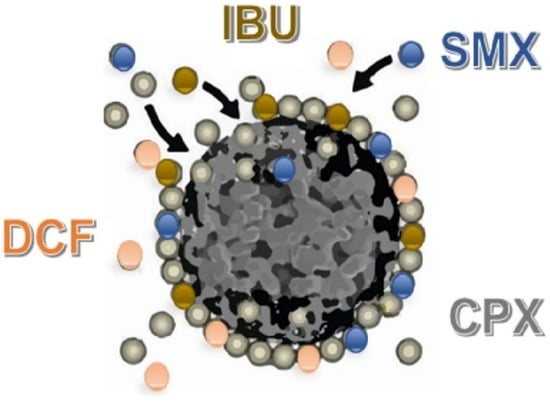
Competitive Adsorption of Drugs from a Multi-Component Mixture on Sugarcane Bagasse
Abstract
1. Introduction
Theory
2. Materials and Methods
2.1. Adsorbent—Sugarcane Bagasse
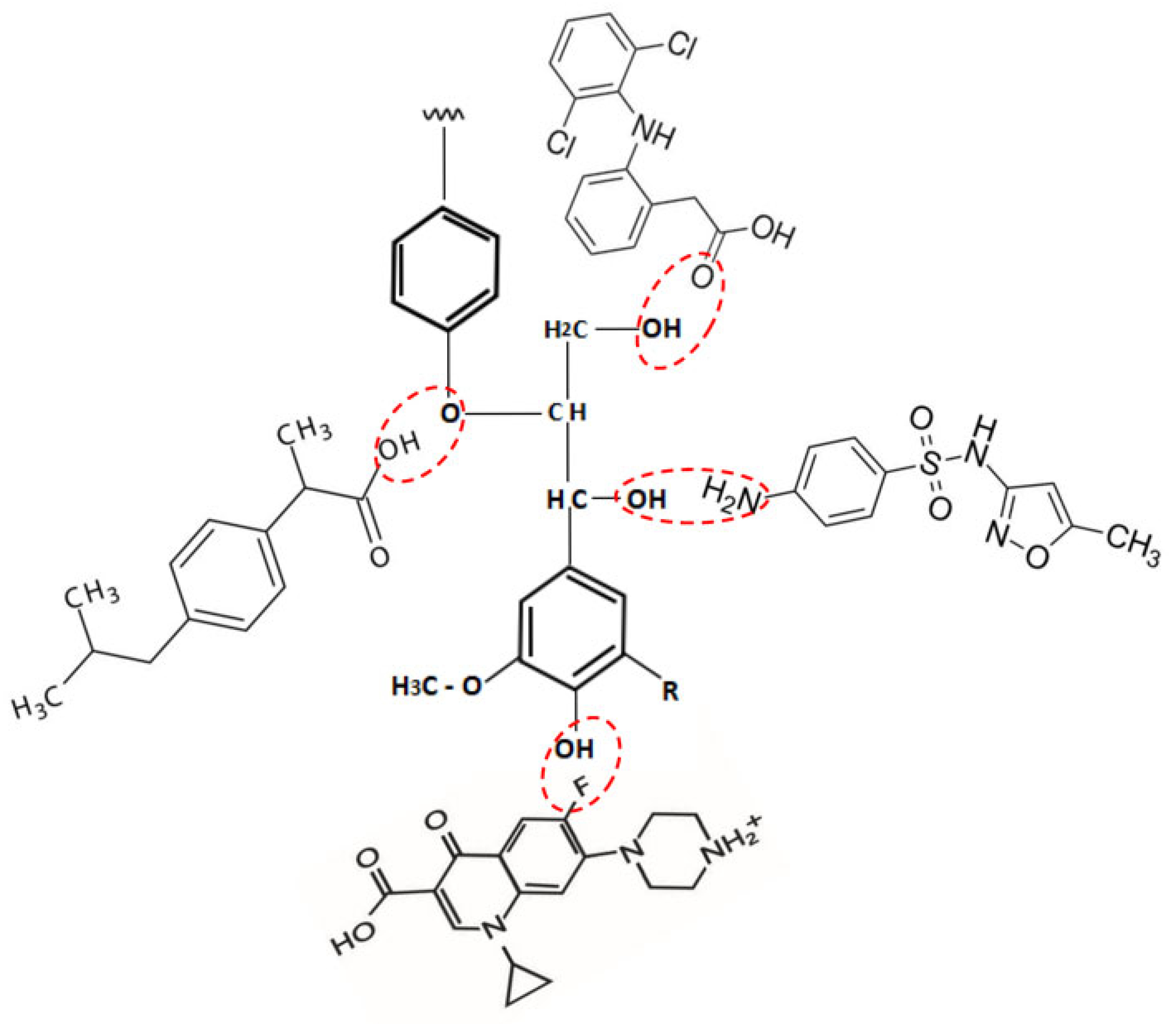
2.2. Adsorbates—Drugs
2.3. Adsorption Experiments for Single- and Multi-Component Drug Solutions
2.4. Characterization of Adsorbents
2.5. Statistics Analysis
3. Results
3.1. Characterization of Sugarcane Bagasse
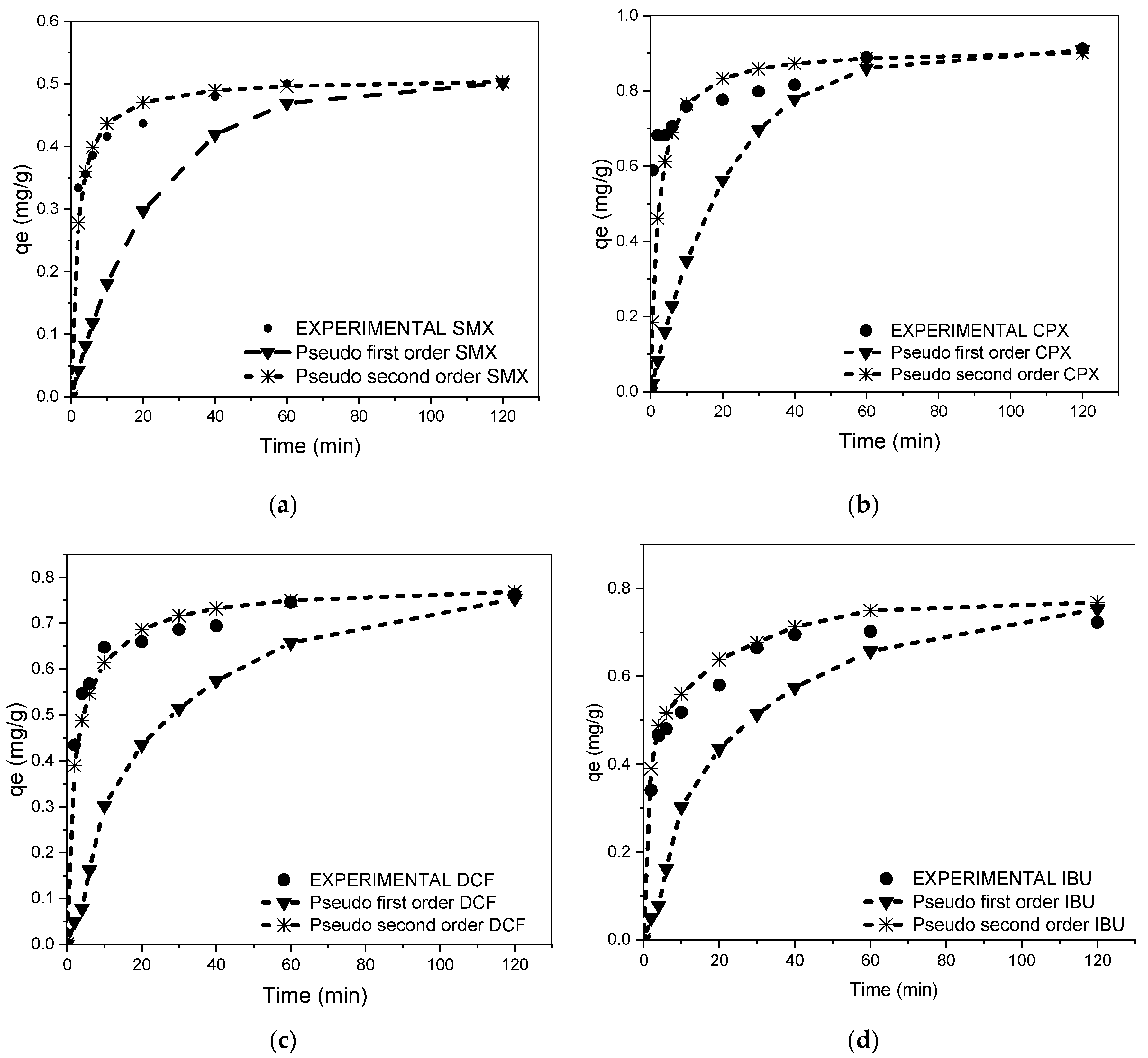
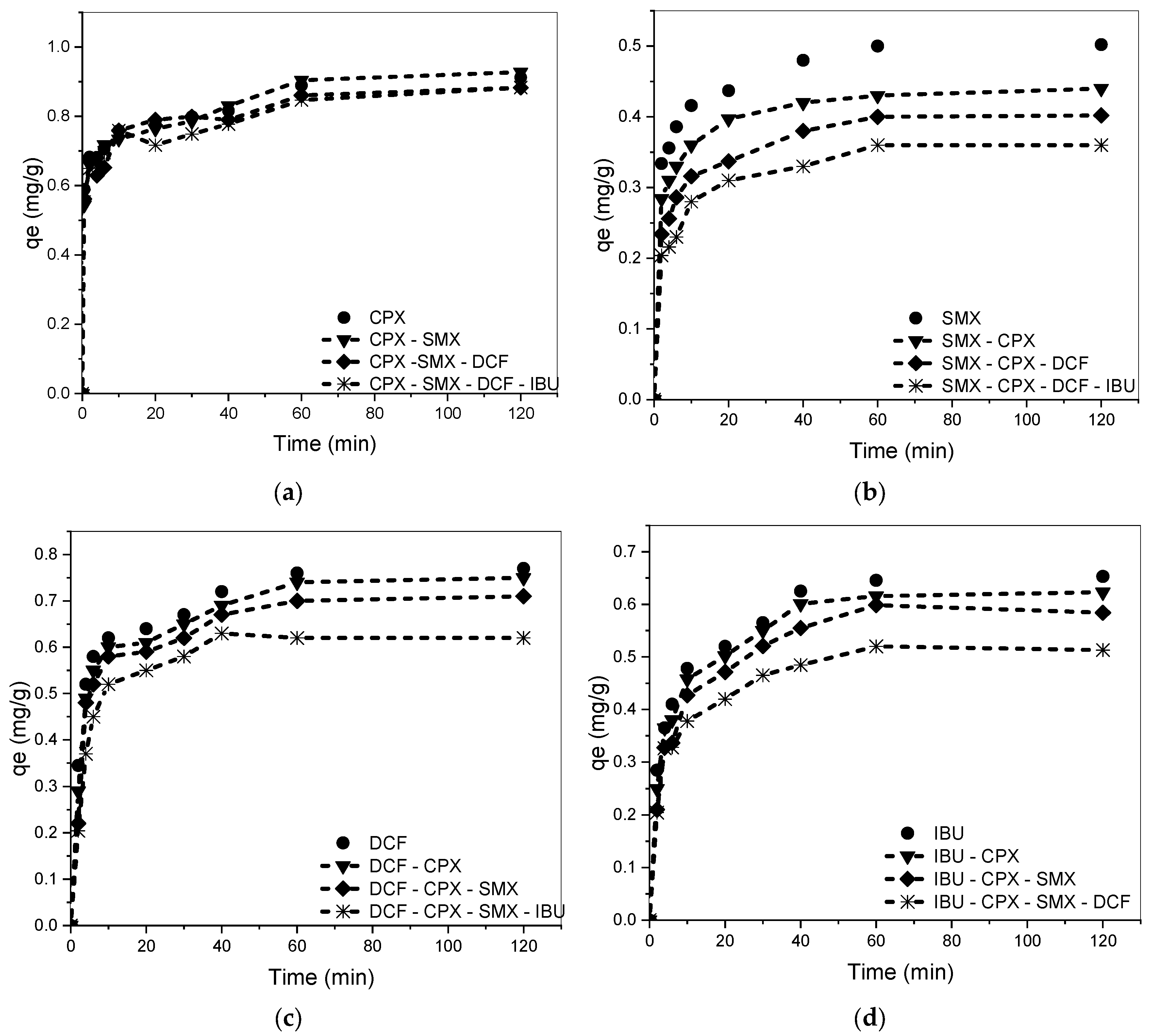
3.2. Kinetic Studies for Single Components
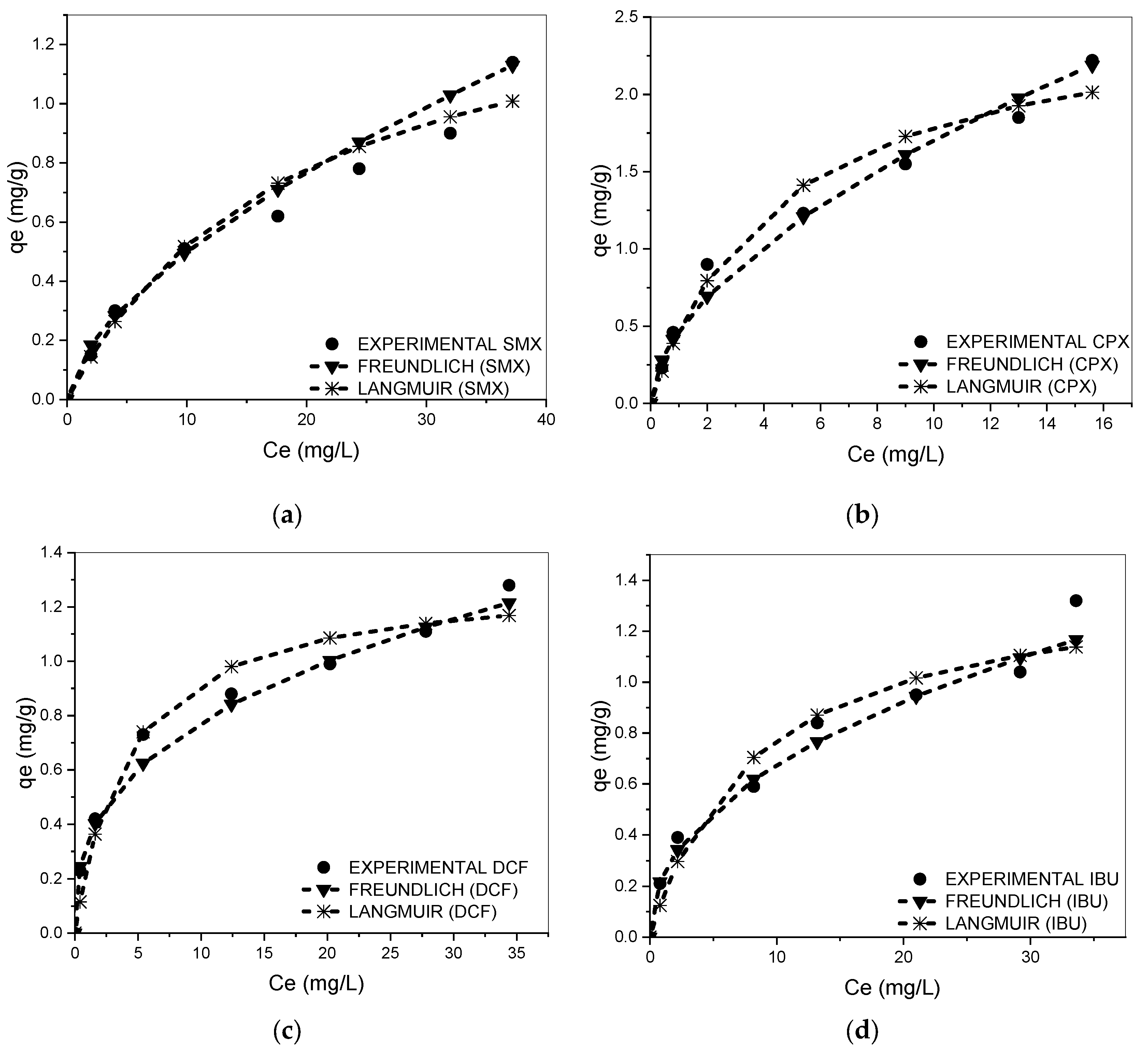
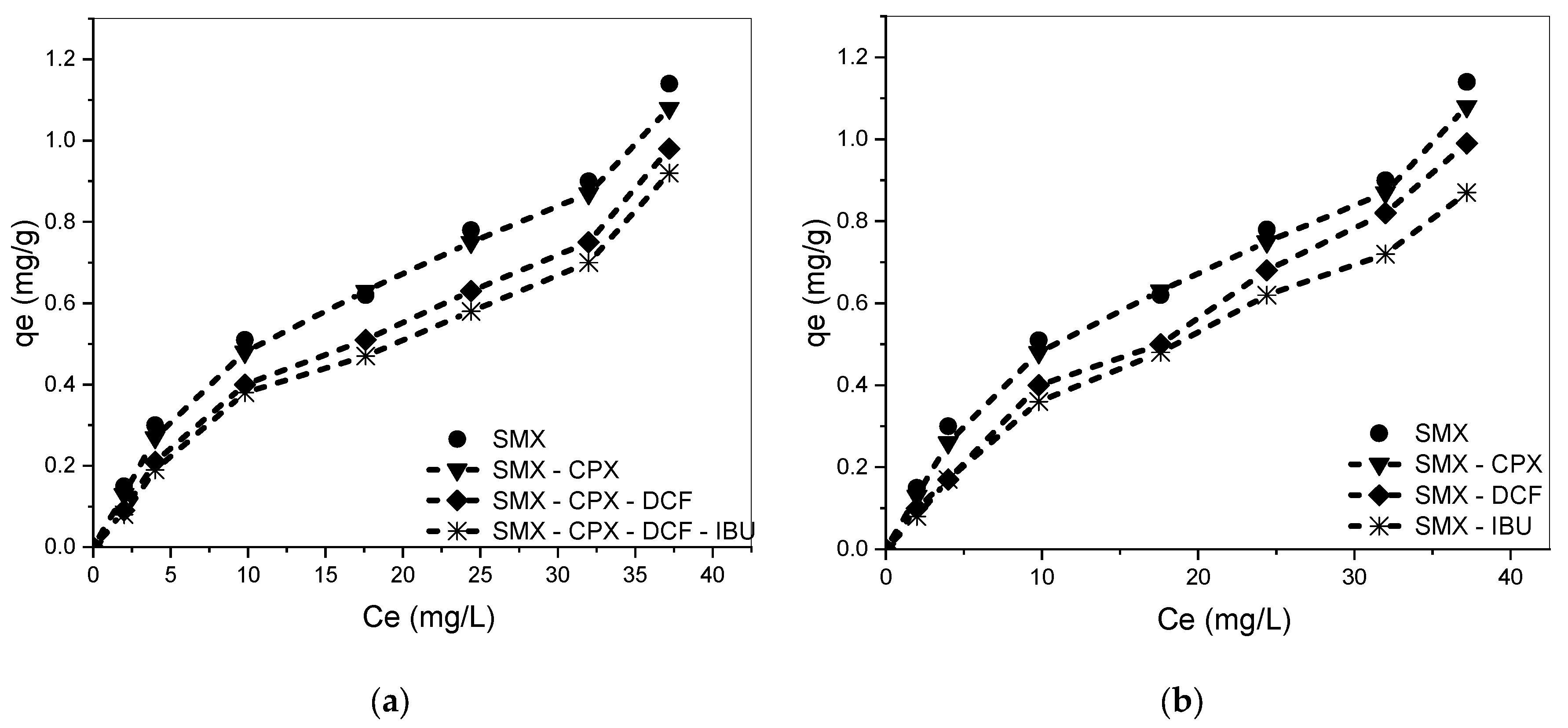
3.3. Equilibrium Adsorption Isotherm for Single Solutes
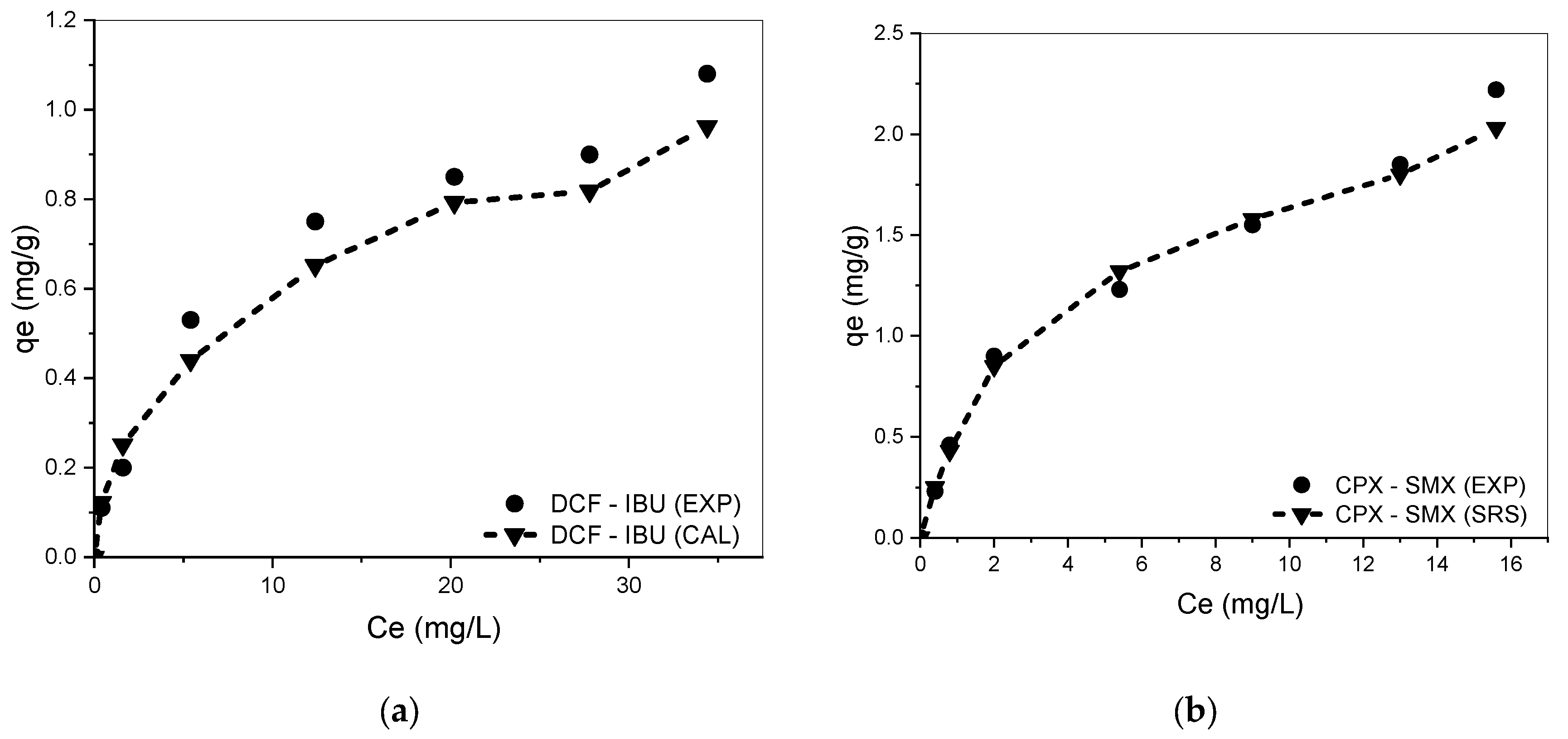
3.4. Competitive Adsorption in Binary, Ternary, and Quaternary Systems
3.5. Influence of the Amount of Each Drug
3.6. Espectro FTIR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M. Emerging contaminants of high concern and their enzyme-assisted biodegradation—A review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial pharmaceuticals in the aquatic environment-occurrence and environmental implications. Eur. J. Pharmacol. 2020, 866, 172813. [Google Scholar] [CrossRef] [PubMed]
- Kutuzova, A.; Dontsova, T.; Kwapinski, W. Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin. Catalysts 2021, 11, 728. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions—A review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [PubMed]
