Simultaneous Determination of PMS, PDS, and H2O2 Concentrations with Multi-Step Iodometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Procedures
2.3. Analytical Methods
3. Results and Discussion
3.1. Unexpected Low Recovery Rate of H2O2 in Peroxide Mixture
3.2. Elevating the Recovery Rate of H2O2 in Peroxide Mixture
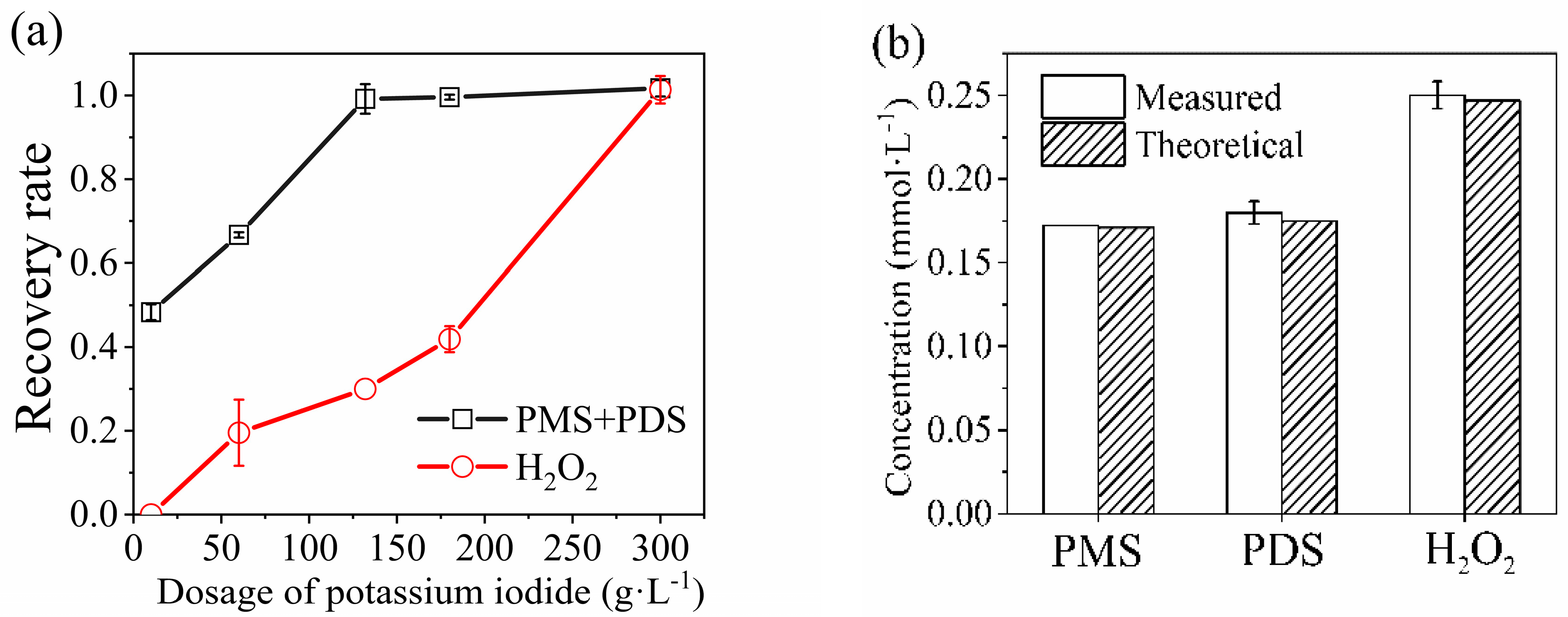
3.2.1. Increasing KI Dosage
3.2.2. Adding Molybdate
3.3. Establishment of Multi-Step Iodometric Titration
3.3.1. Optimization of Operation Parameters in Molybdate–NaHCO3-Buffered Iodometry
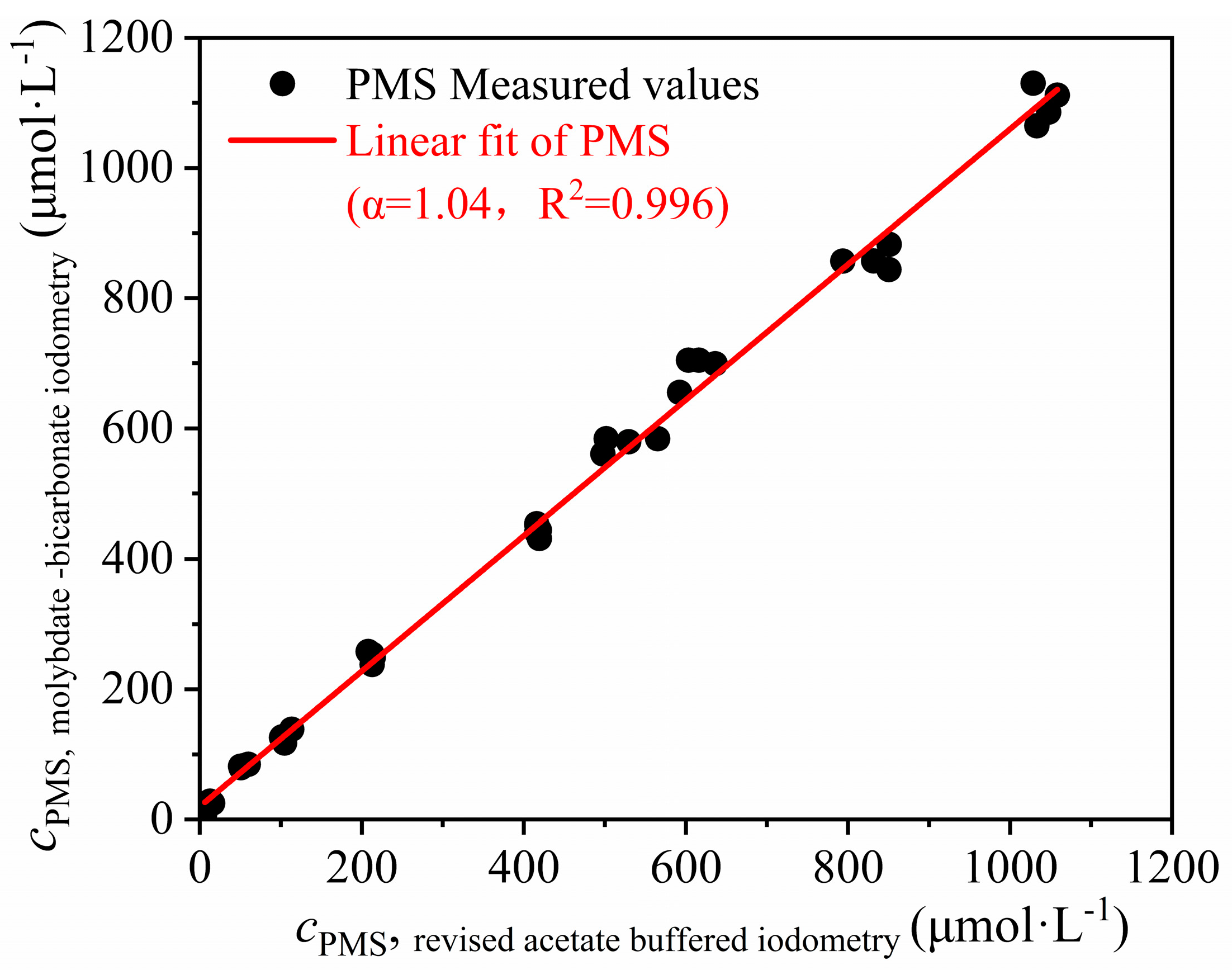
3.3.2. Calibration Coefficient for PMS Concentration Determination with Molybdate–NaHCO3-Buffered Iodometry Referring to Revised Acetate-Buffered Iodometry
3.3.3. Procedure of Multi-Step Iodometric Titration
3.4. Detection Limits and Detection Range of Multi-Step Iodometric Titration
3.5. Influence of Water Matrix on the Determination of Peroxides Coexisting in Sample with Multi-Step Iodometric Titration
3.5.1. Influence of pH
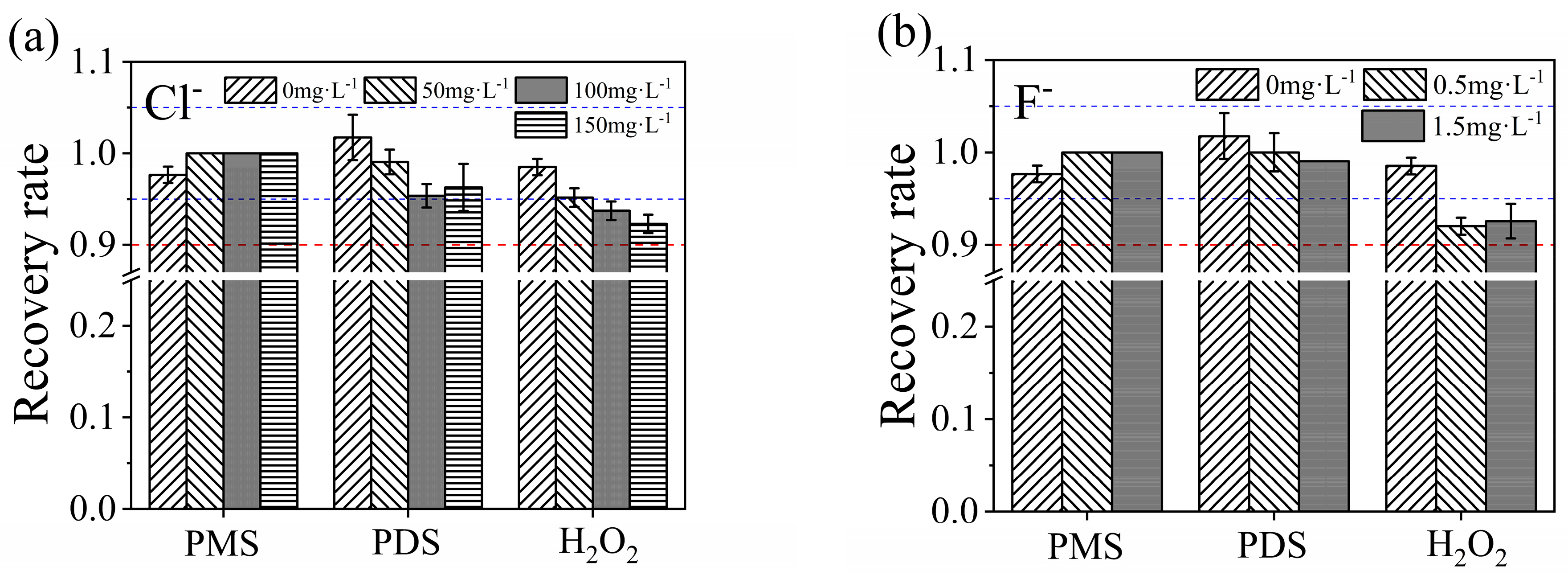
