Origin and Salinization Processes of Groundwater in the Semi-Arid Area of Zagora Graben, Southeast Morocco
Abstract
1. Introduction
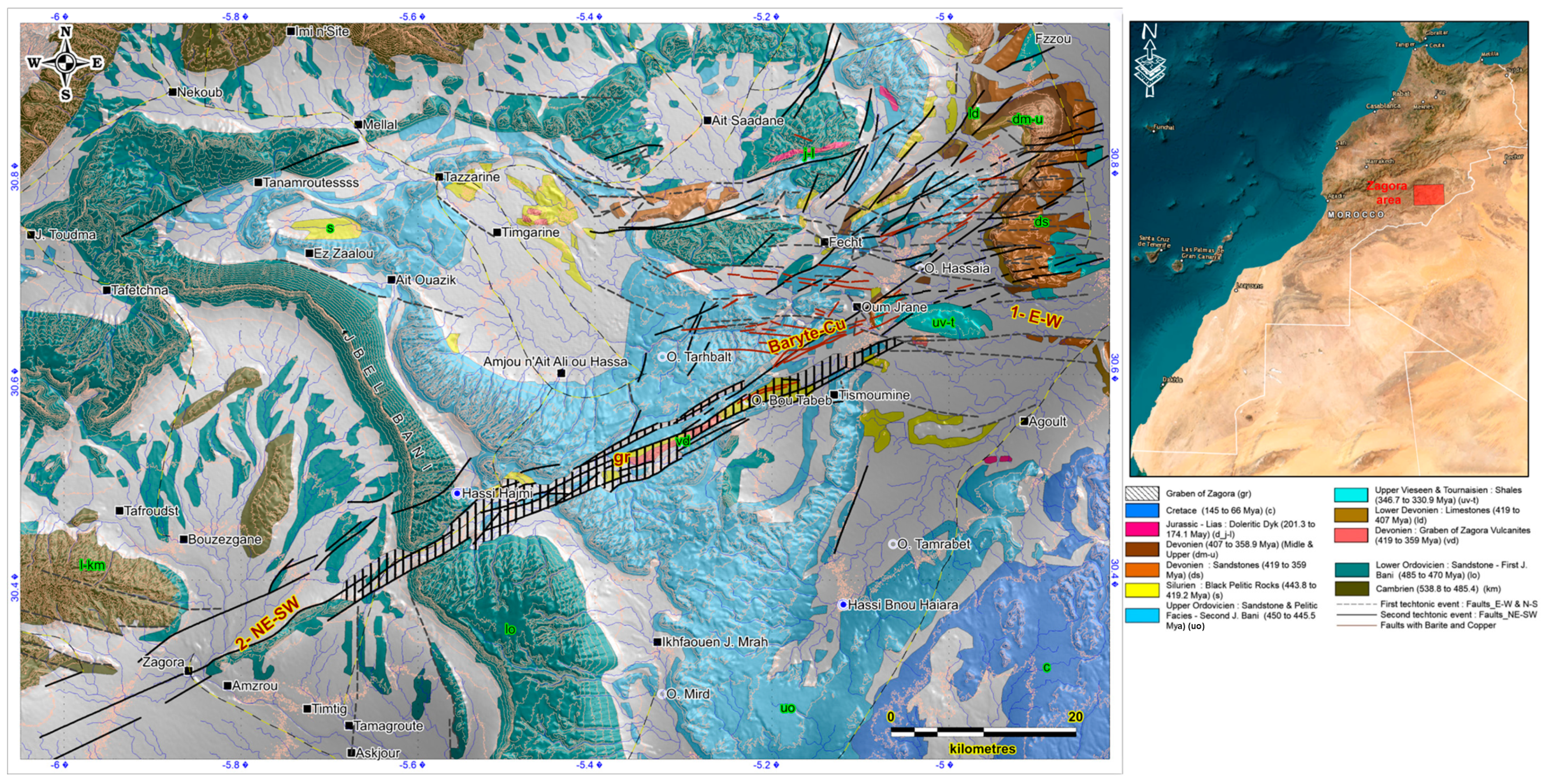
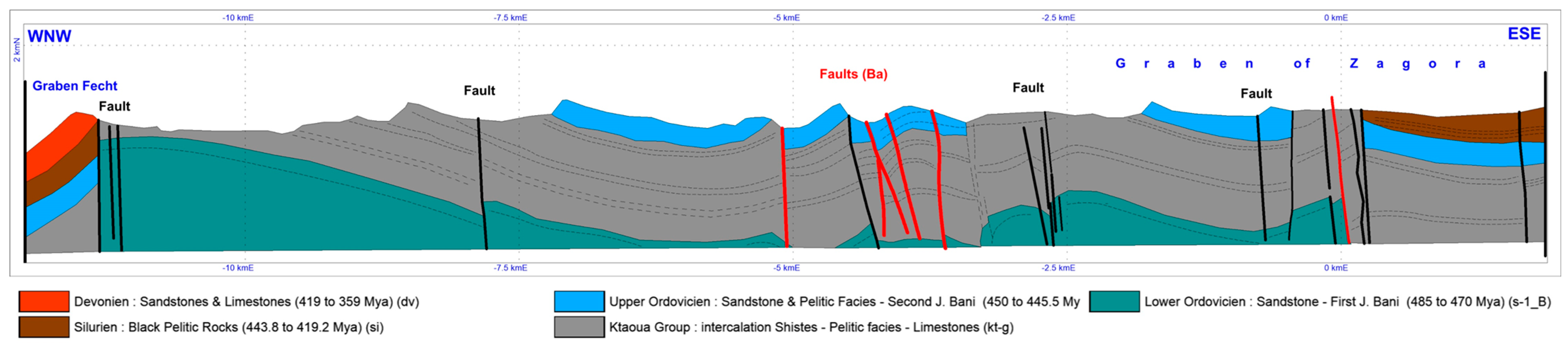
2. Geological Framework
2.1. Field Investigations and Synthetic Map Analysis
- The E–W system, developed exclusively in the northeastern part of the study area. This system of fractures affects mainly the sandstone and the pyelitic facies of the second Jbel Bani.
- The NE–SW system, which is remarkably pronounced within the graben of Zagora. These fractures are characterized with a thickness ranging from 0.5 m to 2 m and are usually filled with quartz-carbonates and, occasionally, with iron oxides.