- Kaya, G.G.; Aznar, E.; Deveci, H.; Martínez-Máñez, R. Low-cost silica xerogels as potential adsorbents for ciprofloxacin removal. Sustain. Chem. Pharm. 2021, 22, 100483. [Google Scholar]
- Ramírez-Malule, H.; Quiñones-Murillo, D.H.; Manotas-Duque, D. Emerging contaminants as global environmental hazards. A bibliometric analysis. Emerg. Contam. 2020, 6, 179–193. [Google Scholar] [CrossRef]
- Silva, C.P.; Jaria, G.; Otero, M.; Esteves, V.I.; Calisto, V. Waste-based alternative adsorbents for the remediation of pharmaceutical contaminated waters: Has a step forward already been taken? Bioresour. Technol. 2018, 250, 888–901. [Google Scholar] [CrossRef]
- Peñafiel, M.E.; Matesanz, J.M.; Vanegas, E.; Bermejo, D.; Ormad, M.P. Corncobs as a potentially low-cost biosorbent for sulfamethoxazole removal from aqueous solution. Sep. Sci. Technol. 2020, 55, 3060–3071. [Google Scholar] [CrossRef]
- Peñafiel, M.E.; Matesanz, J.M.; Vanegas, E.; Bermejo, D.; Mosteo, R.; Ormad, M.P. Comparative adsorption of ciprofloxacin on sugarcane bagasse from Ecuador and on commercial powdered activated carbon. Sci. Total Environ. 2021, 750, 141498. [Google Scholar] [CrossRef]
- Fan, X.; Deng, L.; Li, K.; Lu, H.; Wang, R.; Li, W. Adsorption of malachite green in aqueous solution using sugarcane bagasse-barium carbonate composite. Colloid Interface Sci. Commun. 2021, 44, 100485. [Google Scholar] [CrossRef]
