Growth Performance of Mytilus galloprovincialis Lamarck, 1819 under an Innovative Integrated Multi-Trophic Aquaculture System (IMTA) in the Mar Grande of Taranto (Mediterranean Sea, Italy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Work: Sampling and Processing
2.3. Microbiological Analyses
2.4. Chemical Analyses
2.5. Growth Parameter Analysis
3. Results
3.1. Growth Performance
3.2. Microbiology
3.3. PAHs and PCBs Concentration
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cardellicchio, N.; Annicchiarico, C.; Di Leo, A.; Giandomenico, S.; Spada, L. The Mar Piccolo of Taranto: An interesting marine ecosystem for the environmental problems studies. Environ. Sci. Pollut. Res. 2016, 23, 12495–12501. [Google Scholar] [CrossRef] [PubMed]
- Mossa, M.; Armenio, E.; Meftah, M.B.; Bruno, M.F.; De Padova, D.; De Serio, F. Meteorological and hydrodynamic data in the Mar Grande and Mar Piccolo, Italy, of the Coastal Engineering Laboratory (LIC) Survey, winter and summer 2015. Earth Syst. Sci. Data 2021, 13, 599–607. [Google Scholar] [CrossRef]
- Armenio, E.; Meftah, M.B.; De Padova, D.; De Serio, F.; Mossa, M. Monitoring system in mar grande basin (Ionian Sea). In Proceedings of the 2018 IEEE International Workshop on Metrology for the Sea, Learning to Measure Sea Health Parameters (MetroSea), Bari, Italy, 8–10 October 2018; pp. 104–109. [Google Scholar] [CrossRef]
- Caroppo, C.; Giordano, L.; Palmieri, N.; Bellio, G.; Bisci, A.P.; Portacci, G.; Sclafani, P.; Hopkins, T.S. Progress toward sustainable mussel aquaculture in Mar Piccolo, Italy. Ecol. Soc. 2012, 17, 10. [Google Scholar] [CrossRef]
- Avdelas, L.; Avdic-Mravlje, E.; Borges Marques, A.C.; Cano, S.; Capelle, J.J.; Carvalho, N.; Cozzolino, M.; Dennis, J.; Ellis, T.; Fernandez Polanco, J.M. The decline of mussel aquaculture in the European Union: Causes, economic impacts and opportunities. Rev. Aquac. 2021, 13, 91–118. [Google Scholar] [CrossRef]
- Zgouridou, A.; Tripidaki, E.; Giantsis, I.A.; Theodorou, J.A.; Kalaitzidou, M.; Raitsos, D.E.; Lattos, A.; Mavropoulou, A.; Sofianos, S.; Karagiannis, D. The current situation and potential effects of climate change on the microbial load of marine bivalves of the Greek coastlines: An integrative review. Environ. Microbiol. 2022, 24, 1012–1034. [Google Scholar] [CrossRef] [PubMed]
- Lattos, A.; Papadopoulos, D.K.; Feidantsis, K.; Karagiannis, D.; Giantsis, I.A.; Michaelidis, B. Are Marine Heatwaves Responsible for Mortalities of Farmed Mytilus galloprovincialis? A Pathophysiological Analysis of Marteilia Infected Mussels from Thermaikos Gulf, Greece. Animals 2022, 12, 2805. [Google Scholar] [CrossRef]
- Labarta, U.; Fernández-Reiriz, M.J. The Galician mussel industry: Innovation and changes in the last forty years. Ocean Coast. Manag. 2019, 167, 208–218. [Google Scholar] [CrossRef]
- Georgoulis, I.; Feidantsis, K.; Kouvas, D.; Lattos, A.; Delis, G.A.; Theodoridis, A.; Michaelidis, B.; Giantsis, I.A. The effect of seawater physical parameters in bivalve farming: Could systematic monitoring and early warning prevent negative impacts? A review focused on Vistonikos Gulf, North Aegean Sea. Int. J. Agric. Resour. Gov. Ecol. 2022, 18, 22–37. [Google Scholar] [CrossRef]
- Villasante, S.; Rodríguez-González, D.; Antelo, M.; Rivero-Rodríguez, S.; Lebrancón-Nieto, J. Why are prices in wild catch and aquaculture industries so different? Ambio 2013, 42, 937–950. [Google Scholar] [CrossRef]
- Galli, G.; Solidoro, C.; Lovato, T. Marine heat waves hazard 3D maps and the risk for low motility organisms in a warming Mediterranean Sea. Front. Mar. Sci. 2017, 4, 136. [Google Scholar] [CrossRef]
- Dayan, H.; McAdam, R.; Juza, M.; Masina, S.; Speich, S. Marine heat waves in the Mediterranean Sea: An assessment from the surface to the subsurface to meet national needs. Front. Mar. Sci. 2023, 10, 1–21. [Google Scholar] [CrossRef]
- Frölicher, T.L.; Laufkötter, C. Emerging risks from marine heat waves. Nat. Commun. 2018, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.; Bernard, I.; Villalba, A.; Oden, E.; Burioli, E.A.V.; Allain, G.; Trancart, S.; Bouchart, V.; Houssin, M. High mortality of mussels in northern Brittany–Evaluation of the involvement of pathogens, pathological conditions and pollutants. J. Invertebr. Pathol. 2020, 170, 107308. [Google Scholar] [CrossRef] [PubMed]
- Seuront, L.; Nicastro, K.R.; Zardi, G.I.; Goberville, E. Decreased thermal tolerance under recurrent heat stress conditions explains summer mass mortality of the blue mussel Mytilus edulis. Sci. Rep. 2019, 9, 17498. [Google Scholar] [CrossRef]
- Giordano, L.; Portacci, G.; Caroppo, C. Multidisciplinary tools for sustainable management of an ecosystem service: The case study of mussel farming in the Mar Piccolo of Taranto (Mediterranean, Ionian Sea). Ocean Coast. Manag. 2019, 176, 11–23. [Google Scholar] [CrossRef]
- National Geographic Italy. Acque Bollenti, Moria di Mitili a Taranto. 2015. Available online: http://www.nationalgeographic.it/food/2015/03/25/news/acque_bollenti_moria_di_mitili_a_taranto-2864969/ (accessed on 28 April 2023).