2.2. Litho-Structural Mapping
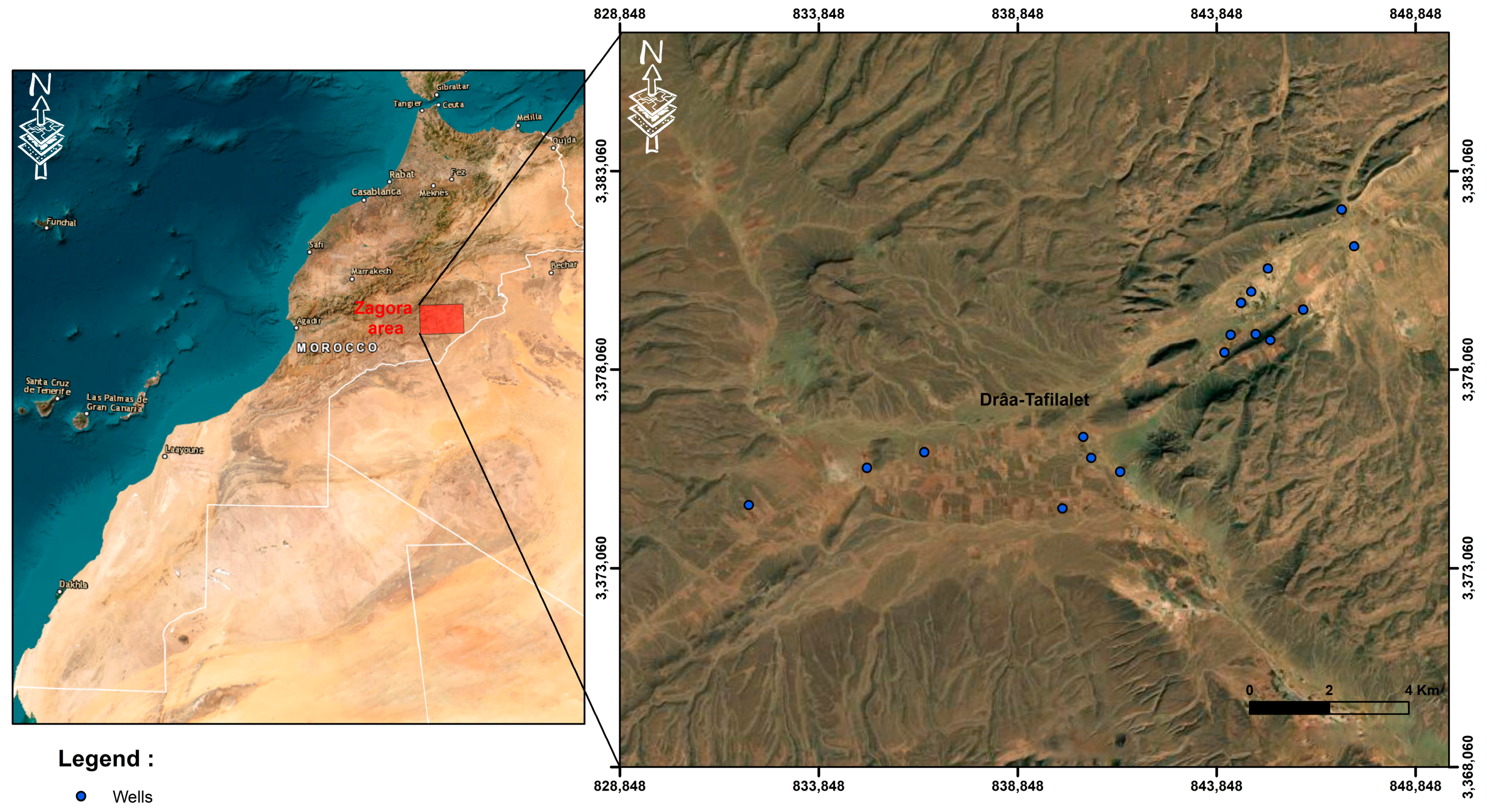
3. Materials and Methods
4. Results and Discussion
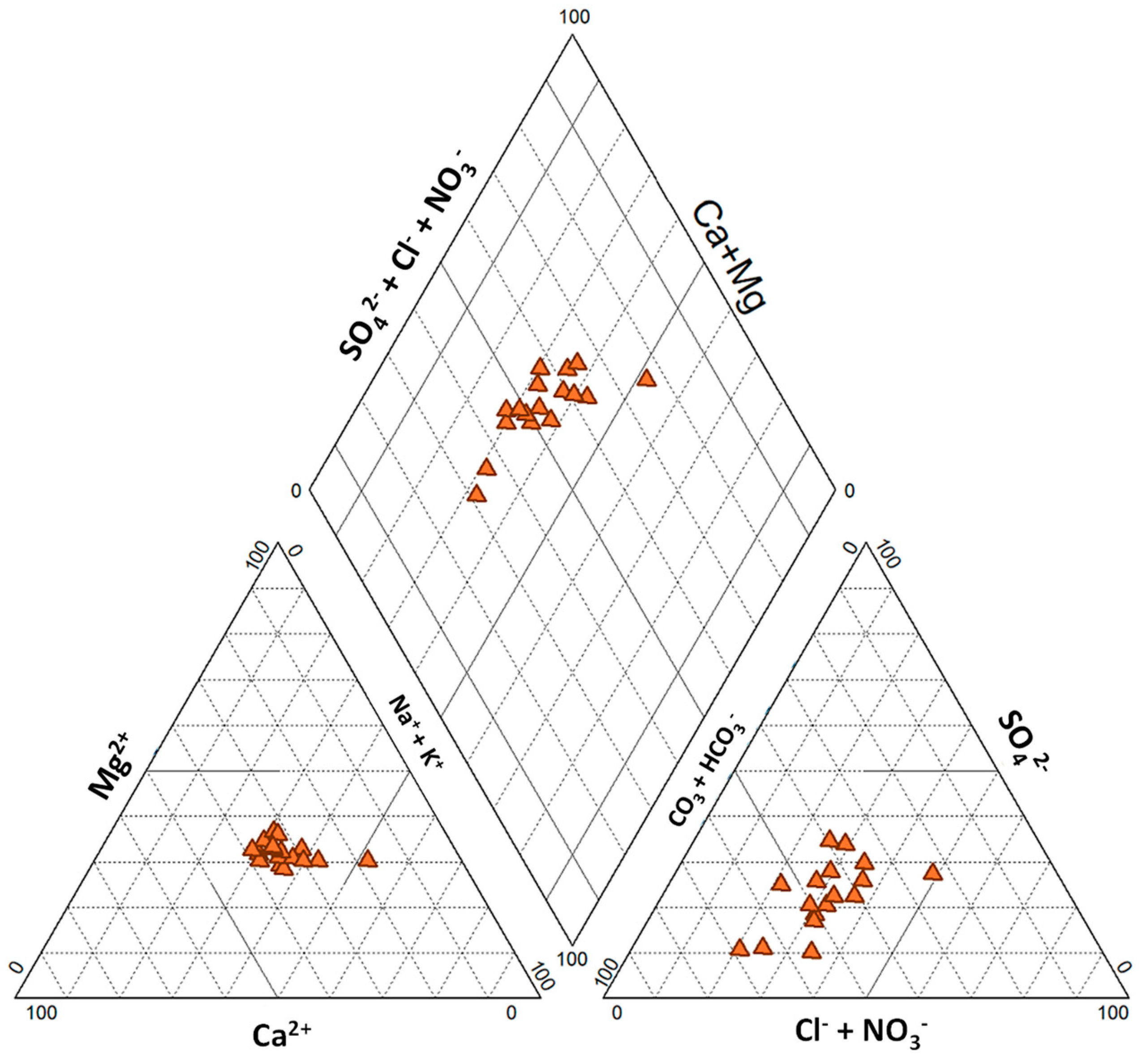
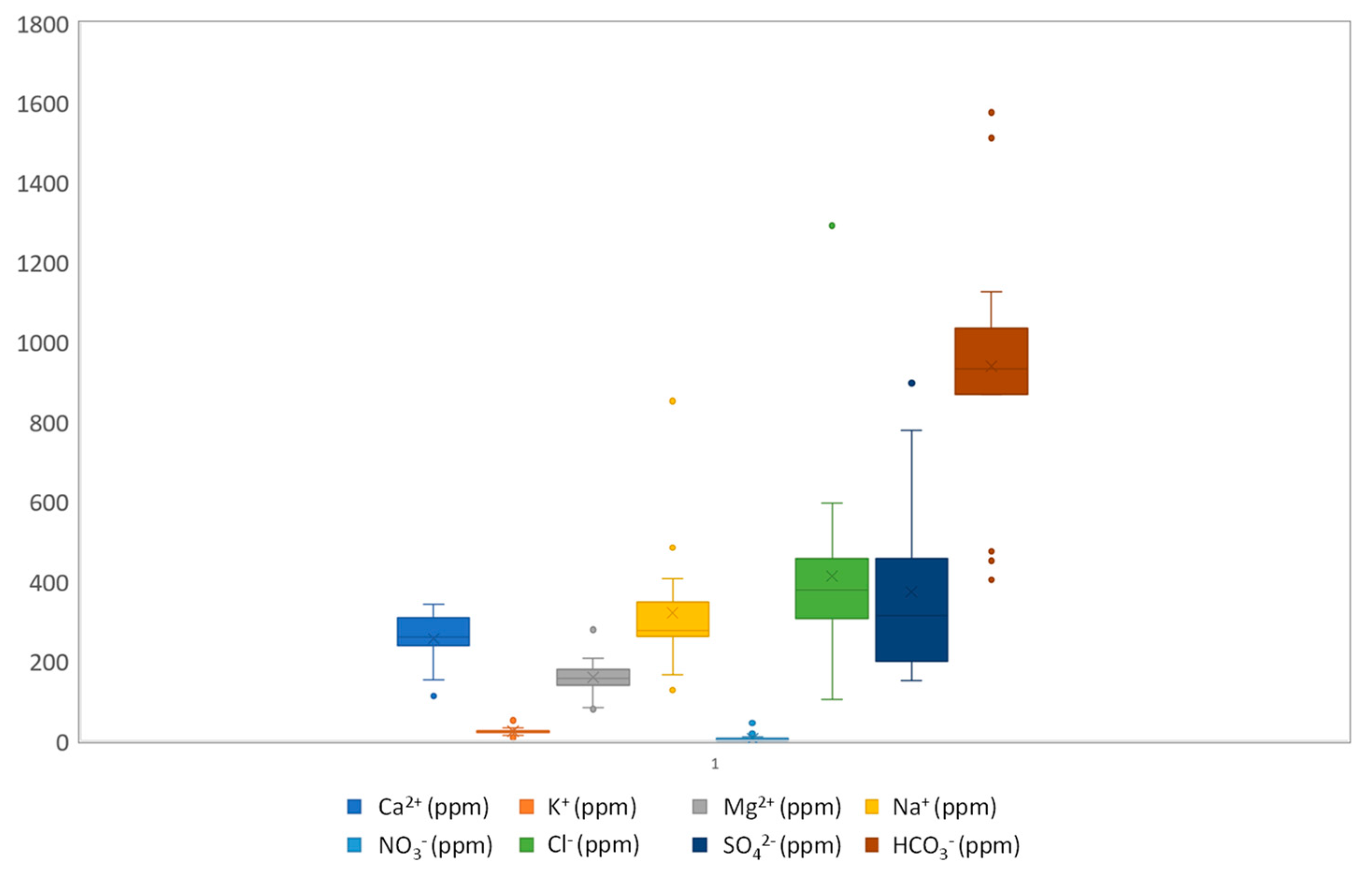
4.1. Hydrochemical Facies
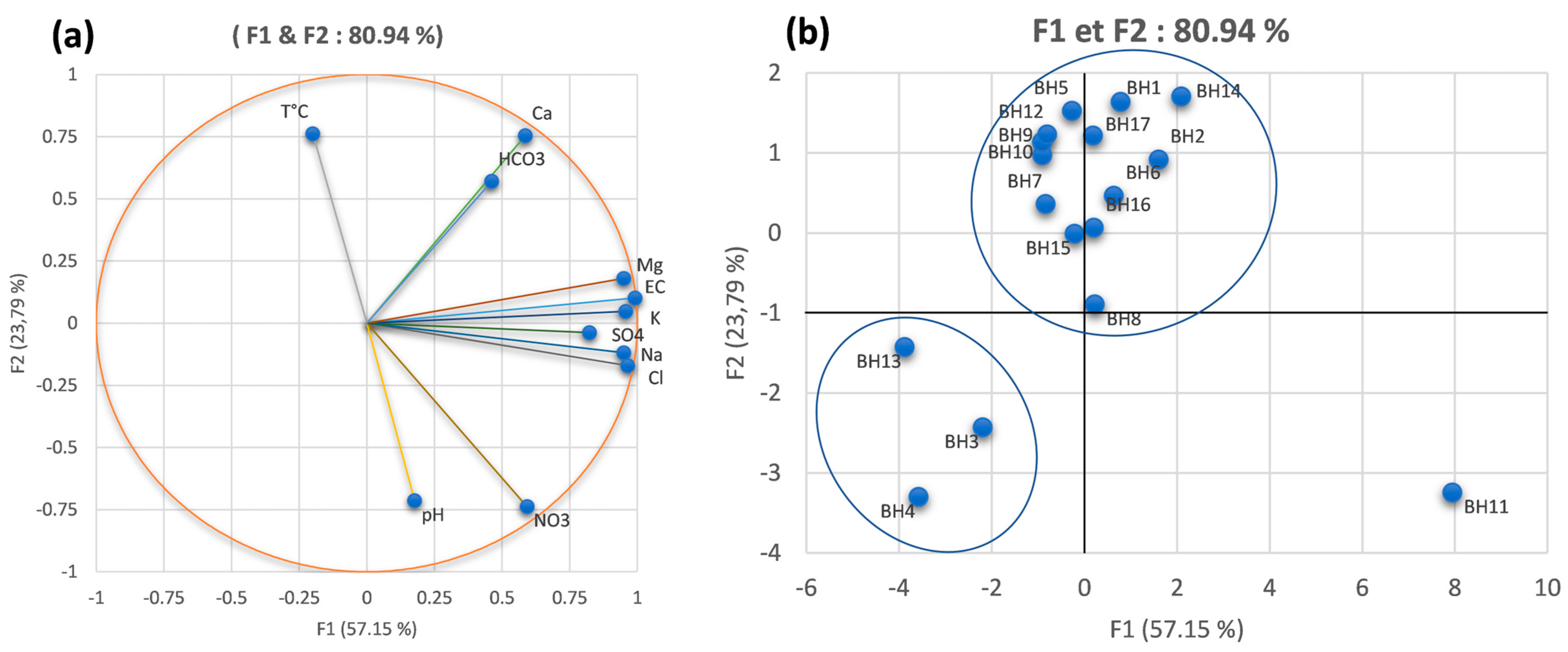
4.2. Principal Component Analysis
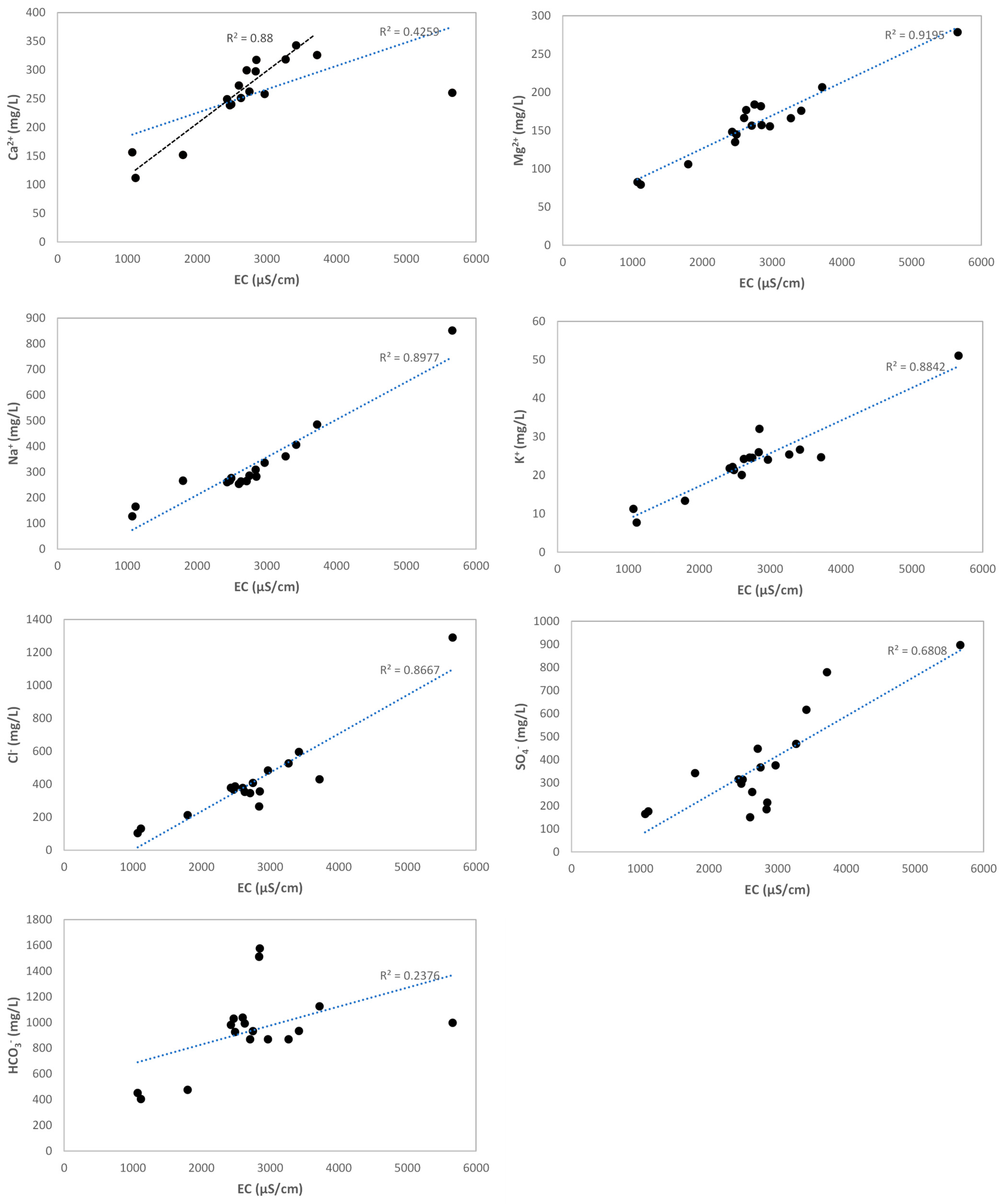
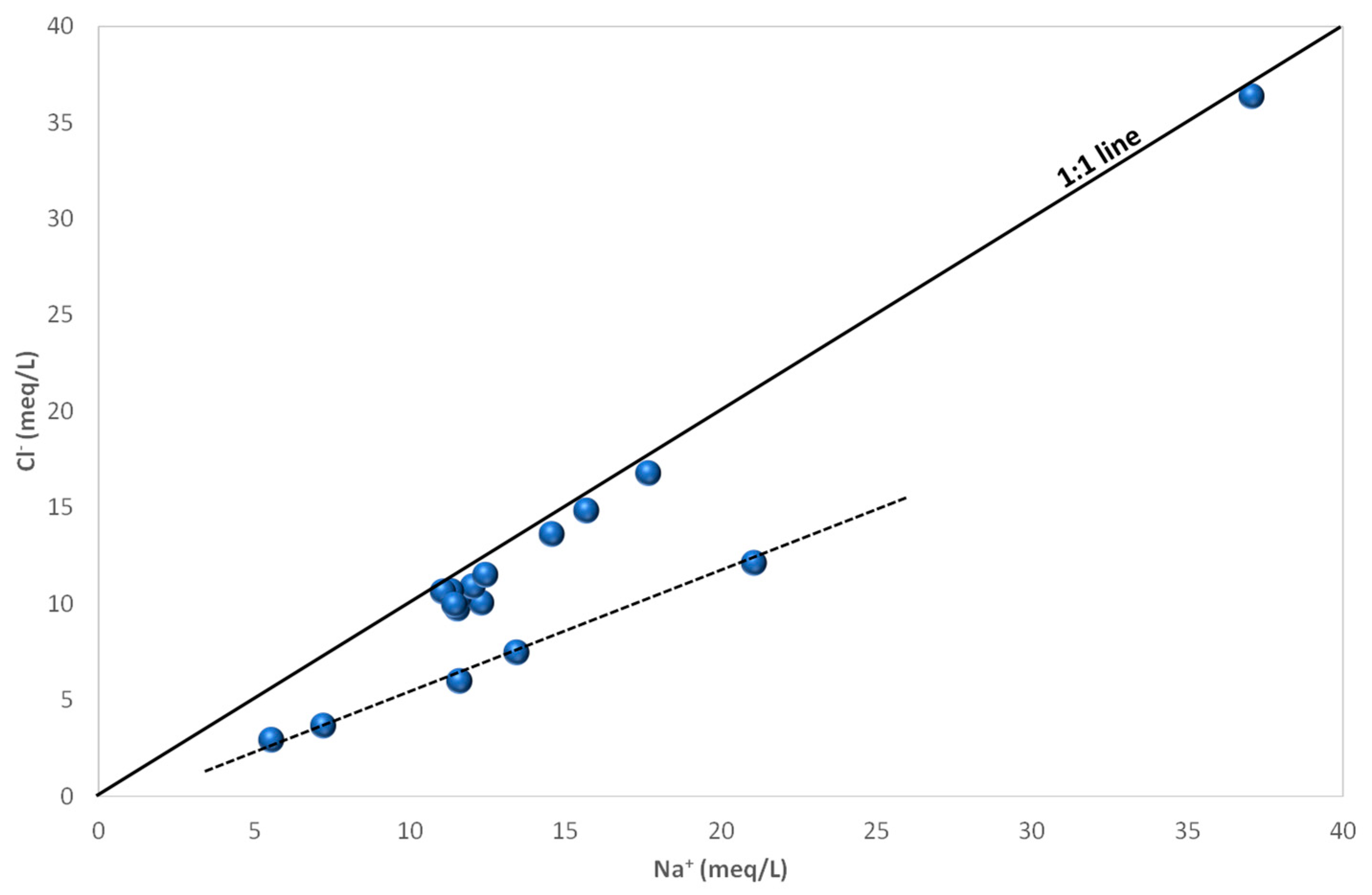
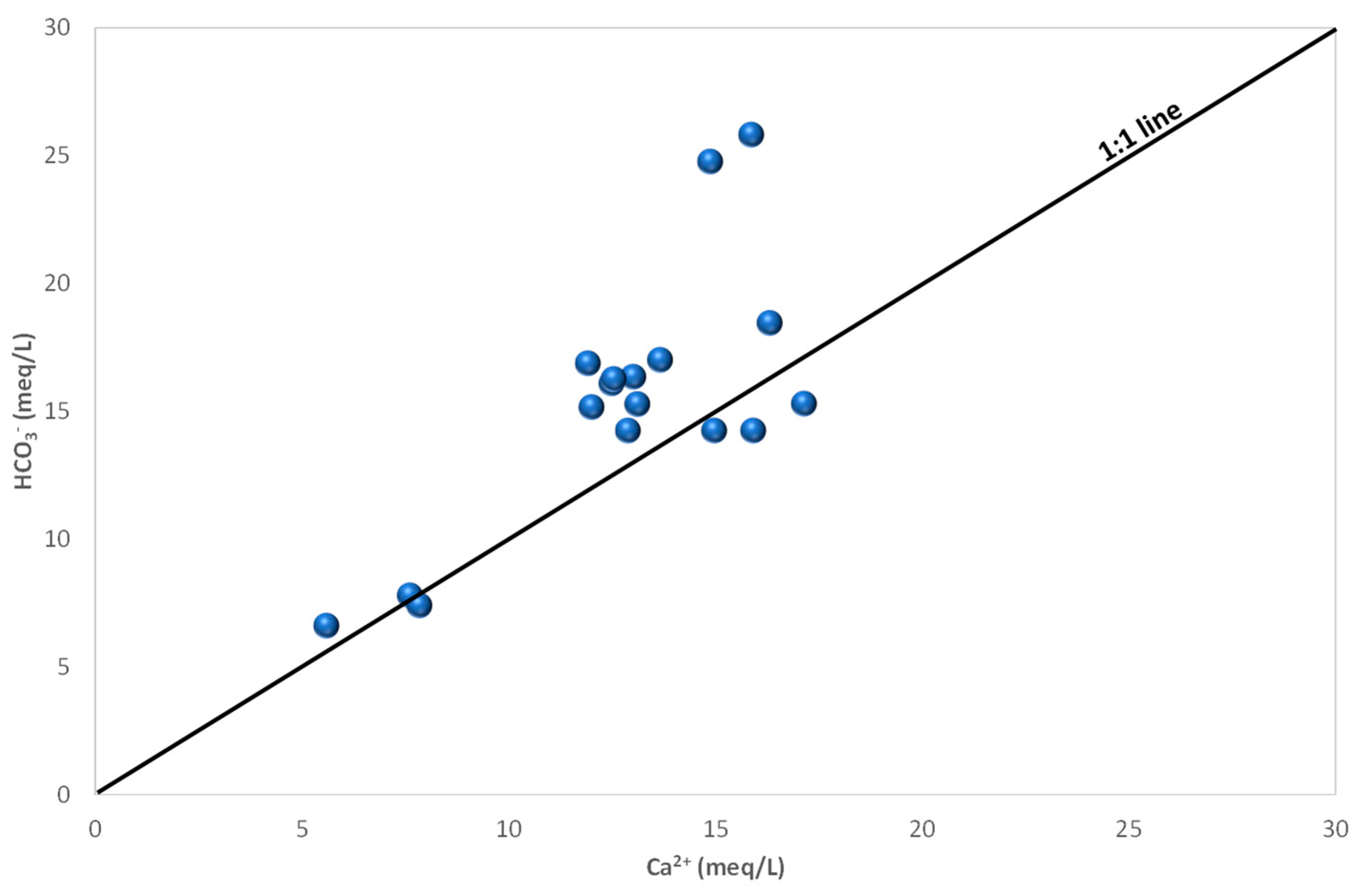
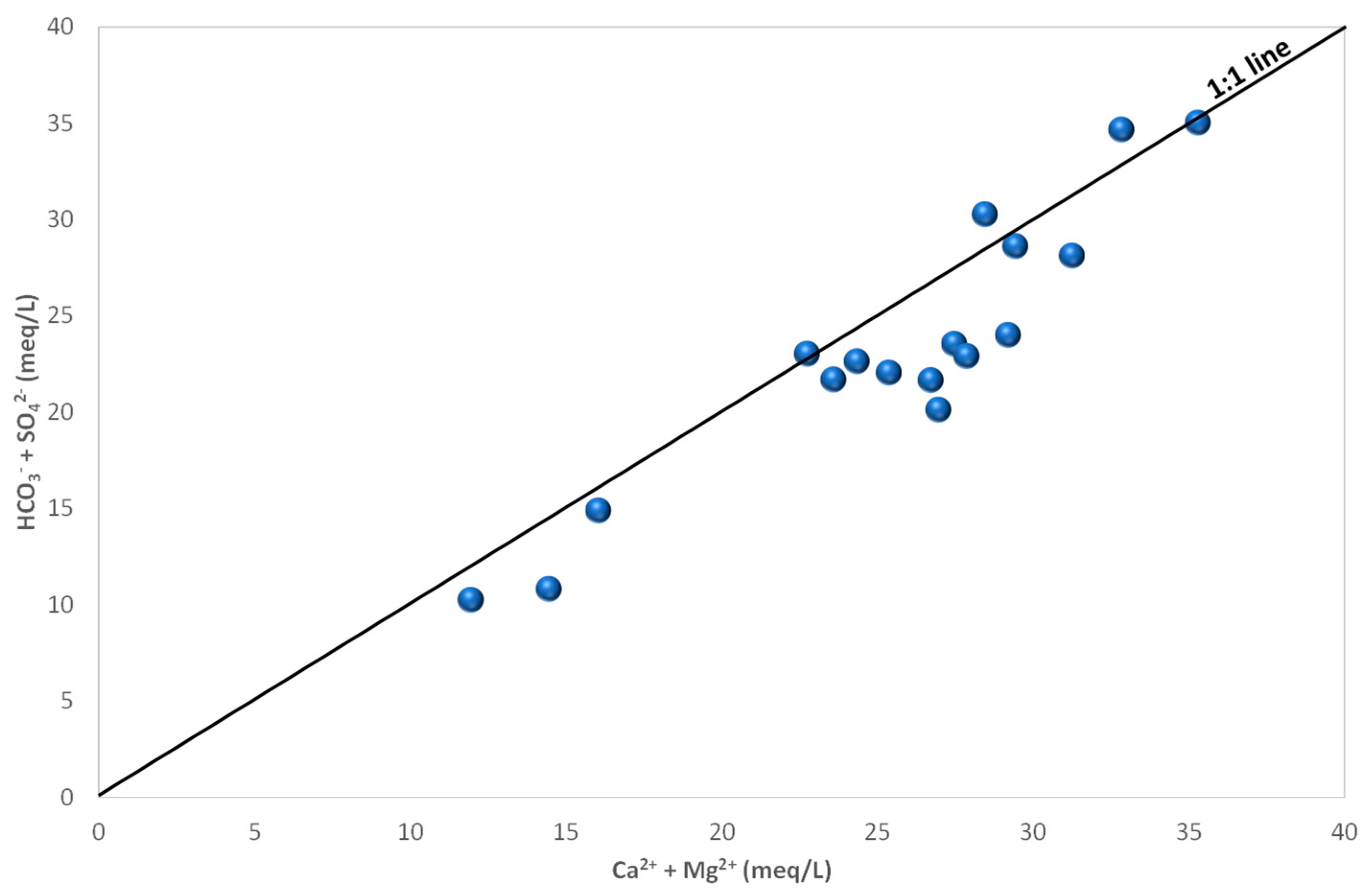
4.3. Groundwater Hydrochemistry and Mineralization Processes
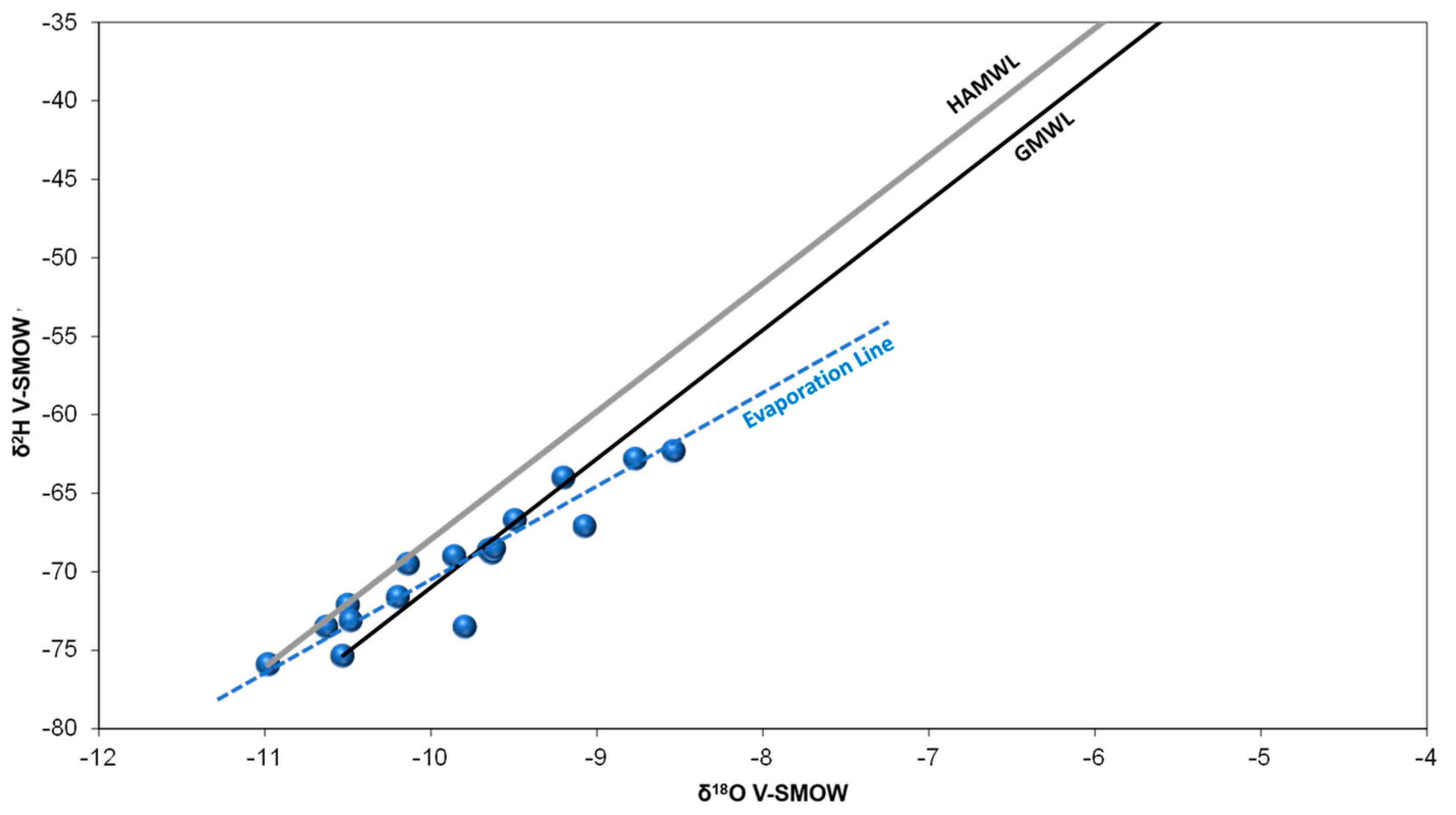
4.4. Isotopic Analysis and Origin of Groundwater
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mays, L.W. Groundwater Resources Sustainability: Past, Present, and Future. Water Resour. Manag. 2013, 27, 4409–4424. [Google Scholar] [CrossRef]
- Born, K.; Fink, A.H.; Paeth, H. Dry and Wet Periods in the Northwestern Maghreb for Present Day and Future Climate Conditions. Meteorol. Z. 2008, 17, 533–551. [Google Scholar] [CrossRef]
- Lgourna, Z.; Warner, N.; Bouchaou, L.; Boutaleb, S.; Hssaisoune, M.; Tagma, T.; Ettayfi, N.; Vengosh, A. Elucidating the Sources and Mechanisms of Groundwater Salinization in the Ziz Basin of Southeastern Morocco. Environ. Earth Sci. 2015, 73, 77–93. [Google Scholar] [CrossRef]
- Hssaisoune, M.; Bouchaou, L.; Sifeddine, A.; Bouimetarhan, I.; Chehbouni, A. Moroccan Groundwater Resources and Evolution with Global Climate Changes. Geosciences 2020, 10, 81. [Google Scholar] [CrossRef]
- Benjmel, K.; Amraoui, F.; Boutaleb, S.; Ouchchen, M.; Tahiri, A.; Touab, A. Mapping of Groundwater Potential Zones in Crystalline Terrain Using Remote Sensing, GIS Techniques, and Multicriteria Data Analysis (Case of the Ighrem Region, Western Anti-Atlas, Morocco). Water 2020, 12, 471. [Google Scholar] [CrossRef]
- Vengosh, A.; Gieskes, J.; Mahn, C. New Evidence for the Origin of Hypersaline Pore Fluids in the Mediterranean Basin. Chem. Geol. 2000, 163, 287–298. [Google Scholar] [CrossRef]
- Hsissou, Y.; Bouchaou, L.; Mudry, J.; Mania, J. Use of Chemical Tracing to Study Acquisition Modalities of the Mineralization and Behaviour of Unconfined Groundwaters under a Semi-Arid Climate: The Case of the Souss Plain (Morocco). Environ. Geol. 2002, 42, 672–680. [Google Scholar] [CrossRef]
