institute: Redlands, CA, USA, 2015.
- Osborne, L.L.; Wiley, M.J. Influence of Tributary Spatial Position on the Structure of Warmwater Fish Communities. Can. J. Fish. Aquat. Sci. 1992, 49, 671–681. [Google Scholar] [CrossRef]
- Benda, L.; Andras, K.; Miller, D.; Bigelow, P. Confluence Effects in Rivers: Interactions of Basin Scale, Network Geometry, and Disturbance Regimes. Water Resour. Res. 2004, 40, W05402. [Google Scholar] [CrossRef]
- Kiffney, P.M.; Greene, C.M.; Hall, J.E.; Davies, J.R. Tributary Streams Create Spatial Discontinuities in Habitat, Biological Productivity, and Diversity in Mainstem Rivers. Can. J. Fish. Aquat. Sci. 2006, 63, 2518–2530. [Google Scholar] [CrossRef]
- Hitt, N.P.; Angermeier, P.L. Evidence for Fish Dispersal from Spatial Analysis of Stream Network Topology. J. N. Am. Benthol. Soc. 2008, 27, 304–320. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P. All-Scale Spatial Analysis of Ecological Data by Means of Principal Coordinates of Neighbour Matrices. Ecol. Model. 2002, 153, 51–68. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P.; Avois-Jacquet, C.; Tuomisto, H. Dissecting the Spatial Structure of Ecological Data at Multiple Scales. Ecology 2004, 85, 1826–1832. [Google Scholar] [CrossRef]
- Dray, S.; Legendre, P.; Peres-Neto, P.R. Spatial Modelling: A Comprehensive Framework for Principal Coordinate Analysis of Neighbour Matrices (PCNM). Ecol. Model. 2006, 196, 483–493. [Google Scholar] [CrossRef]
- Landeiro, V.L.; Magnuson, W.E.; Melo, A.S.; Espírito-Santo, H.M.V.; Bini, L.M. Spatial Eigenfunction Analyses in Stream Networks: Do Watercourse and Overland Distances Produce Different Results? Freshwat. Biol. 2011, 56, 1184–1192. [Google Scholar] [CrossRef]
- Besag, J.; Clifford, P. Generalized Monte Carlo Significance Tests. Biometrika 1989, 76, 633–642. [Google Scholar] [CrossRef]
- Fortin, M.-J.; Drapeau, P.; Jacquez, G.M. Quantification of the Spatial Co-Occurrences of Ecological Boundaries. Oikos 1996, 77, 51–60. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Legendre, P.; Dray, S.; Borcard, D. Variation Partitioning of Species Data Matrices: Estimation and Comparison of Fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef] [PubMed]
- Eakins, R.J. Ontario Freshwater Fishes Life History Database. Version 5.15. Online Database. 2023. Available online: https://www.ontariofishes.ca (accessed on 13 February 2023).
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the Spatial Component of Ecological Variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P. Environmental Control and Spatial Structure in Ecological Communities: An Example Using Oribatid Mites (Acari, Oribatei). Environ. Ecol. Stat. 1994, 1, 37–61. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 3.5.1; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package Version 2.4-3. 2017. Available online: https://CRAN.R-project.org/package=vegan (accessed on 13 February 2023).