- Danioux, C.; Bompais, X.; Loste, C.; Paquotte, P. Offshore mollusc production in the Mediterranean basin. In Mediterranean Offshore Mariculture; Muir, J., Basurco, B., Eds.; Options Méditerranéennes: Série B. Etudes et Recherches; CIHEAM-IAMZ: Zaragoza, Spain, 2000; pp. 115–140. [Google Scholar]
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the art and challenges for offshore integrated multi-trophic aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Danovaro, R.; Gambi, C.; Luna, G.M.; Mirto, S. Sustainable impact of mussel farming in the Adriatic Sea (Mediterranean Sea): Evidence from biochemical, microbial and meiofaunal indicators. Mar. Pollut. Bull. 2004, 49, 325–333. [Google Scholar] [CrossRef]
- Fabi, G.; Manoukian, S.; Spagnolo, A. Impact of an open-sea suspended mussel culture on macrobenthic community (Western Adriatic Sea). Aquaculture 2009, 289, 54–63. [Google Scholar] [CrossRef]
- Gallardi, D. Effects of bivalve aquaculture on the environment and their possible mitigation: A review. Fish. Aquac. J. 2014, 5, 105. [Google Scholar] [CrossRef]
- Kralj, M.; De Vittor, C.; Comici, C.; Relitti, F.; Auriemma, R.; Alabiso, G.; Del Negro, P. Recent evolution of the physical–chemical characteristics of a Site of National Interest—The Mar Piccolo of Taranto (Ionian Sea)—And changes over the last 20 years. Environ. Sci. Pollut. Res. 2016, 23, 12675–12690. [Google Scholar] [CrossRef]
- Cardellicchio, N.; Buccolieri, A.; Giandomenico, S.; Lopez, L.; Pizzulli, F.; Spada, L. Organic pollutants (PAHs, PCBs) in sediments from the mar piccolo in taranto (ionian sea, southern Italy). Mar. Pollut. Bull. 2007, 55, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Fisk, A.T.; Hobson, K.A.; Norstrom, R.J. Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the Northwater Polynya marine food web. Environ. Sci. Technol. 2001, 35, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Okay, O.S.; Karacık, B.; Başak, S.; Henkelmann, B.; Bernhöft, S.; Schramm, K.-W. PCB and PCDD/F in sediments and mussels of the Istanbul strait (Turkey). Chemosphere 2009, 76, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Perugini, M.; Visciano, P.; Giammarino, A.; Manera, M.; Di Nardo, W.; Amorena, M. Polycyclic aromatic hydrocarbons in marine organisms from the Adriatic Sea, Italy. Chemosphere 2007, 66, 1904–1910. [Google Scholar] [CrossRef]
- Storelli, M.M.; Barone, G.; Perrone, V.G.; Storelli, A. Risk characterization for polycyclic aromatic hydrocarbons and toxic metals associated with fish consumption. J. Food Compos. Anal. 2013, 31, 115–119. [Google Scholar] [CrossRef]
- Naso, B.; Perrone, D.; Ferrante, M.C.; Bilancione, M.; Lucisano, A. Persistent organic pollutants in edible marine species from the Gulf of Naples, Southern Italy. Sci. Total Environ. 2005, 343, 83–95. [Google Scholar] [CrossRef]
- Deudero, S.; Box, A.; March, D.; Valencia, J.M.; Grau, A.M.; Tintore, J.; Calvo, M.; Caixach, J. Organic compounds temporal trends at some invertebrate species from the Balearics, Western Mediterranean. Chemosphere 2007, 68, 1650–1659. [Google Scholar] [CrossRef]
- Bajt, O.; Ramšak, A.; Milun, V.; Andral, B.; Romanelli, G.; Scarpato, A.; Mitrić, M.; Kupusović, T.; Kljajić, Z.; Angelidis, M. Assessing chemical contamination in the coastal waters of the Adriatic Sea using active mussel biomonitoring with Mytilus galloprovincialis. Mar. Pollut. Bull. 2019, 141, 283–298. [Google Scholar] [CrossRef]
- De Giovanni, A.; Abondio, P.; Frapiccini, E.; Luiselli, D.; Marini, M. Meta-Analysis of a New Georeferenced Database on Polycyclic Aromatic Hydrocarbons in Western and Central Mediterranean Seafood. Appl. Sci. 2022, 12, 2776. [Google Scholar] [CrossRef]
- Giandomenico, S.; Cardellicchio, N.; Spada, L.; Annicchiarico, C.; Di Leo, A. Metals and PCB levels in some edible marine organisms from the Ionian Sea: Dietary intake evaluation and risk for consumers. Environ. Sci. Pollut. Res. 2016, 23, 12596–12612. [Google Scholar] [CrossRef]
- Annicchiarico, C.; Assennato, G.; Blonda, B.; Cardellicchio, N.; Di Leo, A.; Giandomenico, S.; Lopez, L.; Spada, L.; Umgaro, N. Preliminary results of pollutants biomonitoring in coastal marine and transitional waters of Apulia Region (Southern Italy). Fresenius Env. Bull 2010, 19, 1841–1847. [Google Scholar]
- Biandolino, F.; Parlapiano, I.; Spada, L.; Di Leo, A.; Calò, M.; Fanelli, G.; Prato, E.; Giandomenico, S. Occurrence and patterns of nutritional traits and polycyclic aromatic hydrocarbons (PAHs) in sea cucumber (Holothuria polii) tissues: Benefits and risk for human health. Food Qual. Saf. 2022, 6, fyac005. [Google Scholar] [CrossRef]
- Di Leo, A.; Annicchiarico, C.; Cardellicchio, N.; Giandomenico, S.; Conversano, M.; Castellano, G.; Basile, F.; Martinelli, W.; Scortichini, G.; Spada, L. Monitoring of PCDD/Fs and dioxin-like PCBs and seasonal variations in mussels from the Mar Grande and the Mar Piccolo of Taranto (Ionian Sea, Southern Italy). Environ. Sci. Pollut. Res. 2014, 21, 13196–13207. [Google Scholar] [CrossRef] [PubMed]
