Abstract
Non-indigenous species (NIS) represent one of the greatest threats to biodiversity and ecosystem functioning, altering invaded habitats, competing with native species, and eventually becoming pests. The Mediterranean Sea is a marine biodiversity hotspot, with its coasts being densely populated and its living resources fished since ancient times. As a result of such a long history of exploitation, the whole basin is exposed to a wide array of human pressures, with their combined effects on marine ecosystems being amplified by ongoing climate change. Caulerpa cylindracea Sonder, 1845, is a non-indigenous invasive seaweed widely distributed in the coastal habitats of the Mediterranean Sea, which ultimately affects marine biodiversity and ecosystem functioning. Here, a systematic literature analysis on the consumption of the NIS Caulerpa cylindracea by Mediterranean native and NIS species is provided, focusing on the benefits and drawbacks for the native biota and human health. The present review aims to synthetise knowledge and provide tools to manage the occurrence of the invasive seaweed C. cylindracea in the Mediterranean Sea, encouraging an ecosystem-based approach to the management of the ecological, economic, and social effects of the successful expansion of this NIS.
1. Introduction
The acceleration of the introduction, establishment, and wide expansion of non-indigenous species (NIS) beyond their native range represents a pervasive component of the ongoing global changes. Due to the growing occurrence of NIS worldwide [1], they are considered among the most important factors of change in biodiversity and ecosystem function [2,3]. In the marine coastal system, NIS occur jointly to human-driven impacts such as overfishing, eutrophication, habitat modification and loss, and climate change [4,5,6,7], thereby jeopardizing the integrity of ecosystems and altering biodiversity, ultimately leading to the loss of ecosystem services with consequences in socioecological systems [8,9,10,11,12].
The Mediterranean Sea has reduced biodiversity due to its geological history [13] and exploitation by humans since prehistorical times [14,15]. Nevertheless, it holds 7% of the known marine species [5,16]. The phenomenon called tropicalisation is driven by increasing seawater temperatures, which in turn causes shifts and a decline in temperate species northwards and prompts high abundances of warm-water species [17,18,19]. This is especially the case in eastern and southern areas, where the so-called tropicalisation of the Mediterranean Sea occurs at a high pace when compared with other seas globally [20,21]. The increasing anthropogenic activities at sea, combined with intense marine traffic, the enlargement of the Suez Canal, and the consequent acceleration of the reduction in the salinity barrier represented by the Great Bitter Lake, have prompted the introduction of a growing number of NIS within the Mediterranean Sea [22,23,24]. Ship traffic across the Mediterranean Sea, which accounts for approximately 30% of global cargo shipping [25], represents the main pathway of introduction of NIS [25], followed by aquaculture and aquarium trade [9,26,27,28]. As NIS become invasive, they potentially lead to losses in biological populations that affect ecosystem functioning. This raises societal worries about the maintenance of the delivery of goods and services by marine ecosystems [29,30], and the potential negative consequences on local economies [9,31,32].
In the last decade, important management and policy measures have been adopted to coordinate the prevention and the management of the introduction and spread of invasive NIS in Mediterranean EU and non-EU countries [23]. However, many invasive NIS are well-established, eradication actions are highly expensive, and their outcomes are subjected to substantial uncertainty. Hence, the European Commission actually discourage the application of management measures with disproportionate or excessive costs [33]. Given the practical impossibility to achieve complete NIS eradication, adaptive management becomes crucial, along with monitoring plans and efforts to limit further spread of NIS [34].
In the Mediterranean, the seaweed Caulerpa cylindracea, Sonder, 1845 [35], is among the most successful NIS [36]. This species is a siphonous green alga native to southwestern Australia, first reported in the Mediterranean in the coast of Libya in 1991. From that time, it rapidly spread throughout the basin [37] where it colonised almost every coastal habitat from the surface down to 70 m [38,39]. It tends to form monospecific stands in its range of distribution [40], enhancing sediment and organic matter accumulation in the underneath substrate, thereby creating suitable conditions for itself and facilitating the shift from erect macroalgae to algal turfs [39,41,42].
The high invasiveness of Caulerpa spp. is reported to depend on their biological and functional traits [43], i.e., the presence of toxic metabolites acting as grazing deterrents, wide bathymetric tolerance, rapid growth, large standing biomass, and facility of dispersion [39,44]. However, NIS can also bring positive features, such as introducing novelty in invaded areas, replacing lost ecological functions, adding redundancy, and supporting ecosystem services [45,46].
Here, the potential of the NIS Caulerpa cylindracea as a food resource for Mediterranean species is explored. Indeed, C. cylindracea is selected as a food source by sea urchins [39,47] and fish [48,49,50], despite the presence of grazing-deterrence compounds. These include high levels of caulerpin, a phenazine derivate exhibiting cytotoxic activity [51]. In addition, there are also minor concentrations of caulerpenyne [52], a sesquiterpenoid quickly converted to reactive aldehydes with oxidative activity. Both metabolites make algal tissues unpalatable to marine fauna [53].
The aim of the present work is to assess the consumption of C. cylindracea as a food resource in the Mediterranean by performing a systematic literature analysis on the consumption of this NIS by native species as well as other established NIS. The present review aims to synthetise the available evidence in order to inform place-based management of the invasive C. cylindracea in Mediterranean coastal areas including ecological, economic, and social components.
2. Materials and Methods
The data on the consumption of the NIS Caulerpa cylindracea by marine fauna in the Mediterranean Sea were collected from the published scientific literature, grey literature, and monitoring programs.
The research on the published literature was conducted in the ISI Web of Science (https://www.webofscience.com/wos/alldb/advanced-search, accessed on 30 December 2022) and Scopus (https://www.scopus.com/search/form.uri?display=advanced, accessed on 30 December 2022) for the period between 1985 and 2022. The systematic literature screening was carried out by searching in the “Title”, “Abstract”, and “Keyword” fields the following combination of terms: (“Caulerpa cylindracea” OR “Caulerpa racemosa” OR “Caulerpa”). The grey literature included publications on national journals edited by national associations or institutions (e.g., Italian Society of Marine Biology, Italian Botanical Society), books, unpublished Ph.D. theses, and conference proceedings. We also checked the citation lists of the selected articles for further publications of interest. The full list of publications included in the analysis is reported as Supplementary Material (Table S1).
3. Results
The analysis of the literature showed that 29 marine species were reported to feed on the invasive seaweed C. cylindracea in the Mediterranean Sea. A variety of species belonging to six different Phyla, including Porifera, Annelida, Mollusca, Arthropoda, Echinodermata, and Chordata, used C. cylindracea as a food resource. The analysis revealed that several trophic guilds were involved in C. cylindracea consumption (Figure 1). Consumers of C. cylindracea included deposit feeders (polychaetes, asteroids), detritivores (e.g., sea cucumbers), filter feeders (e.g., sponges, amphipods, molluscs), herbivores (sea urchins, fish, crabs), and omnivores (fish, sea urchins, molluscs).
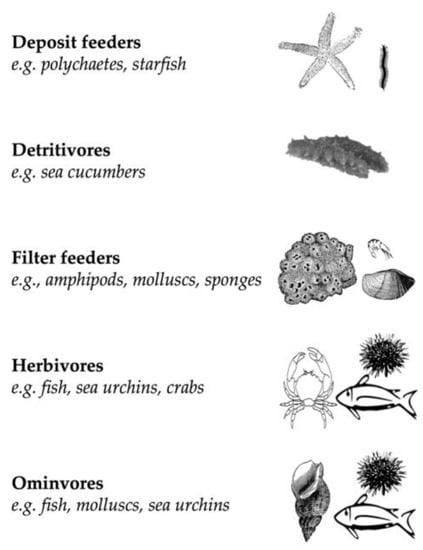
Figure 1.
Feeding guilds potentially consuming the seaweed C. cylindracea.
Among the Porifera, only the sponge Sarcotragus fasciculatus is known to consume the NIS C. cylindracea. Among the annelids, five polychaetes have been identified to feed on this NIS: Eunice vittata, Pelogenia arenosa, Pontogenia chrysocoma, Scoletoma fragilis, and Syllis prolifera (Table 1).

Table 1.
