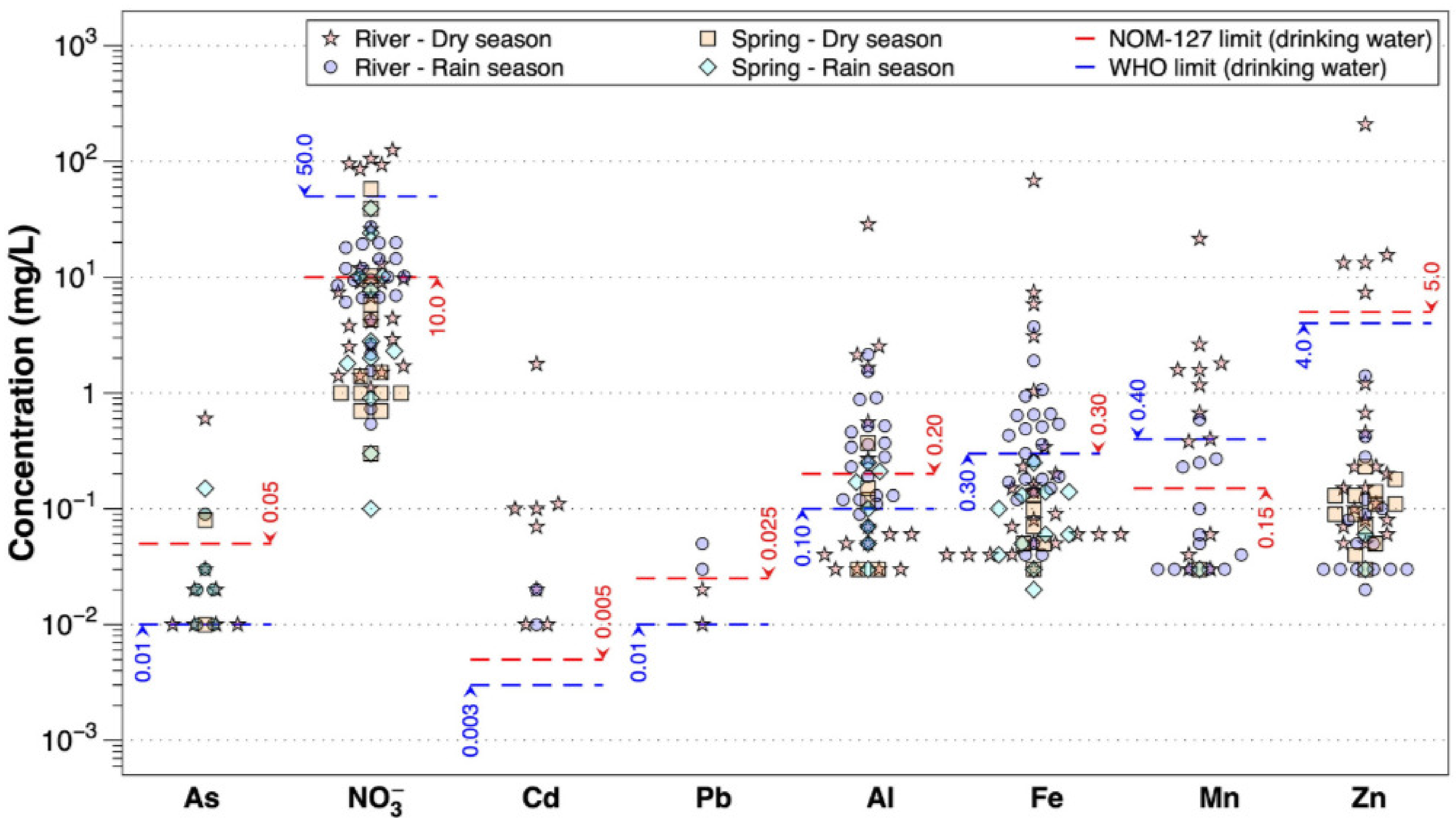
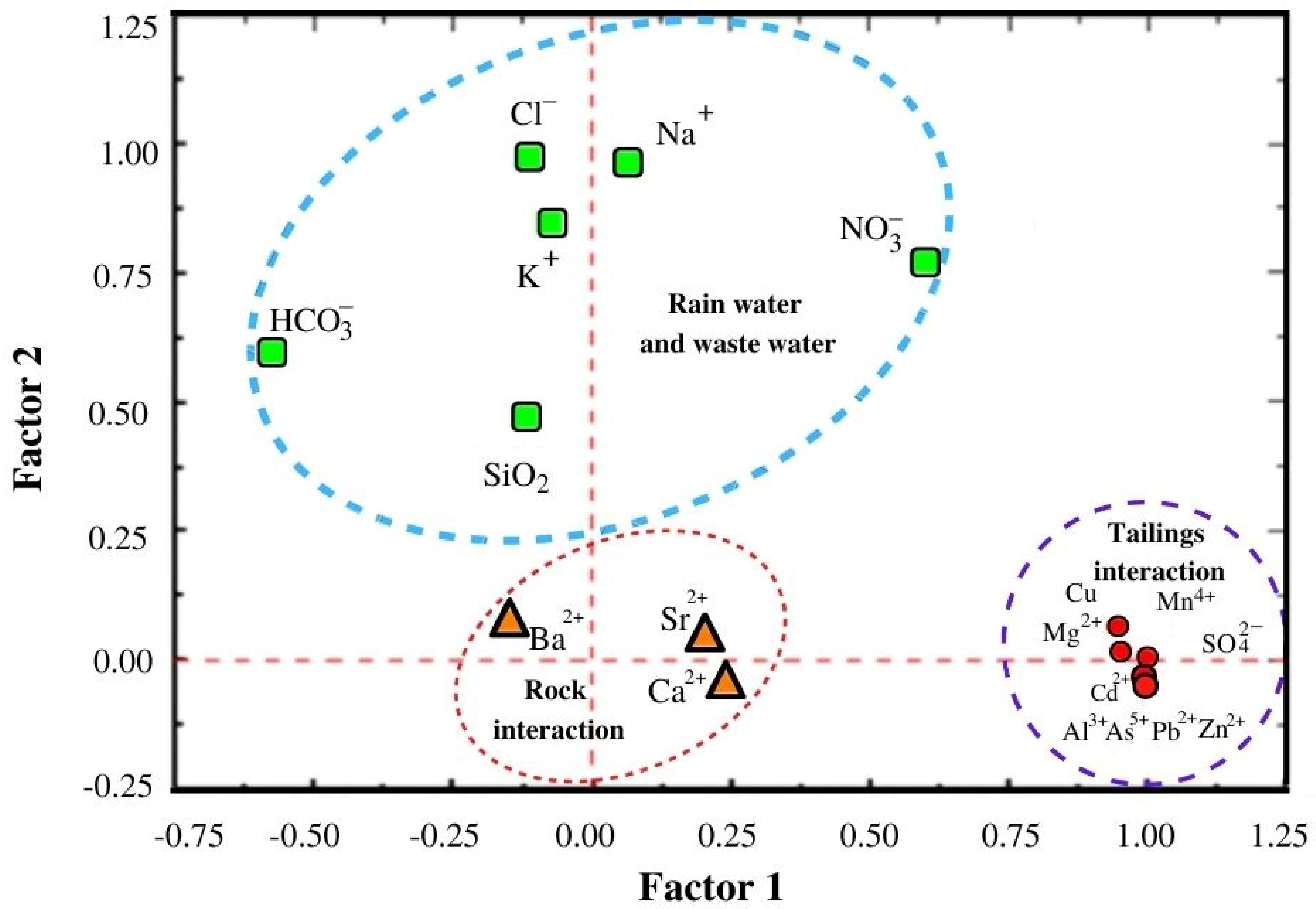
4.1. The Origin of Contaminants in Surface Water
The correlations found between dissolved species reveals that the origin of solutes and the main processes are related to the evolution of the surface water. In
Table 7, a correlation coefficient matrix (
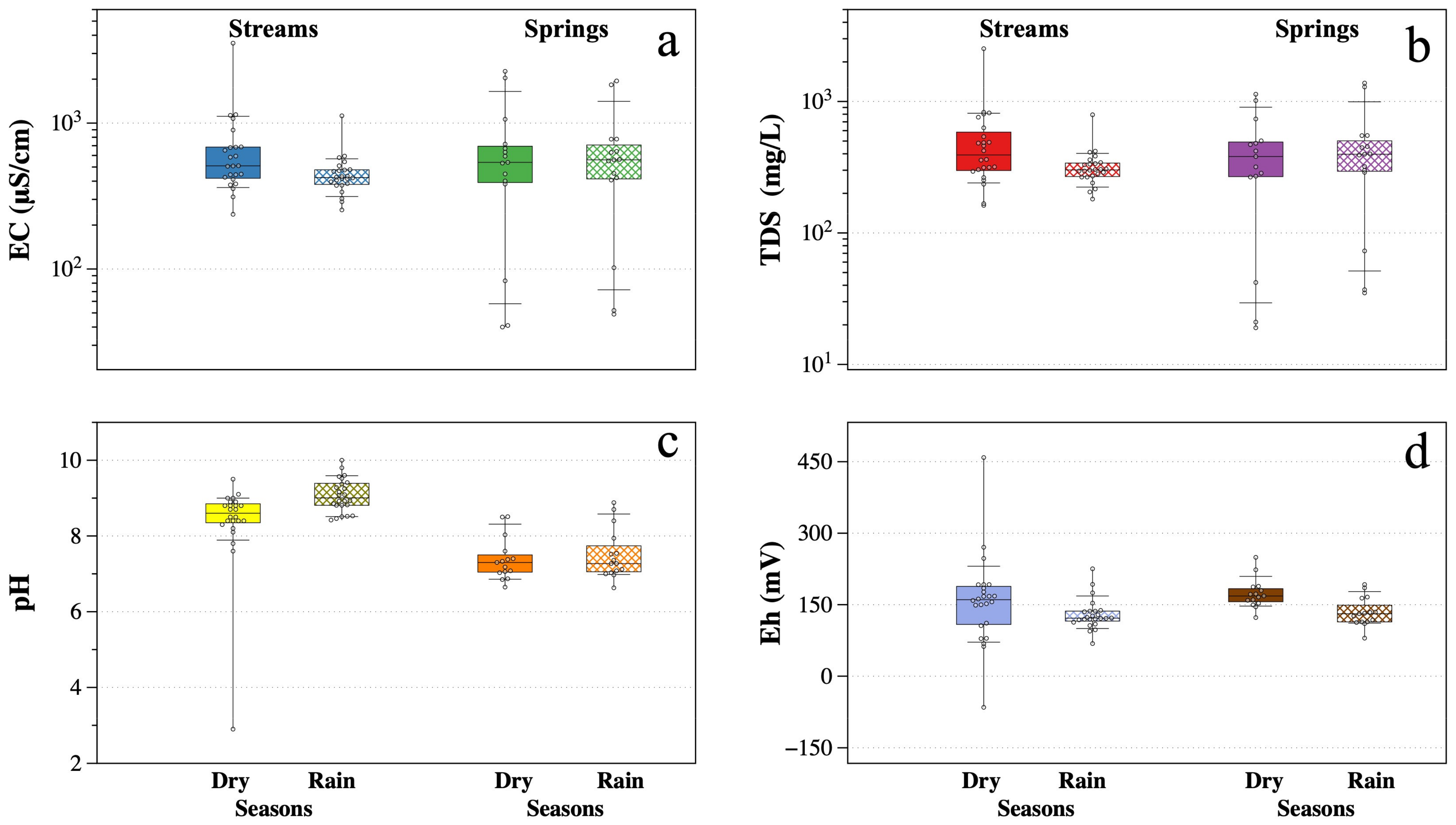
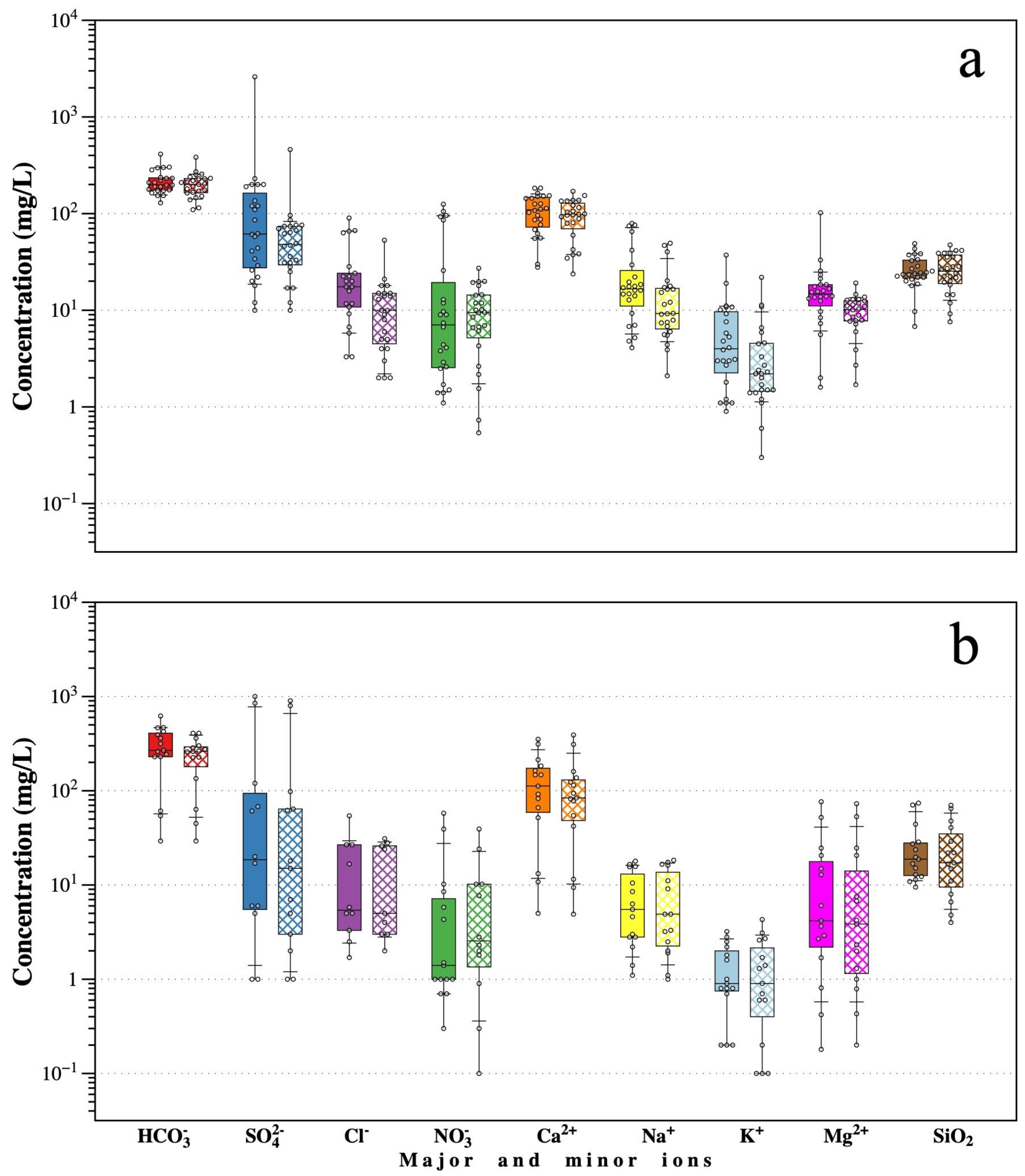
p-value < 0.05) for surface water in the dry season is presented, coinciding with the season with higher HM concentrations. A highly positive correlation (r = 0.97) was observed between EC and TDS. The observed correlation between EC and TDS with major ions
and
was high (r > 0.92), as well as with Al, As, Cd, Cu, Fe, Mn, Pb, and Zn (r > 0.89) and with
ion (r > 0.76). It is considered that pH has an important role in HM release, having a highly negative correlation with Al, As, Cd, Cu, Fe, Mn, Pb, and Zn (r > −0.87), and even higher with
ions (r = −0.95). On the contrary, for the Eh values, the correlations were moderately positive with major ions
and
(r > 0.69), as well as with Al, As, Cd, Cu, Fe, Mn, Pb, and Zn (r = 0.67–0.70). The
ion showed high positive correlations with Al, As, Cd, Cu, Fe, Mn, Pb, and Zn (r > 0.91).
