The Effect of Light on Nitrogen Removal by Microalgae-Bacteria Symbiosis System (MBS)
Abstract
1. Introduction
2. The Effect of Light on Nitrogen-Removal Efficiency in MBS
2.1. Effect of Light Wavelength on Nitrogen-Removal Efficiency of MBS
2.2. Effect of Light Intensity on Nitrogen-Removal Efficiency of MBS
2.3. Effect of Photoperiod on the Efficiency of Nitrogen Removal in MBS
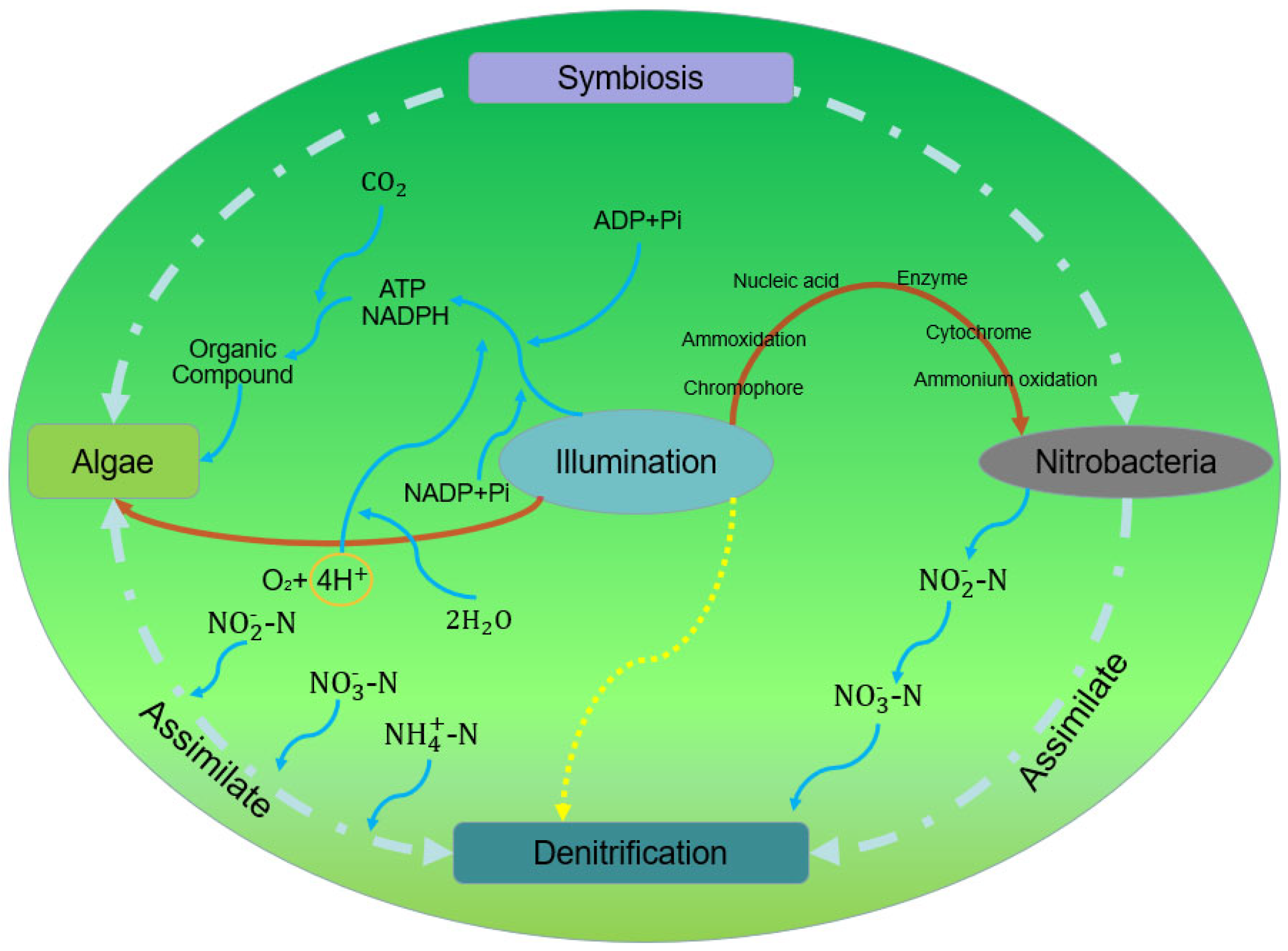
3. The Effect of Light on the Nitrogen-Removal Pathway
4. The Effect of Light on the Mechanism of Nitrogen Removal in the MBS
4.1. Effect of Light on the Physiological Mechanism of Microalgae
4.2. Effect on Nitrogen-Removal Functional Microorganisms
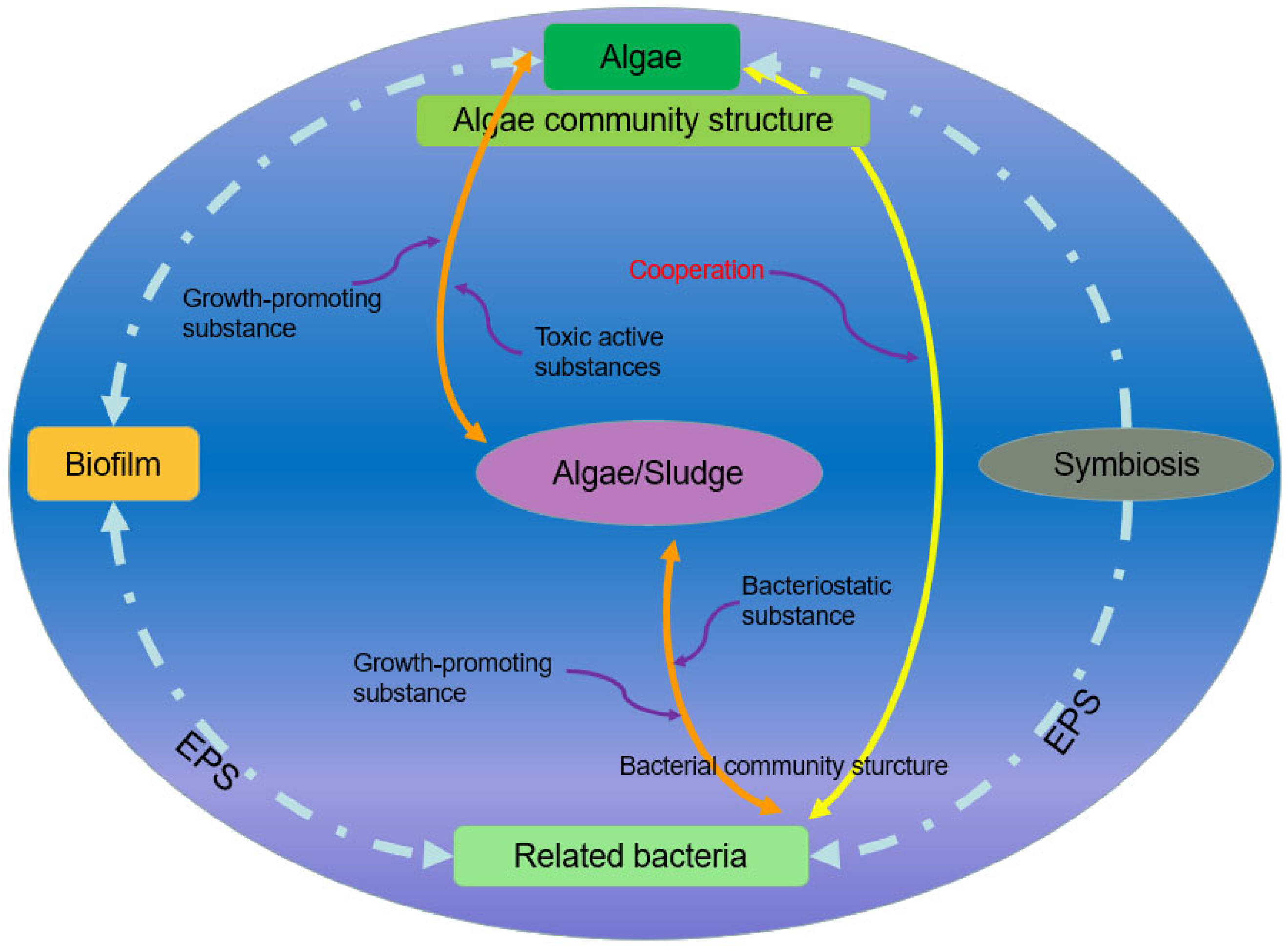
5. The Effect of Light on the Interaction of the MBS
5.1. Extracellular Polymeric Substances
5.2. Physical Effects
6. Summary and Prospects
6.1. Eco-Friendly Lighting Fixtures and Supplementary Lighting Techniques
6.2. Nitrogen-Removal Kinetics of MBS Driven by LED Light
6.3. Interaction Mechanism of MBS under Targeted Light Irradiation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.; Das, P.; Thaher, M.; AbdulQuadir, M.; Mahata, C.; Al Jabri, H. Utilization of nitrogen-rich agricultural waste streams by microalgae for the production of protein and value-added compounds. Curr. Opin. Green Sustain. Chem. 2023, 41, 100797. [Google Scholar] [CrossRef]
- Zhang, L.; Han, J.; Ma, S.; Zhang, Y.; Wang, Y.; Xu, J. Comprehensive evaluation of growth characteristics, nitrogen removal capacity, and nutritional properties of three diet microalgae. Front. Mar. Sci. 2023, 10, 100000. [Google Scholar] [CrossRef]
- Liu, X.; Ji, B.; Li, A. Enhancing biolipid production and self-flocculation of Chlorella vulgaris by extracellular polymeric substances from granular sludge with CO2 addition: Microscopic mechanism of microalgae-bacteria symbiosis. Water Res. 2023, 236, 119960. [Google Scholar] [CrossRef]
- Jupsin, H.; Praet, E.; Vasel, J. Dynamic mathematical model of high rate algal ponds (HRAP). Water Sci. Technol. 2003, 48, 197–204. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Synergistic cooperation between wastewater-born algae and activated sludge for wastewater treatment: Influence of algae and sludge inoculation ratios. Bioresour. Technol. 2012, 105, 67–73. [Google Scholar] [CrossRef]
- Abouhend, A.; McNair, A.; Kuo-Dahab, W.; Watt, C.; Butler, C.; Milferstedt, K.; Hamelin, J.; Seo, J.; Gikonyo, G.J.; El-Moselhy, K.M.; et al. The Oxygenic Photogranule Process for Aeration-Free Wastewater Treatment. Environ. Sci. Technol. 2018, 52, 3503–3511. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, L.; Shi, W.; Zhang, Z.; Lens, P. A novel strategy for rapid development of a self-sustaining symbiotic algal-bacterial granular sludge: Applying algal-mycelial pellets as nuclei. Water Res. 2022, 214, 118210. [Google Scholar] [CrossRef]
- Huang, L.; Lu, Z.; Xie, T.; Wang, L.; Mo, C. Nitrogen and phosphorus removal by coupling Anaerobic ammonia oxidation reaction with algal-bacterial symbiotic system. J. Environ. Chem. Eng. 2022, 10, 108905. [Google Scholar] [CrossRef]
- Lu, R.; Yan, H.; Liu, Y.; Wang, Y.; Cui, X.; Wu, X.; Yu, Z.; Ruan, R.; Zhang, Q. Enhancement of nutrients recovery and cell metabolism in piggery anaerobic digestate by the co-cultivation of indigenous microalgae and bacteria. J. Clean. Prod. 2022, 375, 134193. [Google Scholar] [CrossRef]