Benthic fauna (Phyla: Porifera and Annelida) reported to feed on the NIS C. cylindracea. References are reported in Table S1.
Caulerpa cylindracea is also a food resource for five molluscs: Arca noae, Cerithium vulgatum, Glans trapezia, Hexaplex trunculus, and Pisinna glabrata (Table 2). Among arthropods, the invasive alga C. cylindracea is consumed by Monocorophium sextonae and Percnon gibbesi.

Table 2.
Marine species (Phyla: Mollusca and Arthropoda) reported to consume the NIS C. cylindracea. References are reported in Table S1; * = unassigned order.
Among the echinoderms, two species of sea cucumbers were found to feed upon C. cylindracea, namely Holothuria (Panningothuria) forskali and Holothuria (Roweothuria) poly, as well as three sea urchins (Arbacia lixula, Paracentrotus lividus, and Sphaerechinus granularis), the starfish Echinaster (Echinaster) sepositus, and the ophiurid Ophioderma longicaudum (Table 3).

Table 3.
Echinoderm (Phylum Echinodermata) reported to consume the NIS C. cylindracea. References are reported in Table S1.
The invasive alga C. cylindracea is consumed by several fish species such as the sparids Boops boops, Diplodus annularis, Diplodus sargus, Diplodus vulgaris, Sarpa salpa, and Spondyliosoma cantharus, the spinefoots Siganus luridus, and Siganus rivulatus, and the labrid Thalassoma pavo (Table 4).

Table 4.
Fish species (Phylum Chordata) reported to feed on the NIS C. cylindracea. References are reported in Table S1; * = incertae sedis.
4. Discussion
The reported findings showed that the NIS C. cylindracea appears to be pervasively consumed across different feeding guilds in the Mediterranean Sea, where C. cylindracea is a source of organic matter for benthic consumers [55] and is able to modify the trophic niche of benthic communities in the rocky infralittoral [85]. The presence of C. cylindracea on hard substrates can influence some taxonomic groups of macrozoobenthos communities, such as Amphipoda, Caridea, and Tanaidacea, by creating an extension of both the trophic and edaphic niches and potentially affecting the diversity of the macrozoobenthic system [86]. Up to now, the reported consumers of the NIS C. cylindracea include the sponge Sarcotragus fasciculatus, bivalve filter feeders (Arca noae, and Glans trapezia), a welk (Hexaplex trunculus), detritivores (Holothuria (Panningothuria) forskali and Holothuria (Roweothuria) poli), and benthic deposit feeder invertebrates (Echinaster (Echinaster) sepositus, Eunice vittata, Scoletoma fragili, Ophioderma longicaudum, Pelogenia arenosa, and Pontogenia chrysocoma), in addition to herbivores and omnivores species. Herbivory is expected to be the main pathway of algae to the upper trophic levels in marine webs [87,88]. Recently, omnivory has been recognised to exert considerable control on seaweed abundance [89,90,91,92], and it has been identified as a potential mechanism of biotic resistance against invasive algal species [59]. As far as the NIS C. cylindracea is concerned, the grazing activity seems to be able to limit the early colonisation stages [93]. However, there are still important aspects to be investigated. Up to now, the available information did not show a clear relationship between the herbivory and the variations in the abundance of this NIS [64]. In some cases, in order to face the control exerted by herbivores on C. cylindracea biomass, a compensative strategy by the alga has been hypothesised [94]. In fact, an experimental simulation of the herbivory effects on the NIS C. cylindracea showed, after an initial decrease in the algal abundance, a potential reaction mechanism in the seaweed aimed to stimulate the algal growth rates [94]. It is necessary to keep in mind that the NIS C. cylindracea exhibits a large recovery ability, which allows it to tolerate a wide range of grazing intensities operated by native generalist herbivores, through algal regeneration from detached fragments of several parts of the seaweed such as stolon, rhizoid, and frond [64,94]. However, several context-dependent direct effects deserve to be assessed in order to fully understand the inconsistent responses to the investigated variables in these experiments, and particularly in the attempt to disentangle the contribution of several disturbances whose effects presumably sum up while acting simultaneously on the NIS. Besides the direct effects caused by herbivory on the NIS seaweed, the indirect effects need to be considered, especially when they are coupled with other kinds of environmental and anthropogenic disturbances. For example, in the marine costal systems, the seagrass meadows operate fundamental ecosystem functions and herbivory should theoretically limit the penetration of C. cylindracea by operating a control on the NIS biomass. Nevertheless, herbivores can indirectly influence the spread of the NIS C. cylindracea acting on native seaweed and seagrass habitats. The presence of C. cylindracea represents a threat for the integrity of marine seagrass meadows, since the NIS penetrate them following the reduction in the seagrass resistance inducted by the native herbivores [95] or human-driven impacts, such as clearances produced along the borders of the meadow [96], or within the seagrass meadow, such as by illegal trawling [97] and anchoring [98]. Improving the information about the factors regulating the establishment and spread of C. cylindracea and having an available and exhaustive set of alternative scenarios is crucial to apply management measures, which should be based on mechanisms regulating the spread of the NIS C. cylindracea. The current literature analysis also reported several sea urchin and fish species consuming the NIS C. cylindracea, namely, the sea urchins Arbacia lixula, Paracentrotus lividus, and Sphaerechinus granularis [47,54,57,58,61,62,63,64], and the fish Sarpa salpa, Siganus luridus, and S. rivulatus [64,65,78,81]. In addition to the above-mentioned species, several omnivorous fish species, such as Boops boops, Diplodus sargus, Diplodus annularis, Diplodus vulgaris, Spondyliosoma cantharus, and Thalassoma pavo have occasionally been observed feeding on C. cylindracea [64,74,83]. However, the invasive seaweed C. cylindracea could represent a threat for the native species feeding on it, since the macroalga is recognised to host specific microbial communities [12,99,100], including potential pathogens [100]. Sarpa salpa seemed to prefer C. cylindracea when available along with other macroalgae [78], while the choice of Caulerpa as food appears to be arbitrary for Diplodus annularis [65], Diplodus sargus [65], Spondyliosoma cantharus [65], and Paracentrotus lividus [47,58]. However, the preference for the C. cylindracea by S. salpa requires around 6 years of exposure to be developed, after an initial wariness. Similar to the fish S. salpa, other native species could need time to learn if and how to exploit the new trophic resource, while also developing effective resistance mechanisms against NIS [79]. Santamaria et al. [79] shows how native species, initially not used to interacting with the NIS, start to become gradually familiar and, as a consequences, the strength of the relationship between native consumer and NIS resource reinforces, depending on time and the abundance of the new resource. Moreover, social herbivore species can share information on palatability and handling, increasing consumption in the population and potentially leading to a control of NIS populations. Remarkably, as for S. salpa, the consumers could keep the food preferences also when the resource becomes less abundant, once incorporated in their diet [79]. Further studies could identify other Mediterranean species feeding on the invasive alga and assess their food preference, filling the gap of knowledge on the active choice of this NIS by marine fauna.