- Sharma, B.M.; Bečanová, J.; Scheringer, M.; Sharma, A.; Bharat, G.K.; Whitehead, P.G.; Nizzetto, L. Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India. Sci. Total Environ. 2019, 646, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Sharma, N. Removal of pharmaceutical drugs from binary mixtures by use of ZnO nanoparticles:(Competitive adsorption of drugs). Environ. Technol. Innov. 2019, 15, 100392. [Google Scholar] [CrossRef]
- Jung, C.; Boateng, L.K.; Flora, J.R.; Oh, J.; Braswell, M.C.; Son, A.; Yoon, Y. Competitive adsorption of selected non-steroidal anti-inflammatory drugs on activated biochars: Experimental and molecular modeling study. Chem. Eng. J. 2015, 264, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Wu, X.; Li, M.; Liu, X. Competitive adsorption of tylosin, sulfamethoxazole and Cu (II) on nano-hydroxyapatitemodified biochar in water. Chemosphere 2020, 240, 124884. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kuang, Y.; Chen, J.; Wu, D. Competitive adsorption of phosphate and dissolved organic carbon on lanthanum modified zeolite. J. Colloid Interface Sci. 2020, 574, 197–206. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef] [PubMed]
- Khamash, D.F.; Voskertchian, A.; Tamma, P.D.; Akinboyo, I.C.; Carroll, K.C.; Milstone, A.M. Increasing clindamycin and trimethoprim-sulfamethoxazole resistance in pediatric Staphylococcus aureus infections. J. Pediatr. Infect. Dis. Soc. 2019, 8, 351–353. [Google Scholar] [CrossRef]
- Serwecińska, L. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and to public health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Brown, J.N.; Paxéus, N.; Förlin, L.; Larsson, D.J. Variations in bioconcentration of human pharmaceuticals from sewage effluents into fish blood plasma. Environ. Toxicol. Pharmacol. 2007, 24, 267–274. [Google Scholar] [CrossRef]