- Ji, B.; Fan, S.; Liu, Y. A continuous-flow non-aerated microalgal-bacterial granular sludge process for aquaculture wastewater treatment under natural day-night conditions. Bioresour. Technol. 2022, 350, 126914. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Tang, S.; Lin, K.; Zhao, T.; Chen, X. A review on algal-bacterial symbiosis system for aquaculture tail water treatment. Sci. Total. Environ. 2022, 847, 157620. [Google Scholar] [CrossRef]
- Wang, C.; Tan, Y.; Zhu, L.; Zhou, C.; Yan, X.; Xu, Q.; Ruan, R.; Cheng, P. The intrinsic characteristics of microalgae biofilm and their potential applications in pollutants removal—A review. Algal Res. Biomass Biofuels Bioprod. 2022, 68, 102849. [Google Scholar] [CrossRef]
- Winkler, M.; van Loosdrecht, M. Intensifying existing urban wastewater. Aerobic Granular Sludge Offers Improvements to Treatment Processes. Science 2022, 375, 377–378. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, L.; Liao, Q.; Zhang, Z.; Zhao, Y.; Gao, M.; Jin, C.; She, Z.; Wang, G. Mariculture wastewater treatment with Bacterial-Algal Coupling System (BACS): Effect of light intensity on microalgal biomass production and nutrient removal. Environ. Res. 2021, 201, 111578. [Google Scholar] [CrossRef]
- Akizuki, S.; Kishi, M.; Cuevas-Rodríguez, G.; Toda, T. Effects of different light conditions on ammonium removal in a consortium of microalgae and partial nitrifying granules. Water Res. 2020, 171, 115445. [Google Scholar] [CrossRef]
- Deng, R.; Xu, P.-P.; Fan, P.-P.; Li, B.; Zhang, Y.-H.; Lu, H.-Y.; Bi, Y.-H. Response of photosynthetic activity of Microcystis aeruginosa on short-term exposure to different LED lights. Acta Hydrobiol. Sin. 2023, 47, 71–79. (In Chinese) [Google Scholar] [CrossRef]
- Rossi, S.; Sforza, E.; Pastore, M.; Bellucci, M.; Casagli, F.; Marazzi, F.; Ficara, E. Photo-respirometry to shed light on microalgae-bacteria consortia—A review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 43–72. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, R.; Tan, Y.; Deng, Q.; Zhou, H.; Gao, B.; Zhang, C. Research Progress on LED Quality Regulating Cell Growth and Target Chemicals Accumulation in Microal. Chin. J. Bioprocess. Eng. 2022, 20, 125–136. (In Chinese) [Google Scholar]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total. Environ. 2021, 762, 144590. [Google Scholar] [CrossRef]
- Hülsen, T.; Hsieh, K.; Tait, S.; Barry, E.; Puyol, D.; Batstone, D. White and infrared light continuous photobioreactors for resource recovery from poultry processing wastewater—A comparison. Water Res. 2018, 144, 665–676. [Google Scholar] [CrossRef]
- Jia, H.; Yuan, Q. Ammonium removal using algae–bacteria consortia: The effect of ammonium concentration, algae biomass, and light. Biodegradation 2018, 29, 105–115. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, L.; Luo, X.; Zheng, Z. Effects of various LED light wavelengths and intensities on the performance of purifying synthetic domestic sewage by microalgae at different influent C/N ratios. Ecol. Eng. 2013, 51, 24–32. [Google Scholar] [CrossRef]
- Bahman, M.; Agha Noori, M.; Jalili, H.; Bozorg, A.; Danaee, S.; Bidhendi, M.; Amrane, A. Effect of light intensity and wavelength on nitrogen and phosphate removal from municipal wastewater by microalgae under semi-batch cultivation. Environ. Technol. 2022, 43, 1352–1358. [Google Scholar] [CrossRef]