Interestingly, the non-indigenous crab Perchnon gibbesi as well as the exotic fishes Siganus luridus and Siganus rivulatus were found feeding on C. cylindracea in the Mediterranean. Remarkably, both the two above-mentioned siganid fish and the seaweed investigated here, C. cylindracea, are listed among the ten worst invasive species identified by Tsirintanis et al. [101] within the Mediterranean Sea, considering their negative impact on ecosystem services by using an holistic approach while no information is available on the several types of impacts on marine ecosystem services and human health caused by the cryptogenic species of crab Perchnon gibbesi except for an identified positive impact on the Mediterranean native species Gobius paganellus [102]. A large food contribution of C. cylindracea to the diet of the NIS crab P. gibbesi has been revealed by Maric et al. [56] by using stable isotope analysis, underlining that the NIS C. cylindracea can support the diversity of available prey [56]. In the literature, the interaction between C. cylindracea and other invasive NIS is reported by several studies. In particular, the association of the NIS C. cylindracea with invasive algal components of the algal turf has been described, including the introduced filamentous Rhodophyta Womersleyella setacea (Hollenberg) R.E. Norris and Acrothamnion preissi (Sonder) Wollaston. Remarkably, these NIS are also listed among the ten worst invasive species in the Mediterranean Basin [101], and co-occurrences between C. cylindracea and the other algal NIS seem to stimulate the enhancement of the spread of the NIS C. cylindracea [103,104,105]. The competitive mechanisms between marine invaders in coastal systems deserve further investigations not only based on interaction mechanisms but also including the role of allelochemical mechanisms that remain still understudied. The NIS herbivorous fishes Siganus luridus and Siganus rivulatus have been reported feeding on the NIS C. cylindracea. These rabbitfish were supposedly introduced for the first time off the southern Levantine coast via the Suez Canal between the 1920s and 1950s and, today, they build large fish biomass in rocky habitats in the eastern basin, causing the depletion of macroalgal forests, replacing native herbivorous fish, and altering the marine food chain [106]. As for the non-native fish S. luridus, no caulerpin accumulation has been described in the generalist common two-banded seabream Diplodus vulgaris, as well as in the parrotfish Sparisoma cretense [75]. Efficient pathways of detoxification are supposed to be at play in successfully avoiding caulerpin accumulation in the herbivorous and the NIS Siganus luridus [75]. Instead, due to the trophic relationship between the NIS C. cylindracea and Mediterranean fish, the caulerpin accumulation has been reported in the tissues of several generalist fish species (Figure 2), including the white seabream Diplodus sargus [74], the black seabream Spondyliosoma cantharus [74], and the dream fish Sarpa salpa [76]. For a long time, the phenomenon called Abnormally Tough Specimen (ATS) occurrence has been reported, consisting of a progressive hardness of the meats of some specimens of Diplodus sargus when they are cooked resulting in impaired edibility. As far as S. salpa is concerned, it is a target species of the commercial small-scale fishery in the eastern Adriatic Sea with an annual catch of about 200 tonnes year−1 [107], generally considered a low-value species collected often as a bycatch species by artisanal and recreational fishers. Since the presence of unpleasant palatability factors have been reported in both the white seabream and the salema, recent research focuses on the potential relationship between the accumulation of the main algal secondary metabolite in the fish tissues, the deterioration of their organoleptic profile [108,109], or even the unpleasant taste [66,76]. Although several hypotheses have been formulated about the potential role of organic pollution, chemical contamination, and variations in feeding habits as causes of the observed ATS [110], the exact causes and their underlying mechanisms are still unknown. In fact, a Caulerpa-based diet could lead to these undesirable characteristics and, for this reason, the human consumption of the salema is recommended to be avoided from August to November due to potential, yet unknown, negative effects on human health [66]. Additional studies conducted on fish species D. sargus showed that the use of caulerpin-enriched food can cause alteration in lipid metabolism, behavioural habits [69,70], and cellular and physiological processes [67,68]. In addition, biomagnification of the red pigment caulerpin in the white seabream D. sargus [66] has been suggested along the trophic net through the roles of molluscs and echinoderms. Yet, it has not been settled if D. sargus can assimilate and accumulate compounds derived from the consumption of Caulerpa cylindracea by intermediate generalist herbivores or sediment.
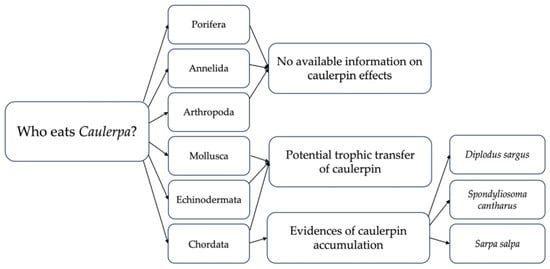
Figure 2.
Potential trophic transfer of the algal metabolite caulerpin.
Understanding the implications of both the positive and negative impacts of NIS on seafood provision and safety is crucial to inform the consumer demand, as well as to create new opportunities for the sustainable exploitation of marine NIS as resources. For example, the NIS Rapa Welk Rapana venosa is an important target for fisheries along the coasts of the environmentally and biologically changing Black Sea [111]. Additional examples are to market some edible NIS rabbitfishes to be targeted and exploited by dedicated fisheries in the Eastern Mediterranean [112], thereby contributing to the adaptive management of NIS in a context of the rapid “tropicalisation” of the local biota. Hence, market opportunity together with control, education, and awareness could modify local consumer behaviour, enabling the marketing of novel products including NIS. In this way, several initiatives have already been undertaken in the Mediterranean with the aim of exploring potential markets for other NIS such as the invasive lionfish [113], jellyfish biomass [114,115,116], and the seagrass Halophila stipulacea [117]. Analogously, the NIS C. cylindracea brings the opportunity to develop therapeutic drugs for treating chronic diseases (e.g., diabetes) due to its content in bioactive compounds, as well as the provision of raw material for a sustainable alternative to plastics [96,118]. The economic exploitation of NIS biomass should be encouraged through the exploration of novel opportunities to be developed through local adaptive management along the Mediterranean coastal territory. It is nonetheless notably the fact that there is not a universal recipe for the management of any given NIS, as is showed by the contrasting consumer attitudes towards rabbitfishes among neighboring countries in the Eastern Mediterranean, which are appreciated and targeted by local fisheries in some areas (e.g., Cyprus, Israel, Lebanon), while considered without any profitable value and discarded in other locations [113]. Control, education, and awareness could modify local consumer behaviour, enabling the marketing of novel products including NIS.
The present review also highlights that other fish and sea urchin species feeding on C. cylindracea constitute fishery targets, hence providing seafood for human consumption. The utilisation of this NIS as a food resource can potentially lead to an accumulation of the secondary metabolite caulerpin in the animal tissues and the amplification of caulerpin in liver, muscle, and brain tissues of consumers such as Sarpa salpa [75,76], Diplodus sargus [50,65,66,67,68,69,70,71,72,73,74], and Spondyliosoma cantharus [75]. Several studies have hypothesised a decrease in the organoleptic quality in Diplodus sargus, possibly due to biomagnification of the caulerpin concentration along the food web [66,71,72,74]. Other studies suggest an anxiolytic-like effect on Diplodus sargus associated with a decrease in aggressiveness [67,70]. The sea urchin Paracentrotus lividus, when exposed to the consumption of C. cylindracea, has reduced motility and coordination [59]. Moreover, the consumption of C. cylindracea also stimulate the production of Heat Shock Proteins [118], and an antioxidant response in the sea urchin P. lividus [60]. In addition, the use of the secondary metabolite caulerpin in the aquaculture sector appears promising [119]. The introduction of the metabolite produced by the invasive alga C. cylindracea into the diet of the model fish Danio rerio can influence the hypothalamic—pituitary—gonadal axis, increasing food intake and improving fish reproductive performances [119]. Future research could offer innovative and sustainable solutions, encouraging the exploitation of NIS as resources for veterinary and aquaculture sectors.
5. Conclusions, Future Directions, and Perspectives
The invasive macroalga C. cylindracea is widely distributed in the Mediterranean Sea and has become a food resource for many native and introduced species, despite containing grazing deterrents such as caulerpin and, to a lesser extent, caulerpenyne [51]. Hence, C. cylindracea expands the diversify of the trophic resources available to several herbivorous and generalist species, both native and NIS. Further study will better determine the ecological impact of this NIS in Mediterranean coastal ecosystems, as well as new perspectives for its exploitation by marine organisms and humans. The last is of particular relevance for food security, since the secondary metabolites caulerpin and caulerpenyne have been found to accumulate in some exploited species and are possibly responsible for the undesired changes in their taste, up to the point of becoming unpalatable in certain cases.
The invasion by Caulerpa cylindracea represents an early and well-known contribution to the ongoing trend of NIS colonisation of the Mediterranean Basin. Despite the fact that Mediterranean species account for a relatively high regional diversity [120], it has a relatively lower diversity of species than that of the Indo-Pacific region by virtue of the much smaller size of the Mediterranean Basin and its geological history that includes its isolation from other water masses, desiccation, and mass mortality of its biota due to heavy hypersaline conditions during the Messinian salinity crisis in late Miocene, followed by the intrusion of continental brackish water later on, and the abrupt reconnection to the Atlantic ocean about 5.33 million years ago, from which the actual native species originated [13,121].