Although the main surface runoff of the study area comprises a total of 80 km, previous studies have focused on the northern part of the hydrological sub-basin. Those studies evaluated pollution from the mining along only 8 km of the Cacalotenango and Taxco Rivers [
3,
12,
13,
33]. However, the interaction of meteoric water with mining tailings produced AMD with high concentrations of As, Pb, Cd, Cu, Fe, Mn, and Zn with pH < 3 [
10,
28,
34]. The AMD is continuously incorporated into the surface drainage near the mining complexes. For instance, sample S2 stood out as a generating point for AMD [
35,
36]. This sampling site is in the northern part of the hydrological recharge zone of the Taxco-Cocula sub-basin. The correlations highlight the common origin of the
anion and the HMs in the northern portion of the sub-basin.
On the other hand, the exclusively high positive correlation between Ba and Sr (r = 0.89) may be related to wáter–rock interaction [
6]. Lack of correlations between
ions and HMs and major ions were found, except for
(r = 0.79),mmight be related to the mixing of surface water and urban wastewater [
37] (
Table 7).
The correlation coefficient matrix obtained for the rainy season is shown in
Table 8. Moderate and high positive correlations among EC and TDS with major ions
and
(r = 0.91 and 0.70) and Cd, Mn, and Zn (r > 0.75), and low moderate correlations with Eh and
(r = 0.53 and 0.52), were observed. Furthermore,
ions presented a moderate positive correlation with
(r = 0.57) and high correlations with Zn (r = 0.95), Mn (r = 0.83), and Cd (r = 0.92). Lower correlations (r < 0.54) between
and the HMs evidenced dilution processes that occur each rainy season. However, high positive correlations between
, Cd, Mn, and Zn (r > 0.83), Al and Mn (r = 0.88), Cd, Fe, and Zn (r > 0.75), Fe and Mn (r = 0.90), and Mn and Zn (r = 0.90) were consistent with the common origin of the HMs coming from mining tailings carried by surface water. In the rainy season high positive correlations between Ba and Sr (r = 0.91) were also observed. Romero et al. [
28] and Talavera Mendoza et al. [
3,
9,
10] showed that tailings exposed to natural runoff from main water tributaries, such as the Taxco River and the Cacalotenango River, had high contents of HMs, due to the oxidation of sulfurous minerals, mainly pyrite. Isotopic studies confirmed that sulfates in the area influence the mineralization of tailings, as well as of Pb and Sr [
3], and that the dry season is marked by evaporation processes and rainfall by dilution that influence the decrease HM concentrations in the area [
3,
12].
Recently, Quevedo-Castañón et al. [
35] suggested that natural mixing of AMD with natural runoff water from streams in the area helped with AMD neutralization in Taxco. This is consistent with the correlation analysis of the rainy season that evidenced this component. A multivariate analysis was performed to improve the contrast between physicochemical variables, major ions, and the HMs in the dry season, by using Principal Component Analysis.
Table S3 (Supplementary Material) shows the concentration values and the percentages of total variance for each variable. Component 1 explained 55.0% of the variance, related to
,
,
,
,
,
,
,
, and
confirming their origins as being from mining tailings. Component 2 explained 20.5% of the variance and the higher weight was associated with
,
,
,
,
, and
variables, suggesting mixing with rainwater and urban wastewater. Component 3 explained 11.2% of the variance, with the higher weight associated with Ba
2+, Sr
2+, and Ca
2+, as suggested in the correlation analysis.