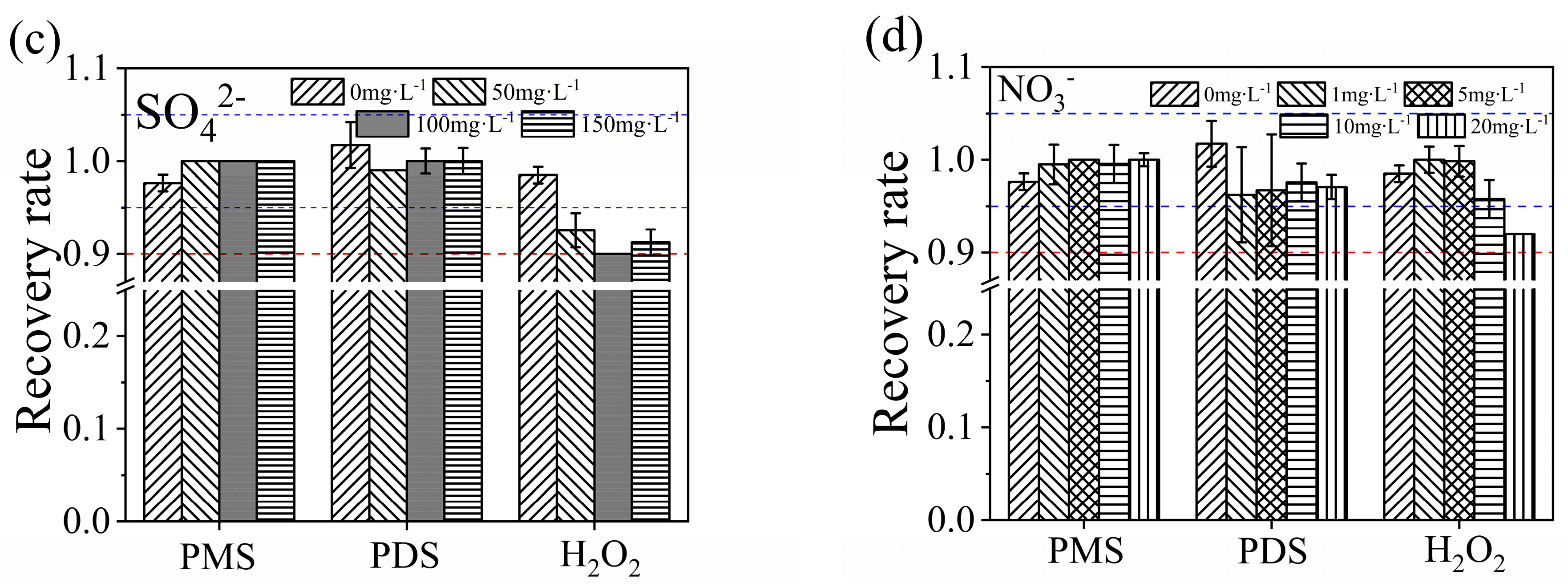
3.5.2. Influences of Anions
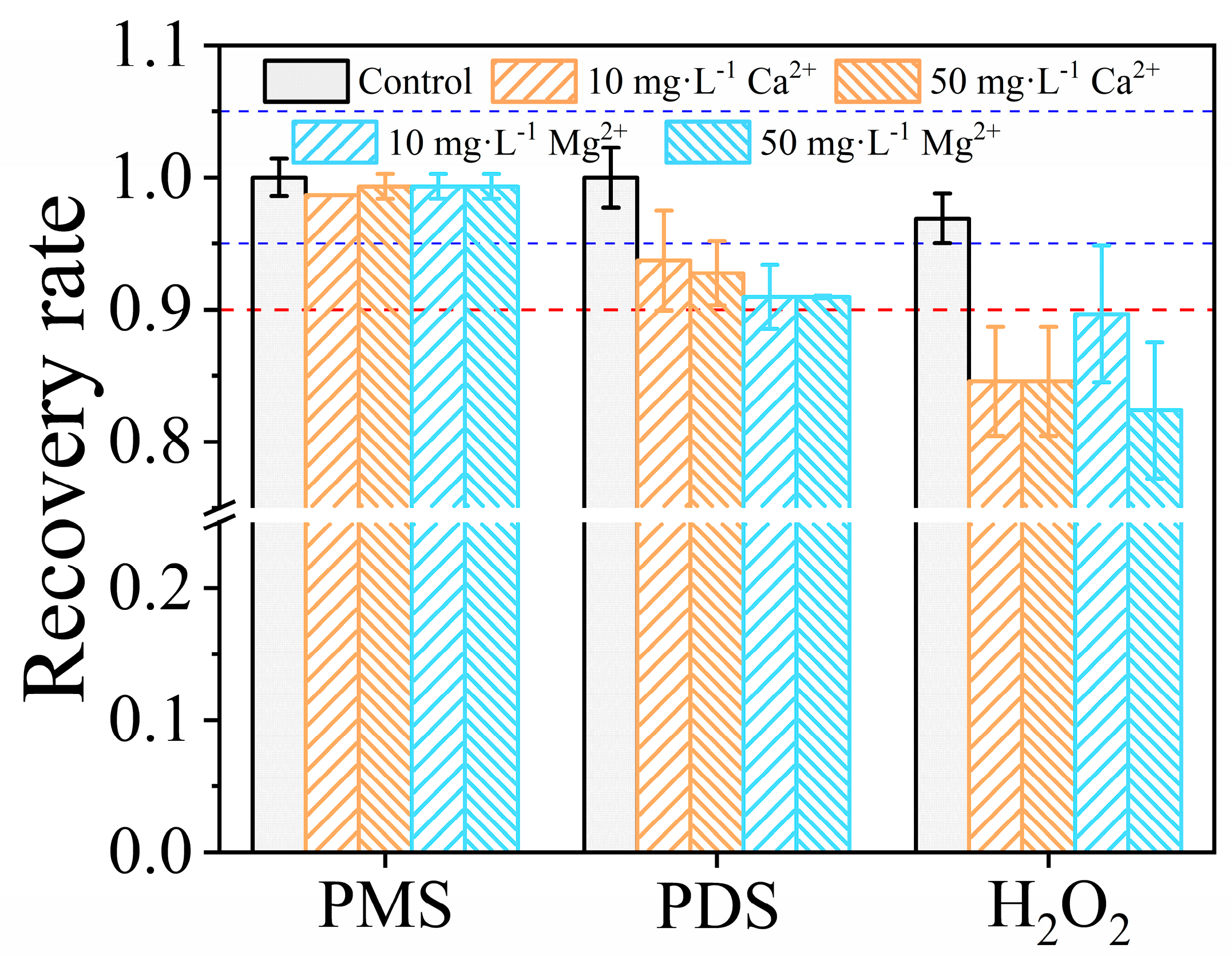
3.5.3. Influences of Ca2+ and Mg2+
3.6. Choice of Buffer
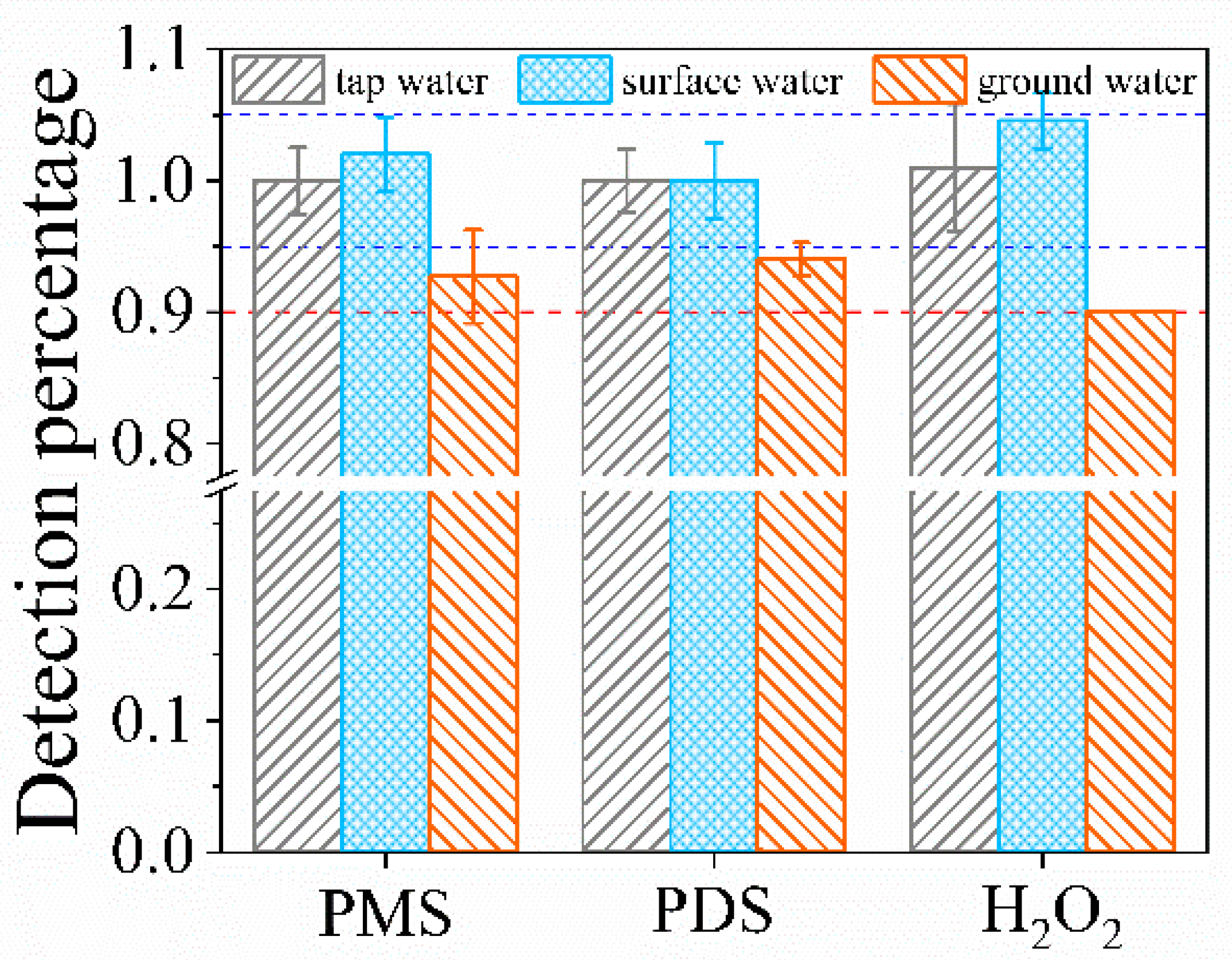
3.7. Applicability in Real Waters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anipsitakis, G.P.; Dionysiou, D.D. Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl. Catal. B. 2004, 54, 155–163. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Guan, Y.-H.; Ma, J.; Li, X.-C.; Fang, J.-Y.; Chen, L.-W. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/Peroxymonosulfate system. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.-H.; Ma, J.; Liu, D.-K.; Ou Z-f Zhang, W.; Gong, X.-L.; Fu, Q.; Crittenden, J.C. Insight into chloride effect on the UV/peroxymonosulfate process. Chem. Eng. J. 2018, 352, 477–489. [Google Scholar] [CrossRef]
- Güpta, Y.K. Iodometric determination of persulphate in sulphuric acid solution. Fresen. J. Anal. Chem. 1961, 180, 260–265. [Google Scholar] [CrossRef]
