Performance and Mechanism of Fe3O4 Loaded Biochar Activating Persulfate to Degrade Acid Orange 7
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Preparation Method of Fe3O4@BC
2.3. Characterization Methods of Carbon-Based Composites
2.4. Experimental Steps for Degradation of AO7 by PS
2.5. Free Radical Quencher and Capture Experiment
2.6. Adsorption Kinetics Theory
2.6.1. Adsorption Kinetics Model
2.6.2. Adsorption Isotherm Model
3. Results
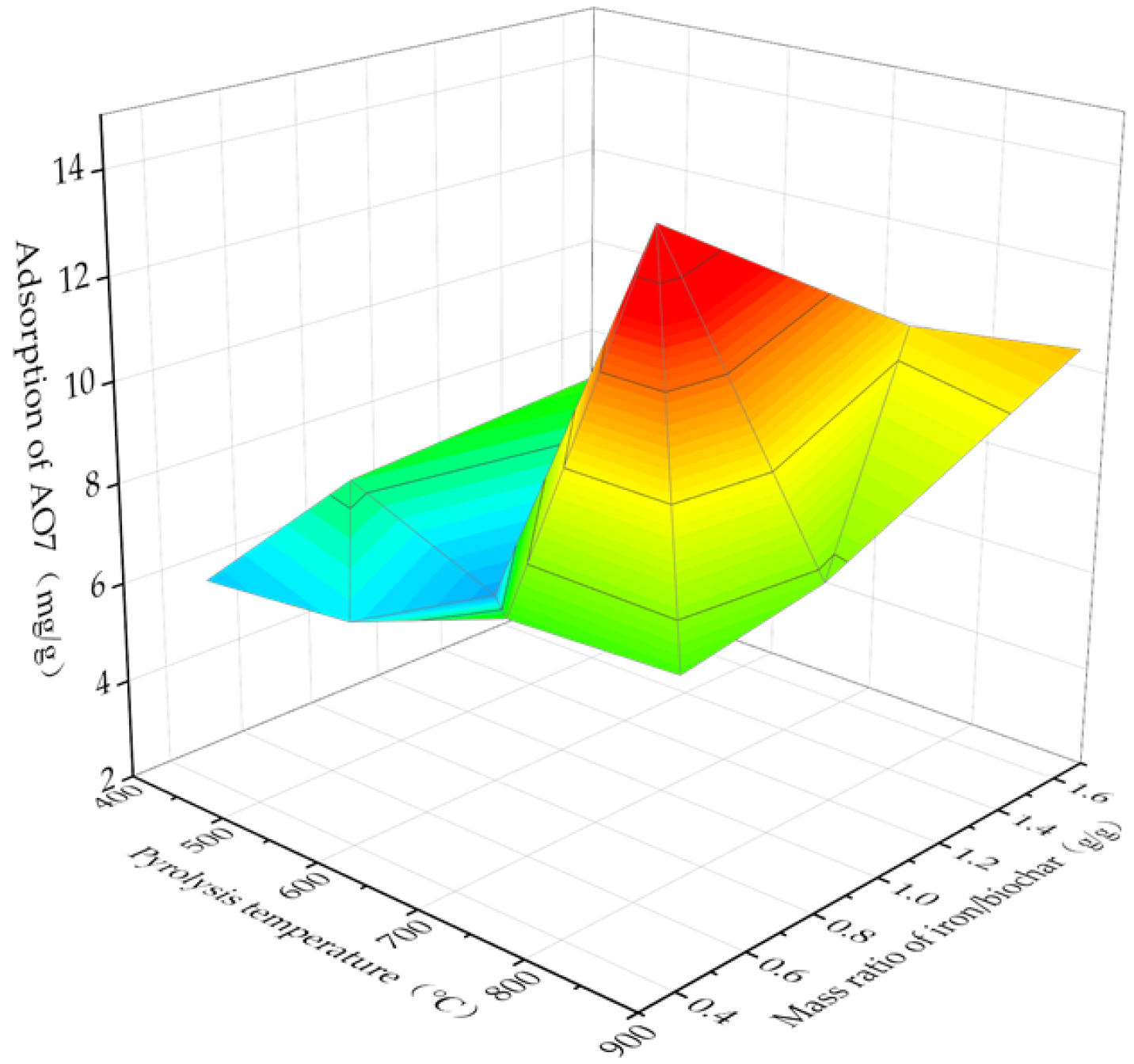
3.1. Determination of Optimal Preparation Conditions for Fe3O4@BC
3.2. Characterization of Fe3O4@BC
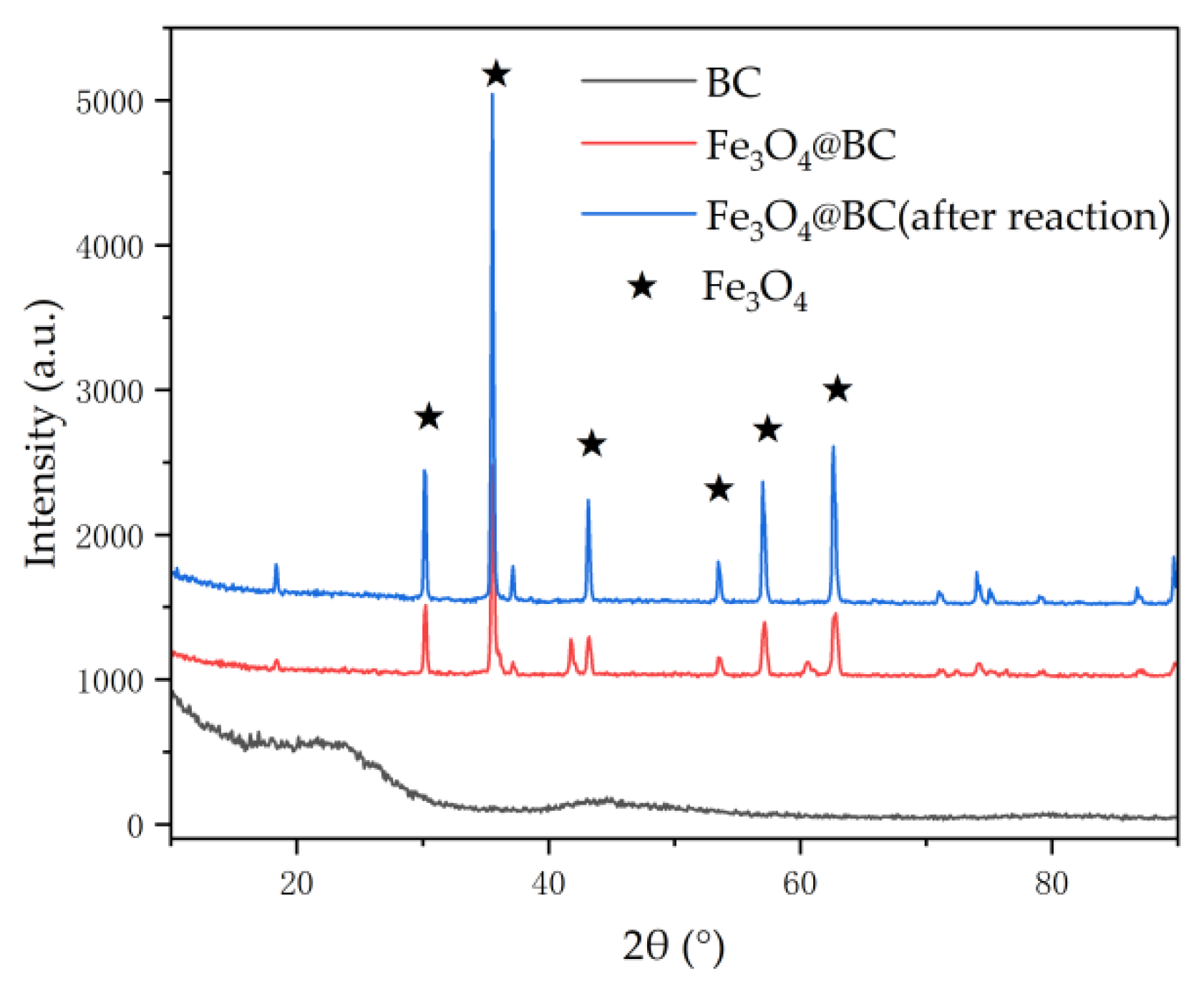
3.2.1. XRD Analysis
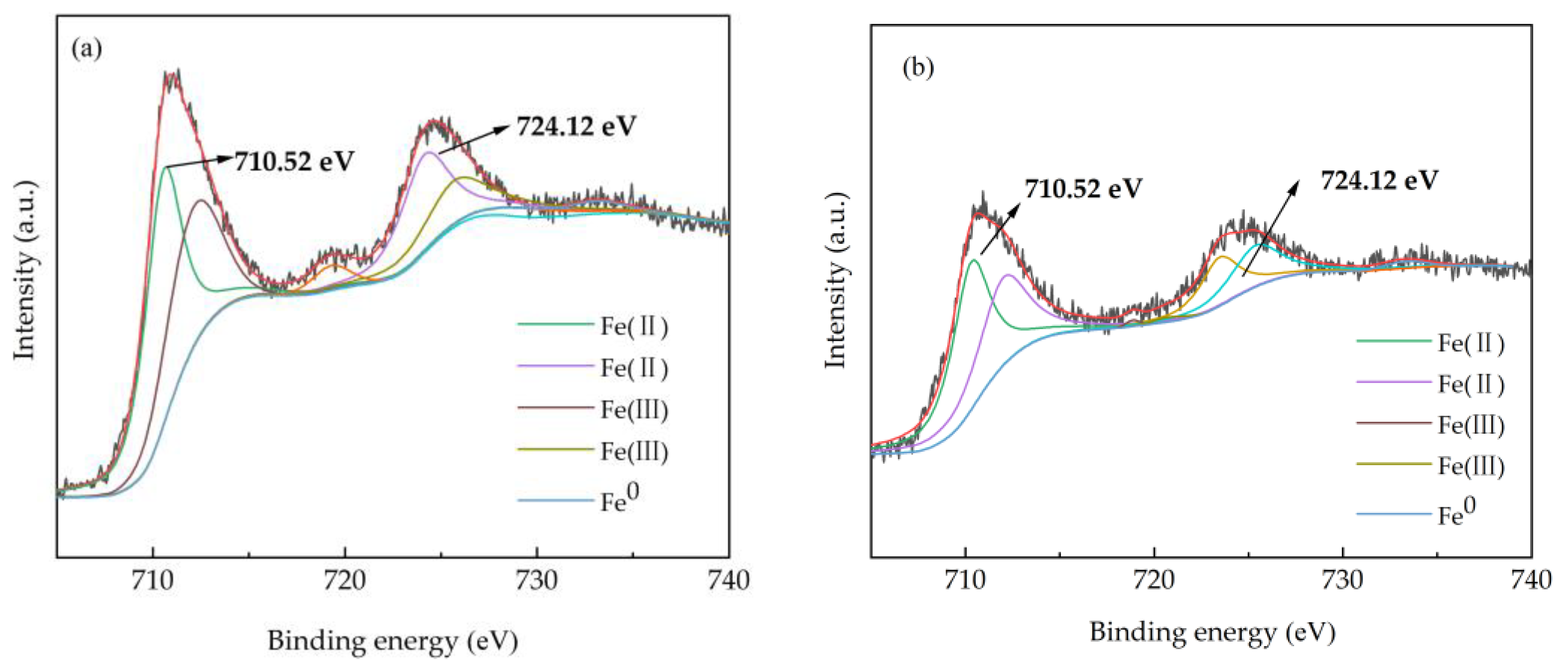
3.2.2. Surface Element Valence Analysis
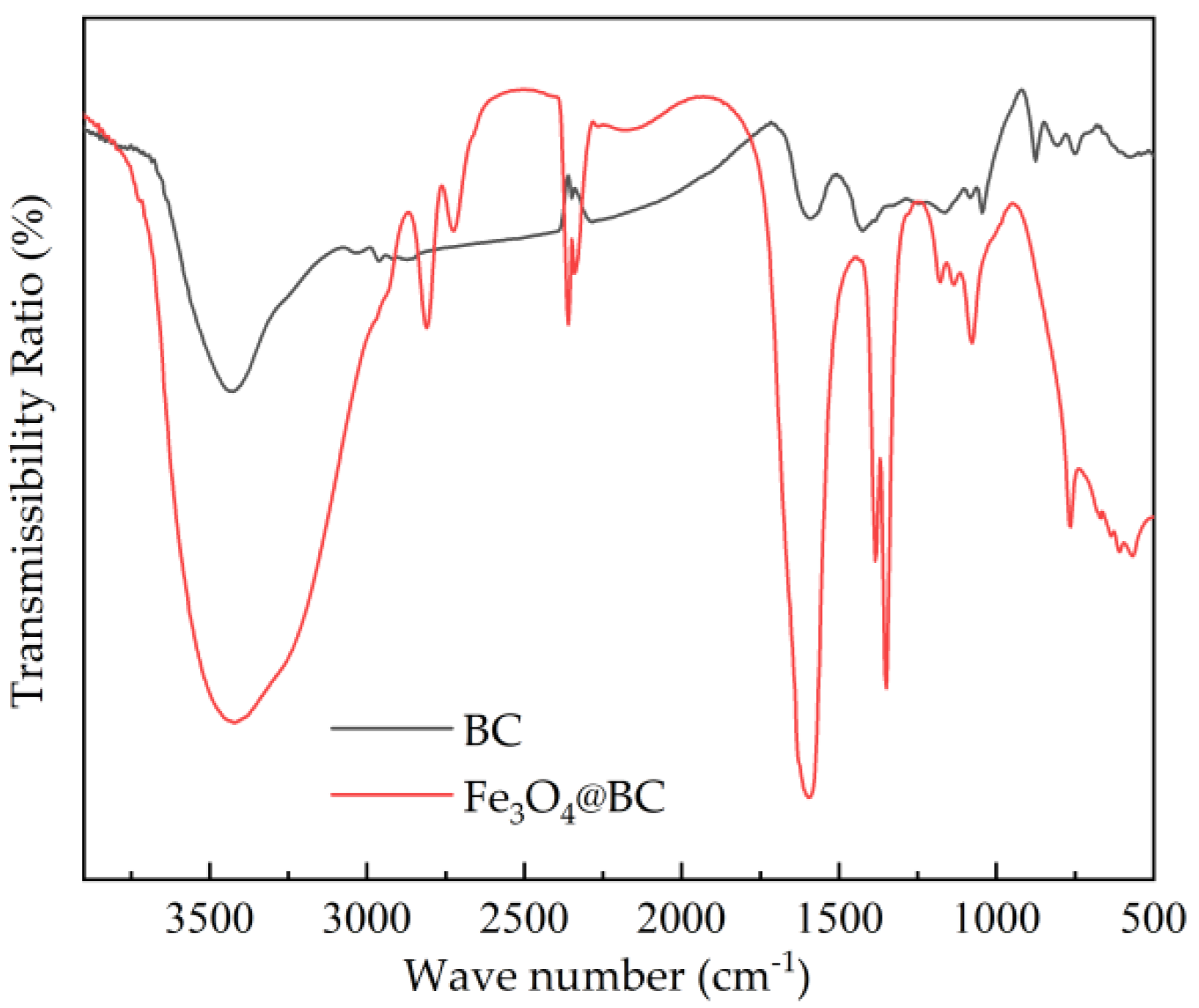
3.2.3. Surface Chemical Functional Group Analysis
3.2.4. Microscopic Morphological Characteristics
3.3. Adsorption Performance of Fe3O4@BC
3.3.1. Analysis of Adsorption Kinetics Fitting Results
3.3.2. Analysis of Fitting Results of Adsorption Isotherm
3.4. Removal Effect for A07 of the PS System Catalyzed by Fe3O4@BC
3.4.1. Effect of PS Dosage
3.4.2. Effect of Fe3O4@BC Dosage
3.4.3. Effect of Initial pH
3.4.4. Effect of AO7 Initial Concentration
3.4.5. Effect of Inorganic Anions on AO7 Removal
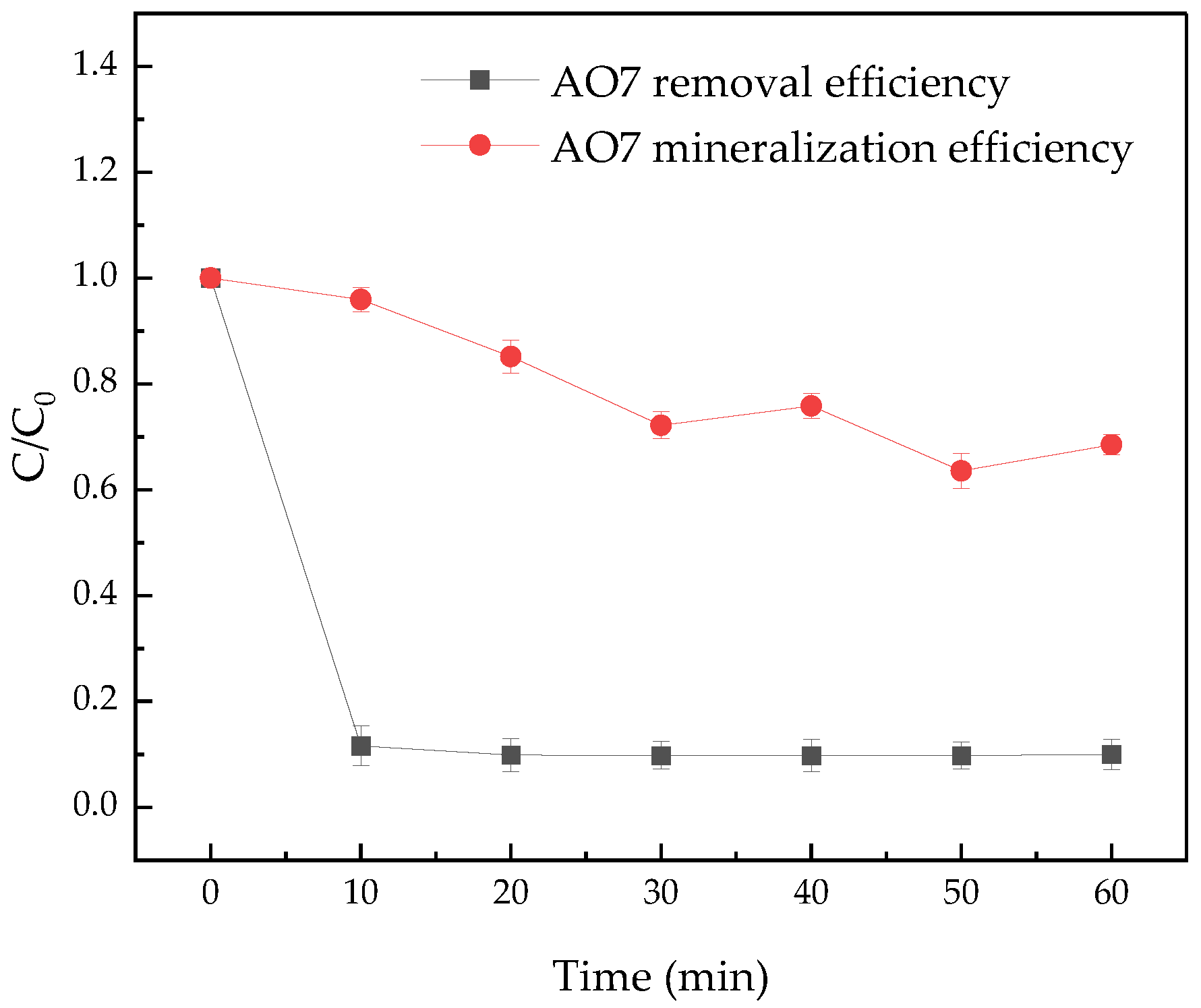
3.5. Changes of TOC in the AO7 Degradation Process
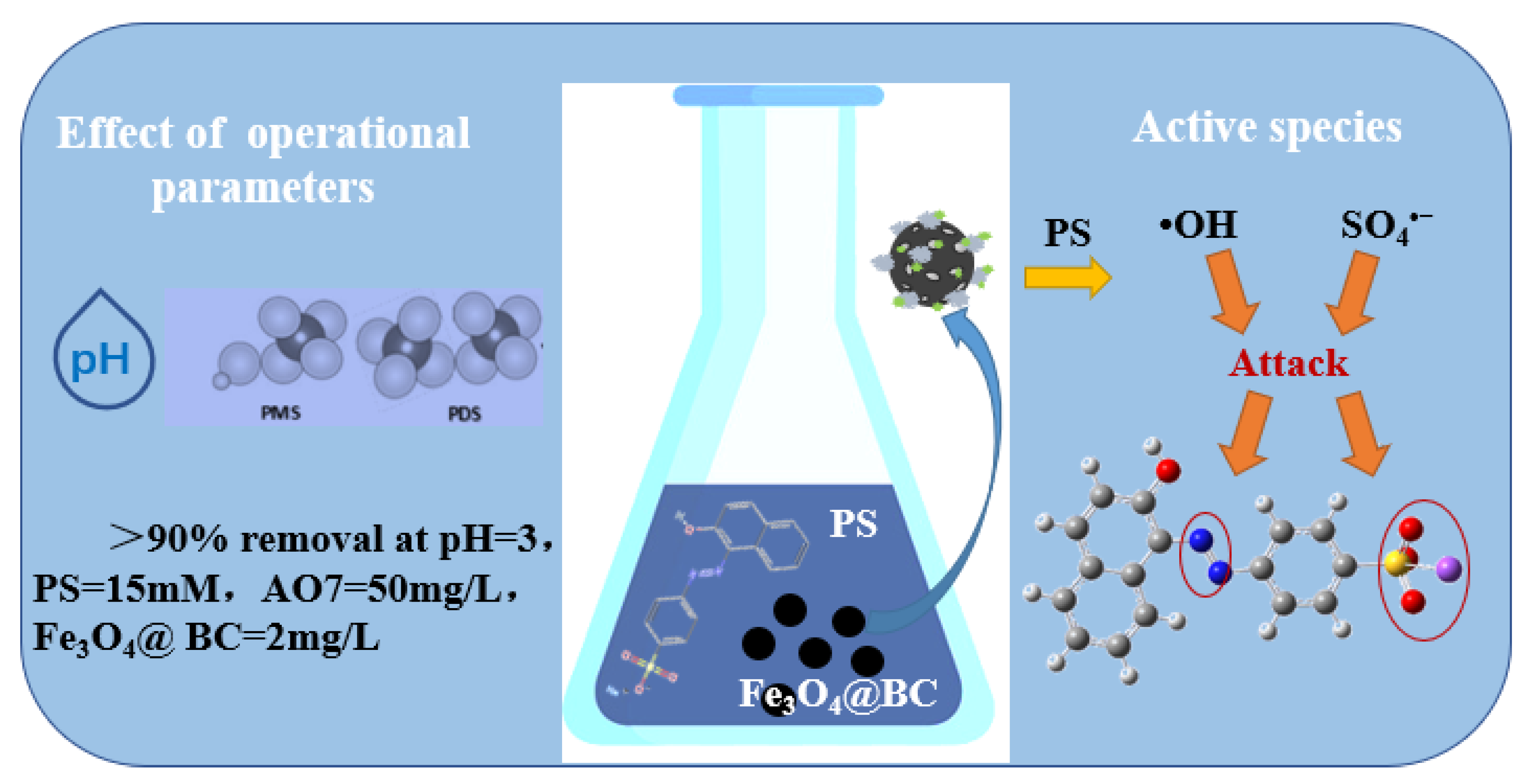
3.6. Degradation Mechanism of AO7 by Fe3O4@BC/PS System
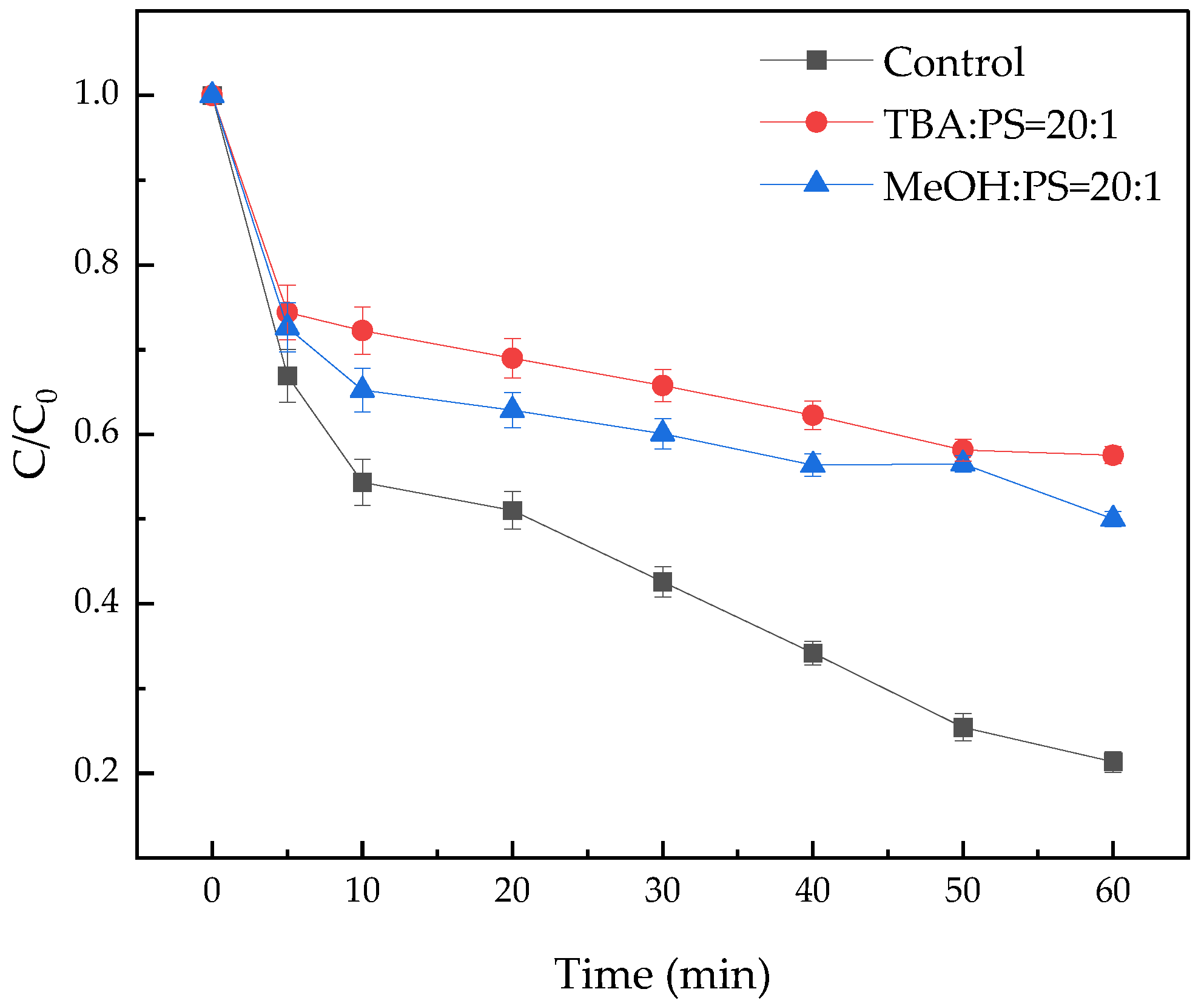
3.6.1. Free Radical Quenching Experiment
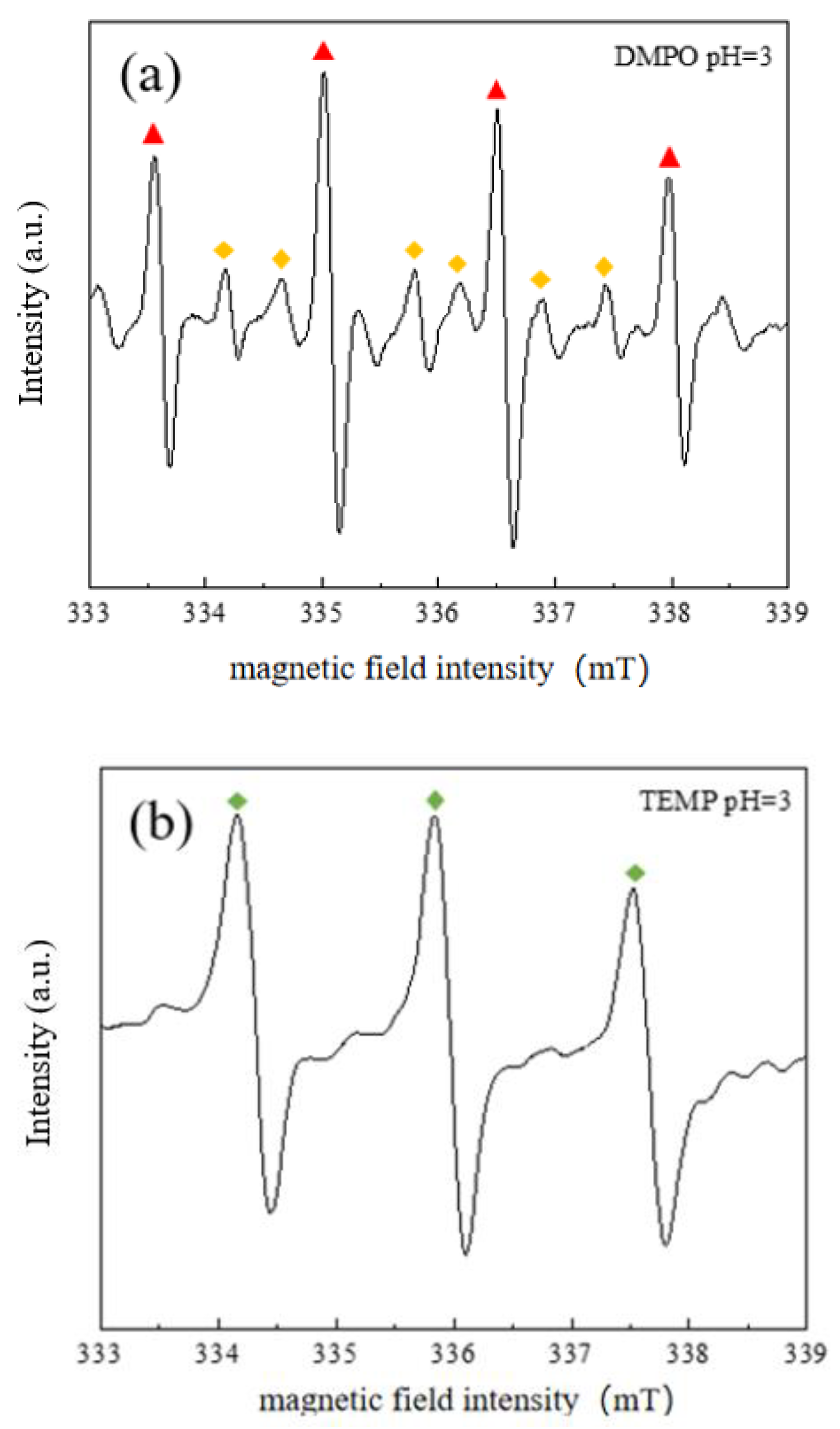
3.6.2. Identification of Free Radicals in the System by EPR Technology
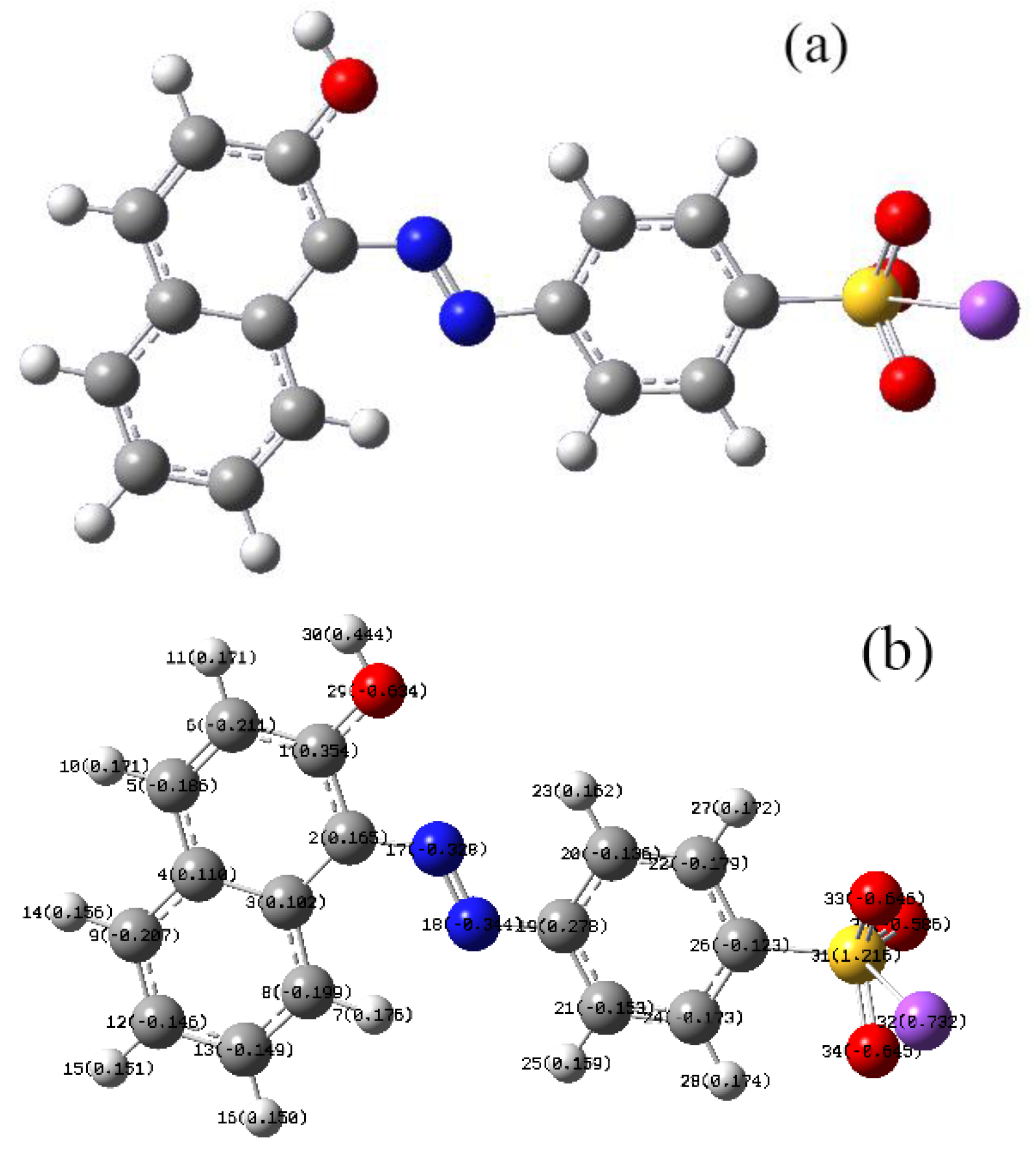
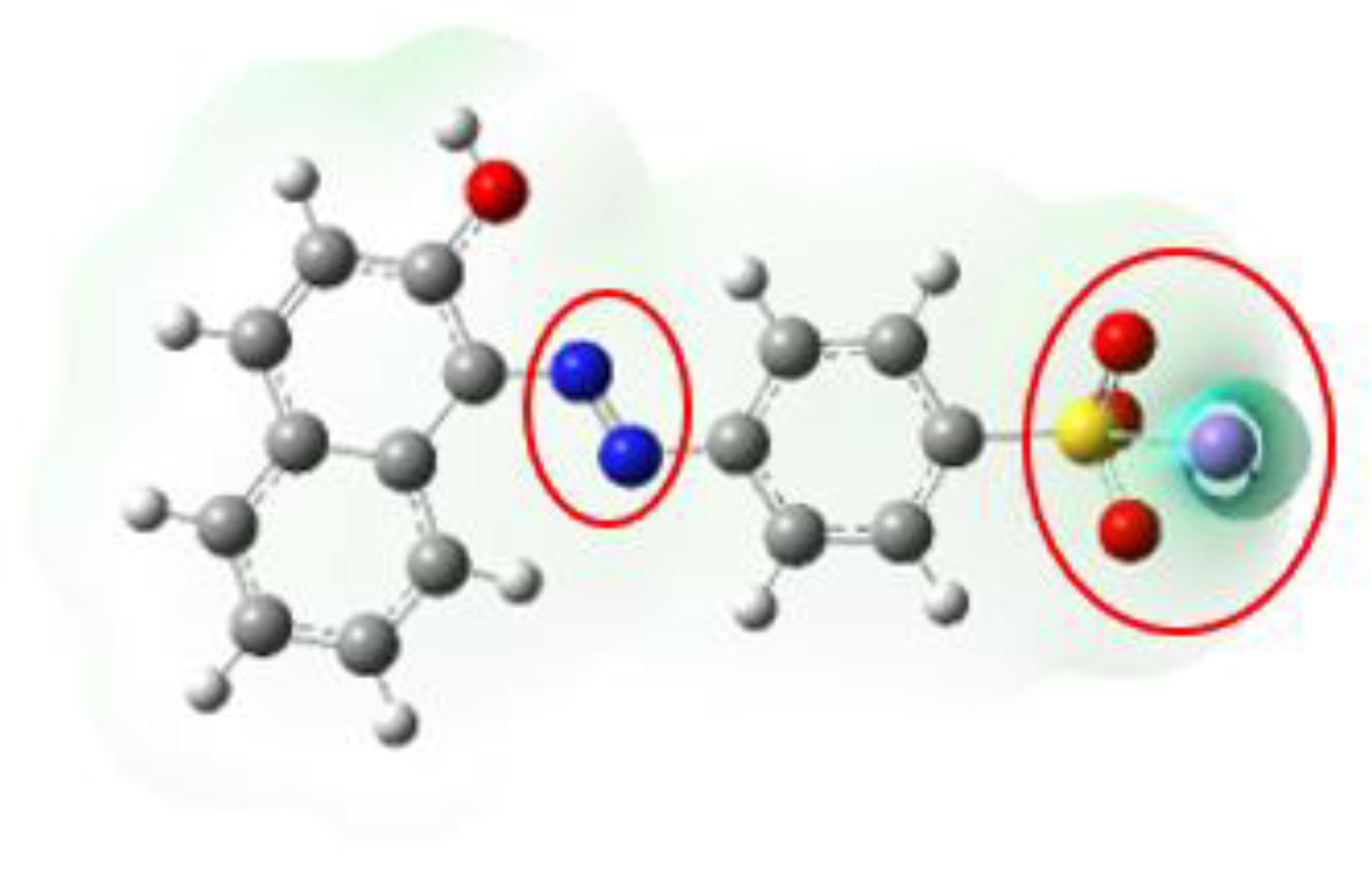
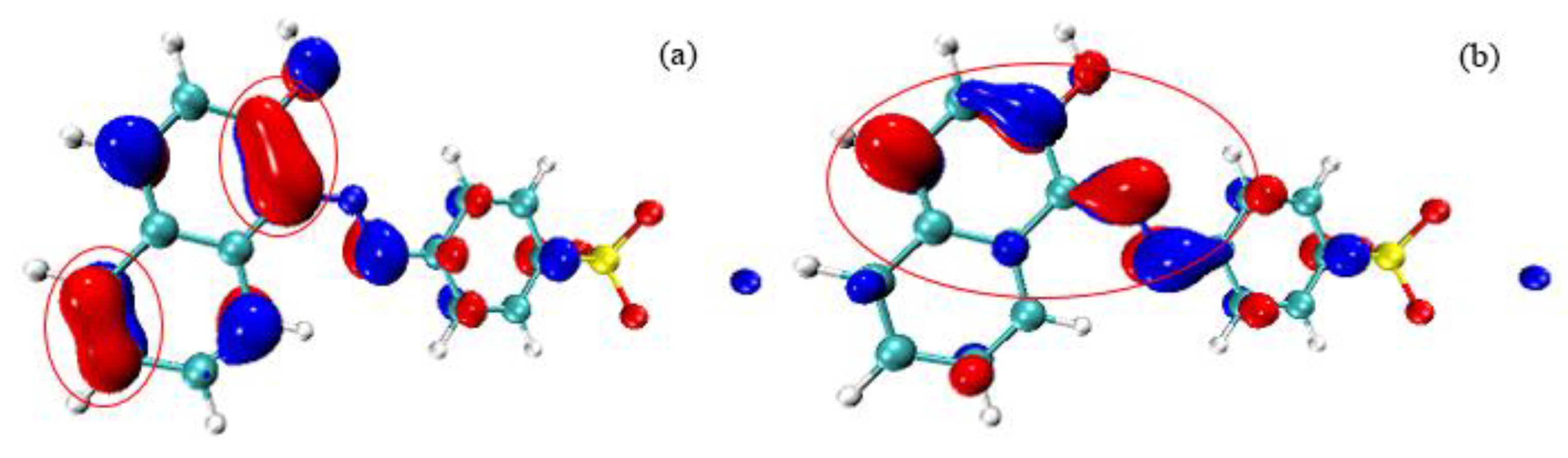
3.6.3. AO7 Density Functional Calculation
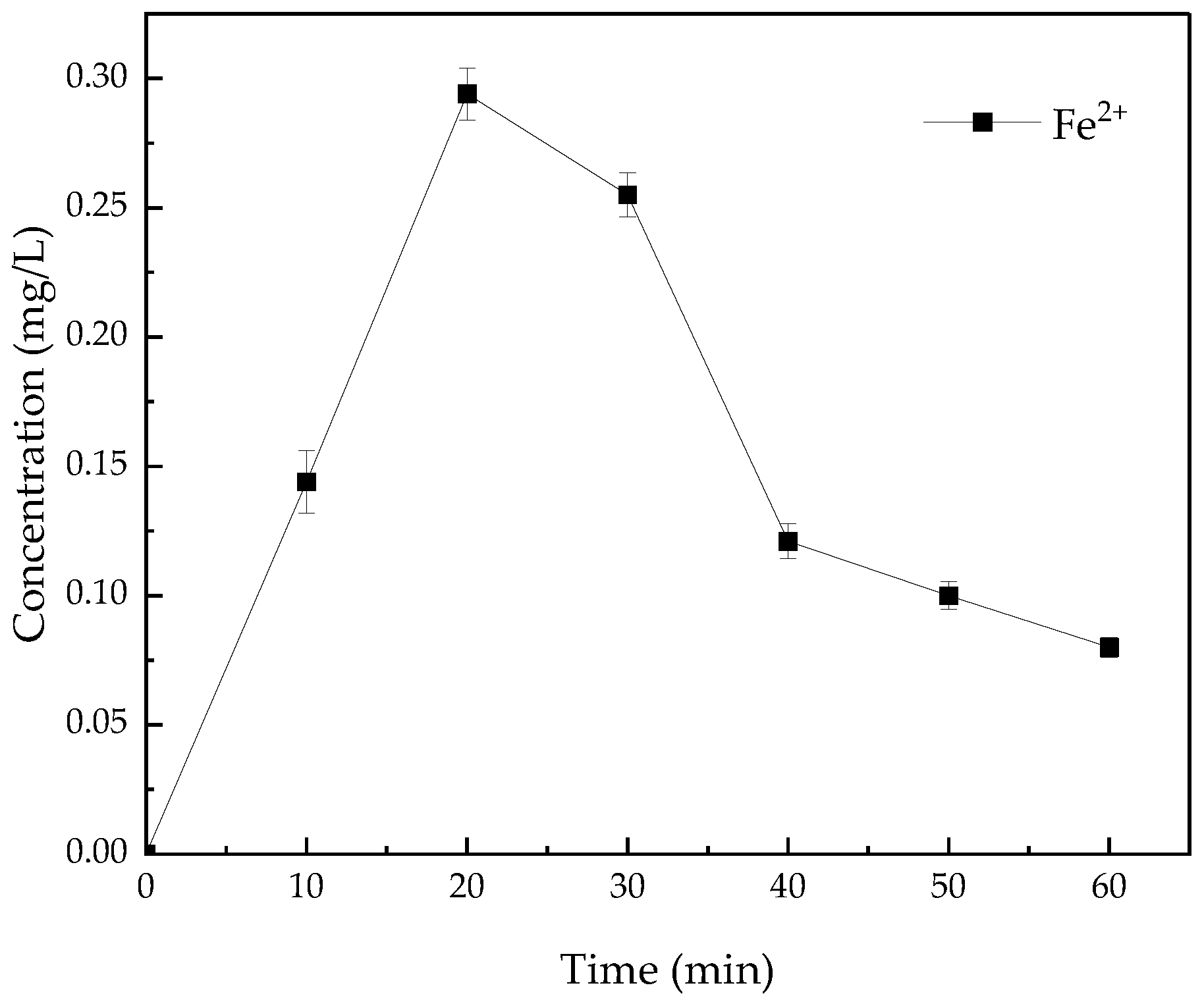
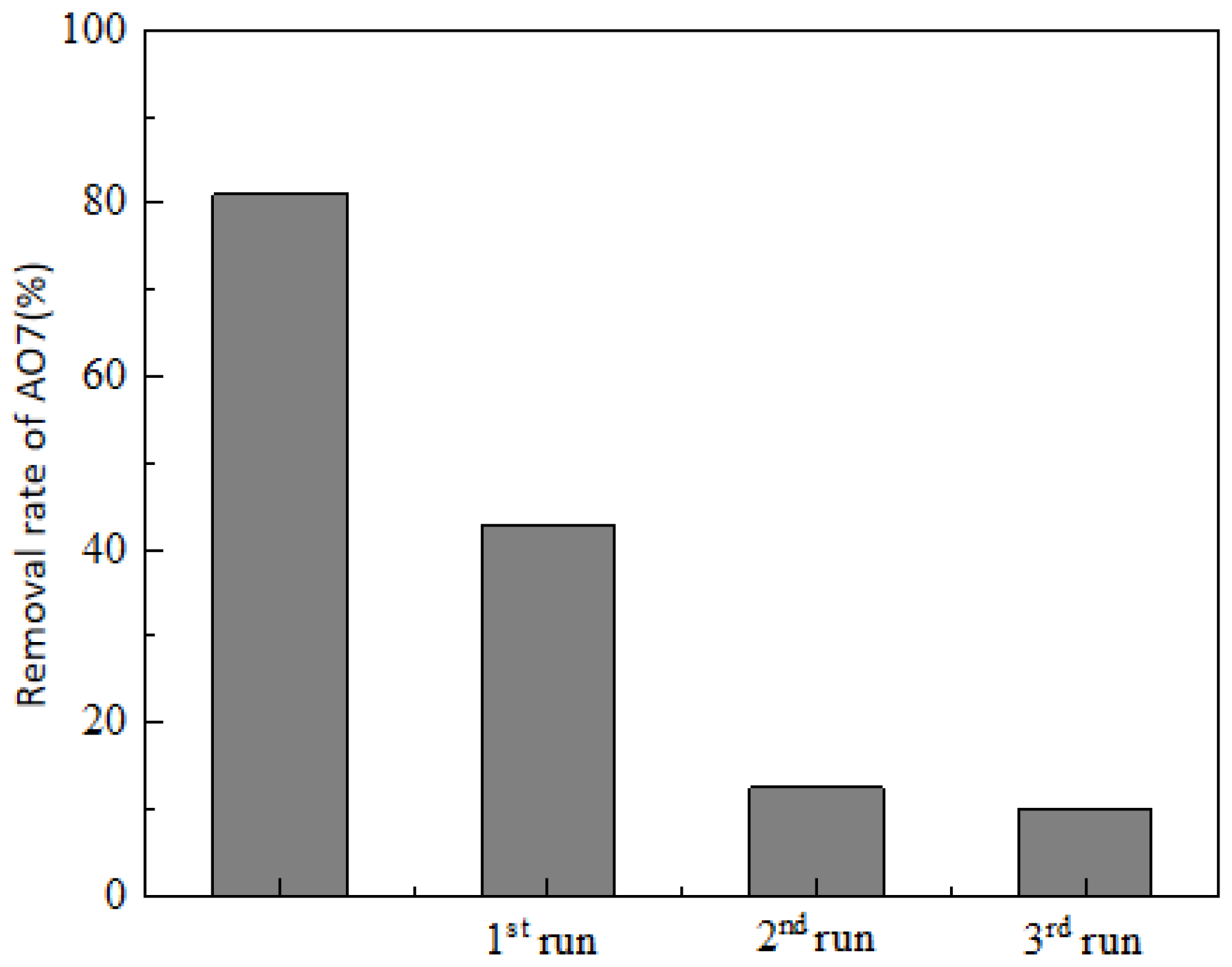
3.7. Leaching of Fe2+ and Stability of Catalyst from the System of Fe3O4@BC/PS Degradation of AO7
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahdi Ahmed, M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A.M. Oxidation of bisoprolol in heated persulfate/H2O systems: Kinetics and products. Chem. Eng. J. 2012, 183, 162–171. [Google Scholar] [CrossRef]