- Bouchaou, L.; Michelot, J.L.; Vengosh, A.; Hsissou, Y.; Qurtobi, M.; Gaye, C.B.; Bullen, T.D.; Zuppi, G.M. Application of Multiple Isotopic and Geochemical Tracers for Investigation of Recharge, Salinization, and Residence Time of Water in the Souss–Massa Aquifer, Southwest of Morocco. J. Hydrol. 2008, 352, 267–287. [Google Scholar] [CrossRef]
- Ettayfi, N.; Bouchaou, L.; Michelot, J.L.; Tagma, T.; Warner, N.; Boutaleb, S.; Massault, M.; Lgourna, Z.; Vengosh, A. Geochemical and Isotopic (Oxygen, Hydrogen, Carbon, Strontium) Constraints for the Origin, Salinity, and Residence Time of Groundwater from a Carbonate Aquifer in the Western Anti-Atlas Mountains, Morocco. J. Hydrol. 2012, 438–439, 97–111. [Google Scholar] [CrossRef]
- Warner, N.; Lgourna, Z.; Bouchaou, L.; Boutaleb, S.; Tagma, T.; Hsaissoune, M.; Vengosh, A. Integration of Geochemical and Isotopic Tracers for Elucidating Water Sources and Salinization of Shallow Aquifers in the Sub-Saharan Drâa Basin, Morocco. Appl. Geochem. 2013, 34, 140–151. [Google Scholar] [CrossRef]
- Huebener, H.; Kerschgens, M. Downscaling of Current and Future Rainfall Climatologies for Southern Morocco. Part II: Climate Change Signals. Int. J. Climatol. 2007, 27, 1065–1073. [Google Scholar] [CrossRef]
- Driouech, F.; Déqué, M.; Sánchez-Gómez, E. Weather Regimes—Moroccan Precipitation Link in a Regional Climate Change Simulation. Glob. Planet. Chang. 2010, 72, 1–10. [Google Scholar] [CrossRef]
- Driouech, F.; Stafi, H.; Khouakhi, A.; Moutia, S.; Badi, W.; ElRhaz, K.; Chehbouni, A. Recent Observed Country-Wide Climate Trends in Morocco. Int. J. Climatol. 2021, 41, E855–E874. [Google Scholar] [CrossRef]
- Baidder, L.; Raddi, Y.; Tahiri, M.; Michard, A. Devonian Extension of the Pan-African Crust North of the West African Craton, and Its Bearing on the Variscan Forelanddeformation: Evidence from Eastern Anti-Atlas (Morocco). Geol. Soc. Lond. Spéc. Publ. 2008, 297, 453–465. [Google Scholar] [CrossRef]
- Boissavy, C. Etude Structurale et Métallogénique Des Filons Cuprifères Du Maider Occidental (Anti–Atlas Marocain). Ph.D. Thesis, Université Pierre et Marie Curie (Paris VI), Paris, France, 1979. [Google Scholar]
- Moumane, A.; El Ghazali, F.E.; Al Karkouri, J.; Delorme, J.; Batchi, M.; Chafiki, D.; Karmaoui, A. Monitoring Spatiotemporal Variation of Groundwater Level and Salinity under Land Use Change Using Integrated Field Measurements, GIS, Geostatistical, and Remote-Sensing Approach: Case Study of the Feija Aquifer, Middle Draa Watershed, Moroccan Sahara. Environ. Monit. Assess 2021, 193, 769. [Google Scholar] [CrossRef] [PubMed]