Figure 7 presents the three main components associated with the multivariate analysis, confirming the processes associated with the observed chemistry on surface water, which were also in accordance with the correlation matrices for both the rainy and dry seasons.
4.2. Contaminant Dispersion Mechanisms on the Taxco-Cocula Sub-Basin
Findings from this research, as well as from research conducted by Armienta et al. [
33,
34], Talavera Mendoza et al. [
9,
10], and Romero et al. [
28], agree that the main influence of the mining is in the north of the TMD. The dispersion of HMs in the sub-basin begins with the rainwater that infiltrates the tailings, leaching and entraining particles. The hydration of this material is the first step in the incorporation of HMs in the nearby rivers and streams, by the weathering of minerals, such as anhydrite to gypsum [
38]. The different tailing deposits are in a surface area of ~25 km
2. Although they have the same origins and are separated from each other, there are differences in their geochemical and environmental behaviors, as evidenced by the HM concentrations.
Acid generating minerals were identified by Bancks et al. [
39] and Dold [
40] in the MS
2 form, as pyrite (FeS
2) and pyrrhotite (Fe1-xS2), and non-acid generating minerals can be generalized in the MS form, as galena (PbS), chalcopyrite (CuFeS
2), sphalerite (ZnS), and arsenopyrite (AsFeS), among others. In this generalization, M
+ represents the divalent cation. For instance, Taxco ore deposit was previously characterized. Minerals, such as Pyrite (10–15%), sphalerite (11%), galena (4%), and other secondary minerals, such as chalcopyrite, argentite, pyrargyrite, pyrolusite, and arsenopyrite, were identified by Talavera Mendoza et al. [
9] and Romero et al. [
28].
Sampling site S2 was close to the Guerrero II tailings, where the last processing plant operated, and the lowest pH value was recorded there (
Table 1,
Figure 1). Thus, the channel that drains the leachates concentrates the oxidation processes of primary, secondary, and gangue minerals that lead to the generation of AMD, evidenced by the physicochemical characteristics and higher concentrations of HMs in the surface water of the entire sub-basin. In 2016, IMMSA (the company in charge of mining liabilities), intensified remediation activities, by reducing slopes in tailing dams, compacting slopes with calcareous gravel, constructing filters to reduce the pollutant discharges from tailings to the main tributaries, and eliminating mine water in rivers and streams, as well as phytoremediation in the zone. The impact of these remediation processes on the tailings could be quantified with new analyses to assess the variations associated with sites with high concentrations of HMs in the Taxco-Cocula sub-basin.