- Ge, Z.; Zhang, H.; Zhang, Y.; Yan, C.; Zhao, Y. Purifying synthetic high-strength wastewater by microalgae Chlorella vulgaris under various light emitting diode wavelengths and intensities. J. Environ. Health Sci. Eng. 2013, 11, 8. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Zhang, H.; Yan, C.; Zhang, Y. Effects of various LED light wavelengths and intensities on microalgae-based simultaneous biogas upgrading and digestate nutrient reduction process. Bioresour. Technol. 2013, 136, 461–468. [Google Scholar] [CrossRef]
- Arcila, J.; Céspedes, D.; Buitrón, G. Influence of the wavelength photoperiods and N/P ratio on wastewater treatment with microalgae–bacteria. Water Sci. Technol. 2021, 84, 712–724. [Google Scholar] [CrossRef]
- Kang, D.; Kim, K.; Jang, Y.; Moon, H.; Ju, D.; Jahng, D. Nutrient removal and community structure of wastewater-borne algal-bacterial consortia grown in raw wastewater with various wavelengths of light. Int. Biodeterior. Biodegradation 2018, 126, 10–20. [Google Scholar] [CrossRef]
- Blair, M.; Kokabian, B.; Gude, V. Light and growth medium effect on Chlorella vulgaris biomass production. Abstr. Pap. Am. Chem. Soc. 2014, 2, 665–674. [Google Scholar] [CrossRef]
- Schulze, P.; Pereira, H.; Santos, T.; Schueler, L.; Guerra, R.; Barreira, L.; Perales, J.; Varela, J. Effect of light quality supplied by light emitting diodes (LEDs) on growth and biochemical profiles of Nannochloropsis oculata and Tetraselmis chuii. Algal Res. Biomass Biofuels Bioprod. 2016, 16, 387–398. [Google Scholar] [CrossRef]
- Kim, T.; Lee, Y.; Han, S.; Hwang, S. The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresour. Technol. 2013, 130, 75–80. [Google Scholar] [CrossRef]
- Sun, J.; Yao, L.; Li, J.; You, F.; Yuan, J.; Wang, D. Effects of LED Light Quality on the Growth and Metabolite Accumulation in Microalgae: A Review. Food Ferment. Ind. 2022, 7, e08525. (In Chinese) [Google Scholar]
- Lee, C.; Lee, S.; Ko, S.; Oh, H.; Ahn, C. Effects of photoperiod on nutrient removal, biomass production, and algal-bacterial population dynamics in lab-scale photobioreactors treating municipal wastewater. Water Res. 2015, 68, 680–691. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Huesemann, M.; Crowe, B.; Waller, P.; Chavis, A.; Hobbs, S.; Edmundson, S.; Wigmosta, M. A validated model to predict microalgae growth in outdoor pond cultures subjected to fluctuating light intensities and water temperatures. Algal Res. Biomass Biofuels Bioprod. 2016, 13, 195–206. [Google Scholar] [CrossRef]
- Patelou, M.; Infante, C.; Dardelle, F.; Randewig, D.; Kouri, E.D.; Udvardi, M.K.; Tsiplakou, E.; Mantecón, L.; Flemetakis, E. Transcriptomic and metabolomic adaptation of Nannochloropsis gaditana grown under different light regimes. Algal Res. Biomass Biofuels Bioprod. 2019, 45, 101735. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Ergas, S.; van der Steen, P. A novel shortcut nitrogen removal process using an algal-bacterial consortium in a photo-sequencing batch reactor (PSBR). Water Res. 2015, 87, 38–48. [Google Scholar] [CrossRef]