- Qian, L.; Kopinke, F.-D.; Scherzer, T.; Griebel, J.; Georgi, A. Enhanced degradation of perfluorooctanoic acid by heat-activated persulfate in the presence of zeolites. Chem. Eng. J. 2022, 429, 132500. [Google Scholar] [CrossRef]
- Ding, X.; Song, X.; Chen, X.; Ding, D.; Xu, C.; Chen, H. Degradation and mechanism of hexafluoropropylene oxide dimer acid by thermally activated persulfate in aqueous solutions. Chemosphere 2022, 286, 131720. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Rizwan, A.D.; Muhammad, N.; Boczkaj, G.; Murtaza, B.; Imran, M.; Khan, H.M.; et al. Solar light driven degradation of norfloxacin using as-synthesized Bi3+ and Fe2+ co-doped ZnO with the addition of HSO5−: Toxicities and degradation pathways investigation. Chem. Eng. J. 2018, 351, 841–855. [Google Scholar] [CrossRef]
- Kordestani, B.; Jalilzadeh Yengejeh, R.; Takdastan, A.; Neisi, A.K. A new study on photocatalytic degradation of meropenem and ceftriaxone antibiotics based on sulfate radicals: Influential factors, biodegradability, mineralization approach. Microchem. J. 2019, 146, 286–292. [Google Scholar] [CrossRef]
- Ni, T.; Yang, Z.; Zhang, H.; Zhou, L.; Guo, W.; Pan, L.; Yang, Z.; Chang, K.; Ge, C.; Liu, D. Peroxymonosulfate activation by Co3O4/SnO2 for efficient degradation of ofloxacin under visible light. J. Colloid Interface Sci. 2022, 615, 650–662. [Google Scholar] [CrossRef]
- Matthaiou, V.; Frontistis, Z.; Petala, A.; Solakidou, M.; Deligiannakis, Y.; Angelopoulos, G.N.; Mantzavinos, D. Utilization of raw red mud as a source of iron activating the persulfate oxidation of paraben. Process Saf. Environ. Prot. 2018, 119, 311–319. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, X.; Guo, R.; Zhang, H.; Cheng, Q.; Xie, M.; Cheng, X. Persulfate activation by magnetic γ-Fe2O3/Mn3O4 nanocomposites for degradation of organic pollutants. Sep. Purif. Technol. 2019, 210, 335–342. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Sun, C.; Sun, J.Q.; Zhou, R. Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe3O4 nanoparticles process. Sep. Purif. Technol. 2015, 142, 182–188. [Google Scholar] [CrossRef]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, H.; Kang, J.; Wang, Y.; Indrawirawan, S.; Wang, S. Insights into Heterogeneous Catalysis of Persulfate Activation on Dimensional-Structured Nanocarbons. ACS Catal. 2015, 5, 4629–4636. [Google Scholar] [CrossRef]