- Schwaiger, J.; Ferling, H.; Mallow, U.; Wintermayr, H.; Negele, R.D. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part I: Histopathological alterations and bioaccumulation in rainbow trout. Aquat. Toxicol. 2004, 68, 141–150. [Google Scholar] [CrossRef]
- Worch, E. Adsorption equilibrium II: Multisolute adsorption. In Adsorption Technology in Water Treatment; De Gruyter: Berlin, Germany, 2012. [Google Scholar]
- DiGiano, F.A.; Baldauf, G.; Frick, B.; Sontheimer, H. A simplified competitive equilibrium adsorption model. Chem. Eng. Sci. 1978, 33, 1667–1673. [Google Scholar] [CrossRef]
- Sheindorf, C.H.; Rebhun, M.; Sheintuch, M. A Freundlich-type multicomponent isotherm. J. Colloid Interface Sci. 1981, 79, 136–142. [Google Scholar] [CrossRef]
- Gutierrez, M.; Fuentes, H.R. Modeling adsorption in multicomponent systems using a Freundlich-type isotherm. J. Contam. Hydrol. 1993, 14, 247–260. [Google Scholar] [CrossRef]
- Linares, R.V.; Yangali-Quintanilla, V.; Li, Z.; Amy, G. Rejection of micropollutants by clean and fouled forward osmosis membrane. Water Res. 2011, 45, 6737–6744. [Google Scholar] [CrossRef] [PubMed]
- Doğan, E.C. Investigation of ciprofloxacin removal from aqueous solution by nanofiltration process. Glob. NEST J. 2016, 18, 291–308. [Google Scholar]
- Varma, A.K.; Mondal, P. Physicochemical characterization and pyrolysis kinetic study of sugarcane bagasse using thermogravimetric analysis. J. Energy Resour. Technol. 2016, 138, 052205. [Google Scholar] [CrossRef]