- González-Camejo, J.; Barat, R.; Pachés, M.; Murgui, M.; Seco, A.; Ferrer, J. Wastewater nutrient removal in a mixed microalgae–bacteria culture: Effect of light and temperature on the microalgae–bacteria competition. Environ. Technol. 2018, 39, 503–515. [Google Scholar] [CrossRef]
- Arun, S.; Ramasamy, S.; Pakshirajan, K. Mechanistic insights into nitrification by microalgae-bacterial consortia in a photo-sequencing batch reactor under different light intensities. J. Clean. Prod. 2021, 321, 128752. [Google Scholar] [CrossRef]
- Gonçalves, A.; Simões, M.; Pires, J. The effect of light supply on microalgal growth, CO2 uptake and nutrient removal from wastewater. Energy Convers. Manag. 2014, 85, 530–536. [Google Scholar] [CrossRef]
- Oruganti, R.; Katam, K.; Show, P.; Gadhamshetty, V.; Upadhyayula, V.; Bhattacharyya, D. A comprehensive review on the use of algal-bacterial systems for wastewater treatment with emphasis on nutrient and micropollutant removal. Bioengineered 2022, 13, 10412–10453. [Google Scholar] [CrossRef]
- Sun, L.; Lei, Y.; Li, H. Exploring the Fundamental Factors Behind Algal-Bacterial Symbiosis and Their Impact on Ecological Interactions. Front. Environ. Sci. 2022, 10, 444. [Google Scholar] [CrossRef]
- Yustinadiar, N.; Manurung, R.; Suantika, G. Enhanced biomass productivity of microalgae Nannochloropsis sp. in an airlift photobioreactor using low-frequency flashing light with blue LED. Bioresour. Bioprocess. 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Lee, C.; Oh, H.; Oh, H.; Kim, H.; Ahn, C. Two-phase photoperiodic cultivation of algal–bacterial consortia for high biomass production and efficient nutrient removal from municipal wastewater. Bioresour. Technol. 2016, 200, 867–875. [Google Scholar] [CrossRef]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef]
- Zhang, B.; Lens, P.; Shi, W.; Zhang, R.; Zhang, Z.; Guo, Y.; Bao, X.; Cui, F. Enhancement of aerobic granulation and nutrient removal by an algal-bacterial consortium in a lab-scale photobioreactor. Chem. Eng. J. 2018, 334, 2373–2382. [Google Scholar] [CrossRef]
- Zhi, R.; Yang, A.; Zhang, G.; Zhu, Y.; Meng, F.; Li, X. Effects of light-dark cycles on photosynthetic bacteria wastewater treatment and valuable substances production. Bioresour. Technol. 2019, 274, 496–501. [Google Scholar] [CrossRef]
- Tang, C.; Zuo, W.; Tian, Y.; Sun, N.; Wang, Z.; Zhang, J. Effect of aeration rate on performance and stability of algal-bacterial symbiosis system to treat domestic wastewater in sequencing batch reactors. Bioresour. Technol. 2016, 222, 156–164. [Google Scholar] [CrossRef]
- De Godos, I.; González, C.; Becares, E.; García-Encina, P.A.; Muñoz, R. Simultaneous nutrients and carbon removal during pretreated swine slurry degradation in a tubular biofilm photobioreactor. Appl. Microbiol. Biotechnol. 2009, 82, 187–194. [Google Scholar] [CrossRef]
- Karya, N.; van der Steen, N.; Lens, P. Photo-oxygenation to support nitrification in an algal–bacterial consortium treating artificial wastewater. Bioresour. Technol. 2013, 134, 244–250. [Google Scholar] [CrossRef]
- Vargas, G.; Donoso-Bravo, A.; Vergara, C.; Ruiz-Filippi, G. Assessment of microalgae and nitrifiers activity in a consortium in a continuous operation and the effect of oxygen depletion. Electron. J. Biotechnol. 2016, 23, 63–68. [Google Scholar] [CrossRef]
- Rada-Ariza, A.; Lopez-Vazquez, C.; van der Steen, N.; Lens, P. Nitrification by microalgal-bacterial consortia for ammonium removal in flat panel sequencing batch photo-bioreactors. Bioresour. Technol. 2017, 245, 81–89. [Google Scholar] [CrossRef]