- Furman, O.S.; Teel, A.L.; Watts, R.J. Mechanism of base activation of persulfate. Environ. Sci. Technol. 2010, 44, 6423–6428. [Google Scholar] [CrossRef]
- Liu, Z.H.; Guo, W.L.; Han, X.M.; Li, X.H.; Zhang, K.; Qiao, Z.M. In situ remediation of ortho-nitrochlorobenzene in soil by dual oxidants (hydrogen peroxide/persulfate). Environ. Sci. Pollut. R. 2016, 23, 19707–19712. [Google Scholar] [CrossRef]
- Yang, Y.; Banerjee, G.; Brudvig, G.W.; Kim, J.H.; Pignatello, J.J. Oxidation of organic compounds in water by unactivated peroxymonosulfate. Environ. Sci. Technol. 2018, 52, 5911–5919. [Google Scholar] [CrossRef]
- Ball, R.E.; Edwards, J.O.; Haggett, M.L.; Jones, P. A kinetic and isotopic study of the decomposition of monoperoxyphthalic acid. J. Am. Chem. Soc. 1967, 89, 2331–2333. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.; Lu, X.; Ma, J.; Liu, Y. Production of sulfate radical and hydroxyl radical by reaction of ozone with peroxymonosulfate: A novel advanced oxidation process. Environ. Sci. Technol. 2015, 49, 7330–7339. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, H.; Croué, J.-P. Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: Efficiency, stability, and mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef]
- Huang, G.X.; Si, J.Y.; Qian, C.; Wang, W.K.; Mei, S.C.; Wang, C.Y.; Yu, H.Q. Ultrasensitive fluorescence detection of peroxymonosulfate based on a sulfate radical-mediated aromatic hydroxylation. Anal. Chem. 2018, 90, 14439–14446. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Huang, C.-F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Klassen, N.V.; Marchington, D.; McGowan, H.C.E. H2O2 determination by the I3− method and by KMnO4 titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Gallard, H.; De Laat, J. Kinetic modelling of Fe(III)/H2O2 oxidation reactions in dilute aqueous solution using atrazine as a model organic compound. Water Res. 2000, 34, 3107–3116. [Google Scholar] [CrossRef]
- Bader, H.; Sturzenegger, V.; Hoigne, J. Photometric method for the determination of low concentrations of hydrogen peroxide by the peroxidase catalyzed oxidation of N,N-diethyl-p-phenylenediamine (DPD). Water Res. 1988, 22, 1109–1115. [Google Scholar] [CrossRef]
- Boudeville, P.E.A. Simultaneous determination of hydrogen peroxide, peroxymonosulfuric acid, and peroxydisulfuric acid by thermometric titrimetry. Anal. Chem. 1983, 55, 612–615. [Google Scholar] [CrossRef]
- Ball, D.L.; Edwards, J.O. The Kinetics and Mechanism of the Decomposition of Caro's Acid. J. Am. Chem. Soc. 1956, 78, 1125–1129. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Washington, DC, USA; Water Environment Federation: Washington, DC, USA, 1999. [Google Scholar]
- Guan, Y.H.; Ma, J.; Ren, Y.M.; Liu, Y.L.; Xiao, J.Y.; Lin, L.Q.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef]
- Ji, Y.; Fan, Y.; Liu, K.; Kong, D.; Lu, J. Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res. 2015, 87, 1–9. [Google Scholar] [CrossRef]
- Li, W.; Orozco, R.; Camargos, N.; Liu, H.Z. Mechanisms on the impacts of alkalinity, pH, and chloride on persulfate-based groundwater remediation. Environ. Sci. Technol. 