- Carrier, M.; Joubert, J.E.; Danje, S.; Hugo, T.; Görgens, J.; Knoetze, J.H. Impact of the lignocellulosic material on fast pyrolysis yields and product quality. Bioresour. Technol. 2013, 150, 129–138. [Google Scholar] [CrossRef]
- Abdolali, A.; Guo, W.S.; Ngo, H.H.; Chen, S.S.; Nguyen, N.C.; Tung, K.L. Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: A critical review. Bioresour. Technol. 2014, 160, 57–66. [Google Scholar] [CrossRef]
- Carrott, P.J.M.; Carrott, M.R. Lignin–from natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar]
- Mansouri, H.; Carmona, R.J.; Gomis-Berenguer, A.; Souissi-Najar, S.; Ouederni, A.; Ania, C.O. Competitive adsorption of ibuprofen and amoxicillin mixtures from aqueous solution on activated carbons. J. Colloid Interface Sci. 2015, 449, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Ovejero, G.; Rodríguez, A.; Álvarez, S.; Galán, J.; García, J. Competitive adsorption studies of caffeine and diclofenac aqueous solutions by activated carbon. Chem. Eng. J. 2014, 240, 443–453. [Google Scholar] [CrossRef]
- Pelekani, C.; Snoeyink, V.L. Competitive adsorption in natural water: Role of activated carbon pore size. Water Res. 1999, 33, 1209–1219. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Seo, P.W.; Jhung, S.H. Adsorption of diclofenac sodium from water using oxidized activated carbon. Chem. Eng. J. 2016, 301, 27–34. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Sun, X.F.; Liu, J.; Song, C.; Wang, S.G.; Javed, A. Enhancement of ciprofloxacin sorption on chitosan/biochar hidrogel beads. Sci. Total Environ. 