- Giannico, O.V.; Desiante, F.; Basile, F.C.; Franco, E.; Baldacci, S.; Fragnelli, G.R.; Diletti, G.; Conversano, M. Dioxins and PCBs contamination in mussels from Taranto (Ionian Sea, Southern Italy): A seven years spatio-temporal monitoring study. Ann. Ist. Super. Sanita 2020, 56, 452–461. [Google Scholar] [PubMed]
- Suplicy, F.M. A review of the multiple benefits of mussel farming. Rev. Aquac. 2020, 12, 204–223. [Google Scholar] [CrossRef]
- Jones, A.R.; Alleway, H.K.; McAfee, D.; Reis-Santos, P.; Theuerkauf, S.J.; Jones, R.C. Climate-friendly seafood: The potential for emissions reduction and carbon capture in marine aquaculture. Bioscience 2022, 72, 123–143. [Google Scholar] [CrossRef]
- Wang, X.; Cuthbertson, A.; Gualtieri, C.; Shao, D. A review on mariculture effluent: Characterization and management tools. Water 2020, 12, 2991. [Google Scholar] [CrossRef]
- Barrington, K.; Chopin, T.; Robinson, S. Integrated multi-trophic aquaculture (IMTA) in marine temperate waters. In Integrated Mariculture: A Global Review; FAO Fisheries and Aquaculture Technical Paper No. 529; FAO: Rome, Italy, 2009; pp. 7–46. [Google Scholar]
- Knowler, D.; Chopin, T.; Martínez-Espiñeira, R.; Neori, A.; Nobre, A.; Noce, A.; Reid, G. The economics of Integrated Multi-Trophic Aquaculture: Where are we now and where do we need to go? Rev. Aquac. 2020, 12, 1579–1594. [Google Scholar] [CrossRef]
- Kerrigan, D.; Suckling, C.C. A meta-analysis of integrated multitrophic aquaculture: Extractive species growth is most successful within close proximity to open-water fish farms. Rev. Aquac. 2018, 10, 560–572. [Google Scholar] [CrossRef]
- Giangrande, A.; Pierri, C.; Arduini, D.; Borghese, J.; Licciano, M.; Trani, R.; Corriero, G.; Basile, G.; Cecere, E.; Petrocelli, A. An innovative IMTA system: Polychaetes, sponges and macroalgae co-cultured in a Southern Italian in-shore mariculture plant (Ionian Sea). J. Mar. Sci. Eng. 2020, 8, 733. [Google Scholar] [CrossRef]
- Sarà, G.; Zenone, A.; Tomasello, A. Growth of Mytilus galloprovincialis (mollusca, bivalvia) close to fish farms: A case of integrated multi-trophic aquaculture within the Tyrrhenian Sea. Hydrobiologia 2009, 636, 129–136. [Google Scholar] [CrossRef]
- Gvozdenović, S.; Mandić, M.; Pešić, V.; Nikolić, M.; Pešić, A.; Ikica, Z. Comparison between IMTA and monoculture farming of mussels (Mytilus galloprovincialis L.) in the Boka Kotorska Bay. Acta Adriat. Int. J. Mar. Sci. 2017, 58, 271–283. [Google Scholar] [CrossRef]
- Gvozdenović, S.; Mandić, M.; Peraš, I. Morphometry and condition index in Mediterranean mussel (Mytilus galloprovincialis Lamarck, 1819) from Boka Kotorska Bay (Montenegro, southeast Adriatic Sea). Stud. Mar. 2020, 33, 15–26. [Google Scholar]
- Chatzivasileiou, D.; Dimitriou, P.D.; Theodorou, J.; Kalantzi, I.; Magiopoulos, I.; Papageorgiou, N.; Pitta, P.; Tsapakis, M.; Karakassis, I. An IMTA in Greece: Co-Culture of Fish, Bivalves, and Holothurians. J. Mar. Sci. Eng. 2022, 10, 776. [Google Scholar] [CrossRef]
- Navarrete-Mier, F.; Sanz-Lázaro, C.; Marín, A. Does bivalve mollusc polyculture reduce marine fin fish farming environmental impact? Aquaculture 2010, 306, 101–107. [Google Scholar] [CrossRef]
- Giangrande, A.; Licciano, M.; Arduini, D.; Borghese, J.; Pierri, C.; Trani, R.; Longo, C.; Petrocelli, A.; Ricci, P.; Alabiso, G. An Integrated Monitoring Approach to the Evaluation of the Environmental Impact of an Inshore Mariculture Plant (Mar Grande of Taranto, Ionian Sea). Biology 2022, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- ISO 16649-3:2015(EN); Microbiology of the Food Chain–Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli–Part 3: Detection and Most Probable Number Technique Using 5-Bromo-4-chloro-3-indolyl-β-D-glucuronide. International Organization for Standardization (ISO): Geneva, Switzerland, 2005.
- ISO 7218:2007; Microbiology of Food and Animal Feeding Stuffs—General Requirements and Guidance for Microbiological Examinations. International Organization for Standardization (ISO): Geneva, Switzerland, 2007.
- Oblinger, J.L.; Koburger, J.A. Understanding and teaching the most probable number technique. J. Milk Food Technol. 1975, 38, 540–545. [Google Scholar] [CrossRef]
- Donovan, T.J.; Gallacher, S.; Andrews, N.J.; Greenwood, M.H.; Graham, J.; Russell, J.E.; Roberts, D.; Lee, R. Modification of the standard method used in the United Kingdom for counting Escherichia coli in live bivalve molluscs. Commun. Dis. Public Health 1998, 1, 188–196. [Google Scholar]
- ISO 4831:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Coliforms—Most Probable Number Technique. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- ISO 6579-1:2017; Microbiology of the Food Chain–Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- U.S. Environmental Protection Agency. Method 8270E (SW-846): Semivolatile Organic Compounds by gas Chromatography/Mass Spectrometry (GC/MS); U.S. Environmental Protection Agency: Washington, DC, USA, 2014.