- Effert-Fanta, E.L.; Fischer, R.U.; Wahl, D.H. Effects of Riparian Forest Buffers and Agricultural Land Use on Macroinvertebrate and Fish Community Structure. Hydrobiologia 2019, 841, 45–64. [Google Scholar] [CrossRef]
- Wang, L.; Lyons, J.; Kanehl, P. Impacts of Urban Land Cover on Trout Streams in Wisconsin and Minnesota. Trans. Am. Fish. Soc. 2003, 132, 825–839. [Google Scholar] [CrossRef]
- Allan, J.D.; Erickson, D.L.; Fay, J. The Influence of Catchment Land Use on Stream Integrity across Multiple Spatial Scales. Freshwat. Biol. 1997, 37, 149–161. [Google Scholar] [CrossRef]
- Richards, C.; Johnson, L.B.; Host, G.E. Landscape-Scale Influences on Stream Habitats and Biota. Can. J. Fish. Aquat. Sci. 1996, 53, 295–311. [Google Scholar] [CrossRef]
- Cottenie, K. Integrating Environmental and Spatial Processes in Ecological Community Dynamics. Ecol. Lett. 2005, 8, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Jager, H.I.; Chandler, J.A.; Lepla, K.B.; Van Winkle, W. A Theoretical Study of River Fragmentation by Dams and Its Effects on White Sturgeon Populations. Environ. Biol. Fish. 2001, 60, 347–361. [Google Scholar] [CrossRef]
- Torrente-Vilara, G.; Zuanon, J.; Leprieur, F.; Oberdorff, T.; Tedesco, P.A. Effects of Natural Rapids and Waterfalls on Fish Assemblage Structure in the Madeira River (Amazon Basin). Ecol. Freshw. Fish 2011, 20, 588–597. [Google Scholar] [CrossRef]
- Gibson-Reinemer, D.K.; Rahel, F.J.; Albeke, S.E.; Fitzpatrick, R.M. Natural and Anthropogenic Barriers to Climate Tracking in River Fishes along a Mountain–Plains Transition Zone. Divers. Distrib. 2017, 23, 761–770. [Google Scholar] [CrossRef]
- Reid, S.M.; Mandrak, N.E.; Carl, L.M.; Wilson, C.C. Influence of Dams and Habitat Condition on the Distribution of Redhorse (Moxostoma) Species in the Grand River Watershed, Ontario. Environ. Biol. Fish. 2008, 81, 111–125. [Google Scholar] [CrossRef]
- MacDougall, T.M.; Wilson, C.C.; Richardson, L.M.; Lavender, M.; Ryan, P.A. Walleye in the Grand River, Ontario: An Overview of Rehabilitation Efforts, Their Effectiveness, and Implications for Eastern Lake Erie Fisheries. J. Great Lakes Res. 2007, 33, 103–117. [Google Scholar] [CrossRef]
- Fausch, K.D.; Torgersen, C.E.; Baxter, C.V.; Li, H.W. Landscapes to Riverscapes: Bridging the Gap between Research and Conservation of Stream Fishes. Bioscience 2002, 52, 483–498. [Google Scholar] [CrossRef]
- Wang, L.; Seelbach, P.W.; Lyons, J. Effects of Levels of Human Disturbance on the Influence of Catchment, Riparian, and Reach-Scale Factors on Fish Assemblages. Am. Fish. Soc. Symp. 2006, 48, 199–219. [Google Scholar]
- Lawler, J.J.; Torgersen, C.E. Assessing the Relative Importance of Factors at Multiple Spatial Scales Affecting Terrestrial and Aquatic Wildlife. Curr. Landsc. Ecol. Rep. 2020, 5, 12–24. [Google Scholar] [CrossRef]
