The Occurrence of Haloacetic Acids and Dalapon in Bottled Waters and an Assessment of Their Health Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Bottled Water Samples
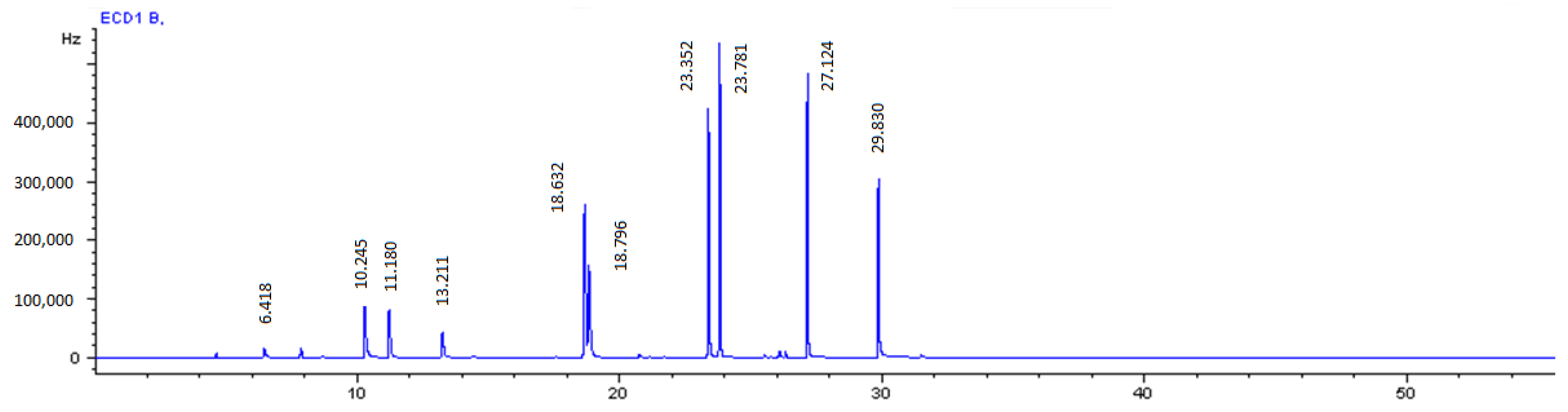
2.2. Analytical Procedure
2.3. Risk Assessment
3. Results
3.1. Physicochemical Properties of Bottle Waters
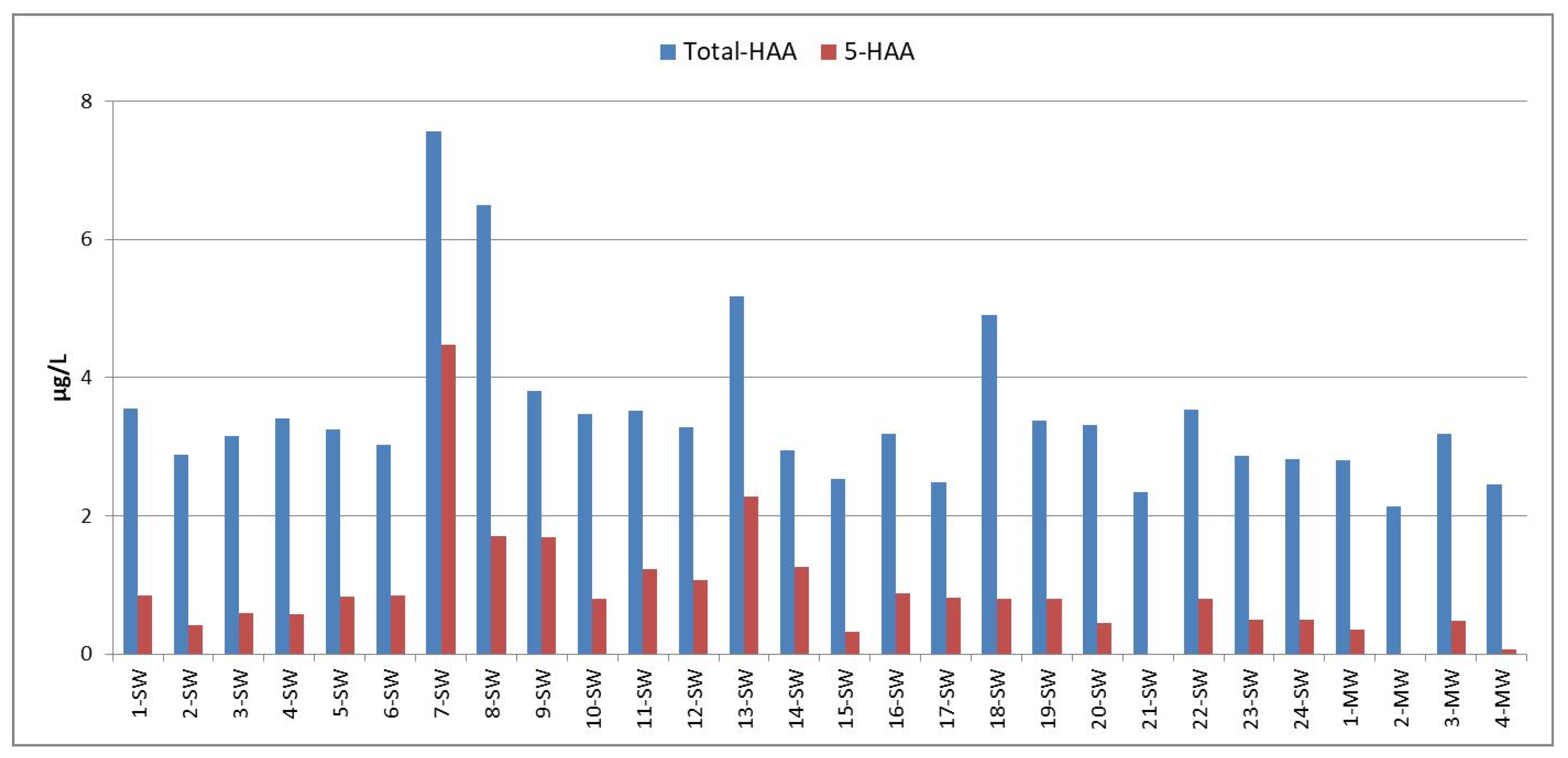
3.2. Concentrations of HAAs and Dalapon in Bottled Water Samples
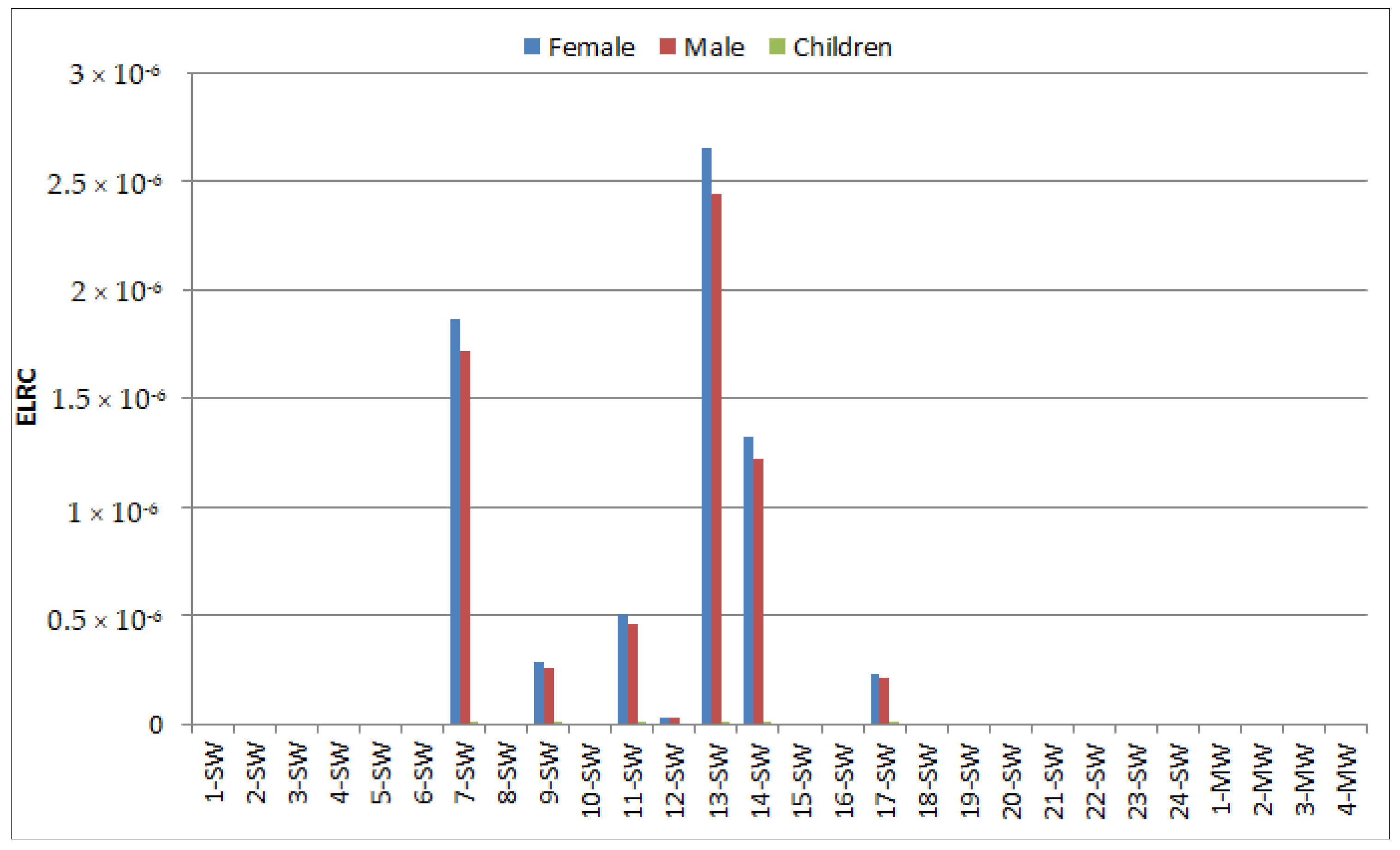
3.3. Risk Assessment
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Srivastav, A.L.; Patel, N.; Chaudhary, V.K. Disinfection by-products in drinking water: Occurrence, toxicity and abatement. Environ. Pollut. 2020, 267, 115474. [Google Scholar] [CrossRef] [PubMed]
- Duana, X.; Liao, X.; Chen, J.; Xie, S.; Qi, H.; Li, F.; Yuan, B. THMs, HAAs and NAs production from culturable microorganisms in pipeline network by ozonation, chlorination, chloramination and joint disinfection strategies. Sci. Total Environ. 2020, 744, 140833. [Google Scholar] [CrossRef] [PubMed]