- Bossenbroek, L.; Ftouhi, H.; Kadiri, Z.; Kuper, M. Watermelons in the Desert in Morocco: Struggles Around a Groundwater Commons-in-the-Making. Water Altern. 2023, 16, 87–107. [Google Scholar]
- Karmaoui, A.; Ifaadassan, I.; Babqiqi, A.; Messouli, M.; Khebiza, M. Analysis of the Water Supply-Demand Relationship in the Middle Draa Valley, Morocco, under Climate Change and Socio-Economic Scenarios. JSRR 2016, 9, 1–10. [Google Scholar] [CrossRef]
- El Mandour, A.; El Yaouti, F.; Fakir, Y.; Zarhloule, Y.; Benavente, J. Evolution of Groundwater Salinity in the Unconfined Aquifer of Bou-Areg, Northeastern Mediterranean Coast, Morocco. Environ. Geol. 2008, 54, 491–503. [Google Scholar] [CrossRef]
- Yaouti, F.E.; Mandour, A.E.; Khattach, D.; Benavente, J.; Kaufmann, O. Salinization Processes in the Unconfined Aquifer of Bou-Areg (NE Morocco): A Geostatistical, Geochemical, and Tomographic Study. Appl. Geochem. 2009, 24, 16–31. [Google Scholar] [CrossRef]
- Elgettafi, M.; Himi, M.; Casas, A.; Elmandour, A. Hydrochemistry Caracterisation of Groundwater Salinity in Kert Aquifer, NE Morocco. Geogr. Tech. 2011, 2, 15–22. [Google Scholar]
- Elgettafi, M.; Elmandour, A.; Himi, M.; Casas, A. The Use of Environmental Markers to Identify Groundwater Salinization Sources in a Neogene Basin, Kert Aquifer Case, NE Morocco. Int. J. Environ. Sci. Technol. 2013, 10, 719–728. [Google Scholar] [CrossRef]
- Krimissa, S.; Michelot, J.-L.; Bouchaou, L.; Mudry, J.; Hsissou, Y. Sur l’origine Par Altération Du Substratum Schisteux de La Minéralisation Chlorurée Des Eaux d’une Nappe Côtière Sous Climat Semi-Aride (Chtouka-Massa, Maroc). Comptes Rendus Geosci. 2004, 336, 1363–1369. [Google Scholar] [CrossRef]
- Mdiker, N.; ElAchheb, A.; El Mandour, A.; Younsi, A.; El Maliki, S. Description Quantitative de La Salinisation Des Eaux Souterraines Côtières (Sahel El Haouzia, Maroc). Afr. Geosci. Rev. 2008, 15, 261–278. [Google Scholar]
- Rochdane, S.; Reddy, D.; El Mandour, A. Hydrochemical and Isotopic Characterisation of Eastern Haouz Plain Groundwater, Morocco. Environ. Earth Sci. 2015, 73, 3487–3500. [Google Scholar] [CrossRef]
- Bucher, K.; Stober, I. Fluids in the Upper Continental Crust. Geofluids 2010, 10, 241–253. [Google Scholar]