- Cooper, S.D.; Diehl, S.; Kratz, K.; Sarnelle, O. Implications of Scale for Patterns and Processes in Stream Ecology. Austral Ecol. 1998, 23, 27–40. [Google Scholar] [CrossRef]
- Paavola, R.; Muotka, T.; Virtanen, R.; Heino, J.; Jackson, D.; Mäki-Petäys, A. Spatial Scale Affects Community Concordance among Fishes, Benthic Macroinvertebrates, and Bryophytes in Streams. Ecol. Appl. 2006, 16, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Heino, J. A Macroecological Perspective of Diversity Patterns in the Freshwater Realm. Freshwat. Biol. 2011, 56, 1703–1722. [Google Scholar] [CrossRef]
- Carpenter, S.R. Microcosm Experiments Have Limited Relevance for Community and Ecosystem Ecology. Ecology 1996, 77, 677–680. [Google Scholar] [CrossRef]
- Schindler, D.W. Whole-Ecosystem Experiments: Replication Versus Realism: The Need for Ecosystem-Scale Experiments. Ecosystems 1998, 1, 323–334. [Google Scholar] [CrossRef]

| Scale | Code | Max# | Description | Source a | GD | GA | T | P |
|---|---|---|---|---|---|---|---|---|
| CAT | CA | 1 | Catchment area (km2) | 1, 11 | X | X | X | X |
| Link | 1 | Shreve link number (count of upstream links) | 1 | X | X | X | X | |
| DamCtDnM | 1 | No. of dams along the downstream mainstem | 1, 2 | X | X b | X | X b | |
| DamCtUp | 1 | No. of dams upstream along all paths | 1, 2 | X | X | X | X | |
| RoadDen | 1 | Total length of road\catchment area (per km) | 3, 11 | X | X | X | X | |
| WSLU_Dev | 1 | Total % of catchment as urban or agriculture (%) | 4, 11 | X | X | X | X | |
| WSLU_For | 1 | Total % of catchment as forest (%) | 4, 11 | X | X | X | X | |
| WSLU_Wet | 1 | Total % catchment as wetland (%) | 4, 11 | X | X | X | X | |
| WSLUC_type | 8 | % of catchment in each land use class (e.g., urban, water, evergreen forest, etc.) (%) | 4, 11 | X | X | X | X | |
| SG_BedR | 1 | Total % catchment as bedrock surficial geology (%) | 5, 11 | X | X b | X | X | |
| SG_Till | 1 | Total % catchment as till surficial geology (%) | 5, 11 | X | X | X | X | |
| SG_Gfluv | 1 | Total % catchment as glaciofluvial deposits surficial geology (%) | 5, 11 | X b | X | X | X | |
| SGC_type | 9 | % of catchment in each surficial geology class (e.g., Maryhill Till, glaciomarine deposits, organic deposits, etc.) (%) | 5, 11 | X | X | X | X | |
| BGC_type | 8 | % of catchment in each bedrock geology class (e.g., gneisses of metasedimentary origin, felsic igneous rocks, etc.) (%) | 6, 11 | X | X b | X | X | |
| SoilDepth | 1 | Area-weighted average of classed depth to bedrock or root restricting layer | 7, 11 | X b | X b | X b | X | |
| SoilAWHC | 1 | Area-weighted average of soil water holding capacity class | 7, 11 | X | X | X b | X | |
| SoilKSAT | 1 | Area-weighted average soil hydraulic conductivity | 7, 11 | X | X | X | X | |