- Giandomenico, S.; Nigro, M.; Parlapiano, I.; Spada, L.; Grattagliano, A.; Prato, E.; Biandolino, F. Effect of in-house cooking in Mytilus galloprovincialis and Trachurus trachurus: Lipid and fatty acids quality and polycyclic aromatic hydrocarbons formation. Food Chem. Toxicol. 2023, 173, 113606. [Google Scholar] [CrossRef]
- Boscolo, R.; Cornello, M.; Giovanardi, O. A condition index applied to mussel cultured mussel in the Northen Adriatic: A preliminary study. Biol. Mar. Mediterr. 2002, 11, 243–254. [Google Scholar]
- Borghese, J.; Arduini, D.; Calabrese, C.; Licciano, M.; Giangrande, A.; Migoni, D.; Longo, C.; Stabili, L. Monitoring biomass quality of by-products from an imta system: Evaluation of heavy metals. In Proceedings of the Aquaculture Europe 2022: Innovative Solutions in a Changing World, Rimini, Italy, 27–30 September 2022. [Google Scholar]
- Arduini, D.; Borghese, J.; Gravina, M.F.; Trani, R.; Longo, C.; Pierri, C.; Giangrande, A. Biofouling role in mariculture environment restoration: An example in the Mar Grande of Taranto (Mediterranean Sea). Front. Mar. Sci. 2022, 9, 842616. [Google Scholar] [CrossRef]
- Borghese, J.; Musco, L.; Arduini, D.; Tamburello, L.; Del Pasqua, M.; Giangrande, A. A Comparative Approach to Detect Macrobenthic Response to the Conversion of an Inshore Mariculture Plant into an IMTA System in the Mar Grande of Taranto (Mediterranean Sea, Italy). Water 2023, 15, 68. [Google Scholar] [CrossRef]
- MacDonald, B.A.; Robinson, S.M.C.; Barrington, K.A. Feeding activity of mussels (Mytilus edulis) held in the field at an integrated multi-trophic aquaculture (IMTA) site (Salmo salar) and exposed to fish food in the laboratory. Aquaculture 2011, 314, 244–251. [Google Scholar] [CrossRef]
- Mazzola, A.; Sarà, G. The effect of fish farming organic waste on food availability for bivalve molluscs (Gaeta Gulf, Central Tyrrhenian, MED): Stable carbon isotopic analysis. Aquaculture 2001, 192, 361–379. [Google Scholar] [CrossRef]
- Ning, Z.; Liu, S.; Zhang, G.; Ning, X.; Li, R.; Jiang, Z.; Fang, J.; Zhang, J. Impacts of an integrated multi-trophic aquaculture system on benthic nutrient fluxes: A case study in Sanggou Bay, China. Aquac. Environ. Interact. 2016, 8, 221–232. [Google Scholar] [CrossRef]
- Doglioli, A.M.; Magaldi, M.G.; Vezzulli, L.; Tucci, S. Development of a numerical model to study the dispersion of wastes coming from a marine fish farm in the Ligurian Sea (Western Mediterranean). Aquaculture 2004, 231, 215–235. [Google Scholar] [CrossRef]
- Sanderson, M.G.; Dentener, F.J.; Fiore, A.M.; Cuvelier, C.; Keating, T.J.; Zuber, A.; Atherton, C.S.; Bergmann, D.J.; Diehl, T.; Doherty, R.M. A multi-model study of the hemispheric transport and deposition of oxidised nitrogen. Geophys. Res. Lett. 2008, 35, L17815. [Google Scholar] [CrossRef]
- Cranford, P.J.; Reid, G.K.; Robinson, S.M.C. Open water integrated multi-trophic aquaculture: Constraints on the effectiveness of mussels as an organic extractive component. Aquac. Environ. Interact. 2013, 4, 163–173. [Google Scholar] [CrossRef]
- Troell, M.; Norberg, J. Modelling output and retention of suspended solids in an integrated salmon–mussel culture. Ecol. Modell. 1998, 110, 65–77. [Google Scholar] [CrossRef]
- Sarà, G.; Scilipoti, D.; Milazzo, M.; Modica, A. Use of stable isotopes to investigate dispersal of waste from fish farms as a function of hydrodynamics. Mar. Ecol. Prog. Ser. 2006, 313, 261–270. [Google Scholar] [CrossRef]
- Sarà, G.; Scilipoti, D.; Mazzola, A.; Modica, A. Effects of fish farming waste to sedimentary and particulate organic matter in a southern Mediterranean area (Gulf of Castellammare, Sicily): A multiple stable isotope study (δ13C and δ15N). Aquaculture 2004, 234, 199–213. [Google Scholar] [CrossRef]
- Irisarri, J.; Fernández-Reiriz, M.J.; Cranford, P.J.; Labarta, U. Effects of seasonal variations in phytoplankton on the bioenergetic responses of mussels (Mytilus galloprovincialis) held on a raft in the proximity of red sea bream (Pagellus bogaraveo) net-pens. Aquaculture 2014, 428, 41–53. [Google Scholar] [CrossRef]
- Mitch, A.A.; Gasner, K.C.; Mitch, W.A. Fecal coliform accumulation within a river subject to seasonally-disinfected wastewater discharges. Water Res. 2010, 44, 4776–4782. [Google Scholar] [CrossRef]
- Mok, J.S.; Lee, T.S.; Kim, P.H.; Lee, H.J.; Ha, K.S.; Shim, K.B.; Lee, K.J.; Jung, Y.J.; Kim, J.H. Bacteriological quality evaluation of seawater and oysters from the Hansan-Geojeman area in Korea, 2011–2013: Impact of inland pollution sources. Springerplus 2016, 5, 1–16. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, P.H.; Jung, Y.J.; Lee, K.J.; Kim, M.S.; Go, K.R.; Park, S.G.; Kwon, S.J.; Yang, J.H.; Mok, J.S. Seasonal variation of physicochemical factor and fecal pollution in the Hansan-Geojeman area, Korea. Fish. Aquat. Sci. 2016, 19, 1–9. [Google Scholar] [CrossRef]
- Stabili, L.; Acquaviva, M.I.; Cavallo, R.A. Mytilus galloprovincialis filter feeding on the bacterial community in a Mediterranean coastal area (Northern Ionian Sea, Italy). Water Res. 2005, 39, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Mok, J.S.; Lee, K.J.; Kim, P.H.; Lee, T.S.; Lee, H.J.; Jung, Y.J.; Kim, J.H. Bacteriological quality evaluation of seawater and oysters from the Jaranman-Saryangdo area, a designated shellfish growing area in Korea: Impact of inland pollution sources. Mar. Pollut. Bull. 2016, 108, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Oh, S.J.; Shin, Y.; Kim, Y.; Oh, E.-G.; So, J.-S. Impact of inland pollution sources on the bacteriological water quality of the Southern Ganghwado Bay Area, South Korea. Urban Water J. 2017, 14, 69–73. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, K.B.; Shin, S.B.; Park, K.; Oh, E.G.; Son, K.T.; Yu, H.; Lee, H.J.; Mok, J.S. Comparison of bioaccumulation and elimination of Escherichia coli and male-specific bacteriophages by ascidians and bivalves. Environ. Sci. Pollut. Res. 2017, 24, 28268–28276. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–26. [Google Scholar]
- NZFSA (New Zealand Food Safety Authority). Animal Products (Specifications for Bivalve Molluscan Shellfish). 2006. Available online: https://water.wa.gov.au/__data/assets/pdf_file/0004/5737/NZFSA-Shellfish-2006.pdf (accessed on 20 June 2016).
