Prokaryotic and Eukaryotic Communities Characteristic in the Water Column and Sediment along the Xiangjiang River, China
Abstract
1. Introduction
2. Material and Methods
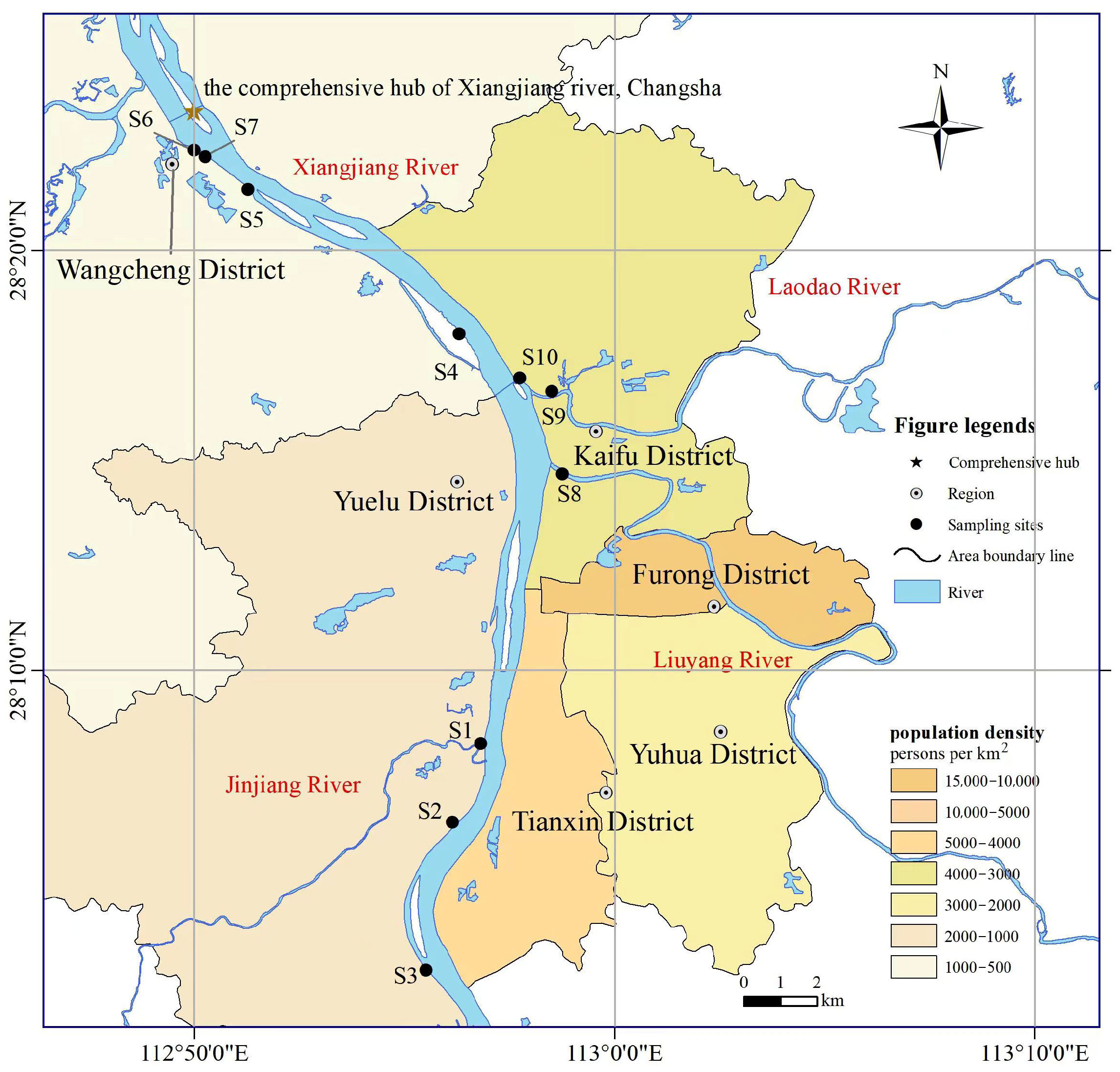
2.1. Study Area and Sampling
2.2. Chemical Analysis
2.3. Biological Analysis