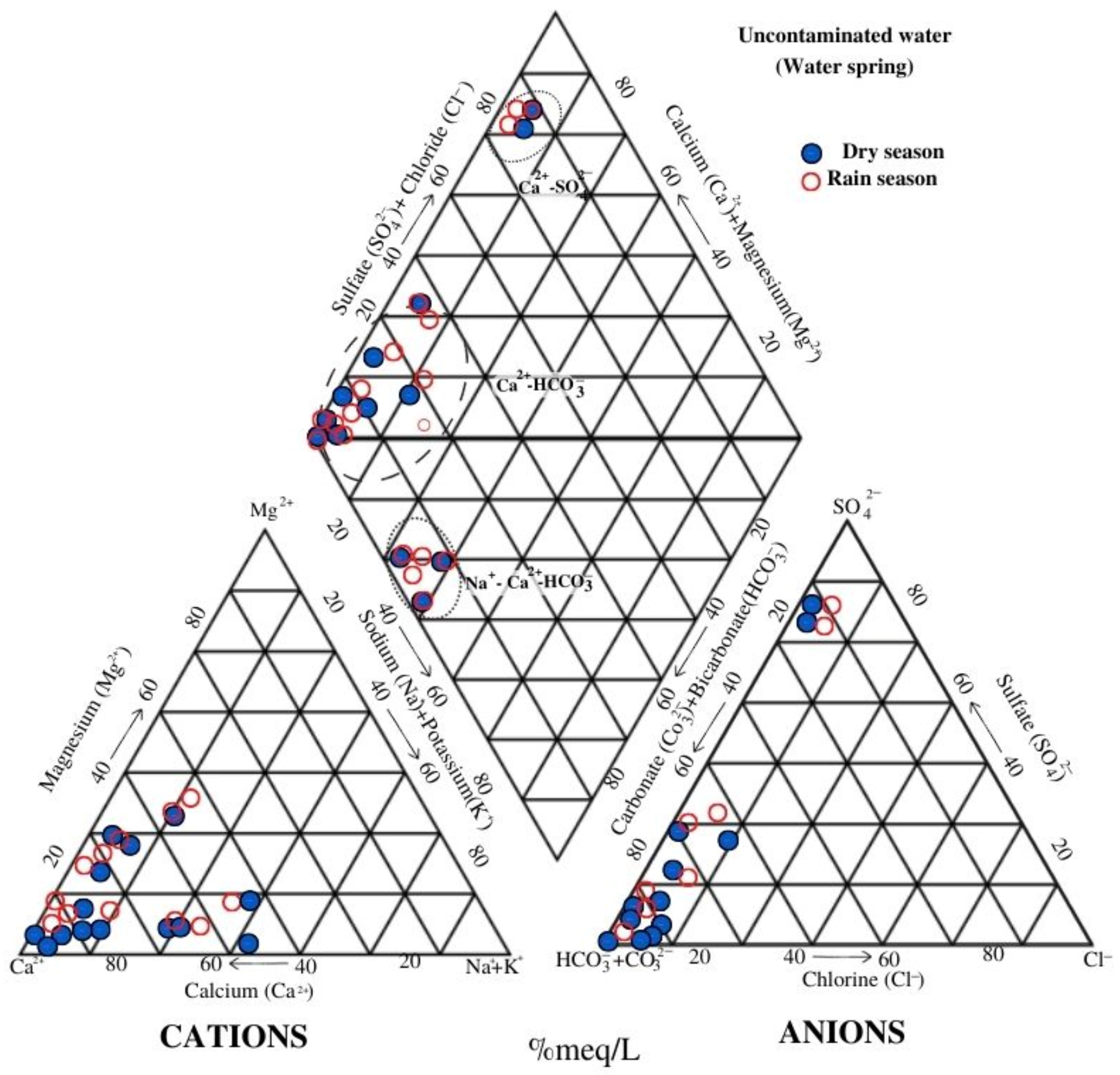
By contrast, the measurements of the same parameters in springs in the dry and rainy seasons did not present significant changes and were related to peripheral water, evidenced by the high concentrations of
. The differences between the chemical facies are due to the geology in which the springs are located as their hydrogeochemical characteristics in the dry and rainy seasons do not vary significantly, in congruence with the physical-chemical parameters measured in situ. The geology of the zone plays a key role in diminishing the hazardous effects of AMD [
36,
41] (
Table 3,
Figure 1).
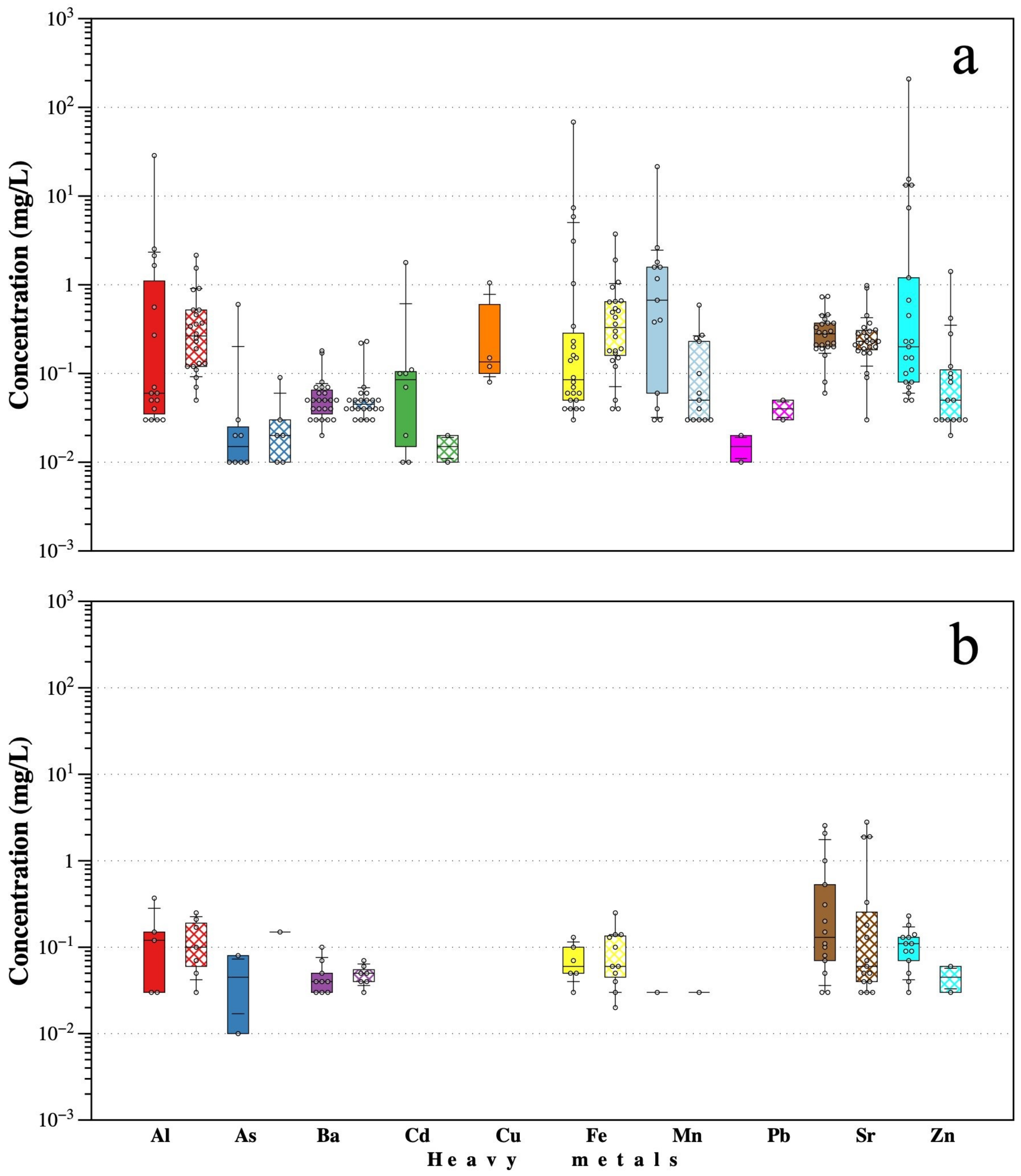
According to previous results, one can conclude that the highest concentrations of the analyzed HMs (Al, As, Ba, Cd, Cu, Fe, Mn, Pb, Sr, and Zn) are mainly found in the Cacalotenango River, having concentrations in the range of the values reported by Talavera Mendoza et al. [
9]. Moreover, in Taxco River, Quevedo-Castañón et al. [
35], reported on the behavior involved in the generation of AMD in a small tributary of the Taxco River, with extreme values of pH < 3.0 and high concentrations of HMs measured in total concentration and in soluble elements. They also reported data on Pb isotopes, which showed chemical signatures similar to the mineral deposits of Taxco, as well as the reactive mineral phases that control AMD. The results of this work showed that in both the Taxco and Cacalotenango Rivers, metal leaching was magnified during the rainy season.
4.3. Speciation and Saturation Index (SI)
Tables S4 and S5 (Supplementary Material) show the behaviors at each sampled point for the dry and rainy seasons, respectively. The spatial and temporal chemical variations of the surface water of the Taxco-Cocula sub-basin were consistent with the diversity of reactive phases, which control the concentration and distribution of HMs downstream of the main source of pollutant emissions (S2).