- Li, J.; Ou, R.; Liao, H.; Ma, J.; Sun, L.; Jin, Q.; He, D.; Wang, Q. Natural lighting enhancing the algae proliferation and nitrogen removal in membrane-aerated bacterial-algal biofilm reactor. Sci. Total. Environ. 2022, 851, 158063. [Google Scholar] [CrossRef]
- Van der Steen, P.; Rahsilawati, K.; Rada-Ariza, A.; Lopez-Vazquez, C.; Lens, P. A new photo-activated sludge system for nitrification by an algal-bacterial consortium in a photo-bioreactor with biomass recycle. Water Sci. Technol. 2015, 72, 443–450. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, W.; Zeng, W.; Chen, R.; Lin, D.; Li, G.; Liang, H. Bacterial-algae biofilm enhance MABR adapting a wider COD/N ratios wastewater: Performance and mechanism. Sci. Total. Environ. 2021, 781, 146663. [Google Scholar] [CrossRef]
- Rossi, S.; Díez-Montero, R.; Rueda, E.; Cascino, F.; Parati, K.; García, J.; Ficara, E. Free ammonia inhibition in microalgae and cyanobacteria grown in wastewaters: Photo-respirometric evaluation and modelling. Bioresour. Technol. 2020, 305, 123046. [Google Scholar] [CrossRef]
- González, C.; Marciniak, J.; Villaverde, S.; García-Encina, P.; Muñoz, R. Microalgae-based processes for the biodegradation of pretreated piggery wastewaters. Appl. Microbiol. Biotechnol. 2008, 80, 891–898. [Google Scholar] [CrossRef]
- Gong, J.; Liu, Z.; Zou, D. Growth and photosynthetic characteristics of Gracilaria lemaneiformis (Rhodophyta) and Ulva lactuca (Chlorophyta) cultured under fluorescent light and different LED light. J. Appl. Phycol. 2020, 32, 3265–3272. [Google Scholar] [CrossRef]
- Lamare, P.; Aguillon, N.; Sainte-Marie, J.; Grenier, J.; Bonnefond, H.; Bernard, O. Gradient-based optimization of a rotating algal biofilm process. Automatica 2019, 105, 80–88. [Google Scholar] [CrossRef]
- You, X.; Zhang, Z.; Guo, L.; Liao, Q.; Wang, Y.; Zhao, Y.; Jin, C.; Gao, M.; She, Z.; Wang, G. Integrating acidogenic fermentation and microalgae cultivation of bacterial-algal coupling system for mariculture wastewater treatment. Bioresour. Technol. 2021, 320, 124335. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Wu, T.; Li, Y.; Lee, Y.; Liu, J.; Chen, F. The synergistic energy and carbon metabolism under mixotrophic cultivation reveals the coordination between photosynthesis and aerobic respiration in Chlorella zofingiensis. Algal Res. Biomass Biofuels Bioprod. 2017, 25, 109–116. [Google Scholar] [CrossRef]
- Nair, A.; Chakraborty, S. Synergistic effects between autotrophy and heterotrophy in optimization of mixotrophic cultivation of Chlorella sorokiniana in bubble-column photobioreactors. Algal Res. Biomass Biofuels Bioprod. 2020, 46, 101799. [Google Scholar] [CrossRef]
- Kuo, F.; Chien, Y.; Chen, C. Effects of light sources on growth and carotenoid content of photosynthetic bacteria Rhodopseudomonas palustris. Bioresour. Technol. 2012, 113, 315–318. [Google Scholar] [CrossRef]
- Govarthanan, M.; Kamala-Kannan, S.; Selvankumar, T.; Mythili, R.; Srinivasan, P.; Kim, H. Effect of blue light on growth and exopolysaccharides production in phototrophic Rhodobacter sp. BT18 isolated from brackish water. Int. J. Biol. Macromol. 2019, 131, 74–80. [Google Scholar] [CrossRef]