The aperture of the Suez Canal in 1869 connected the Mediterranean biota with the huge biodiversity pool of the Indo-Pacific region, resulting in an influx of tropical species towards the Mediterranean. The rate of colonisation of the Mediterranean Sea by tropical species was further prompted by the progressive decrease in the salinity barrier represented by the Great Bitter Lake, which was further accelerated by the successive enlargements of the route to accommodate the ever-increasing flux of carriers. This picture has been probably reinforced by the increase in the temperature driven by global warming, which will proceed faster in the Mediterranean as a consequence of its smaller thermal inertia with respect to the larger oceanic water masses [120].
Commercial shipping is supposed to be the major vector of NIS introduction into the Mediterranean Sea, through which nearly a third of the world commercial ship traffic transits [25]. Given the fundamental role of commercial shipping for NIS in the Mediterranean Sea, as well as for the global economy, the trend of NIS introductions in the Mediterranean was expected to grow following the recent increase in the capacity of the Suez Canal [22]. In addition, the redistribution of shipping traffic should be expected following the year-round aperture of the Arctic shipping route as a result of the ongoing reduction in the ice cover, the enhanced capacity to predict ice presence, and the enhanced shipping facilities and services along the coasts [122]. In addition, geopolitical instability in the South China Sea resulting from the growing attempts to control resources and secure national sovereignty claims by emerging geopolitical players such as China and India adds uncertainty to future shipping routes in the area [123], with possible repercussions on the transport of potentially invasive species.
This overview provided an in-depth analysis on trophic relationships based on the known consumers of the NIS C. cylindracea, highlighting multiple issues that are still under-investigated or almost unknown and indicating the main goals to reach in further scientific investigations. The NIS C. cylindracea has been one of the most studied marine invaders, and the overall knowledge acquired has allowed for identifying a wide taxonomic range of marine fauna that has exploited the newly available trophic resource. The present review highlights that complex interactions among biotic factors affect Caulerpa-based trophic chains, with direct and indirect effects on food webs, and more generally on the whole marine coastal ecosystem. Although further studies will be necessary to understand the complex mechanisms involved in the introduction of the invasive C. cylindracea in the Mediterranean trophic webs and its related effects at multiple scales (i.e., species, populations, assemblages, and ecosystems), the patterns emerging from the present review indicate that the NIS C. cylindracea is a source of organic matter for benthic consumers and is able to modify the trophic niches of benthic species in Mediterranean infralittoral communities.
Herbivory represents the main pathway of the NIS seaweed to upper trophic levels in marine webs; however, omnivory has recently been recognised to exert considerable control on seaweed abundance. It is necessary to take into account that C. cylindracea exhibits great spread and regeneration abilities that allow it to tolerate a wide range of grazing intensities operated by marine consumers since it can easily recover its dense standing biomass by regenerating from fragments of several parts of the thallium, such as stolon, rhizoid, and frond. Additional complex compensative strategies of C. cylindracea could stimulate the algal growth rates and determinate the colonisation success of this NIS. In addition, several context-dependent effects deserve to be assessed in order to fully understand the inconsistent responses to the investigated variables across experimental research, trying to untangle the contribution of environmental and anthropogenic disturbances, often acting simultaneously, to the observed patterns. This issue remains an important aspect to be investigated, since the data available up to now did not show a clear relationship between the herbivory, omnivory, environmental conditions, and variations in the NIS biomass.
Furthermore, the consumption of the NIS C. cylindracea among Mediterranean marine herbivores and omnivores can cause the cumulation of the secondary metabolite caulerpin, a compound present in the Caulerpa genus [51], which was identified as the cause of detrimental effects in some native Mediterranean fish [66,71,72,74]. Some invasive fish species could have mechanisms of detoxification for the metabolites produced by Caulerpa [124], while these are at present unknown among Mediterranean native species. The collateral negative effects of Caulerpa introduction in the Mediterranean marine food webs include potential negative effects on human health as a consequence of consumption of the caulerpin accumulated in seafood. The mechanisms of accumulation and detoxification of caulerpin deserve further research to fully understand the processes regulating the adaptation to new invasive compound products.
Importantly, taking into account that Caulerpa cylindracea extracts and bioactive compounds are recognised as candidate pharmaceutical and nutraceutical products is needed; this deserves careful evaluation in order to ascertain their potential marketing, thereby contributing to control of this NIS through exploitation of its standing biomass by different industries from biotechnological, nutraceutical, and aquaculture fields.
Lastly, the present review will prove useful in the study of NIS, to propose the hypotheses that ad hoc studies should test C. cylindracea as well as other species, in order to promptly manage their ecological effects, forecast possible management scenarios, and obtain potential benefits from their exploitation as new marine resources.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15112115/s1, Table S1: List of the selected papers analysed in the literature review.
Author Contributions
Conceptualization, L.R.; resources, L.R.; writing—original draft preparation, L.R.; writing—review and editing, L.R. and T.V.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the “ON Foods—Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods” project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3—Call for proposals No. 341 of 15 March 2022 of the Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Project code PE00000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP D93C22000890001.
Data Availability Statement
Data are contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pysek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; Garcia-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Cassey, P.; García-Díaz, P.; Lockwood, J.L.; Blackburn, T.M. Invasion biology: Searching for predictions and prevention, and avoiding lost causes. Invasion. Biol. Hypotheses Evid. 2018, 9, 3–13. [Google Scholar] [CrossRef]