An SI value of zero, with an associated uncertainty (±0.1), indicates that mineral precipitation (supersaturation) is possible, while a value less than zero indicates that mineral dissolution (sub-saturation) is possible. These calculations assume that the dissolved species in surface water are in chemical equilibrium [
42].
The main saturated phases were aragonite, calcite (CaCO3), goethite (FeOOH), quartz (SiO2), barite (BaSO4) and zincite (ZnO), which appear to control the concentration and partition of the HMs in all areas of the sub-basin. The aragonite and calcite in all samples were saturated, except in sample S2, where AMD was reported.
Jarosite [(K,Na,H)Fe
3(SO
4)
2(OH)
6] and goethite (FeOOH) were subsaturated in sample S2. However, at sites S3, S8 and S10 the saturation indices were >0 for jarosite, so this phase could precipitate at these sites, which are close to S2 (
Figure 1). All samples after S11 through to S24 were unsaturated in Jarosite, indicating the aqueous phase. Goethite was saturated in all other samples, even in the rainy season.
Talavera Mendoza et al. [
9] and Romero et al. [
43] identified fluorescent minerals and gypsum as evidence of proton neutralization by calcite. These fluorescent minerals were observed in the dry season at points S2, S3, and S4 and were identified near the sampling sites. For the Taxco-Cocula River segment at site S2, cuprousferrite (CuFeO
2) was supersaturated, while Anglesite (PbSO
4), tenorite (CuO), and compounds of As
2O
3, and CdSO
4 were subsaturated. These behaviors were related to pH = 2.9 and Eh 458.7 mV. The S3 site near the AMD generation site was the only site where reducing potential was recorded (−65.4 mV), and here the compounds were saturated, Otavita (CdCO
3) Tenorite (CuO), AlOOH, and Ba
3(AsO
4)
2.
After sample S8 the compounds changed, highlighting the carbonates of cadmium, manganese, and magnesium, in addition to Ba3(AsO4)2, and AlOOH, that occurred mainly up to sample S14. From samples S16 to S23, the presence of willemite (Zn2SiO4) was present in a saturated form, while the MgCO3 saturation index was near equilibrium, and siderite (FeCO3) and SrSO4 were unsaturated.
For the Cacalotenango stream segment, in samples S4, S5, S6, and S7, the change in compounds, where willemite was saturated, stood out, as well as AlOOH and Al (OH)3, while siderite and MgCO3 were close to equilibrium.
For the Buenavista stream segment, in samples S17, S18, S19, S20, and S20, carbonate compounds, such as siderite, MgCO3, MnCO3, were subsaturated, as well as SrSO4, while compounds Ba3(AsO4)2, AlOOH and Zn2SiO4 were saturated.