- U.S. FDA (Food and Drug Administration). National Shellfish Sanitation Program (NSSP), Guide for the Control of Molluscan Shellfish.; 2015. Available online: https://www.fda.gov/Food/GuidanceRegulation/FederalStateFoodPrograms/ucm2006754.html (accessed on 20 June 2016).
- Ministry of Ocean and Fisheries (MOF). Korean Shellfish Sanitation Program (KSSP) Annual Report; Ministry of Oceans and Fisheries: Sejong, Republic of Korea, 2015.
- Korea Ministry of Food and Drug Safety (KMFDS). Korea Food Code. 2015. Available online: http://www.sernapesca.cl/sites/default/files/food_code_2015_0.pdf (accessed on 20 June 2016).
- European Commission. Regulation (EC) No. 854/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Rules for the Organisation of Official Controls on Products of Animal Origin Intended for Human Consumption. Off. J. Eur. Union 2004, L226, 83–127. [Google Scholar]
- European Commission. Regulation No. 2015/2285 of 8 December 2015 amending annex II to Regulation (EC) No 854/ 2004 of the European Parliament and of the Council laying down specific rules for the organisation of official controls on products of animal origin intended for hum. Off. J. Eur. Union 2015, L323, 2–4. [Google Scholar]
- European Commission. Regulation No. 835/2011 of 19 August 2011 amending Regulation No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, L215, 4–8. [Google Scholar]
- Binelli, A.; Provini, A. POPs in edible clams from different Italian and European markets and possible human health risk. Mar. Pollut. Bull. 2003, 46, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Barone, G.; Perrone, V.G.; Giacominelli-Stuffler, R. Polychlorinated biphenyls (PCBs), dioxins and furans (PCDD/Fs): Occurrence in fishery products and dietary intake. Food Chem. 2011, 127, 1648–1652. [Google Scholar] [CrossRef]
- Storelli, M.M.; Dambrosio, A.; Storelli, A.; Barone, G.; Ioanna, F.; Normanno, G. Levels of polychlorinated biphenyls (PCBs) in marine gastropod Hexaplex trunculus: Compliance with European Union legislation. J. Food Compos. Anal. 2014, 36, 35–39. [Google Scholar] [CrossRef]