2.3.1. DNA Extraction
2.3.2. Illumina Sequencing of 16S rRNA Gene Amplicons
2.3.3. Data Analysis
3. Results
3.1. Water Quality of Sampling Sites
3.2. 16S rRNA Sequencing
3.3. 18S rRNA Sequencing
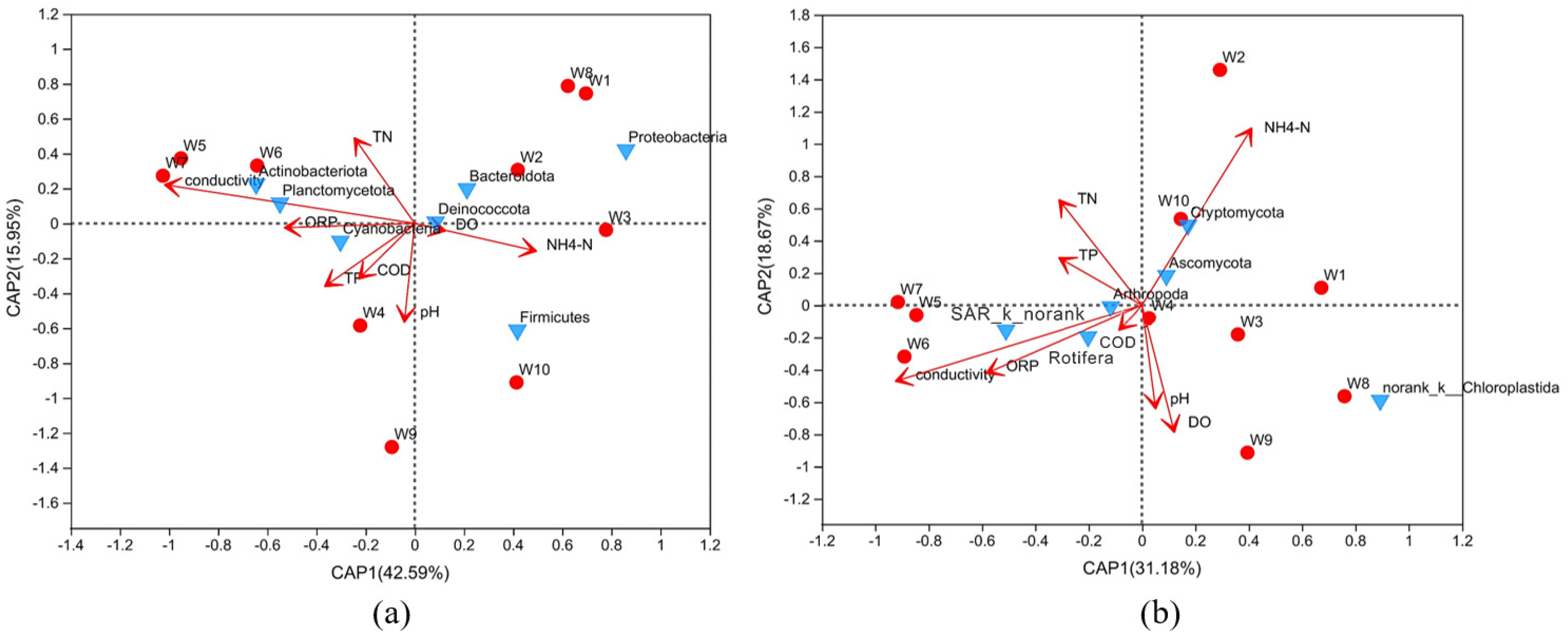
3.4. Redundancy Analysis
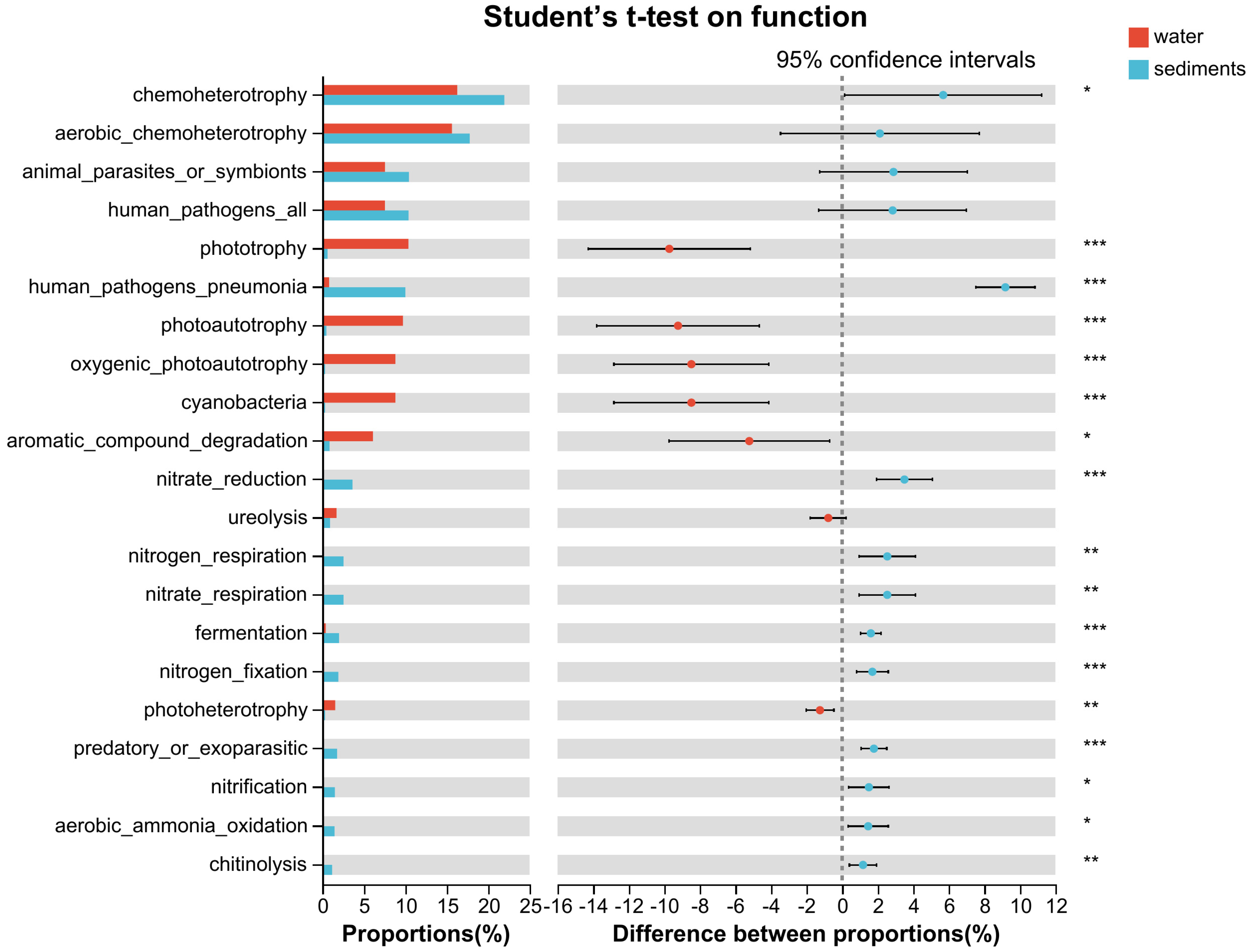
3.5. Functional Annotation
4. Discussion
4.1. Prokaryotic Variations
4.2. Eukaryotic Variations
4.3. Variations of Ecological Function
5. Conclusions
- Conductivity, TN, and NH4+-N were the main environmental parameters influencing the distribution of microbial communities in the XJ River water column.