- Chu, Z.; Huang, X.; Su, Y.; Yu, H.; Rong, H.; Wang, R.; Zhang, L. Low-dose Ultraviolet-A irradiation selectively eliminates nitrite oxidizing bacteria for mainstream nitritation. Chemosphere 2020, 261, 128172. [Google Scholar] [CrossRef]
- Merbt, S.; Stahl, D.; Casamayor, E.; Martí, E.; Nicol, G.; Prosser, J.I. Differential photoinhibition of bacterial and archaeal ammonia oxidation. FEMS Microbiol. Lett. 2012, 327, 41–46. [Google Scholar] [CrossRef]
- Hyman, M.; Arp, D. 14C2H2- and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J. Biol. Chem. 1992, 267, 1534–1545. [Google Scholar] [CrossRef]
- Sforza, E.; Simionato, D.; Giacometti, G.; Bertucco, A.; Morosinotto, T. Adjusted Light and Dark Cycles Can Optimize Photosynthetic Efficiency in Algae Growing in Photobioreactors. PLoS ONE 2012, 7, e38975. [Google Scholar] [CrossRef]
- Ye, J.; Liang, J.; Wang, L.; Markou, G.; Jia, Q. Operation optimization of a photo-sequencing batch reactor for wastewater treatment: Study on influencing factors and impact on symbiotic microbial ecology. Bioresour. Technol. 2018, 252, 7–13. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, B.; Liu, Y.; Feng, X.; Shi, W. Revealing the influencing mechanisms of polystyrene microplastics (MPs) on the performance and stability of the algal-bacterial granular sludge. Bioresour. Technol. 2022, 354, 127202. [Google Scholar] [CrossRef]
- Mota, R.; Guimarães, R.; Buttel, Z.; Rossi, F.; Colica, G.; Silva, C.; Santos, C.; Gales, L.; Zille, A.; De Philippis, R.; et al. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohydr. Polym. 2013, 92, 1408–1415. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y.; Lens, P.; Zhang, Z.; Shi, W.; Cui, F.; Tay, J. Effect of light intensity on the characteristics of algal-bacterial granular sludge and the role of N-acyl-homoserine lactone in the granulation. Sci. Total. Environ. 2019, 659, 372–383. [Google Scholar] [CrossRef]
- Dejsungkranont, M.; Chisti, Y.; Sirisansaneeyakul, S. Simultaneous production of C-phycocyanin and extracellular polymeric substances by photoautotrophic cultures of Arthrospira platensis. J. Chem. Technol. Biotechnol. 2017, 92, 2709–2718. [Google Scholar] [CrossRef]
- Katsuda, T.; Lababpour, A.; Shimahara, K.; Katoh, S. Astaxanthin production by Haematococcus pluvialis under illumination with LEDs. Enzym. Microb. Technol. 2004, 35, 81–86. [Google Scholar] [CrossRef]
- Dahalan, F.; Abdullah, N.; Yuzir, A.; Olsson, G.; Salmiati; Hamdzah, M.; Din, M.; Ahmad, S.; Khalil, K.; Anuar, A.; et al. A proposed aerobic granules size development scheme for aerobic granulation process. Bioresour. Technol. 2015, 181, 291–296. [Google Scholar] [CrossRef]
- Wei, K.; Amano, Y.; Machida, M. Impacts of different extracellular polysaccharides on colony formation and buoyancy of Microcystis aeruginosa. Ann. Limnol. Int. J. Limnol. 2020, 56, 28. [Google Scholar] [CrossRef]
- He, Q.; Chen, L.; Zhang, S.; Chen, R.; Wang, H.; Zhang, W.; Song, J. Natural sunlight induced rapid formation of water-born algal-bacterial granules in an aerobic bacterial granular photo-sequencing batch reactor. J. Hazard. Mater. 2018, 359, 222–230. [Google Scholar] [CrossRef]
| Pigment | Algae | Absorption Spectra/nm |
|---|---|---|
| Chlorophyll a | All algae | 436, 670~690 |
| Chlorophyll b | Chlamydomonas, green algae, Euglena, and diatoms | 455, 650~660 |