| MaxTemp | 1 | Average maximum temp of the warmest month (°C) | 8, 11 | X | X | X | X | |
| TempVar | 1 | Average temperature seasonality (StDev × 100) (°C) | 8, 11 | X | X | X | X | |
| AnnPrec | 1 | Average annual precipitation (mm) | 8, 11 | X | X | X | X | |
| PrecVar | 1 | Average precipitation seasonality (Coefficient of Variation) | 8, 11 | X | X | X | X | |
| VAL | RipLU_Dev | 1 | Total % of local riparian buffer as urban or agriculture (%) | 4, 11 | X | X | X | X |
| RipLU_For | 1 | Total % of local riparian buffer as forest (%) | 4, 11 | X | X | X | X | |
| RipLU_Wet | 1 | Total % of local riparian buffer as wetland (%) | 4, 11 | X | X | X | X | |
| RipLUC_type | 9 | % of local riparian buffer in each land use class (%) | 4, 11 | X | X | X | X | |
| TribCt | 1 | Total # of tributaries entering mainstem in buffer | 1, 11 | X | X | X | X | |
| MajorTribCt | 1 | Total # of major tributaries entering mainstem in buffer | 1, 11 | X | X | X | X | |
| Sinuos | 1 | Local channel sinuosity in buffer | 1, 11 | X | X | X | X | |
| Slope | 1 | Local channel slope in buffer | 1, 9, 11 | X | X | X | X | |
| MSHabPtch | 1 | Total length of mainstem habitat between dams (km) | 1, 2 | X | X b | X | X b | |
| TotHabPtch | 1 | Total length of all stream habitat between dams (km) | 1, 2 | X | X b | X | X b | |
| ISH | CDepth | 1 | Average depth in channel habitat (m) | 10 | X | X | X | |
| CDepthCV | 1 | Coefficient of variation (CV) of depth in channel habitat (%) | 10 | X | X | X | ||
| SDepth | 1 | Average depth in shoreline habitat (m) | 10 | X | X | X | ||
| SDepthCV | 1 | CV of depth in shoreline habitat (%) | 10 | X | X | X | ||
| Depth | 1 | Average depth (m) | 10 | X | X | X | X | |
| DepthCV | 1 | CV of depth (%) | 10 | X | X | X | X | |
| Width | 1 | Average river width (m) | 10 | X | X | X | X | |
| WidthCV | 1 | CV of river width (%) | 10 | X | X | X | ||
| CVel | 1 | Average surface velocity in channel habitat (m/s) | 10 | X | X | X | ||
| CVelCV | 1 | CV of surface velocity in channel habitat (%) | 10 | X | X | X | ||
| SVel | 1 | Average surface velocity in shoreline habitat (m/s) | 10 | X | X | X | ||
| SVelCV | 1 | CV of surface velocity in shoreline habitat (%) | 10 | X | X | X | ||
| Vel | 1 | Average surface velocity (m/s) | 10 | X | X | X | ||
| VelCV | 1 | CV of surface velocity (%) | 10 | X | X | X | ||
| HydHdAve | 1 | Average hydraulic head (mm) | 10 | X | ||||
| HydHdCV | 1 | CV of hydraulic head (%) | 10 | X | ||||
| CSubs | 1 | Proportion hard substrate in channel habitat | 10 | X | X | |||
| CSubsCV | 1 | CV of proportion hard substrate in channel habitat (%) | 10 | X | X | |||
| SSubs | 1 | Proportion hard substrate in shoreline habitat | 10 | X | X | |||
| SSubsCV | 1 | CV of proportion hard substrate in shoreline habitat (%) | 10 | X | X | |||
| Subs | 1 | Proportion hard substrate | 10 | X | X | |||