- Cartwright, I.; Weaver, T.R.; Cendón, D.I.; Fifield, L.K.; Tweed, S.O.; Petrides, B.; Swane, I. Constraining Groundwater Flow, Residence Times, Inter-Aquifer Mixing, and Aquifer Properties Using Environmental Isotopes in the Southeast Murray Basin, Australia. Appl. Geochem. 2012, 27, 1698–1709. [Google Scholar] [CrossRef]
- Dogramaci, S.; Skrzypek, G.; Dodson, W.; Grierson, P.F. Stable Isotope and Hydrochemical Evolution of Groundwater in the Semi-Arid Hamersley Basin of Subtropical Northwest Australia. J. Hydrol. 2012, 475, 281–293. [Google Scholar] [CrossRef]
- Siebert, C.; Rödiger, T.; Mallast, U.; Gräbe, A.; Guttman, J.; Laronne, J.B.; Storz-Peretz, Y.; Greenman, A.; Salameh, E.; Al-Raggad, M.; et al. Challenges to Estimate Surface- and Groundwater Flow in Arid Regions: The Dead Sea Catchment. Sci. Total Environ. 2014, 485–486, 828–841. [Google Scholar] [CrossRef]
- Robert–Charrue, C. Géologie Structurale de l’Anti–Atlas Oriental, Maroc; Université de Neuchâtel: Neuchâtel, Switzerland, 2006. [Google Scholar]
- Baidder, L.; Michard, A.; Soulaimani, A.; Fekkak, A.; Eddebbi, A.; Rjimati, E.-C.; Raddi, Y. Fold Interference Pattern in Thick-Skinned Tectonics; a Case Study from the External Variscan Belt of Eastern Anti-Atlas, Morocco. J. Afr. Earth Sci. 2016, 119, 204–225. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Ma, H.; Wang, L.; Martín, J.D. Identification of the Hydrogeochemical Processes and Assessment of Groundwater Quality Using Classic Integrated Geochemical Methods in the Southeastern Part of Ordos Basin, China. Environ. Pollut. 2016, 218, 879–888. [Google Scholar] [CrossRef]
- Chang, J.; Wang, G. Major Ions Chemistry of Groundwater in the Arid Region of Zhangye Basin, Northwestern China. Environ. Earth Sci. 2010, 61, 539–547. [Google Scholar] [CrossRef]
- Al-Mashreki, M.H.; Eid, M.H.; Saeed, O.; Székács, A.; Szűcs, P.; Gad, M.; Abukhadra, M.R.; AlHammadi, A.A.; Alrakhami, M.S.; Alshabibi, M.A.; et al. Integration of Geochemical Modeling, Multivariate Analysis, and Irrigation Indices for Assessing Groundwater Quality in the Al-Jawf Basin, Yemen. Water 2023, 15, 1496. [Google Scholar] [CrossRef]
- Gad, M.; Gaagai, A.; Eid, M.H.; Szűcs, P.; Hussein, H.; Elsherbiny, O.; Elsayed, S.; Khalifa, M.M.; Moghanm, F.S.; Moustapha, M.E.; et al. Groundwater Quality and Health Risk Assessment Using Indexing Approaches, Multivariate Statistical Analysis, Artificial Neural Networks, and GIS Techniques in El Kharga Oasis, Egypt. Water 2023, 15, 1216. [Google Scholar] [CrossRef]