| Chlorophyll c | Heterokonts, excluding diatoms | 442~444, 630 |
| Chlorophyll d | Cyanobacteria (blue-green algae) and red algae | 380, 440, 700~720 |
| Chlorophyll f | Cyanobacteria (blue-green algae) | 700~760 |
| Carotenoids | All algae | 420~470 |
| Lutein | All algae | 410~500 (540) |
| Phycocyanin | Dinoflagellates, Diatoms, Red algae | 610~635 (PC) 495~560 (PE) |
| Inoculum | Sewage Source | Light Source | Light Quality | Initial Ammonia Nitrogen Concentration (mg-N/L) | Total Ammonia Nitrogen-Removal Rate (%) | Reference |
|---|---|---|---|---|---|---|
| Algae, bacteria | Poultry processing wastewater | LED, Fluorescent lamp | Red light White light | 200.00 | 45.70 ± 2.00 36.20 ± 0.70 | [21] |
| Cyanobacteria, green algae, Activated sludge | High-strength synthetic wastewater containing ammonia | LED | Blue light Red light Cold white light Natural white light | 800.00 | 53.00 ± 2.00 41.00 ± 3.71 51.00 ± 3.00 50.00 ± 3.00 | [22] |
| C. vulgaris, bacteria | Synthetic domestic sewage | LED | Red light white light Yellow light Purple light Blue light Green light | 184.00 | 75.08 ± 3.65 71.36 ± 2.63 67.59 ± 1.45 49.42 ± 1.78 47.37 ± 2.64 29.63 ± 1.72 | [23] |
| Spirulina platensis | Artificial urban wastewater | Artificial light source | Blue light Red light Purple light White light | 37.00 | 18.00 56.00 68.00 60.00 | [24] |
| C. vulgaris | Synthetic high-carbon wastewater | LED | Red light White light Yellow light Blue light | 53.82 ± 7.21 | 76.04 ± 8.39 61.31 ± 5.79 35.72 ± 4.06 17.35 ± 3.92 | [25] |
| Chlorella sp. | Biogas slurry | LED | Red light White light Yellow light Blue light | 51.34 ± 1.85 | 54.94 ± 2.09 49.32 ± 3.61 46.64 ± 3.57 41.53 ± 4.05 | [26] |
| Microalgal mixture, Active sludge | Synthetic wastewater | LED | Blue light Green light | 15.10 ± 3.10 | 68.00 ± 1.00 60.00 | [27] |
| Algal-Bacterial Consortia | Real domestic sewage | LED | Blue light Green light Red light White light | 31.80 ± 3.40 | 56.90 ± 2.50 60.40 ± 1.60 88.30 ± 0.70 79.00 ± 2.00 | [28] |
| Inoculum | Sewage Source | Light Source | Light Intensity (μmol/m2/s) | The Initial Concentration of Ammonia Nitrogen (mg-N/L) | Total Ammonia Nitrogen-Removal Rate (%) | Reference |
|---|---|---|---|---|---|---|
| Chlorella sp., Chlamydomonas, Stichococcus | Digested pig manure | White fluorescent lamp | 74.5 105.0 | 301.00 ± 16.00 | 65.00 ± 6.00 93.00 ± 2.00 | [37] |
| Chlorella sorokiniana, Activated sludge | Synthetic wastewater containing ammonia | LED | 0.0 100.0 450.0 1600.0 | 43.00 | 66.40 61.60 5.20 −10.00 | [15] |
| C. vulgaris | Synthetic high-carbon wastewater | LED | 500.0 1000.0 1500.0 2000.0 2500.0 3000.0 | 53.82 ± 7.21 | 12.00 ± 1.00 54.00 ± 1.00 64.00 ± 1.00 75.00 ± 1.00 80.00 ± 1.00 14.00 ± 1.00 | [25] |
| Microalgae, bacteria (including Cyanobacteria) | The effluent of an AnMBR pilot plant | Fluorescent lamps | 45.0 85.0 125.0 | 57.40 ± 2.20 | 97.20 ± 2.30 99.90 ± 0.20 99.50 ± 0.20 | [38] |
| Chlorella sorokiniana, nitrifying bacterial culture | Synthetic wastewater | Warm white lamp | 0.0 150.0 500.0 1500.0 2000.0 | 100.00 | 7.20 100.00 100.00 6.20 −2.20 | [39] |
| C. vulgaris | Simulating domestic sewage | Fluorescent light | 36.0 60.0 120.0 180.0 | 250.00 | 42.30 ± 1.60 53.60 ± 1.00 76.40 ± 4.00 86.20 ± 1.70 | [40] |
| Chlorella sp. | Biogas slurry | LED | 800 1200 1600 2000 | 51.34 ± 1.85 | 44.12 ± 2.40 51.03 ± 2.23 53.81 ± 1.96 54.94 ± 2.09 | [26] |