- Claudet, J.; Fraschetti, S. Human-driven impacts on marine habitats: A regional meta-analysis in the Mediterranean Sea. Biol. Conserv. 2010, 143, 2195–2206. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.R.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Lacoue-Labarthe, T.; Nunes, P.A.; Ziveri, P.; Cinar, M.; Gazeau, F.; Hall-Spencer, J.M.; Hilmi, N.; Moschella, P.; Safa, A.; Sauzade, D. Impacts of ocean acidification in a warming Mediterranean Sea: An overview. Reg. Stud. Mar. Sci. 2016, 5, 1–11. [Google Scholar] [CrossRef]
- Liquete, C.; Piroddi, C.; Macías, D.; Druon, J.-N.; Zulian, G. Ecosystem services sustainability in the Mediterranean Sea: Assessment of status and trends using multiple modelling approaches. Sci. Rep. 2016, 6, 34162. [Google Scholar] [CrossRef]
- Essl, F.; Lenzner, B.; Bacher, S.; Bailey, S.; Capinha, C.; Daehler, C.; Dullinger, S.; Genovesi, P.; Hui, C.; Hulme, P.E.; et al. Drivers of future alien species impacts: An expert-based assessment. Glob. Chang. Biol. 2020, 26, 4880–4893. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Zenetos, A.; Belchior, C.; Cardoso, A.C. Invading European Seas: Assessing pathways of introduction of marine aliens. Ocean Coast Manag. 2013, 76, 64–74. [Google Scholar] [CrossRef]
- Pusceddu, A.; Fraschetti, S.; Scopa, M.; Rizzo, L.; Danovaro, R. Meiofauna communities, nematode diversity and C degradation rates in seagrass (Posidonia oceanica L.) and unvegetated sediments invaded by the algae Caulerpa cylindracea (Sonder). Mar. Environ. Res. 2016, 119, 88–99. [Google Scholar] [CrossRef]
- Rizzo, L.; Pusceddu, A.; Bianchelli, S.; Fraschetti, S. Potentially combined effect of the invasive seaweed Caulerpa cylindracea (Sonder) and sediment deposition rates on organic matter and meiofaunal assemblages. Mar. Environ. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Pusceddu, A.; Stabili, L.; Alifano, P.; Fraschetti, S. Potential effects of an invasive seaweed (Caulerpa cylindracea, Sonder) on sedimentary organic matter and microbial metabolic activities. Sci. Rep. 2017, 7, 12113. [Google Scholar] [CrossRef] [PubMed]
- Krijgsman, W.; Hilgen, F.J.; Raffi, I.; Sierro, F.J.; Wilson, D.S. Chronology, causes and progression of the Messinian salinity crisis. Nature 1999, 400, 652–655. [Google Scholar] [CrossRef]
- Morales-Muñiz, A.; Roselló-Izquierdo, E. Twenty thousand years of fishing in the strait. In Human Impacts on Ancient Marine Ecosystems, a Global Perspective; Rick, T.C., Erlandson, J.M., Eds.; University of California Press: Berkeley, CA, USA, 2008; pp. 243–277. [Google Scholar]
- Theodoropoulou, T. Fishing together, fishing on its own: Fish exploitation patterns at the Neolithic Alepotrypa cave (Diros, Greece) and Aegean prehistoric fishing traditions. Int. J. Osteoarchaeol. 2019, 29, 395–406. [Google Scholar] [CrossRef]
- Boudouresque, C.-F. Marine biodiversity in the Mediterranean: Status of species, populations and communities. Trav. Sci. Parc. Natl. Port-Cros 2004, 20, 97–146. [Google Scholar]
- Kalogirou, S.; Azzurro, E.; Bariche, M. The ongoing shift of Mediterranean coastal fish assemblages and the spread of non-indigenous species. In Biodiversity Enrichment in a Diverse World; Lameed, G.A., Ed.; InTech: Rijeka, Croatia, 2012; p. 11. [Google Scholar]
- Givan, O.; Edelist, D.; Sonin, O.; Belmaker, J. Thermal affinity as the dominant factor changing Mediterranean fish abundances. Glob. Chang. Biol. 2018, 24, e80–e89. [Google Scholar] [CrossRef]
- Azzurro, E.; Sbragaglia, V.; Cerri, J.; Bariche, M.; Bolognini, L.; Souissi, J.B.; Busoni, G.; Coco, S.; Chryssanthi, A.; Garrabou, J. The shifting distribution of Mediterranean fishes: A spatio-temporal assessment based on Local Ecological Knowledge. Glob. Chang. Biol. 2019, 25, 2779–2792. [Google Scholar] [CrossRef]
- Last, P.R.; White, W.T.; Gledhill, D.C.; Hobday, A.J.; Brown, R.; Edgar, G.J.; Pecl, G. Long-term shifts in abundance and distribution of a temperate fish fauna: A response to climate change and fishing practices. Glob. Ecol. Biogeogr. 2011, 20, 58–72. [Google Scholar] [CrossRef]
- Cheung, W.W.; Watson, R.; Pauly, D. Signature of ocean warming in global fisheries catch. Nature 2013, 497, 365. [Google Scholar] [CrossRef]
- Galil, B.S.; Marchini, A.; Occhipinti-Ambrogi, A. Mare Nostrum, Mare Quod Invaditur—The History of Bioinvasions in the Mediterranean Sea. In Histories of Bioinvasions in the Mediterranean; Queiroz, A., Pooley, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 21–49. [Google Scholar]
- Galil, B.S.; Marchini, A.; Occhipinti-Ambrogi, A. East is east and West is west? Management of marine bioinvasions in the Mediterranean Sea. Estuar. Coast Shelf Sci. 2018, 201, 7–16. [Google Scholar] [CrossRef]
- Galil, B.S.; Danovaro, R.; Rothman, S.; Gevili, R.; Goren, M. Invasive biota in the deep-sea Mediterranean: An emerging issue in marine conservation and management. Biol. Invasions 2019, 21, 281–288. [Google Scholar] [CrossRef]
- Dobler, J.P. Analysis of shipping patterns in the Mediterranean and Black seas. In Proceedings of the Alien Marine Organisms Introduced by Ships, Istanbul, Turkey, 6–9 November 2002; CIESM Workshop Monographs. 2002; Volume 20, pp. 19–28. [Google Scholar]
- Kalogirou, S. Alien Fish Species in the Eastern Mediterranean Sea: Invasion Biology in Coastal Ecosystems; Department of marine ecology, University of Gothenburg: Gothenburg, Sweden, 2011; p. 140. [Google Scholar]
- Hulme, P.E. Invasion pathways at a crossroad: Policy and research challenges for managing alien species introductions. J. Appl. Ecol. 2015, 52, 1418–1424. [Google Scholar] [CrossRef]
- Gewing, M.-T.; Shenkar, N. Monitoring the magnitude of marine vessel infestation by non-indigenous ascidians in the Mediterranean. Mar. Pollut. Bull. 2017, 121, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.; Fitter, A. The varying success of invaders. Ecology 1996, 77, 1661–1666. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Biological pollution in the Mediterranean Sea: Invasive versus introduced macrophytes. Mar. Pollut. Bull. 2002, 44, 32–38. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Gaertner, M.; Biggs, R.; Te Beest, M.; Hui, C.; Molofsky, J.; Richardson, D.M. Invasive plants as drivers of regime shifts: Identifying high-priority invaders that alter feedback relationships. Divers. Distrib. 2014, 20, 733–744. [Google Scholar] [CrossRef]
- Tsiamis, K.; Azzurro, E.; Bariche, M.; Çinar, M.E.; Crocetta, F.; De Clerck, O.; Galil, B.; Gomez, F.; Hoffman, R.; Jensen, K.R. Prioritizing marine invasive alien species in the European Union through horizon scanning. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 794–845. [Google Scholar] [CrossRef]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C. Management priorities for marine invasive species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef]
- Belton, G.S.; Phomme van Reine, W.F.; Huisman, J.M.; Draisma, S.G.A.; Gurgel, C.F.D. Resolving phenotypic plasticity and species designation in the morphology challenging Caulerpa racemosa-peltata complex (Caulerpaceae, Chlorophyta). J. Phycol. 2014, 50, 32–54. [Google Scholar] [CrossRef]
- Streftaris, N.; Zenetos, A. Alien marine species in the Mediterranean-the 100 ‘Worst Invasives’ and their impact. Mediterr. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef]