- Farid, I.; Zouari, K.; Rigane, A.; Beji, R. Origin of the Groundwater Salinity and Geochemical Processes in Detrital and Carbonate Aquifers: Case of Chougafiya Basin (Central Tunisia). J. Hydrol. 2015, 530, 508–532. [Google Scholar] [CrossRef]
- Ben Moussa, A.; Zouari, K.; Oueslati, N. Geochemical Study of Groundwater Mineralization in the Grombalia Shallow Aquifer, North-Eastern Tunisia: Implication of Irrigation and Industrial Waste Water Accounting. Environ. Geol. 2009, 58, 555–566. [Google Scholar] [CrossRef]
- John, B.; Das, S. Role of Electrical Conductivity on Salinity and Mineralization Due to Groundwater Level Fluctuations in Kolkata City. IOP Conf. Ser. Earth Environ. Sci. 2020, 505, 012021. [Google Scholar] [CrossRef]
- Elango, L.; Kannan, R. Chapter 11 Rock–Water Interaction and Its Control on Chemical Composition of Groundwater. In Concepts and Applications in Environmental Geochemistry; Sarkar, D., Datta, R., Hannigan, R., Eds.; Developments in Environmental Science; Elsevier: Amsterdam, The Netherlands, 2007; Volume 5, pp. 229–243. [Google Scholar]
- Medjani, F.; Djidel, M.; Labar, S.; Bouchagoura, L.; Rezzag Bara, C. Groundwater Physico-Chemical Properties and Water Quality Changes in Shallow Aquifers in Arid Saline Wetlands, Ouargla, Algeria. Appl. Water Sci 2021, 11, 82. [Google Scholar] [CrossRef]
- Ravikumar, P.; Somashekar, R.K. Principal Component Analysis and Hydrochemical Facies Characterization to Evaluate Groundwater Quality in Varahi River Basin, Karnataka State, India. Appl. Water Sci 2017, 7, 745–755. [Google Scholar] [CrossRef]
- Ait Lemkademe, A.; Michelot, J.-L.; Benkaddour, A.; Hanich, L.; Maliki, A. Isotopic and Hydrochemical Approach to the Functioning of an Aquifer System in the Region of Marrakech (Morocco): Isotopic and Hydrochemical Approach to the Functioning of an Aquifer. Rapid Commun. Mass Spectrom. 2011, 25, 2785–2792. [Google Scholar] [CrossRef]
- Ait Lemkademe, A.; Michelot, J.-L.; Benkaddour, A.; Hanich, L.; Heddoun, O. Origin of Groundwater Salinity in the Draa Sfar Polymetallic Mine Area Using Conservative Elements (Morocco). Water 2022, 15, 82. [Google Scholar] [CrossRef]
- Henderson, T. Geochemistry of Ground-Water in Two Sandstone Aquifer Systems in the Northern Great Plains in Parts of Montana and Wyoming, North Dakota, and South Dakota; Professional Paper; United States Government Printing Office: Reston, VA, USA, 1985. [Google Scholar]