2017, 51, 3948–3959. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Kurita, S.; Ochiai, A.; Sueki, K.; Utsunomiya, S. Nano-scaled calcium molybdate particle formation on egg phosphatidylcholine liposome surface. Chem. Lett. 2019, 48, 1480–1483. [Google Scholar]
- Ge, F.; Li, Q.; Liu, H.-N.; Wu, Z.-J. Progress in preparation and application of calcium peroxide. Inorganic Chem. Ind. 2010, 42, 1–4. [Google Scholar]
- Crutchik, D.; Garrido, J.M. Struvite crystallization versus amorphous magnesium and calcium phosphate precipitation during the treatment of a saline industrial wastewater. Water Sci. Technol. 2011, 64, 2460–2467. [Google Scholar] [CrossRef]



| H2O2 | PDS | PMS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Scenarios | Theoretical Concentration (μmol∙L−1) | Recovery Rate (%) | Standard Deviation (%) | Theoretical Concentration (μmol∙L−1) | Recovery Rate (%) | Standard Deviation (%) | Theoretical Concentration (μmol∙L−1) | Recovery Rate (%) | Standard Deviation (%) |
| 1 | 13.40 | 97.83 | 3.37 | 10.49 | 102.54 | 4.31 | 10.78 | 100.00 | 0.00 |