- Verlaque, M.; Afonso-Carrillo, J.; Gil-Rodriguez, M.C.; Durand, C.; Boudouresque, C.F.; Le Parco, Y. Blitzkrieg in a marine invasion: Caulerpa racemosa var. cylindracea (Bryopsidales, Chlorophyta) reaches the Canary Islands (north-east Atlantic). Biol. Invasions 2004, 6, 269–281. [Google Scholar] [CrossRef]
- Klein, J.; Verlaque, M. The Caulerpa racemosa invasion: A critical review. Mar. Pollut. Bull. 2008, 56, 205–225. [Google Scholar] [CrossRef]
- Piazzi, L.; Balata, D.; Bulleri, F.; Gennaro, P.; Ceccherelli, G. The invasion of Caulerpa cylindracea in the Mediterranean: The known, the unknown and the knowable. Mar. Biol. 2016, 163, 1–14. [Google Scholar] [CrossRef]
- Papini, A.; Mosti, S.; Santosuosso, U. Tracking the origin of the invading Caulerpa (Caulerpales, Chlorophyta) with Geographic Profiling, a criminological technique for a killer alga. Biol. Invasions 2013, 15, 1613–1621. [Google Scholar] [CrossRef]
- Bulleri, F.; Balata, D.; Bertocci, I.; Tamburello, L.; Benedetti-Cecchi, L. The seaweed Caulerpa racemosa on Mediterranean rocky reefs: From passenger to driver of ecological change. Ecology 2010, 91, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Bulleri, F.; Badalamenti, F.; Iveša, L.; Mikac, B.; Musco, L.; Jaklin, A.; Rattray, A.; Vega Fernández, T.; Benedetti-Cecchi, L. The effects of an invasive seaweed on native communities vary along a gradient of land-based human impacts. PeerJ 2016, 4, e179. [Google Scholar] [CrossRef]
- van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef]
- Parker, J.D.; Burkepile, D.E.; Hay, M.E. Opposing effects of native and exotic herbivores on plant invasions. Science 2006, 311, 1459–1461. [Google Scholar] [CrossRef]
- Chaffin, B.C.; Garmestani, A.S.; Angeler, D.G.; Herrmann, D.L.; Stow, C.A.; Nystrom, M.; Sendzimir, J.; Hopton, M.E.; Kolasa, J.; Allen, C.R. Biological invasions, ecological resilience and adaptive governance. J. Environ. Manag. 2016, 183, 399–407. [Google Scholar] [CrossRef]
- Sfriso, A.; Buosi, A.; Wolf, M.A.; Sfriso, A.A. Invasion of alien macroalgae in the Venice Lagoon, a pest or a resource? Aquat. Invasions 2020, 15, 245–270. [Google Scholar] [CrossRef]
- Noe, S.; Badalamenti, F.; Bonaviri, C.; Musco, L.; Vega Fernandez, T.; Vizzini, S.; Gianguzza, P. Food selection of a generalist herbivore exposed to native and alien seaweeds. Mar. Pollut. Bull. 2018, 129, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Holmer, M.; Marbà, N.; Lamote, M.; Duarte, C.M. Deterioration of Sediment Quality in Seagrass Meadows (Posidonia oceanica) Invaded by Macroalgae (Caulerpa sp.). Estuar. Coast. 2009, 32, 456–466. [Google Scholar] [CrossRef]
- Cebrian, E.; Linares, C.; Marchal, C.; Garrabou, J. Exploring the effects of invasive algae on the persistence of gorgonian populations. Biol. Invasions 2012, 14, 2647–2656. [Google Scholar] [CrossRef]
- Felline, S.; Caricato, R.; Cutignano, A.; Gorbi, S.; Lionetto, M.G.; Mollo, E.; Regoli, F.; Terlizzi, A. Subtle effects of biological invasions: Cellular and physiological responses of fish eating the exotic pest Caulerpa racemosa. PLoS ONE 2012, 7, e38763. [Google Scholar] [CrossRef]
- Schwede, J.G.; Cardellina, J.H.; Grode, S.H.; James, T.R.; Blackman, A.J. Distribution of the pigment caulerpin in species of the green alga Caulerpa. Phytochemistry 1986, 26, 155–158. [Google Scholar] [CrossRef]
- Box, A.; Sureda, A.; Tauler, P.; Terrados, J.; Marbà, N.; Pons, A.; Deudero, S. Seasonality of caulerpenyne content in native Caulerpa prolifera and invasive C. taxifolia and C. racemosa var. cylindracea in the western Mediterranean Sea. Bot. Mar. 2010, 53, 367–375. [Google Scholar] [CrossRef]
- Paul, V.J.; Puglisi, M.P. Chemical mediation of interactions among marine organisms. Nat. Prod. Rep. 2004, 21, 189–209. [Google Scholar] [CrossRef]
- Deudero, S.; Box, A.; Alos, J.; Arroyo, N.L.; Marba, N. Functional changes due to invasive species: Food web shifts at shallow Posidonia oceanica seagrass beds colonized by the alien macroalga Caulerpa racemosa. Estuar. Coast. Shelf Sci. 2011, 93, 106–116. [Google Scholar] [CrossRef]
- Casu, D.; Ceccherelli, G.; Sechi, N.; Rumolo, P.; Sara, G. Caulerpa racemosa var. cylindracea as a potential source of organic matter for benthic consumers: Evidences from a stable isotope analysis. Aquat. Ecol. 2009, 43, 1023–1029. [Google Scholar] [CrossRef]
- Maric, M.; De Troch, M.; Occhipinti-Ambrogi, A.; Olenin, S. Trophic interactions between indigenous and non-indigenous species in Lampedusa Island, Mediterranean Sea. Mar. Environ. Res. 2016, 120, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Bulleri, F.; Tamburello, L.; Benedetti-Cecchi, L. Loss of consumers alters the effects of resident assemblages on the local spread of an introduced macroalga. Oikos 2009, 118, 269–279. [Google Scholar] [CrossRef]
- Pusceddu, A.; Mikhno, M.; Giglioli, A.; Secci, M.; Pasquini, V.; Moccia, D.; Addis, P. Foraging of the sea urchin Paracentrotus lividus (Lamarck, 1816) on invasive allochthonous and autochthonous algae. Mar. Environ. Res. 2021, 170, 105428. [Google Scholar] [CrossRef] [PubMed]
- Vega Fernandez, T.; Badalamenti, F.; Bonaviri, C.; Di Trapani, F.; Gianguzza, P.; Noe, S.; Musco, L. Synergistic reduction of a native key herbivore performance by two non-indigenous invasive algae. Mar. Pollut. Bull. 2019, 141, 649–654. [Google Scholar] [CrossRef]
- Tejada, S.; Deudero, S.; Box, A.; Sureda, A. Physiological response of the sea urchin Paracentrotus lividus fed with the seagrass Posidonia oceanica and the alien algae Caulerpa racemosa and Lophocladia lallemandii. Mar. Environ. Res. 2013, 83, 48–53. [Google Scholar] [CrossRef]
- Tomas, F.; Box, A.; Terrados, J. Effects of invasive seaweeds on feeding preference and performance of a keystone Mediterranean herbivore. Biol. Invasions 2011, 13, 1559–1570. [Google Scholar] [CrossRef]
- Cebrian, E.; Ballesteros, E.; Linares, C.; Tomas, F. Do native herbivores provide resistance to Mediterranean marine bioinvasions? A seaweed example. Biol. Invasions 2011, 13, 1397–1408. [Google Scholar] [CrossRef]
- Zuljevic, A.; Nikolic, V.; Despalatovic, M.; Antolic, B. Experimental in situ feeding of the sea urchin Paracentrotus lividus with invasive algae Caulerpa racemosa var. cylindracea and Caulerpa taxifolia in the Adriatic sea. Fresenius Environ. Bull. 2008, 17, 2098–2102. [Google Scholar]
- Ruitton, S.; Verlaque, M.; Aubin, G.; Boudouresque, C.F. Grazing on Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) in the Mediterranean Sea by herbivorous fishes and sea urchins. Vie Milieu-Life Environ. 2006, 56, 33–41. [Google Scholar]
- Santamaria, J.; Tomas, F.; Ballesteros, E.; Cebrian, E. Herbivory on the Invasive Alga Caulerpa cylindracea: The Role of Omnivorous Fishes. Front. Mar. Sci. 2021, 8, 702492. [Google Scholar] [CrossRef]
- Miccoli, A.; Mancini, E.; Boschi, M.; Provenza, F.; Lelli, V.; Tiralongo, F.; Renzi, M.; Terlizzi, A.; Bonamano, S.; Marcelli, M. Trophic, Chemo-Ecological and Sex-Specific Insights on the Relation Between Diplodus sargus (Linnaeus, 1758) and the Invasive Caulerpa cylindracea (Sonder, 1845). Front. Mar. Sci. 2021, 8, 680787. [Google Scholar] [CrossRef]