- Madrigal-Solís, H.; Jiménez-Gavilán, P.; Vadillo-Pérez, I.; Fonseca-Sánchez, A.; Calderón-Sánchez, H.; Quesada-Hernández, L.; Gómez-Cruz, A. Discriminant Model and Hydrogeochemical Processes for Characterizing Preferential Flow Paths in Four Interconnected Volcanic Aquifers in Costa Rica. Hydrogeol. J. 2022, 30, 2315–2340. [Google Scholar] [CrossRef]
- Su, Y. A Study of Shallow Groundwater Quality Distribution in the Ordos Cretaceous Artisan Basin Based on GIS. Hydrogeol. Eng. Geol. J. 2009, 36, 24–29. [Google Scholar]
- Herczeg, A.L.; Edmunds, W.M. Inorganic Ions as Tracers. In Environmental Tracers in Subsurface Hydrology; Springer: Boston, MA, USA, 2000; pp. 31–77. [Google Scholar]
- Choi, B.-Y.; Yun, S.-T.; Kim, K.-H.; Choi, H.-S.; Chae, G.-T.; Lee, P.-K. Geochemical Modeling of CO2–Water–Rock Interactions for Two Different Hydrochemical Types of CO2-Rich Springs in Kangwon District, Korea. J. Geochem. Explor. 2014, 144, 49–62. [Google Scholar] [CrossRef]
- Koh, Y.-K.; Choi, B.-Y.; Yun, S.-T.; Choi, H.-S.; Mayer, B.; Ryoo, S.-W. Origin and Evolution of Two Contrasting Thermal Groundwaters (CO2-Rich and Alkaline) in the Jungwon Area, South Korea: Hydrochemical and Isotopic Evidence. J. Volcanol. Geotherm. Res. 2008, 178, 777–786. [Google Scholar] [CrossRef]
- Raibi, F.; Benkaddour, A.; Hanich, L.; Chehbouni, A. Caractérisation Hydrogéochimique et Isotopique Des Eaux de Surface et Des Eaux Souterraines Du Bassin Versant de Tensift. In Proceedings of the Gestion Intégrée des Ressources en Eaux et Défis du Développement Dura-ble (GIRE3D), Marrakesh, Marocco, 23–25 May 2006. [Google Scholar]
| Name | EC (µS/cm) | pH | T °C | Ca2+ (mg/L) | K+ (mg/L) | Mg2+ (mg/L) | Na+ (mg/L) | NO3− (mg/L) | Cl− (mg/L) | SO42− (mg/L) | HCO3− (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BH1 | 3270 | 5.70 | 27 | 318 | 25 | 166 | 361 | 0 | 490 | 469 | 869 |
| BH2 | 3420 | 5.84 | 26 | 343 | 27 | 176 | 406 | 3 | 536 | 616 | 933 |
| BH3 | 1799 | 6.10 | 25 | 152 | 13 | 106 | 266 | 10 | 213 | 341 | 475 |
| BH4 | 1118 | 6.68 | 26 | 112 | 8 | 79 | 166 | 18 | 131 | 175 | 403 |
| BH5 | 2710 | 5.74 | 27 | 299 | 25 | 156 | 265 | 0 | 346 | 447 | 869 |
| BH6 | 2850 | 6.76 | 26 | 317 | 32 | 157 | 283 | 0 | 357 | 214 | 1575 |
| BH7 | 2470 | 5.86 | 26 | 239 | 22 | 135 | 266 | 0 | 367 | 296 | 1029 |
| BH8 | 2970 | 6.26 | 25 | 258 | 24 | 155 | 335 | 0 | 452 | 375 | 869 |