| 2 | 17.00 | 97.81 | 5.32 | 13.93 | 101.75 | <0.01 | 14.17 | 99.28 | 0.00 |
| 3 | 20.10 | 104.99 | 4.50 | 15.74 | 101.60 | 5.74 | 16.17 | 98.87 | 0.00 |
| 4 | 28.20 | 100.40 | 3.23 | 20.54 | 103.76 | 4.99 | 21.88 | 101.47 | 2.08 |
| 5 | 70.50 | 103.60 | 1.44 | 51.35 | 100.42 | 2.04 | 54.70 | 98.82 | 1.66 |
| 6 | 137.05 | 99.31 | 1.77 | 101.36 | 98.02 | 1.65 | 103.59 | 102.47 | 1.31 |
| 7 | 252.71 | 97.64 | 0.89 | 184.88 | 101.55 | 1.76 | 162.56 | 100.71 | 1.38 |
| 8 | 320.93 | 98.82 | 1.57 | 255.00 | 101.00 | 1.78 | 253.40 | 100.63 | 0.00 |
| 9 | 718.57 | 99.85 | 0.31 | 545.65 | 105.00 | 0.47 | 503.60 | 99.53 | 0.45 |
| 10 | 507.00 | 99.65 | 0.63 | 52.33 | 103.44 | 4.32 | 47.96 | 99.99 | 0.00 |
| 11 | 55.86 | 97.31 | 5.72 | 52.33 | 96.00 | 6.22 | 527.59 | 100.30 | 0.42 |
| 12 | 55.86 | 103.03 | <0.01 | 549.97 | 98.22 | 0.43 | 47.96 | 96.66 | 4.71 |
| 13 | 0.00 | 0.00 | 0.00 | 52.33 | 97.33 | 4.32 | 47.96 | 99.99 | 0.00 |
| 14 | 55.86 | 100.17 | 4.04 | 52.33 | 97.76 | 0.00 | 0.00 | 0.00 | 0.00 |