The behaviors of the main reactive phases (aragonite, calcite, goethite, quartz, barite, and zincite) in the rainy season for the Taxco-Cocula sub-basin were like those o9f the dry season, except for samples S2 and S3; and other minerals were undersaturated or close to equilibrium.
Based on the results of this study, it is possible to affirm that the main geochemical process of AMD generation derives from the chemical oxidation of sulfides (especially pyrite). The process can be summarized in the following three steps: (1) pyrite oxidation in the presence of atmospheric oxygen and water is the main process generating acidity (H
+) [
44,
45,
46,
47]. Usually, a decrease in pH is associated with an increase in TDS, Fe
2+, and
, which were documented in the areas studied [
9,
35]; (2) if the surrounding environment is sufficiently oxidizing, ferrous ion oxidizes to ferric ion and Fe
3+ ions in solution can further oxidize additional pyrite and generate more acidity and release of
ions [
44]; (3) hydrolysis and precipitation of ferric complexes, being chemical reactions producing most of the acidity in generation of the AMD process, generate three moles of H
+ for one mole of pyrite [
40,
48]. The above does not consider the bacterial action that plays an important role in the oxidation process of Fe
+2 to Fe
+3 ions, based on sulfides. Chemolithotrophic bacteria act as catalysts, accelerating the oxidation process, decreasing pH and increasing ferric ion mobility in restricted environments [
49,
50,
51].
The generation of secondary minerals, such as jarosite and hematite, releases protons and some metastable minerals transform into more stable phases, such as goethite, again producing protons and releasing sulfate ions [
52,
53]. Thus, hydroxide precipitation and hydrolysis are identified as part of the geochemical sequence of AMD production. If the pH is close to 2, ferric hydrolysis products, such as Fe(OH)
3, are not stable and the Fe
3+ ions remain in the solution [
40]. Under these AMD conditions, minerals such as aluminosilicates can hydrolyze and release trivalent cations in the presence of aluminum in the main streams, evidenced by the high concentrations of HMs measured in the northern part of the recharge zone of the sub-basin (
Figure 1).
The oxidation of MS-type minerals is responsible for the release of divalent metal and sulfate anions, without the production of acidity MS + 2O
2 = M
2+ +
and, in the case of sphalerite, leach dissolved Zn and
, with variable amounts of Cd, while galena produces secondary anglesite (PbSO
4) in equilibrium with a Pb
2+ and
solution [
28,
40,
47]. Thus, the oxidation of MS-type minerals in acidic environments releases water-soluble ions, as reported in this work (
Tables S4 and S5, Supplementary Material).
The generation of AMD is a complex phenomenon that combines physical, chemical, and biological processes promoting the release and/or mobility of contaminants in restricted environments. Close to the tailings, such as at sampling site S2, there are no carbonate minerals (
Tables S4 and S5) due to the low pH (<3.0). After sample S3, the pH value increased to circumneutral until sample S23.
The release of H
+ in tailings depends on the number of minerals with the capacity to generate acidity, as well as minerals capable of neutralizing it, such as carbonates and silicates, which result in increase in pH from neutral to alkaline [
40]. On the other hand, calcite is one of the most common carbonate minerals, with rapid neutralization capacity. As a result of neutralization processes, secondary minerals, such as gypsum, and Fe-Mn hydroxides, such as jarosite and goethite, are obtained, which also play important roles in buffering, acidification, and sorption processes that can seasonally retain HM mobility. Quevedo-Castañón et al. [
35] reinforced the fact that the mobility of HMs in the Xochula stream, where AMD originates (near S2), are mainly controlled by Fe and Mn oxyhydroxides, as well as by minerals, such as otavite, cuprousferrite, tenorite, and willemite, identified in this work. HM sorption and desorption reactions were identified by Méndez-Ramírez and Armienta Hernández [
13] and Armienta et al. [
33].
The processes described above justify the neutral to alkaline pH values in the main streams of the sub-basin. The buffering action of bicarbonate, the dilution of rainwater, the minerals forming the rocks, and the new secondary minerals, allow neutralization processes to take place. Therefore, acidic pH values were not reported beyond the S2 sampling point, where there were AMD generation processes.
The sorption and desorption reactions activated throughout the dry and rainy seasons are means of transport of HMs, due to the low energy of drag and decrease in the water flow in the dry season, favoring the deposition of sediments. Subsequently, the high drag energy in the rainy season erodes the particles and promotes movement of ions in the solution [
35], and, thus, dispersion mechanisms are activated in each seasonal period.
The dispersion of HMs through these mechanisms leads to basin-wide concentrations in different proportions, as shown in this work, so that chemical processes of dissolution, oxidation, hydration, hydrolysis, neutralization, precipitation, sorption, and desorption can take place at the same time at different scales and at different locations.
Hydrogeological components, such as climate, topography, geology, and geomorphological features, determine the energy available for water movement in streams. Therefore, the chemical, physical and kinetic processes activated in each season could continue to act along the main river and a decrease in pollution could be reflected in the medium or long term. The result of this research shows the distribution of the spatial and temporal changes of mining origin contaminants along the Taxco-Cocula sub-basin.