- Tang, L.; Liu, Y.; Wang, J.; Zeng, G.; Deng, Y.; Dong, H.; Feng, H.; Wang, J.; Peng, B. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl. Catal. B Environ. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Zhao, C.; Zhao, Q.; Niu, T.; Pan, L.; Xu, P.; Zhang, F.; Wu, W.; Ni, T. Facile synthesis of three-dimensional hollow porous carbon doped polymeric carbon nitride with highly efficient photocatalytic performance. Chem. Eng. J. 2022, 438, 135623. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Ao, Z.; Zhou, L.; Wang, G.; Wang, S. Unveiling the active sites of graphene-catalyzed peroxymonosulfate activation. Carbon 2016, 107, 371–378. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Gao, R.; Zhao, Q.; Yang, Z.; Gao, X.; Wang, Z.; Zhang, F.; Wu, W. Enhanced visible light photoelectrocatalytic degradation of tetracycline hydrochloride by I and P co-doped TiO2 photoelectrode. J. Hazard. Mater. 2021, 406, 124309. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Wang, H. Superior fenton-like degradation of tetracycline by iron loaded graphitic carbon derived from microplastics: Synthesis, catalytic performance, and mechanism. Sep. Purif. Technol. 2021, 270, 118773. [Google Scholar] [CrossRef]
- Chokejaroenrat, C.; Sakulthaew, C.; Angkaew, A.; Satapanajaru, T.; Poapolathep, A.; Chirasatienpon, T. Remediating sulfadimethoxine-contaminated aquaculture wastewater using ZVI-activated persulfate in a flow-through system. Aquac. Eng. 2019, 84, 99–105. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Liu, G.; Zheng, Q.; Zhang, G. Catalytic degradation of p-nitrophenol by magnetically recoverable Fe3O4 as a persulfate activator under microwave irradiation. Chemosphere 2020, 240, 124977. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, W.; Zhou, T.; Huang, M.; Wang, C.; Wu, X.; Mao, J.; Wang, P. Visible light induced efficient activation of persulfate by a carbon quantum dots (CQDs) modified γ-Fe2O3 catalyst. Chin. Chem. Lett. 2020, 31, 2757–2761. [Google Scholar] [CrossRef]