- Rodrıguez-Ariza, A.; Rodrıguez-Ortega, M.J.; Marenco, J.L.; Amezcua, O.; Alhama, J.; Lopez-Barea, J. Uptake and clearance of PCB congeners in Chamaelea gallina: Response of oxidative stress biomarkers. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 134, 57–67. [Google Scholar] [CrossRef]
- Bihari, N.; Fafanđel, M.; Piškur, V. Polycyclic aromatic hydrocarbons and ecotoxicological characterization of seawater, sediment, and mussel Mytilus galloprovincialis from the Gulf of Rijeka, the Adriatic Sea, Croatia. Arch. Environ. Contam. Toxicol. 2007, 52, 379–387. [Google Scholar] [CrossRef]
- Perugini, M.; Cavaliere, M.; Giammarino, A.; Mazzone, P.; Olivieri, V.; Amorena, M. Levels of polychlorinated biphenyls and organochlorine pesticides in some edible marine organisms from the Central Adriatic Sea. Chemosphere 2004, 57, 391–400. [Google Scholar] [CrossRef]
- El Nemr, A.; Said, T.O.; Khaled, A.; El Sikaily, A.; Abd-Allah, A.M.A. Polychlorinated biphenyls and chlorinated pesticides in mussels collected from the Egyptian Mediterranean Coast. Bull. Environ. Contam. Toxicol. 2003, 71, 290–297. [Google Scholar] [CrossRef]
- Nesto, N.; Romano, S.; Moschino, V.; Mauri, M.; Da Ros, L. Bioaccumulation and biomarker responses of trace metals and micro-organic pollutants in mussels and fish from the Lagoon of Venice, Italy. Mar. Pollut. Bull. 2007, 55, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.; Fontaine, C.; Callier, M.; Roque d’orbcastel, E. Blue mussel (Mytilus edulis) bouchot culture in Mont-St Michel Bay: Potential mitigation effects on climate change and eutrophication. Int. J. Life Cycle Assess. 2018, 23, 1030–1041. [Google Scholar] [CrossRef]
- Carranza, A.; Zu Ermgassen, P.S.E. A global overview of restorative shellfish mariculture. Front. Mar. Sci. 2020, 7, 722. [Google Scholar] [CrossRef]
- Smaal, A.C.; Ferreira, J.G.; Grant, J.; Petersen, J.K.; Strand, Ø. Goods and Services of Marine Bivalves; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Bunting, S.W.; Pretty, J. Aquaculture Development and Global Carbon Budgets: Emissions, Sequestration and Management Options; Centre for Environment and Society Occasional Paper 2007-1; University of Essex: Colchester, UK, 2007. [Google Scholar]