2018, 639, 560–569. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Fan, X.; Quan, X.; Tan, F.; Zhang, Y.; Gao, J. Adsorption of ciprofloxacin, bisphenol and 2-chlorophenol on electrospun carbon nanofibers: In comparison with powder activated carbon. J. Colloid Interface Sci. 2015, 447, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.H.; Guo, W.; Zhang, J.; Liang, S.; Ton-That, C.; Zhang, X. Typical low-cost biosorbents for adsorptive removal of specific organic pollutants from water. Bioresour. Technol. 2015, 182, 353–363. [Google Scholar]
- Rosales, E.; Meijide, J.; Pazos, M.; Sanromán, M.A. Challenges and recent advances in biochar as low-cost biosorbent: From batch assays to continuous-flow systems. Bioresour. Technol. 2017, 246, 176–192. [Google Scholar] [CrossRef]
- Tonucci, M.C.; Gurgel, L.V.A.; de Aquino, S.F. Activated carbons from agricultural byproducts (pine tree and coconut shell), coal, and carbon nanotubes as adsorbents for removal of sulfamethoxazole from spiked aqueous solutions: Kinetic and thermodynamic studies. Ind. Crops Prod. 2015, 74, 111–121. [Google Scholar] [CrossRef]
- Seader, J.D.; Henley, E.J.; Roper, D.K. Adsorption, Ion Exchange, Chromatography, and Electrophoresis. In Separation Process Principles, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1998; Volume 25, pp. 568–648. [Google Scholar]
- Vijayaraghavan, K.; Balasubramanian, R. Single and binary biosorption of cerium and europium onto crab shell particles. Chem. Eng. J. 2010, 163, 337–343. [Google Scholar] [CrossRef]