- River sediments had higher diversity and richness than water samples.
- Proteobacteria, Acidobacteria, and Actinobacteria were the dominant bacteria in sediments. The most abundant taxa in the water samples were Proteobacteria, Actinobacteria, and Firmicutes.
- Eukaryotes in sediments are much spatially stochastic, while in the water column are dominated by Chloroplastida.
- Sediments are more active in the global nitrogen cycle, while the overlying water plays an important role in oxygenic photosynthesis.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, J.; Zhao, C.; Luo, Y.; Liu, C.; Kyzas, G.Z.; Luo, Y.; Zhao, D.; An, S.; Zhu, H. Heavy metals in surface sediments of the Jialu River, China: Their relations to environmental factors. J. Hazard. Mater. 2014, 270, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Kiersztyn, B.; Chróst, R.; Kaliński, T.; Siuda, W.; Bukowska, A.; Kowalczyk, G.; Grabowska, K. Structural and functional microbial diversity along a eutrophication gradient of interconnected lakes undergoing anthropopressure. Sci. Rep. 2019, 9, 11144. [Google Scholar] [CrossRef] [PubMed]
- Savio, D.; Sinclair, L.; Ijaz, U.Z.; Parajka, J.; Reischer, G.H.; Stadler, P.; Blaschke, A.P.; Blöschl, G.; Mach, R.L.; Kirschner, A.K.T.; et al. Bacterial diversity along a 2600 km river continuum. Environ. Microbiol. 2015, 17, 4994–5007. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Dyble, J.; Moisander, P.H.; Noble, R.T.; Piehler, M.F.; Pinckney, J.L.; Steppe, T.F.; Twomey, L.; Valdes, L.M. Microbial indicators of aquatic ecosystem change: Current applications to eutrophication studies. FEMS Microbiol. Ecol. 2003, 46, 233–246. [Google Scholar] [CrossRef]
- Xie, G.; Tang, X.; Shao, K.; Zhu, G.; Gao, G. Bacterial diversity, community composition and metabolic function in Lake Tianmuhu and its dammed river: Effects of domestic wastewater and damming. Ecotoxicol. Environ. Saf. 2021, 213, 112069. [Google Scholar] [CrossRef]
- Newton, R.J.; Jones, S.E.; Eiler, A.; McMahon, K.D.; Bertilsson, S. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev. MMBR 2011, 75, 14–49. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Liu, M.; Li, L.; Zhang, W.; Wang, D.; Ma, T. Bacterial community structure in the surface sediments of different habitats of Baiyangdian Lake, Northern China: Effects of nutrient conditions. J. Soils Sediments 2021, 21, 1866–1874. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, D. Integrated assessment of water quality characteristics and ecological compensation in the Xiangjiang River, south-central China. Ecol. Indic. 2020, 110, 105922. [Google Scholar] [CrossRef]
- Han, B.; Addo, F.G.; Mu, X.; Zhang, L.; Zhang, S.; Lv, X.; Li, X.; Wang, P.; Wang, C. Epiphytic bacterial community shift drives the nutrient cycle during Potamogeton malaianus decomposition. Chemosphere 2019, 236, 124253. [Google Scholar] [CrossRef]
- Chen, M.; Chen, F.; Zhao, B.; Wu, Q.L.; Kong, F. Seasonal variation of microbial eukaryotic community composition in the large, shallow, subtropical Taihu Lake, China. Aquat. Ecol. 2010, 44, 1–12. [Google Scholar] [CrossRef]
- Lim, E.L.; Dennett, M.R.; Caron, D.A. Identification of Heterotrophic Nanoflagellates by Restriction Fragment Length Polymorphism Analysis of Small Subunit Ribosomal DNA. J. Eukaryot. Microbiol. 2001, 48, 247–257. [Google Scholar] [CrossRef]