| BH9 | 2490 | 5.83 | 27 | 240 | 21 | 145 | 276 | 0 | 367 | 314 | 925 |
| BH10 | 2430 | 5.83 | 27 | 249 | 22 | 148 | 261 | 0 | 329 | 315 | 981 |
| BH11 | 5660 | 6.68 | 25 | 260 | 51 | 279 | 852 | 45 | 1171 | 897 | 997 |
| BH12 | 2600 | 5.98 | 27 | 273 | 20 | 166 | 254 | 0 | 378 | 150 | 1037 |
| BH13 | 1071 | 6.18 | 26 | 157 | 11 | 83 | 128 | 0 | 104 | 164 | 451 |
| BH14 | 3720 | 5.92 | 27 | 326 | 25 | 207 | 485 | 1 | 431 | 779 | 1126 |
| BH15 | 2630 | 6.08 | 26 | 251 | 24 | 177 | 263 | 6 | 354 | 260 | 992 |
| BH16 | 2750 | 5.98 | 26 | 262 | 25 | 184 | 286 | 8 | 389 | 366 | 932 |
| BH17 | 2840 | 6.09 | 26 | 298 | 26 | 182 | 309 | 0 | 266 | 185 | 1511 |
| Name | δ18O ‰ | δ2H ‰ |
|---|---|---|
| BH1 | −9.5 | −66.7 |
| BH2 | −9.65 | −68.6 |
| BH3 | −9.63 | −68.8 |
| BH4 | −9.62 | −68.5 |
| BH5 | −8.77 | −62.8 |
| BH6 | −10.14 | −69.5 |
| BH7 | −10.53 | −75.35 |
| BH8 | −10.98 | −75.9 |
| BH9 | −9.8 | −73.5 |
| BH10 | −10.2 | −71.6 |
| BH11 | −8.54 | −62.3 |
| BH12 | −9.2 | −64 |
| BH13 | −10.63 | −73.5 |
| BH14 | −10.5 | −72.1 |
| BH15 | −9.86 | −69 |
| BH16 | −9.07 | −67.1 |
| BH17 | −10.48 | −73.1 |
| Name | δ18O ‰ | Altitude of Recharge |
|---|---|---|
| BH6 | −10.14 | 2598 |
| BH8 | −10.98 | 2813 |
| BH13 | −10.63 | 2723 |
| BH14 | −10.52 | 2695 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Lemkademe, A.; El Ghorfi, M.; Zouhri, L.; Heddoun, O.; Khalil, A.; Maacha, L. Origin and Salinization Processes of Groundwater in the Semi-Arid Area of Zagora Graben, Southeast Morocco. Water 2023, 15, 2172. https://doi.org/10.3390/w15122172
Ait Lemkademe A, El Ghorfi M, Zouhri L, Heddoun O, Khalil A, Maacha L. Origin and Salinization Processes of Groundwater in the Semi-Arid Area of Zagora Graben, Southeast Morocco. Water. 2023; 15(12):2172. https://doi.org/10.3390/w15122172
Chicago/Turabian StyleAit Lemkademe, Anasse, Mustapha El Ghorfi, Lahcen Zouhri, Ouissal Heddoun, Abdessamad Khalil, and Lhou Maacha. 2023. "Origin and Salinization Processes of Groundwater in the Semi-Arid Area of Zagora Graben, Southeast Morocco" Water 15, no. 12: 2172. https://doi.org/10.3390/w15122172
APA StyleAit Lemkademe, A., El Ghorfi, M., Zouhri, L., Heddoun, O., Khalil, A., & Maacha, L. (2023). Origin and Salinization Processes of Groundwater in the Semi-Arid Area of Zagora Graben, Southeast Morocco. Water, 15(12), 2172. https://doi.org/10.3390/w15122172