- Magliozzi, L.; Maselli, V.; Almada, F.; Di Cosmo, A.; Mollo, E.; Polese, G. Effect of the algal alkaloid caulerpin on neuropeptide Y (NPY) expression in the central nervous system (CNS) of Diplodus sargus. J. Comp. Physiol. 2019, 205, 203–210. [Google Scholar] [CrossRef]
- Vitale, R.M.; D’Aniello, E.; Gorbi, S.; Martella, A.; Silvestri, C.; Giuliani, M.E.; Fellous, T.; Gentile, A.; Carbone, M.; Cutignano, A.; et al. Fishing for Targets of Alien Metabolites: A Novel Peroxisome Proliferator-Activated Receptor (PPAR) Agonist from a Marine Pest. Mar. Drugs 2018, 16, 431. [Google Scholar] [CrossRef] [PubMed]
- Del Coco, L.; Felline, S.; Girelli, C.R.; Angile, F.; Magliozzi, L.; Almada, F.; D’Aniello, B.; Mollo, E.; Terlizzi, A.; Fanizzi, F.P. H-1 NMR Spectroscopy and MVA to Evaluate the Effects of Caulerpin-Based Diet on Diplodus sargus Lipid Profiles. Mar. Drugs 2018, 16, 390. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, L.; Almada, F.; Robalo, J.; Mollo, E.; Polese, G.; Goncalves, E.J.; Felline, S.; Terlizzi, A.; D’Aniello, B. Cryptic effects of biological invasions: Reduction of the aggressive behaviour of a native fish under the influence of an invasive biomolecule. PLoS ONE 2017, 12, e0185620. [Google Scholar] [CrossRef] [PubMed]
- De Pascali, S.A.; Del Coco, L.; Felline, S.; Mollo, E.; Terlizzi, A.; Fanizzi, F.P. H-1 NMR Spectroscopy and MVA Analysis of Diplodus sargus Eating the Exotic Pest Caulerpa cylindracea. Mar. Drugs 2015, 13, 3550–3566. [Google Scholar] [CrossRef] [PubMed]
- Felline, S.; Mollo, E.; Ferramosca, A.; Zara, V.; Regoli, F.; Gorbi, S.; Terlizzi, A. Can a marine pest reduce the nutritional value of Mediterranean fish flesh? Mar. Biol. 2014, 161, 1275–1283. [Google Scholar] [CrossRef]
- Gorbi, S.; Giuliani, M.E.; Pittura, L.; d’Errico, G.; Terlizzi, A.; Felline, S.; Grauso, L.; Mollo, E.; Cutignano, A.; Regoli, F. Could molecular effects of Caulerpa racemosa metabolites modulate the impact on fish populations of Diplodus sargus? Mar. Environ. Res. 2014, 96, 2–11. [Google Scholar] [CrossRef]
- Terlizzi, A.; Felline, S.; Lionetto, M.G.; Caricato, R.; Perfetti, V.; Cutignano, A.; Mollo, E. Detrimental physiological effects of the invasive alga Caulerpa racemosa on the Mediterranean white seabream Diplodus sargus. Aquat. Biol. 2011, 12, 109–117. [Google Scholar] [CrossRef]
- Felline, S.; Mollo, E.; Cutignano, A.; Grauso, L.; Andaloro, F.; Castriota, L.; Consoli, P.; Falautano, M.; Sinopoli, M.; Terlizzi, A. Preliminary observations of caulerpin accumulation from the invasive Caulerpa cylindracea in native Mediterranean fish species Aquat. Biol. 2017, 26, 27–31. [Google Scholar] [CrossRef]
- Turhan, S.; Cavas, L. The threat on your plate: Do we just eat Sarpa salpa or more? Reg. Stud. Mar. Sci. 2019, 29, 100697. [Google Scholar] [CrossRef]
- Marco-Mendez, C.; Ferrero-Vicente, L.M.; Prado, P.; Sanchez-Lizaso, J.L. Epiphytes and nutrient contents influence Sarpa salpa herbivory on Caulerpa spp vs. seagrass species in Mediterranean meadows. Estuar. Coast. Shelf Sci. 2017, 184, 54–66. [Google Scholar] [CrossRef]
- Tomas, F.; Cebrian, E.; Ballesteros, E. Differential herbivory of invasive algae by native fish in the Mediterranean Sea. Estuar. Coast. Shelf Sci. 2011, 92, 27–34. [Google Scholar] [CrossRef]
- Santamaría, J.; Golo, R.; Verdura, J.; Tomas, F.; Ballesteros, E.; Alcoverro, T.; Arthur, R.; Cebrian, E. Learning takes time: Biotic resistance by native herbivores increases through the invasion process. Ecol. Lett. 2022, 25, 2525–2539. [Google Scholar] [CrossRef] [PubMed]
- Bariche, M. Diet of the Lessepsian Fishes, Siganus rivulatus and S. luridus (Siganidae) in the Eastern Mediterranean: A Bibliographic Analysis. Cybium 2006, 30, 41–49. Available online: https://sfi-cybium.fr/sites/default/files/pdfs-cybium/10-Bariche%20275.pdf (accessed on 30 December 2022).
- Azzurro, E.; Fanelli, E. Preliminary data on feeding habits of dusky spinefoot Siganus luridus in the Sicily Channel (Central Mediterranean). Biol. Mar. Mediterr. 2004, 11, 145. [Google Scholar]
- Lundberg, B.; Payiatas, G.; Argyrou, M. Notes on the diet of the Lessepsian migrant herbivorous fishes, Siganus luridus and S. rivulatus, in Cyprus. Isr. J. Zool. 1999, 45, 127–134. [Google Scholar]
- Box, A.; Deudero, S.; Sureda, A.; Blanco, A.; Alos, J.; Terrados, J.; Grau, A.M.; Riera, F. Diet and physiological responses of Spondyliosoma cantharus (Linnaeus, 1758) to the Caulerpa racemosa var. cylindracea invasion. J. Exp. Mar. Biol. Ecol. 2009, 380, 11–19. [Google Scholar] [CrossRef]
- Vazquez-Luis, M.; Sanchez-Jerez, P.; Bayle-Sempere, J.T. Effects of Caulerpa racemosa var. cylindracea on prey availability: An experimental approach to predation of amphipods by Thalassoma pavo (Labridae). Hydrobiologia 2010, 654, 147–154. [Google Scholar] [CrossRef]
- Alomar, C.; Deudero, S.; Andaloro, F.; Castriota, L.; Consoli, P.; Falautano, M.; Sinopoli, M. Caulerpa cylindracea Sonder invasion modifies trophic niche in in-fralittoral rocky benthic community. Mar. Environ. Res. 2016, 120, 86–92. [Google Scholar] [CrossRef]
- Sinopoli, M.; Allegra, A.; Andaloro, F.; Consoli, P.; Esposito, V.; Falautano, M.; Mangano, M.C.; Nicastro, A.; Scotti, G.; Castriota, L. Assessing the effect of the alien seaweed Caulerpa cylindracea on infralittoral rocky benthic invertebrate community: Evidence from a Mediterranean Marine Protected Area. Reg. Stud. Mar. Sci. 2020, 38, 101372. [Google Scholar] [CrossRef]
- Kimbro, D.L.; Cheng, B.S.; Grosholz, E.D. Biotic resistance in marine environments. Ecol. Lett. 2013, 16, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Papacostas, K.J.; Freestone, A.L. Stronger predation in a subtropical community dampens an invasive species-induced trophic cascade. Biol. Invasions 2019, 21, 203–215. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Hoey, A.S. Sleeping functional group drives coral-reef recovery. Curr. Biol. 2006, 16, 2434–2439. [Google Scholar] [CrossRef]
- Mendes, T.C.; Cordeiro, C.A.M.M.; Ferreira, C.E.L. An experimental evaluation of macroalgal consumption and selectivity by nominally herbivorous fishes on subtropical rocky reefs. J. Exp. Mar. Bio. Ecol. 2015, 471, 146–152. [Google Scholar] [CrossRef]
- Mendes, T.C.; Quimbayo, J.P.; Bouth, H.F.; Silva, L.P.S.; Ferreira, C.E.L. The omnivorous triggerfish Melichthys niger is a functional herbivore on an isolated Atlantic oceanic island. J. Fish Biol. 2019, 95, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Tebbett, S.B.; Hoey, A.S.; Depczynski, M.; Wismer, S.; Bellwood, D.R. Macroalgae removal on coral reefs: Acemose ecosystem functions transcend biogeographic locations. Coral Reefs 2020, 39, 203–214. [Google Scholar] [CrossRef]
- Caronni, S.; Calabretti, C.; Delaria, M.A.; Bernardi, G.; Navone, A.; Occhipinti-Ambrogi, A.; Panzalis, P.; Ceccherelli, G. Consumer depletion alters seagrass resistance to an invasive macroalga. PLoS ONE 2015, 10, e0115858. [Google Scholar] [CrossRef]
- Bulleri, F.; Malquori, F. High tolerance to simulated herbivory in the clonal seaweed, Caulerpa cylindracea. Mar. Environ. Res. 2015, 107, 61–65. [Google Scholar] [CrossRef]
- Tamburello, L.; Bulleri, F.; Balata, D.; Benedetti-Cecchi, L. The role of overgrazing and anthropogenic disturbance in shaping spatial patterns of distribution of an invasive seaweed. J. Appl. Ecol. 2014, 51, 406–414. [Google Scholar] [CrossRef]