- Yin, R.; Sun, J.; Xiang, Y.; Shang, C. Recycling and reuse of rusted iron particles containing core-shell Fe-FeOOH for ibuprofen removal: Adsorption and persulfate-based advanced oxidation. J. Clean. Prod. 2018, 178, 441–448. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Lin, L.; Li, Y.; Avtar, R.; Dzarfan Othman, M.H.; Li, F. Arsenic removal in aqueous solutions using FeS2. J. Env. Manag. 2021, 286, 112246. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Han, L.; Gao, W.; Xue, S.; Chen, M. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef]
- Huong, P.T.; Jitae, K.; Al Tahtamouni, T.M.; Le Minh Tri, N.; Kim, H.-H.; Cho, K.H.; Lee, C. Novel activation of peroxymonosulfate by biochar derived from rice husk toward oxidation of organic contaminants in wastewater. J. Water Process Eng. 2020, 33, 101037. [Google Scholar] [CrossRef]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Ouyang, D.; Yan, J.; Qian, L.; Chen, Y.; Han, L.; Su, A.; Zhang, W.; Ni, H.; Chen, M. Degradation of 1,4-dioxane by biochar supported nano magnetite particles activating persulfate. Chemosphere 2017, 184, 609–617. [Google Scholar] [CrossRef]
- Xu, L.; Gao, H.; Shi, Y.; Zhao, Y. The heterogeneous volume-volatility relations in the exchange-traded fund market: Evidence from China. Econ. Model. 2020, 85, 400–408. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, Y.; Yan, J.; Qian, L.; Han, L.; Chen, M. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: Important role of biochar defect structures. Chem. Eng. J. 2019, 370, 614–624. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Han, D.; Cao, W.; Zheng, L.; Xie, Z.; Liu, H. Identification of Streptococcus mutans genes involved in fluoride resistance by screening of a transposon mutant library. Mol. Oral Microbiol. 2020, 35, 260–270. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Yuan, X.; Li, Y.; Han, L. Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar. Bioresour. Technol. 2020, 296, 122318. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, J.; Jia, C.; Shao, J.; Yang, Q.; Chen, Y.; Yang, H.; Wang, X.; Chen, H. Preparation of furfural by catalytic pyrolysis of cellulose based on nano Na/Fe-solid acid. Fuel 2019, 258, 116089. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yuan, S.; Liu, J.; Zhang, Y.; Du, H.; Wu, C.; Zhao, P.; Chen, H.; Pei, Y. Synergistic effect and mechanism of mass transfer and catalytic oxidation of octane degradation in yolk-shell Fe3O4@C/Fenton system. Chem. Eng. J. 2020, 379, 122262. [Google Scholar] [CrossRef]