- Filgueira, R.; Byron, C.J.; Comeau, L.A.; Costa-Pierce, B.; Cranford, P.J.; Ferreira, J.G.; Grant, J.; Guyondet, T.; Jansen, H.M.; Landry, T. An integrated ecosystem approach for assessing the potential role of cultivated bivalve shells as part of the carbon trading system. Mar. Ecol. Prog. Ser. 2015, 518, 281–287. [Google Scholar] [CrossRef]
- Sea, M.A.; Hillman, J.R.; Thrush, S.F. The influence of mussel restoration on coastal carbon cycling. Glob. Chang. Biol. 2022, 28, 5269–5282. [Google Scholar] [CrossRef]
- Tamburini, E.; Turolla, E.; Lanzoni, M.; Moore, D.; Castaldelli, G. Manila clam and Mediterranean mussel aquaculture is sustainable and a net carbon sink. Sci. Total Environ. 2022, 848, 157508. [Google Scholar] [CrossRef]
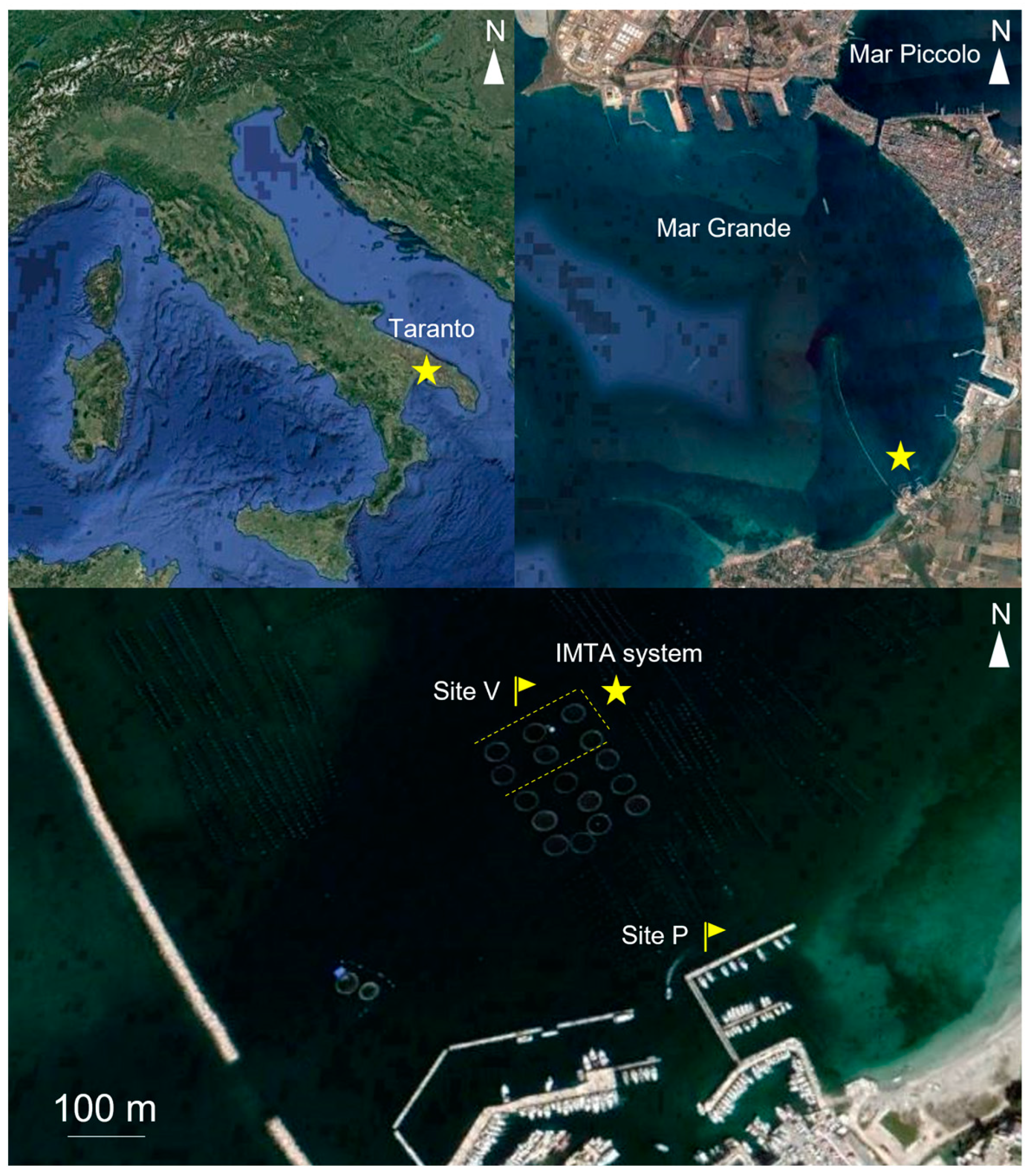
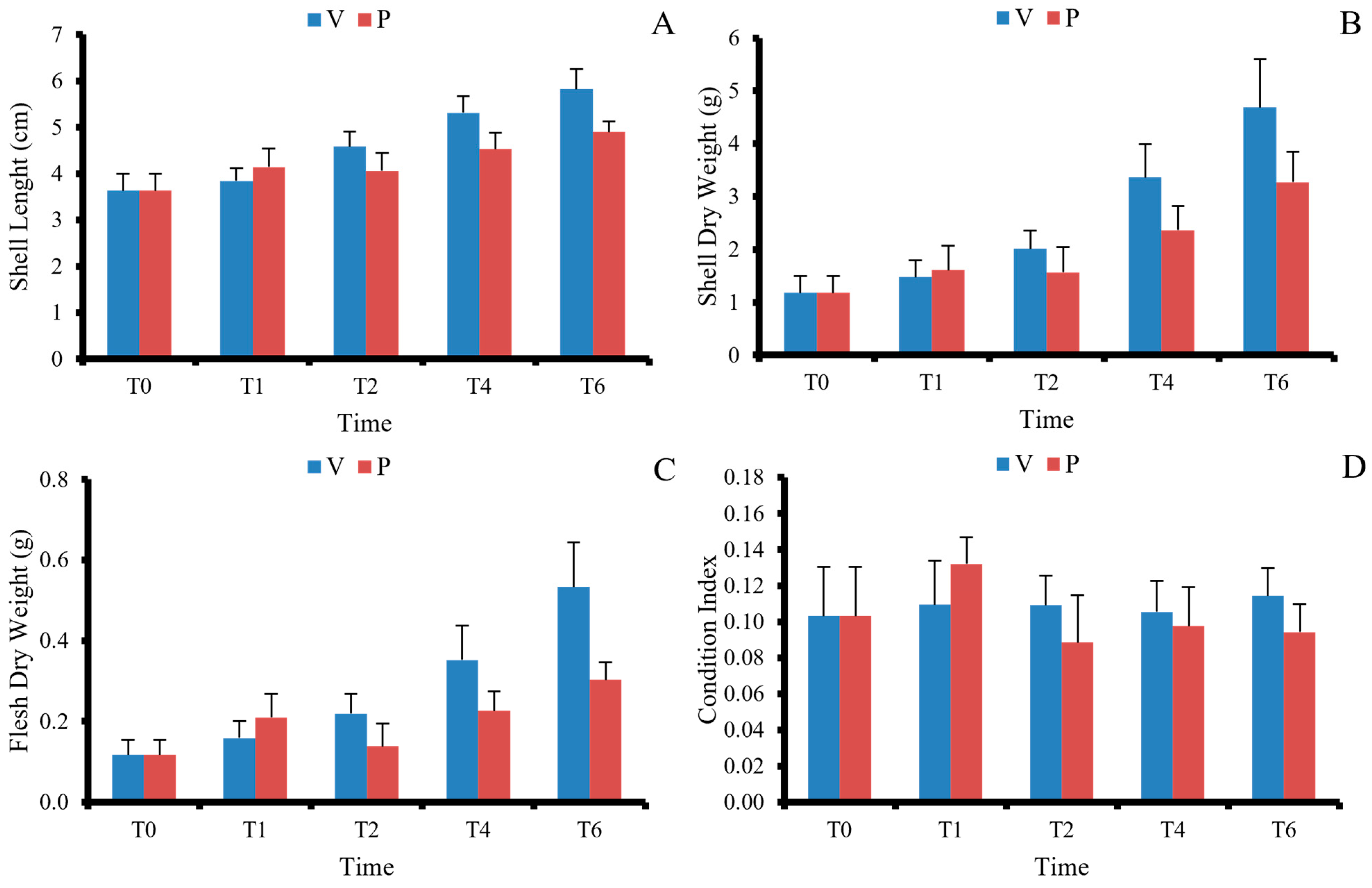

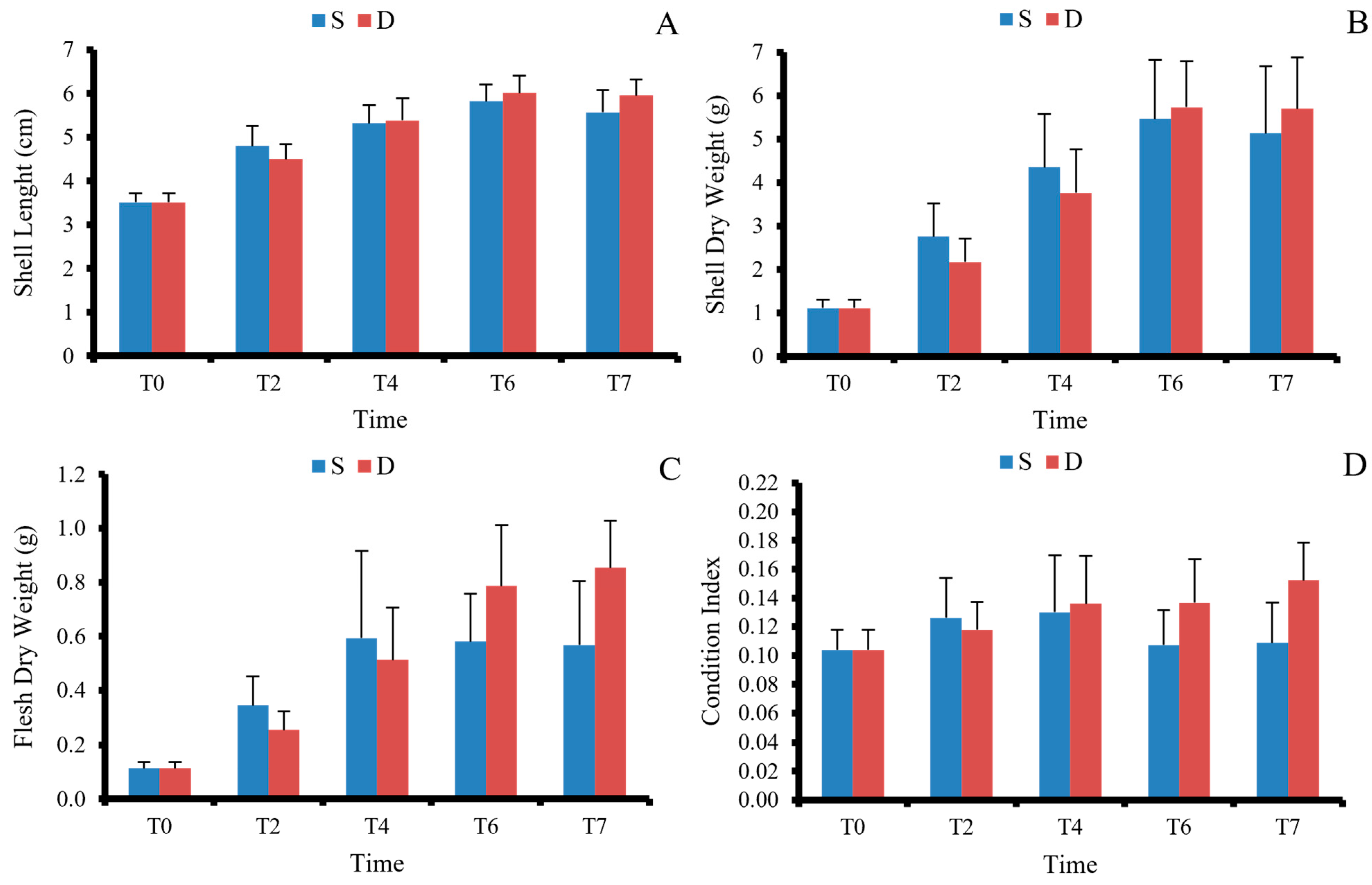
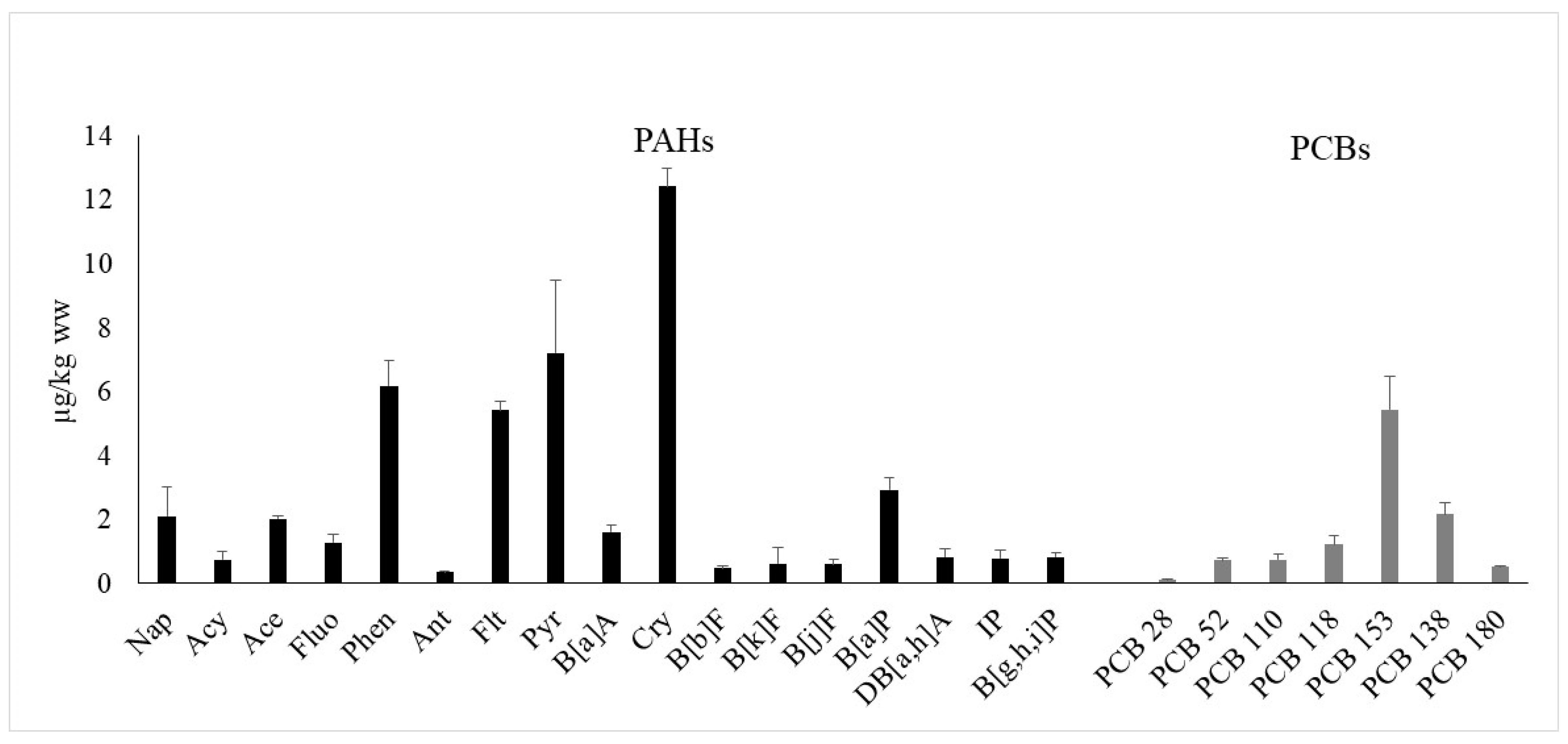
| Site | AMBI (Status) | M-AMBI (Status) | Microtox STI (%Bioluminescence Inhibition) | Escherichia coli (MPN/g) | Salmonella spp. (+/−) |
|---|---|---|---|---|---|
| IMTA- converted | 4.81 (Poor) | 0.41 (Moderate) | 0.33 ± 0.01 (Hormensis) | 40.0 ± 9.4 | Absent |
| Control | 2.78 (Good) | 0.95 (High) | 0.13 ± 0.01 (Hormensis) | 40.0 ± 9.4 | Absent |
| Production Cycle | Experiment | Number of Mussel Nets | Study Period | Site | Distance from the Cages | Depth |
|---|---|---|---|---|---|---|
| 2018/2019 | Traditional | 307 | T0–T7 (1) | V | 1 m | 1 m |
| 2020/2021 | Horizontal distance | 16 | T0–T6 (2) | V/P | 1 m/300 m | 1 m |
| 2021/2022 | Vertical distance | 16 | T0–T7 (3) | V | 1 m | 1 m/12 m |
| Horizontal Distance | Vertical Distance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Growth Parameter | Site V | Site P | df | t | p | Surface | Depth | df | t | p |
| L increase (cm) | 2.19 ± 0.48 | 1.27 ± 0.39 | 37 | 6.64 | <0.001 | 2.06 ± 0.52 | 2.44 ± 0.34 | 32 | 2.77 | 0.009 |
| SDW increase (g) | 3.51 ± 0.94 | 2.09 ± 0.68 | 35 | 5.47 | <0.001 | 4.03 ± 1.53 | 4.59 ± 1.10 | 34 | 1.33 | 0.190 |