| 15 | 55.86 | 97.31 | 4.05 | 0.00 | 0.00 | 0.00 | 47.96 | 96.67 | 4.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhou, Y.; Yang, S.; Jiang, X.; Jiang, X.; Xing, Z.; Guan, Y. Simultaneous Determination of PMS, PDS, and H2O2 Concentrations with Multi-Step Iodometry. Water 2023, 15, 2190. https://doi.org/10.3390/w15122190
Wang M, Zhou Y, Yang S, Jiang X, Jiang X, Xing Z, Guan Y. Simultaneous Determination of PMS, PDS, and H2O2 Concentrations with Multi-Step Iodometry. Water. 2023; 15(12):2190. https://doi.org/10.3390/w15122190
Chicago/Turabian StyleWang, Mingxuan, Yuehan Zhou, Songyu Yang, Xinxin Jiang, Xue Jiang, Zhenxiang Xing, and Yinghong Guan. 2023. "Simultaneous Determination of PMS, PDS, and H2O2 Concentrations with Multi-Step Iodometry" Water 15, no. 12: 2190. https://doi.org/10.3390/w15122190
APA StyleWang, M., Zhou, Y., Yang, S., Jiang, X., Jiang, X., Xing, Z., & Guan, Y. (2023). Simultaneous Determination of PMS, PDS, and H2O2 Concentrations with Multi-Step Iodometry. Water, 15(12), 2190. https://doi.org/10.3390/w15122190