- Li, K.; Hu, J.; Li, T.; Liu, F.; Tao, J.; Liu, J.; Zhang, Z.; Luo, X.; Li, L.; Deng, Y.; et al. Microbial abundance and diversity investigations along rivers: Current knowledge and future directions. WIREs Water 2021, 8, e1547. [Google Scholar] [CrossRef]
- Lepère, C.; Domaizon, I.; Humbert, J.-F.; Jardillier, L.; Hugoni, M.; Debroas, D. Diversity, spatial distribution and activity of fungi in freshwater ecosystems. PeerJ 2019, 7, e6247. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, C.; Liu, M.; Zhao, W.; Li, Y.; Meng, X.-Z.; Cai, M. Occurrence of organophosphate esters in surface water and sediment in drinking water source of Xiangjiang River, China. Sci. Total. Environ. 2021, 781, 146734. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Bråte, J.; Logares, R.; Berney, C.; Ree, D.K.; Klaveness, D.; Jakobsen, K.S.; Shalchian-Tabrizi, K. Freshwater Perkinsea and marine-freshwater colonizations revealed by pyrosequencing and phylogeny of environmental rDNA. ISME J. 2010, 4, 1144–1153. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Huang, R.; Zhang, W.; He, W.; Deng, Z.; Han, Y.; Xiao, B.; Luo, H.; Qu, W. Effects of temperature and total solid content on biohydrogen production from dark fermentation of rice straw: Performance and microbial community characteristics. Chemosphere 2022, 286, 131655. [Google Scholar] [CrossRef]
- Davis, K.E.; Sangwan, P.; Janssen, P.H. Acidobacteria, Rubrobacteridae and Chloroflexi are abundant among very slow-growing and mini-colony-forming soil bacteria. Environ. Microbiol. 2011, 13, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Betiku, O.C.; Sarjeant, K.C.; Ngatia, L.W.; Aghimien, M.O.; Odewumi, C.O.; Latinwo, L.M. Evaluation of microbial diversity of three recreational water bodies using 16S rRNA metagenomic approach. Sci. Total Environ. 2021, 771, 144773. [Google Scholar] [CrossRef] [PubMed]
- Korajkic, A.; Parfrey, L.W.; McMinn, B.R.; Baeza, Y.V.; VanTeuren, W.; Knight, R.; Shanks, O.C. Changes in bacterial and eukaryotic communities during sewage decomposition in Mississippi river water. Water Res. 2015, 69, 30–39. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, M.; Zhang, W.; Ni, Z.; Hashidoko, Y.; Shen, W. Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment. Sci. Total Environ. 2018, 624, 407–415. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Song, S.; Zhou, Y.; Zhang, J. Bacterial diversity patterns differ in different patch types of mixed forests in the upstream area of the Yangtze River Basin. Appl. Soil Ecol. 2021, 161, 103868. [Google Scholar] [CrossRef]
- Yergeau, E.; Bokhorst, S.; Kang, S.; Zhou, J.; Greer, C.W.; Aerts, R.; Kowalchuk, G.A. Shifts in soil microorganisms in response to warming are consistent across a range of Antarctic environments. ISME J. 2012, 6, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.M.; Singleton, C.; Clegg, L.A.; Petriglieri, F.; Nielsen, P.H. High Diversity and Functional Potential of Undescribed “Acidobacteriota” in Danish Wastewater Treatment Plants. Front. Microbiol. 2021, 12, 643950. [Google Scholar] [CrossRef]
- Koblížek, M. Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol. Rev. 2015, 39, 854–870. [Google Scholar] [CrossRef]