- Sun, H.W.; Chen, T.; Kong, L.J.; Cai, Q.; Xiong, Y.; Tian, S.H. Potential of Sludge Carbon as New Granular Electrodes for Degradation of Acid Orange 7. Ind. Eng. Chem. Res. 2015, 54, 5468–5474. [Google Scholar] [CrossRef]
- Biswas, P.; Ainabayev, A.; Zhussupbekova, A.; Jose, F.; O’Connor, R.; Kaisha, A.; Walls, B.; Shvets, I.V. Tuning of oxygen vacancy-induced electrical conductivity in Ti-doped hematite films and its impact on photoelectrochemical water splitting. Sci. Rep. 2020, 10, 7463. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Li, Y.-F.; Chen, M.-J.; Chen, Y.-C.; Kuo, J.; Lo, S.-L. Efficient decomposition of perfluorooctanic acid by persulfate with iron-modified activated carbon. Water Res. 2020, 174, 115618. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Kuwano, N.; Bt Ahmad Khairudin, N.B.; Bt Mohamad, S.E.; Lee, K.X. Green Synthesis of Magnetite (Fe3O4) Nanoparticles Using Seaweed (Kappaphycus alvarezii) Extract. Nanoscale Res. Lett. 2016, 11, 276. [Google Scholar] [CrossRef]
- Radoń, A.; Drygała, A.; Hawełek, Ł.; Łukowiec, D. Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 2017, 131, 148–156. [Google Scholar] [CrossRef]
- Feng, Z.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B.; Chen, H. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: A review. Sci. Total Environ. 2021, 765, 142673. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, S.-S.; Geng, Y.; Zhen, J.; Zhan, J.; Cao, C.; Ni, S.-Q. Synergistic catalysis by Fe3O4-biochar/peroxymonosulfate system for the removal of bisphenol a. Sep. Purif. Technol. 2021, 276, 119351. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Duan, X.; Ang, H.M.; Tadé, M.O.; Wang, S. A new magnetic nano zero-valent iron encapsulated in carbon spheres for oxidative degradation of phenol. Appl. Catal. B Environ. 2015, 172–173, 73–81. [Google Scholar] [CrossRef]
- Bourahla, S.; Nemchi, F.; Belayachi, H.; Belayachi, A.; Harrats, C.; Belhakem, M. Removal of the AO7 dye by adsorption on activated carbon based on grape marc: Equilibrium, regeneration, and FTIR spectroscopy. J. Iran. Chem. Soc. 2023, 20, 669–681. [Google Scholar] [CrossRef]