- Wu, C.H.; Kuo, C.Y.; Lin, C.F.; Lo, S.L. Modeling competitive adsorption of molybdate, sulfate, selenate, and selenite using a Freundlich-type multi-component isotherm. Chemosphere 2002, 47, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Zermane, F.; Bouras, O.; Baudu, M.; Basly, J.P. Cooperative coadsorption of 4-nitrophenol and basic yellow 28 dye onto an iron organo–inorgano pillared montmorillonite clay. J. Colloid Interface Sci. 2010, 350, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Hamidouche, S.; Bouras, O.; Zermane, F.; Cheknane, B.; Houari, M.; Debord, J.; Baudu, M. Simultaneous sorption of 4-nitrophenol and 2-nitrophenol on a hybrid geocomposite based on surfactant-modified pillared-clay and activated carbon. Chem. Eng. J. 2015, 279, 964–972. [Google Scholar] [CrossRef]



| Drug | Class | Molecular Weight (g·mol−1) | Log Kow | Dissociation Constant (pKa) | Size (nm) | Λmax (nm) |
|---|---|---|---|---|---|---|
| IBU | Analgesic | 206.28 | 3.97 | 4.5 | 1.39 × 0.73 [26] | 222 |
| CPX | Antibiotic | 331.34 | 0.28 | 6.0–8.7 | 0.825 [27] | 271 |
| DCF | Analgesic | 318.10 | 5.71 | 4.2 | 0.97 × 0.96 | 276 |
| SMX | Antibiotic | 253.28 | 0.89 | 1.9–5.7 | 0.56 × 1.03 | 261 |
| Properties | |
|---|---|
| SBET (m2 g −1) | 2.5 |
| Desorption average pore diameter (nm) | 10.6 |
| pHPZC | 6.1 |
| Drug | Pseudo-First-Order Models | ||||
|---|---|---|---|---|---|
| K1 | R2 | MPSD | |||
| SMX | 0.51 (±0.03) | 0.50 (±0.02) | 0.045 | 0.77 | 20.2 |
| CPX | 0.98 (±0.01) | 0.95 (±0.01) | 0.028 | 0.78 | 11.7 |
| DCF | 0.77 (±0.02) | 0.55 (±0.02) | 0.026 | 0.75 | 14.3 |