| SubsCV | 1 | CV of proportion hard substrate (%) | 10 | X | X | |||
| MNPSAve | 1 | Mean particle size (cm) | 10 | X | ||||
| MNPSCV | 1 | CV of mean particle size (%) | 10 | X | ||||
| MXPSAve | 1 | Maximum particle size (cm) | 10 | X | ||||
| MXPSCV | 1 | CV of max particle size (%) | 10 | X | ||||
| CVegProp | 1 | Proportion of channel habitat with vegetation | 10 | X | ||||
| SVegProp | 1 | Proportion of shoreline habitat with vegetation | 10 | X | ||||
| VegProp | 1 | Proportion of river habitat with vegetation | 10 | X | ||||
| WTemp | 1 | Water temperature (°C) | 10 | X | X | |||
| Cond | 1 | Conductivity (μS/cm) | 10 | X | ||||
| Qcms | 1 | River discharge (cms) | 10 | X |
| Summary Variable | GD | GA | T | P |
|---|---|---|---|---|
| Catchment (CAT): | ||||
| CA (km2) | 5088 (3387, 6645) | 110 (11,269) | 10,040 (7318, 12,552) | 3486 (3164, 4130) |
| Link (#) | 328 (252,434) | 41 (7, 76) | 411 (303, 502) | 274 (261, 289) |
| WSLUC_Urban (%) | 9.1 (8.5, 10.1) | 2.2 (0.8, 3.2) | 2.9 (2.6, 3.2) | 0.1 (0.1, 0.3) |
| WSLUC_Agriculture (%) | 71.8 (70.0, 72.8) | 51.2 (42.9, 59.1) | 26.1 (23.2, 29.0) | 0 (0, 0.1) |
| SG_Gfluv (%) | 0 | 71.4 (46.3, 100) | 7.2 (6.2, 8.1) | 29.9 (28.8, 30.2) |
| DamCtDnM (#) | 3 (1, 5) | 1 | 12 (0, 24) | 0 |
| Valley (VAL): | ||||
| RipLU_Urban (%) | 23 (1.0, 94.8) | 3.4 (0.1, 11.1) | 23.2 (1.0, 95.1) | 3.8 (0.0, 52.8) |
| RipLU_Wet (%) | 8.7 (0.0, 29.2) | 17.0 (7.4, 29.9) | 25.2 (0.0, 86.9) | 0.1 (0.0, 0.3) |
| Slope (%) | 0.1 (0.0, 0.3) | 0.5 (0.1, 1.4) | 0.1 (0.0, 0.5) | 0.1 (0.0, 0.5) |
| MSHabPtch (river km) | 36.8 (15.0, 54.7) | 51.8 | 12.6 (0.8, 62.1) | 72.7 |
| Instream Habitat (ISH): | ||||
| Width (m) | 116 (55, 210) | 8 (3, 16) | 188 (69, 604) | 160 (73, 398) |
| Depth (m) | 1.8 (0.7, 3.6) | 0.3 (0.1, 0.5) | 4.0 (1.7, 6.4) | 4.0 (2, 9.5) |
| Fish community: | ||||
| Species richness (by basin) | 47 | 26 | 38 | 25 |
| Species richness (by site) | 11.0 (2,16) | 10.8 (3, 17) | 13.2 (4, 23) | 9 (6,15) |
| Intolerant species (% by basin) | 13 | 23 | 11 | 13 |
| Tolerant species (% by basin) | 26 | 18 | 27 | 17 |
| Most common fish species | GRED | BTRT | ROCK | SBAS |
| CARP | RAIN | PUNK | PUNK | |
| SBAS | SLIM | SBAS | ROCK | |
| COMM | KING | BLUE | LOGP | |
| GRDH | WSUK | BNOS | BNOS |
| River | Variable Pool | p (F) | %Exp | Adj %Exp | Included Variables (Scale) | Marg % Exp | Cond % Exp |
|---|---|---|---|---|---|---|---|
| GD | ALL | 0.019 (4.0) | 42.1 | 31.6 | Link (CAT) | 9.8 ** | 21.0 ** |
| MSHabPtch (VAL) | 8.3 * | 9.1 | |||||
| SoilAWHC (CAT) | 6.2 | 6.2 | |||||
| CVel (ISH) | 5.8 * | 5.8 * | |||||
| CAT | 0.037 (3.8) | 33.4 | 24.7 | Link | 17.7 ** | 21.0 ** | |
| SoilAWHC | 6.9 | 6.7 | |||||
| WSLU_Dev | 5.7 | 5.7 | |||||
| VAL | 0.019 (3.7) | 33.4 | 24.7 | MSHabPtch | 14.9 ** | 18.1 ** | |
| RipLUC_Emergent Wetland | 8.5 ** | 8.6 * | |||||