- Scheufele, F.B.; Módenes, A.N.; Borba, C.E.; Ribeiro, C.; Espinoza-Quiñones, F.R.; Bergamasco, R.; Pereira, N.C. Monolayer–multilayer adsorption phenomenological model: Kinetics, equilibrium and thermodynamics. Chem. Eng. J. 2016, 284, 1328–1341. [Google Scholar] [CrossRef]
- Li, J.; Du, Y.; Deng, B.; Zhu, K.; Zhang, H. Activated carbon adsorptive removal of azo dye and peroxydisulfate regeneration: From a batch study to continuous column operation. Environ. Sci. Pollut. Res. 2017, 24, 4932–4941. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Jian, X.; Dong, Y.; Lu, X.; Liu, X.; Xiang, H.; Cui, X.; Deng, J.; Gao, H. Activation of peroxymonosulfate by a novel EGCE@Fe3O4 nanocomposite: Free radical reactions and implication for the degradation of sulfadiazine. Chem. Eng. J. 2019, 359, 594–603. [Google Scholar] [CrossRef]
- Muhammad, S.; Shukla, P.R.; Tadé, M.O.; Wang, S. Heterogeneous activation of peroxymonosulphate by supported ruthenium catalysts for phenol degradation in water. J. Hazard. Mater. 2012, 215–216, 183–190. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; Deng, J.; Zhou, S.; Li, J.; Xin, X. Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate. J. Hazard. Mater. 2014, 276, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yue, M.; Natarajan, V.; Kong, L.; Ma, L.; Zhang, Y.; Zhao, Q.; Zhan, J. Efficient activation of persulfate by Fe3O4@β-cyclodextrin nanocomposite for removal of bisphenol A. RSC Adv. 2018, 8, 14879–14887. [Google Scholar] [CrossRef]
- Tang, J.; Ma, Y.; Zeng, C.; Yang, L.; Cui, S.; Zhi, S.; Yang, F.; Ding, Y.; Zhang, K.; Zhang, Z. Fe-Al bimetallic oxides functionalized-biochar via ball milling for enhanced adsorption of tetracycline in water. Bioresour. Technol. 2023, 369, 128385. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Huang, X.; Wu, Q.P.; Yan, C.H.; Lu, J.F. Efficient peroxymonosulfate activation by Zn/Fe metal-organic framework-derived ZnO/Fe3O4@carbon spheres for the degradation of Acid Orange 7. Water Environ. Res. 2019, 91, 634–641. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Chen, S.; Cai, M.; Liu, Y.; Zhang, L.; Feng, L. Effects of water matrices on the degradation of naproxen by reactive radicals in the UV/peracetic acid process. Water Res. 2019, 150, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zheng, W.; Ji, Y.; Zhang, J.; Zeng, C.; Zhang, Y.; Wang, Q.; Yang, X. Ferrous-activated persulfate oxidation of arsenic(III) and diuron in aquatic system. J. Hazard. Mater. 