| IBU | 0.61 (±0.02) | 0.64 (±0.03) | 0.035 | 0.82 | 12.6 |
| Drug | Pseudo-Second-Order Models | ||||
| ) | K2 | R2 | MPSD | ||
| SMX | 0.51 ± (0.02) | 1.17 | 0.99 | 12.2 | |
| CPX | 0.97 ± (0.01) | 0.09 | 0.99 | 6.7 | |
| DCF | 0.78 ± (0.02) | 0.40 | 0.99 | 8.3 | |
| IBU | 0.62 ± (0.03) | 0.98 | 0.98 | 10.6 | |
| Drug | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| qm (SD) | KL | R2 | MPSD | KF | n | R2 | MPSD | |
| (mg·g−1) | (L·mg−1) | (mg/g)(mg/L)−1/n | ||||||
| SMX | 1.43 (±0.08) | 0.05 | 0.90 | 18.5 | 0.12 | 1.53 | 0.98 | 8.5 |
| CPX | 2.61(±0.12) | 0.22 | 0.94 | 7.5 | 1.47 | 1.78 | 0.98 | 5.6 |
| DCF | 1.81(±0.09) | 0.24 | 0.96 | 12.5 | 0.34 | 2.78 | 0.98 | 10.2 |
| IBU | 1.62(±0.09) | 0.12 | 0.93 | 23.2 | 0.24 | 2.22 | 0.98 | 13.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñafiel, M.E.; Flores, D. Competitive Adsorption of Drugs from a Multi-Component Mixture on Sugarcane Bagasse. Water 2023, 15, 2127. https://doi.org/10.3390/w15112127
Peñafiel ME, Flores D. Competitive Adsorption of Drugs from a Multi-Component Mixture on Sugarcane Bagasse. Water. 2023; 15(11):2127. https://doi.org/10.3390/w15112127
Chicago/Turabian StylePeñafiel, Maria E., and Damián Flores. 2023. "Competitive Adsorption of Drugs from a Multi-Component Mixture on Sugarcane Bagasse" Water 15, no. 11: 2127. https://doi.org/10.3390/w15112127
APA StylePeñafiel, M. E., & Flores, D. (2023). Competitive Adsorption of Drugs from a Multi-Component Mixture on Sugarcane Bagasse. Water, 15(11), 2127. https://doi.org/10.3390/w15112127