| RipLUC_Agriculture | 6.0 * | 6.0 * | |||||
| ISH | 0.019 (2.9) | 19.7 | 13.0 | Vel | 12.8 ** | 14.3 ** | |
| SDepth | 5.4 | 5.4 | |||||
| GA | ALL | 0.033 (9.0) | 71.0 | 63.1 | Link (CAT) | 8.3 * | 39.6 ** |
| RipLU_For (VAL) | 12.7 | 17.4 | |||||
| SoilAWHC (CAT) | 6.0 | 6.0 | |||||
| CAT | 0.033 (6.9) | 65.4 | 55.9 | Link | 18.8 ** | 41.4 ** | |
| MaxTemp | 11.7 | 15.8 | |||||
| SoilAWHC | 8.2 | 8.2 | |||||
| VAL | 0.067 (7.1) | 54.0 | 46.3 | Slope | 33.1 ** | 32.7 ** | |
| RipLUC_Mixed Forest | 21.3 | 21.3 | |||||
| ISH | 0.067 (2.7) | 32.2 | 20.9 | Width | 20.4 | 13.8 ** | |
| HydHdAve | 17.5 * | 17.5* | |||||
| T | ALL | 0.01 (6.3) | 34.1 | 28.7 | SSubs (ISH) | 6.3 *** | 13.9 *** |
| CA (CAT) | 9.7 ** | 9.8 *** | |||||
| WSLUC_Urban (CAT) | 6.5 *** | 6.6 *** | |||||
| SVegProp (ISH) | 3.8 ** | 3.8 ** | |||||
| CAT | 0.01 (6.4) | 20.0 | 16.8 | SoilAWHC | 12.0 ** | 11.0 *** | |
| WSLUC_Mixed Forest | 9.0 * | 9.0 * | |||||
| VAL | 0.04 (5.4) | 17.3 | 14.1 | RipLU_Wet | 10.6 *** | 12.4 ** | |
| MSHabPtch | 5.0 ** | 5.0 | |||||
| ISH | 0.01 (4.6) | 27.2 | 21.2 | SSubs | 6.4 *** | 13.9 *** | |
| SVegProp | 5.7 *** | 5.6 ** | |||||
| SSubsCV | 4.9 ** | 4.9 ** | |||||
| SVel | 2.9 * | 2.9 * | |||||
| P | ALL | 0.07 (2.4) | 41.6 | 24.0 | WSLU_Wet (CAT) | 19.1 | 16.4 ** |
| SSubs (ISH) | 13.9 | 14.3 | |||||
| CDepth (ISH) | 10.8 | 10.8 | |||||
| CAT | 0.002 (2.4) | 30.1 | 17.4 | WSLU_Wet | 15.5 | 16.4 | |
| PrecVar | 13.7 | 13.7 | |||||
| VAL | 0.04 (2.4) | 30.4 | 17.7 | RipLUC_Water | 15.2 | 14.5 | |
| RipLU_Dev | 14.9 * | 11.2 | |||||
| ISH | 0.11 (1.8) | 24.8 | 11.1 | SSubs | 15.3 | 14.4 | |
| DepthCV | 10.4 | 10.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparks-Jackson, B.L.; Esselman, P.C.; Wilson, C.; Carl, L.M. Using Multiscale Environmental and Spatial Analyses to Understand Natural and Anthropogenic Influence on Fish Communities in Four Canadian Rivers. Water 2023, 15, 2213. https://doi.org/10.3390/w15122213
Sparks-Jackson BL, Esselman PC, Wilson C, Carl LM. Using Multiscale Environmental and Spatial Analyses to Understand Natural and Anthropogenic Influence on Fish Communities in Four Canadian Rivers. Water. 2023; 15(12):2213. https://doi.org/10.3390/w15122213
Chicago/Turabian StyleSparks-Jackson, Beth L., Peter C. Esselman, Chris Wilson, and Leon M. Carl. 2023. "Using Multiscale Environmental and Spatial Analyses to Understand Natural and Anthropogenic Influence on Fish Communities in Four Canadian Rivers" Water 15, no. 12: 2213. https://doi.org/10.3390/w15122213
APA StyleSparks-Jackson, B. L., Esselman, P. C., Wilson, C., & Carl, L. M. (2023). Using Multiscale Environmental and Spatial Analyses to Understand Natural and Anthropogenic Influence on Fish Communities in Four Canadian Rivers. Water, 15(12), 2213. https://doi.org/10.3390/w15122213