2013, 263, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Z.; Huang, F.; Liu, Y.; Feng, L.; Jiang, J.; Zhang, L.; Qi, F.; Liu, C. Carbonized polyaniline activated peroxymonosulfate (PMS) for phenol degradation: Role of PMS adsorption and singlet oxygen generation. Appl. Catal. B Environ. 2021, 286, 119921. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Si, Q.; Liu, B.; Zhao, Q.; Luo, H.; Ren, N. Non-covalent doping of carbon nitride with biochar: Boosted peroxymonosulfate activation performance and unexpected singlet oxygen evolution mechanism. Chem. Eng. J. 2021, 418, 129504. [Google Scholar] [CrossRef]
- Long, Y.; Li, S.; Yang, P.; Chen, X.; Liu, W.; Zhan, X.; Xue, C.; Liu, D.; Huang, W. Synthesis of ZIF-67 derived honeycomb porous Co/NC catalyst for AO7 degradation via activation of peroxymonosulfate. Sep. Purif. Technol. 2022, 286, 120470. [Google Scholar] [CrossRef]
- Xu, Y.; Ai, J.; Zhang, H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef]
- Wang, C.; Shi, P.; Cai, X.; Xu, Q.; Zhou, X.; Zhou, X.; Yang, D.; Fan, J.; Min, Y.; Ge, H.; et al. Synergistic Effect of Co3O4 Nanoparticles and Graphene as Catalysts for Peroxymonosulfate-Based Orange II Degradation with High Oxidant Utilization Efficiency. J. Phys. Chem. C 2016, 120, 336–344. [Google Scholar] [CrossRef]
- Zhong, H.; Brusseau, M.L.; Wang, Y.; Yan, N.; Quig, L.; Johnson, G.R. In-situ activation of persulfate by iron filings and degradation of 1,4-dioxane. Water Res. 2015, 83, 104–111. [Google Scholar] [CrossRef]
- Temiz, K.; Olmez-Hanci, T.; Arslan-Alaton, I. Zero-valent iron-activated persulfate oxidation of a commercial alkyl phenol polyethoxylate. Environ. Technol. 2016, 37, 1757–1767. [Google Scholar] [CrossRef]
- Yi, X.-H.; Ji, H.; Wang, C.-C.; Li, Y.; Li, Y.-H.; Zhao, C.; Wang, A.; Fu, H.; Wang, P.; Zhao, X.; et al. Photocatalysis-activated SR-AOP over PDINH/MIL-88A(Fe) composites for boosted chloroquine phosphate degradation: Performance, mechanism, pathway and DFT calculations. Appl. Catal. B Environ. 2021, 293, 120229. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J.; Chen, X.; Wang, Z.; Ji, H.; Chen, L.; Liu, W.; Wang, C.