| FDW increase (g) | 0.42 ± 0.12 | 0.19 ± 0.06 | 27 | 7.85 | <0.001 | 0.45 ± 0.24 | 0.74 ± 0.17 | 34 | 4.31 | <0.001 |
| CI | 0.11 ± 0.02 | 0.09 ± 0.02 | 38 | 4.13 | <0.001 | 0.11 ± 0.03 | 0.15 ± 0.03 | 38 | 5.08 | <0.001 |
| Samples | Total Coliforms | Fecal Coliforms | Escherichia coli | Salmonella spp. |
|---|---|---|---|---|
| MPN/100 g | MPN/100 g | MPN/100 g | Presence/Absence | |
| Mytilus galloprovincialis | 430 | 430 | 18 | Absence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arduini, D.; Portacci, G.; Giangrande, A.; Acquaviva, M.I.; Borghese, J.; Calabrese, C.; Giandomenico, S.; Quarta, E.; Stabili, L. Growth Performance of Mytilus galloprovincialis Lamarck, 1819 under an Innovative Integrated Multi-Trophic Aquaculture System (IMTA) in the Mar Grande of Taranto (Mediterranean Sea, Italy). Water 2023, 15, 1922. https://doi.org/10.3390/w15101922
Arduini D, Portacci G, Giangrande A, Acquaviva MI, Borghese J, Calabrese C, Giandomenico S, Quarta E, Stabili L. Growth Performance of Mytilus galloprovincialis Lamarck, 1819 under an Innovative Integrated Multi-Trophic Aquaculture System (IMTA) in the Mar Grande of Taranto (Mediterranean Sea, Italy). Water. 2023; 15(10):1922. https://doi.org/10.3390/w15101922
Chicago/Turabian StyleArduini, Daniele, Giuseppe Portacci, Adriana Giangrande, Maria Immacolata Acquaviva, Jacopo Borghese, Claudio Calabrese, Santina Giandomenico, Elisa Quarta, and Loredana Stabili. 2023. "Growth Performance of Mytilus galloprovincialis Lamarck, 1819 under an Innovative Integrated Multi-Trophic Aquaculture System (IMTA) in the Mar Grande of Taranto (Mediterranean Sea, Italy)" Water 15, no. 10: 1922. https://doi.org/10.3390/w15101922
APA StyleArduini, D., Portacci, G., Giangrande, A., Acquaviva, M. I., Borghese, J., Calabrese, C., Giandomenico, S., Quarta, E., & Stabili, L. (2023). Growth Performance of Mytilus galloprovincialis Lamarck, 1819 under an Innovative Integrated Multi-Trophic Aquaculture System (IMTA) in the Mar Grande of Taranto (Mediterranean Sea, Italy). Water, 15(10), 1922. https://doi.org/10.3390/w15101922