| Inoculum | Sewage Source | Light Source | Photoperiod | Initial Ammonia Nitrogen Concentration (mg-N/L) | Total Ammonia Nitrogen-Removal Rate (%) | Reference |
|---|---|---|---|---|---|---|
| Scenedesmus sp. activated sludge | urban sewage | LED | 12:12 12:60 | 40.6 ± 1.3 | 75.40 ± 3.80 54.50 ± 1.80 | [44] |
| P. subcapitata | Simulated municipal wastewater | fluorescent light | 10:14 14:10 24:0 | 250.0 | 43.50 ± 0.70 74.40 ± 2.90 88.00 ± 2.70 | [40] |
| Cyanobacteria, green algae, Activated sludge | Concentrated wastewater with high ammonium content | LED | 24:0 16:8 2:1 | 800.0 | 65.00 ± 5.00 | [22] |
| Microalgae, Activated sludge | Municipal wastewater | Fluorescent lamp | 12:12 12:36 12:60 | 39.5 | 87.85 64.56 35.19 | [33] |
| Microbial aggregates (microalgae, bacteria, and other microorganisms) | Municipal wastewater | Fluorescent lamp | 12:12 18:6 | 41.6 ± 11.7 | 99.74 99.66 | [45] |
| Chlorophyta, Trebouxiophyceae, Bacillariophyceae, activated sludge | Synthetic wastewater | warm white light | 0:24 12:12 | 200.0 | 96.90 99.00 | [46] |
| Inoculum | Sewage Source | Initial Ammonia Nitrogen Concentration | Light Source | Light Intensity | Photoperiod | Ammonia Nitrogen-Removal Rate Pathway | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| (Lx) | (μmol/m2/s) | Nitrification | Microalgae Assimilation | ||||||
| Chlorella sorokiniana, Mixed bacterial cultures | Pretreated pigmanure | 60 150 290 650 | Fluorescent lamps | 10,000.0 | 180.0 | 24 | 0.00 7.00 23.00 8.00 | 100.0 93.00 77.00 92.00 | [49] |
| Chlorella sorokiniana Activated sludge | Synthetic wastewater | 100 | LED | - | 135.0 | - | 61.00 | 39.00 | [15] |
| S. quadricauda Activated sludge | BG-11 medium improves artificial wastewater | 50 | Warm white light | - | 60.0 | - | 80.00 | 20.00 | [50] |
| Microalgae Aerobic sludge | Synthetic wastewater | 1400 | Artificial light source | - | 67.5 | - | 60.00 | 40.00 | [51] |
| Microbiota (microalgae, bacteria, and other microorganisms) | Municipal wastewater | 41.6 ± 17.1 | Fluorescent lamps | - | 45.0 | 12:12 18:6 | 72.00 83.00 | 28.00 17.00 | [45] |
| Chlorella spp. Chlamydomonas, Stichococcus, | Treated pig manure | 301 ± 16 | Fluorescent lamps | - | 74.0 ± 5.0 | - | 85.05 | 14.95 | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Guo, Z.; Ding, X.; Li, L.; Jin, Z.; Zhang, C.; Liu, S.; Zhou, Y.; Fan, G. The Effect of Light on Nitrogen Removal by Microalgae-Bacteria Symbiosis System (MBS). Water 2023, 15, 1991. https://doi.org/10.3390/w15111991
Wang S, Guo Z, Ding X, Li L, Jin Z, Zhang C, Liu S, Zhou Y, Fan G. The Effect of Light on Nitrogen Removal by Microalgae-Bacteria Symbiosis System (MBS). Water. 2023; 15(11):1991. https://doi.org/10.3390/w15111991
Chicago/Turabian StyleWang, Shumin, Zhenghao Guo, Xiaofan Ding, Linling Li, Zhongyou Jin, Chengcai Zhang, Shouping Liu, Yan Zhou, and Gongduan Fan. 2023. "The Effect of Light on Nitrogen Removal by Microalgae-Bacteria Symbiosis System (MBS)" Water 15, no. 11: 1991. https://doi.org/10.3390/w15111991
APA StyleWang, S., Guo, Z., Ding, X., Li, L., Jin, Z., Zhang, C., Liu, S., Zhou, Y., & Fan, G. (2023). The Effect of Light on Nitrogen Removal by Microalgae-Bacteria Symbiosis System (MBS). Water, 15(11), 1991. https://doi.org/10.3390/w15111991