- Zhang, S.; Merino, N.; Wang, N.; Ruan, T.; Lu, X. Impact of 6:2 fluorotelomer alcohol aerobic biotransformation on a sediment microbial community. Sci. Total. Environ. 2017, 575, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Sutton, N.B.; Maphosa, F.; Morillo, J.A.; Abu Al-Soud, W.; Langenhoff, A.A.M.; Grotenhuis, T.; Rijnaarts, H.H.M.; Smidt, H. Impact of Long-Term Diesel Contamination on Soil Microbial Community Structure. Appl. Environ. Microbiol. 2013, 79, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Kasana, R.C.; Pandey, C.B. Exiguobacterium: An overview of a versatile genus with potential in industry and agriculture. Crit. Rev. Biotechnol. 2018, 38, 141–156. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, M.; Li, X.; Lu, W.; Li, J. River bacterial community structure and co-occurrence patterns under the influence of different domestic sewage types. J. Environ. Manag. 2020, 266, 110590. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Yuan, Y.; Liu, D.; Zhu, C.; Zheng, D.; Li, G.; Wei, Y.; Sun, D. Different response of bacterial community to the changes of nutrients and pollutants in sediments from an urban river network. Front. Environ. Sci. Eng. 2020, 14, 28. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, Y.; Huang, X.; Ni, P.; Wu, Y.; Deng, Y.; Zhan, A. Adaptive shifts of bacterioplankton communities in response to nitrogen enrichment in a highly polluted river. Environ. Pollut. 2019, 245, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.G.; Lissemore, L.; Sibley, P.K.; Solomon, K.R. Effects of Monensin on Zooplankton Communities in Aquatic Microcosms. Environ. Sci. Technol. 2007, 41, 6620–6626. [Google Scholar] [CrossRef] [PubMed]



| Sampling Site | Conductivity | ORP (mV) | DO (mg/L) | pH | TP (mg/L) | NH4+-N (mg/L) | TN (mg/L) | CODcr (mg/L) |
|---|---|---|---|---|---|---|---|---|
| #1 | 244 | 205 | 6.45 | 7.43 | 1.32 | 0.86 | 0.67 | 16.16 |
| #2 | 248 | 265 | 7.42 | 7.55 | 0.48 | 0.39 | 1.13 | 17.15 |
| #3 | 251 | 261 | 6.45 | 7.77 | 0.52 | 0.30 | 1.09 | 1.97 |
| #4 | 232 | 231 | 6.8 | 7.38 | 0.44 | 0.76 | 0.90 | 11.43 |
| #5 | 215 | 234 | 6.7 | 7.91 | 0.66 | 0.44 | 0.74 | 10.64 |
| #6 | 224 | 235 | 6.71 | 7.95 | 0.82 | 1.07 | 0.85 | 5.32 |
| #7 | 224 | 235 | 7.47 | 7.95 | 0.68 | 0.43 | 1.00 | 7.09 |
| #8 | 190.5 | 215 | 5.81 | 7.02 | 0.76 | 1.36 | 1.13 | 12.22 |
| #9 | 172.3 | 216 | 6.95 | 7.48 | 0.72 | 0.71 | 0.63 | 10.64 |
| #10 | 237 | 236 | 7.6 | 7.42 | 1.22 | 0.91 | 1.00 | 49.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zhao, W.; Liu, M.; Gao, F.; Chen, H. Prokaryotic and Eukaryotic Communities Characteristic in the Water Column and Sediment along the Xiangjiang River, China. Water 2023, 15, 2189. https://doi.org/10.3390/w15122189
Wu S, Zhao W, Liu M, Gao F, Chen H. Prokaryotic and Eukaryotic Communities Characteristic in the Water Column and Sediment along the Xiangjiang River, China. Water. 2023; 15(12):2189. https://doi.org/10.3390/w15122189
Chicago/Turabian StyleWu, Sha, Wenyu Zhao, Mengyue Liu, Fei Gao, and Hong Chen. 2023. "Prokaryotic and Eukaryotic Communities Characteristic in the Water Column and Sediment along the Xiangjiang River, China" Water 15, no. 12: 2189. https://doi.org/10.3390/w15122189
APA StyleWu, S., Zhao, W., Liu, M., Gao, F., & Chen, H. (2023). Prokaryotic and Eukaryotic Communities Characteristic in the Water Column and Sediment along the Xiangjiang River, China. Water, 15(12), 2189. https://doi.org/10.3390/w15122189