- Ceccherelli, G.; Pinna, S.; Cusseddu, V.; Bulleri, F. The role of disturbance in promoting the spread of the invasive seaweed Caulerpa racemosa in seagrass meadows. Biol. Invasions 2014, 16, 2737–2745. [Google Scholar] [CrossRef]
- Kiparissis, S.; Fakiris, E.; Papatheodorou, G.; Geraga, M.; Kornaros, M.; Kapareliotis, A.; Ferentinos, G. Illegal trawling and induced invasive algal spread as collaborative factors in a Posidonia oceanica meadow degradation. Biol. Inv. 2011, 13, 669–678. [Google Scholar] [CrossRef]
- Montefalcone, M.; Lasagna, R.; Bianchi, C.N.; Morri, C.; Albertelli, G. Anchoring damage on Posidonia oceanica meadow cover: A case study in Prelo Cove (Ligurian Sea, NW Mediterranean). Chem. Ecol. 2006, 22 (Suppl. S1), S207–S217. [Google Scholar] [CrossRef]
- Stabili, L.; Rizzo, L.; Pizzolante, G.; Alifano, P.; Fraschetti, S. Spatial distribution of the culturable bacterial community associated with the invasive alga Caulerpa cylindracea in the Mediterranean Sea. Mar. Environ. Res. 2017, 125, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Fraschetti, S.; Alifano, P.; Tredici, M.S.; Stabili, L. Association of Vibrio community with the Atlantic Mediterranean invasive alga Caulerpa cylindracea. J. Exp. Mar. Biol. Ecol. 2016, 475, 129–136. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 2022, 3, 308–352. [Google Scholar] [CrossRef]
- Tiralongo, F.; Messina, G.; Lombardo, B.M. Invasive Species Control: Predation on the Alien Crab Percnon gibbesi (H. Milne Edwards, 1853) (Malacostraca: Percnidae) by the Rock Goby, Gobius paganellus Linnaeus, 1758 (Actinopterygii: Gobiidae). J. Mar. Sci. Eng. 2021, 9, 393. [Google Scholar] [CrossRef]
- Ceccherelli, G.; Piazzi, L.; Balata, D. Spread of introduced Caulerpa species in macroalgal habitats. J. Exp. Mar. Biol. Ecol. 2002, 280, 1–11. [Google Scholar] [CrossRef]
- Piazzi, L.; Ceccherelli, G.; Balata, D.; Cinelli, F. Early patterns of Caulerpa racemosa recovery in the Mediterranean Sea: The influence of algal turfs. J. Mar. Biol. Assoc. 2003, 83, 27–29. [Google Scholar] [CrossRef]
- Gennaro, P.; Piazzi, L. The indirect role of nutrients in enhancing the invasion of Caulerpa racemosa var. cylindracea. Biol. Invasion 2014, 16, 1709–1717. [Google Scholar] [CrossRef]
- Galil, B.S. A Sea, a Canal, a Disaster: The Suez Canal and the Transformation of the Mediterranean Biota. In The Suez Canal: Past Lessons and Future Challenges; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; pp. 199–215. [Google Scholar] [CrossRef]
- Jardas, I. Adriatic Ichthyofauna; Školska knjiga: Zagreb, Croatia, 1996; 535p. (In Croatian) [Google Scholar]
- Buñuel, X.; Alcoverro, T.; Romero, J.; Ruiz, J.M.; Arthur, R. The dominant seagrass herbivore Sarpa salpa shifts its shoaling and feeding strategies as they grow. Sci. Rep. 2020, 10, 10622. [Google Scholar] [CrossRef] [PubMed]
- Pallaoro, A.; Dulčić, J.; Matić-Skoko, S.; Kraljević, M.; Jardas, I. Biology of the salema, Sarpa salpa (L. 1758) (Pisces, Sparidae) from the middle-eastern Adriatic. J. Appl. Ichthyol. 2008, 24, 276–281. [Google Scholar] [CrossRef]
- Casadevall, M.; Rodríguez-Prieto, C.; Pueyo, J.; Martí, C.; Merciai, R.; Verlaque, M.; Real, E.; Torres, J.; Richir, J. The strange case of tough white seabream (Diplodus sargus, Teleostei: Sparidae): A first approach to the extent of the phenomenon in the mediterranean. Front. Mar. Sci. 2020, 7, 387. [Google Scholar] [CrossRef]
- Demirel, N.; Ulman, A.; Yıldız, T.; Ertör-Akyazi, P. A moving target: Achieving good environmental status and social justice in the case of an alien species, Rapa whelk in the Black Sea. Mar. Pol. 2021, 132, 104687. [Google Scholar] [CrossRef]
- Jimenez-Munoz, L.; Quintanilla, M.; Filomena, A. Managing the lionfish: Influence of high intensity ultrasound and binders on textural and sensory properties of lionfish (Pterois volitans) surimi patties. J. Food Sci. Technol. 2019, 56, 2167–2174. [Google Scholar] [CrossRef]
- Kleitou, P.; Crocetta, F.; Giakoumi, S.; Giovos, I.; Hall-Spencer, J.M.; Kalogirou, S.; Kletou, D.; Moutopoulos, D.K.; Rees, S. Fishery reforms for the management of non-indigenous species. J. Environ. Manag. 2021, 280, 111690. [Google Scholar] [CrossRef]
- Angilè, F.; Del Coco, L.; Girelli, C.R.; Basso, L.; Rizzo, L.; Piraino, S.; Stabili, L.; Fanizzi, F.P. 1H NMR metabolic profile of scyphomedusa Rhizostoma pulmo (Scyphozoa, Cnidaria) in Female gonads and somatic tissues: Preliminary results. Molecules 2020, 25, 806. [Google Scholar] [CrossRef]
- Stabili, L.; Rizzo, L.; Fanizzi, F.P.; Angilè, F.; Del Coco, L.; Girelli, C.R.; Lomartire, S.; Piraino, S.; Basso, L. The jellyfish Rhizostoma pulmo (Cnidaria): Biochemical composition of ovaries and antibacterial lysozyme-like activity of the oocyte lysate. Mar. Drugs 2018, 17, 17. [Google Scholar] [CrossRef]
- Basso, L.; Papadia, P.; Rizzo, L.; Migoni, D.; Fanizzi, F.P.; Piraino, S. Trace metals do not accumulate over time in the edible Mediterranean jellyfish Rhizostoma pulmo (Cnidaria, Scyphozoa) from urban coastal waters. Water 2021, 13, 1410. [Google Scholar] [CrossRef]
- Bel Mabrouk, S.; Reis, M.; Sousa, M.L.; Ribeiro, T.; Almeida, J.R.; Pereira, S.; Antunes, J.; Rosa, F.; Vasconcelos, V.; Achour, L. The marine seagrass Halophila stipulacea as a source of bioactive metabolites against obesity and biofouling. Mar. Drugs 2020, 18, 88. [Google Scholar] [CrossRef]
- Gianguzza, P.; Musco, L.; Russo, D.; Bonaviri, C.; Vega Fernández, T.; Arizza, V. Stress response, induced by the invasive algae Caulerpa taxifolia var. distichophylla and C. cylindracea, in the sea urchin Paracentrotus lividus. In Proceedings of the XXIV Congresso Società Italiana di Ecologia, Ferrara, Italy, 15–17 September 2014; p. 101. [Google Scholar]
- Schiano, V.; Cutignano, A.; Maiello, D.; Carbone, M.; Ciavatta, M.L.; Polese, G.; Fioretto, F.; Attanasio, C.; Palladino, A.; Felline, S.; et al. An Alkaloid from a Highly Invasive Seaweed Increases the Voracity and Reproductive Output of a Model Fish Species. Mar. Drugs 2022, 20, 513. [Google Scholar] [CrossRef] [PubMed]
- Lejeusne, C.; Chevaldonné, P.; Pergent-Martini, C.; Boudouresque, C.F.; Pérez, T. Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Roveri, M.; Flecker, R.; Krijgsman, W.; Lofi, J.; Lugli, S.; Manzi, V.; Sierro, F.J.; Bedrtini, A.; Camerlenghi, A.; De Lange, G.; et al. The Messinian Salinity Crisis: Past and future of a great challenge for marine sciences. Mar. Geol. 2014, 352, 25–58. [Google Scholar] [CrossRef]
- Schøyen, H.; Bråthen, S. The Northern Sea Route versus the Suez Canal: Cases from bulk shipping. J. Transp. Geogr. 2011, 19, 977–983. [Google Scholar] [CrossRef]
- Macaraig, C.E.; Fenton, A.J. Analyzing the causes and effects of the South China Sea dispute: Natural resources and freedom of navigation. J. Territ. Marit. Stud. 2021, 8, 42–58. [Google Scholar]
- Regoli, F.; Giuliani, M.E.; Benedetti, M.; Arukwe, A. Molecular and biochemical biomarkers in environmental monitoring: A comparison of biotransformation and antioxidant defense systems in multiple tissues. Aquat. Toxicol. 2011, 105 (Suppl. S3–S4), 56–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).