-C. Bifunctional Bi12O17Cl2/MIL-100(Fe) composites toward photocatalytic Cr(VI) sequestration and activation of persulfate for bisphenol A degradation. Sci. Total Environ. 2021, 752, 141901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, X.; Shi, C.; Yang, S. Decolorization of Acid Orange 7 with peroxymonosulfate oxidation catalyzed by granular activated carbon. Chem. Eng. J. 2013, 232, 259–265. [Google Scholar] [CrossRef]
- Li, F.; Duan, F.; Ji, W.; Gui, X. Biochar-activated persulfate for organic contaminants removal: Efficiency, mechanisms and influencing factors. Ecotoxicol. Environ. Saf. 2020, 198, 110653. [Google Scholar] [CrossRef] [PubMed]
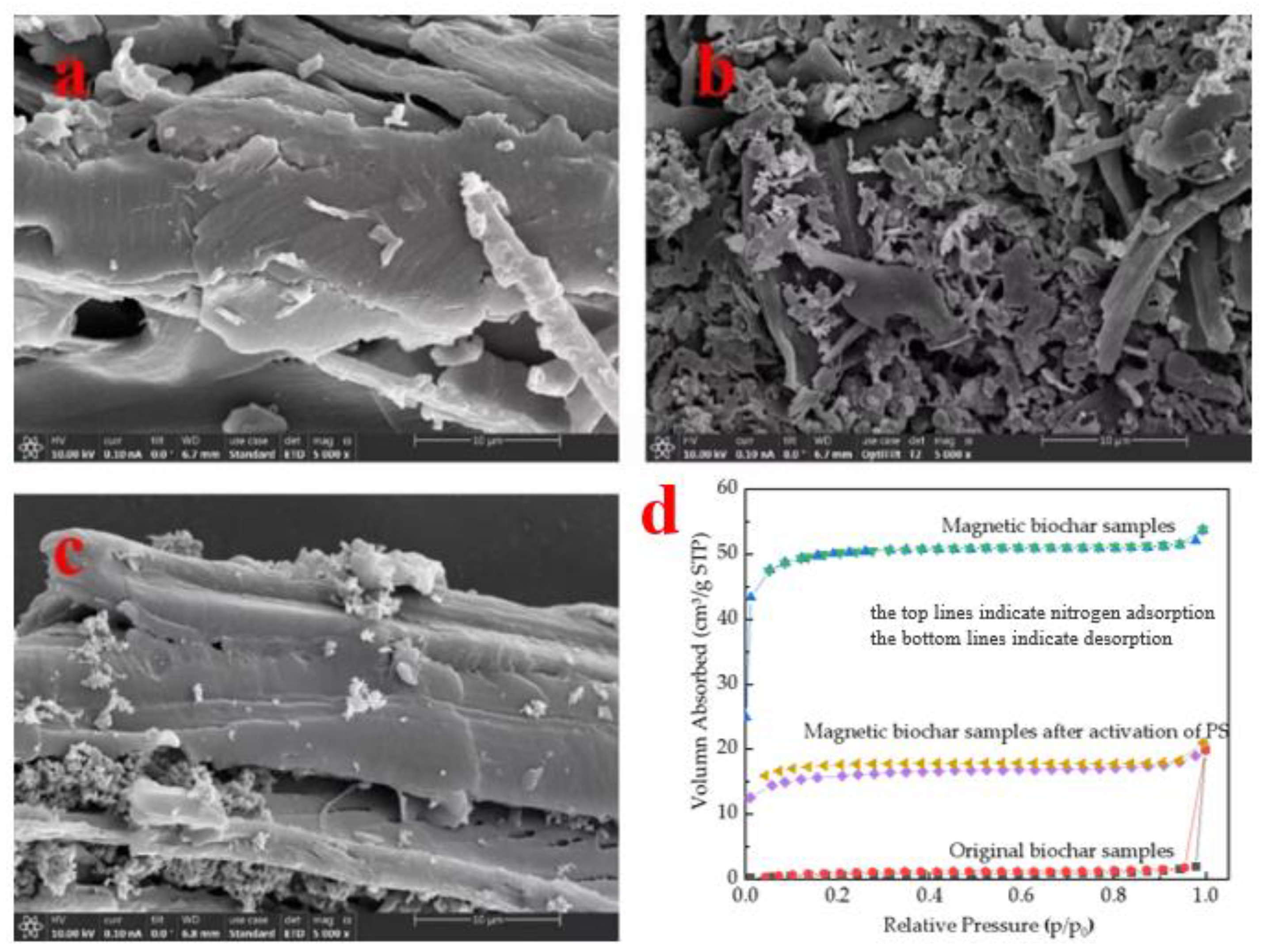
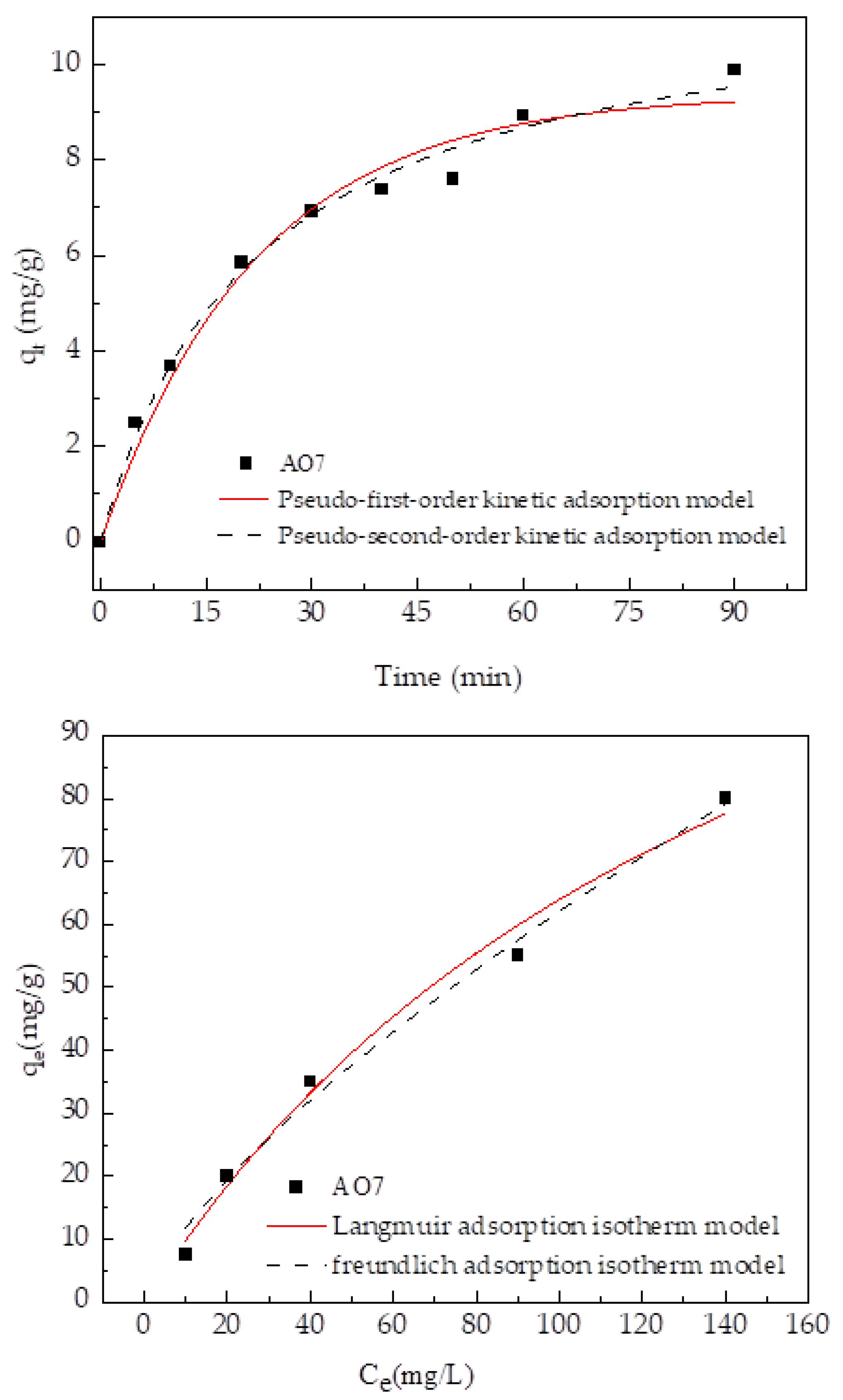
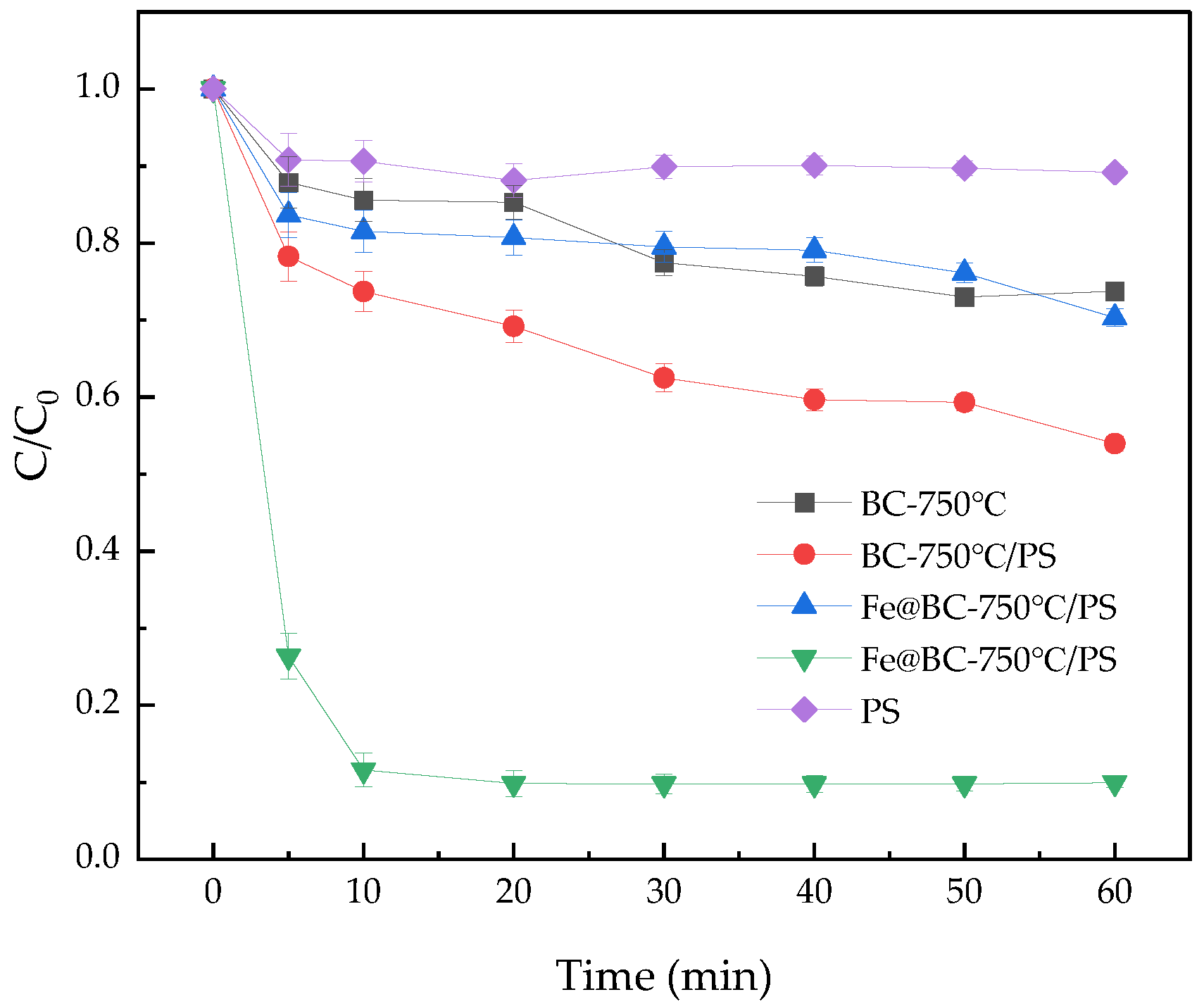
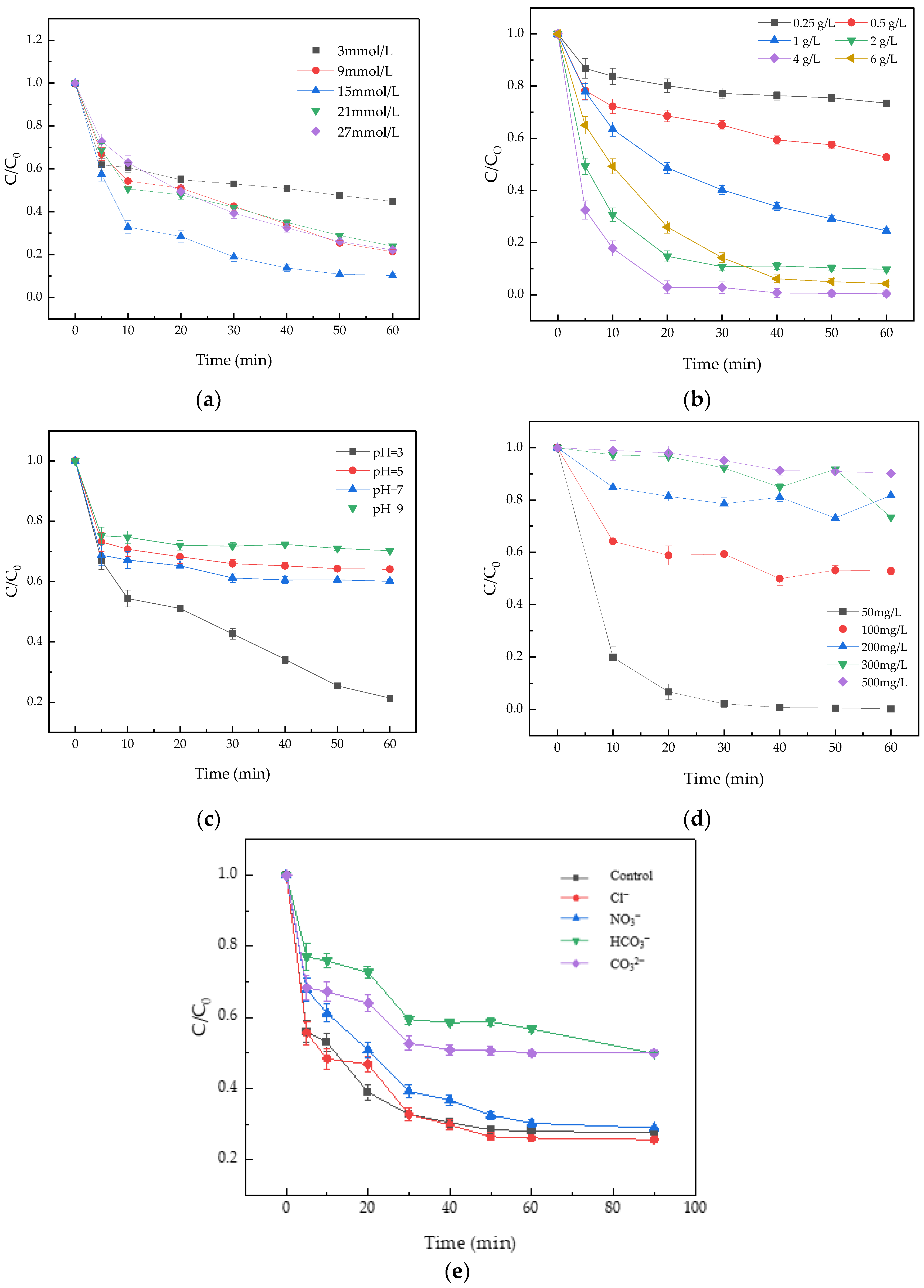
| Oxidants | F2 | O3 | S2O82− | HSO5− | H2O2 | MnO4− | O2 |
| Redox potential (V) | 2.87 | 2.07 | 2.01 | 1.82 | 1.78 | 1.67 | 1.23 |
| Model | Parameter | AO7 | |
|---|---|---|---|
| kinetic model | pseudo-first-order kinetic adsorption model | k1 (min−1) | 0.045 |
| qe (mg/g) | 9.37 | ||
| R2 | 0.97 | ||
| pseudo-second-order kinetic adsorption model | k2 (min−1) | 0.0038 | |
| qe (mg/g) | 11.83 | ||
| R2 | 0.98 | ||
| isotherms model | Langmuir adsorption isotherm model | KL (min−1) | 0.006 |
| n | 165.8 | ||
| R2 | 0.98 | ||
| Freundlich adsorption isotherm model | KF (min−1) | 2.23 | |
| n | 1.38 | ||
| R2 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Zhang, K.; Xu, J.; Wei, X.; Shi, L.; Sumita; Li, C.; Lichtfouse, E. Performance and Mechanism of Fe3O4 Loaded Biochar Activating Persulfate to Degrade Acid Orange 7. Water 2023, 15, 1849. https://doi.org/10.3390/w15101849
Zhu Q, Zhang K, Xu J, Wei X, Shi L, Sumita, Li C, Lichtfouse E. Performance and Mechanism of Fe3O4 Loaded Biochar Activating Persulfate to Degrade Acid Orange 7. Water. 2023; 15(10):1849. https://doi.org/10.3390/w15101849
Chicago/Turabian StyleZhu, Qijia, Kai Zhang, Jiani Xu, Xinyu Wei, Lixia Shi, Sumita, Cong Li, and Eric Lichtfouse. 2023. "Performance and Mechanism of Fe3O4 Loaded Biochar Activating Persulfate to Degrade Acid Orange 7" Water 15, no. 10: 1849. https://doi.org/10.3390/w15101849