- Richardsona, S.D.; Plewa, M.J. To regulate or not to regulate? What to do with more toxic disinfection by-products? J. Environ. Chem. Eng. 2020, 8, 103939. [Google Scholar] [CrossRef]
- Sun, X.; Chen, M.; Wei, D.; Du, Y. Research progress of disinfection and disinfection by-products in China. J. Environ. Sci. 2019, 81, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Gilca, A.F.; Teodosiu, C.; Fiore, S.; Musteret, C.P. Emerging disinfection byproducts: A review on their occurrence and control in drinking water treatment processes. Chemosphere 2020, 259, 127476. [Google Scholar] [CrossRef]
- Kanokkantapong, V.; Marhaba, T.F.; Pavasant, P.; Panyapinyophol, B. Characterization of haloacetic acid precursors in source water. J. Environ. Manag. 2006, 80, 214–221. [Google Scholar] [CrossRef]
- Zhang, X.; Saini, C.; Pohl, C.; Liu, Y. Fast determination of nine haloacetic acids, bromate and dalapon in drinking water samples using ion chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A 2020, 1621, 461052. [Google Scholar] [CrossRef]
- Malliarou, E.; Collins, C.; Graham, N.; Nieuwenhuijsen, M.J. Haloacetic acids in drinking water in the United Kingdom. Water Res. 2005, 39, 2722–2730. [Google Scholar] [CrossRef]
- Sinha, R.; Gupta, A.K.; Ghosal, P.S. A review on Trihalomethanes and Haloacetic acids in drinking water: Global status, health impact, insights of control and removal Technologies. J. Environ. Chem. Eng. 2021, 9, 106511. [Google Scholar] [CrossRef]
- Xue, R.; Donovan, A.; Shi, H.; Yang, J.; Hua, B.; Inniss, E.; Eichholz, T. Rapid simultaneous analysis of 17 haloacetic acids and related halogenated water contaminants by high-performance ion chromatography-tandem mass spectrometry. ABC 2016, 408, 6613–6622. [Google Scholar] [CrossRef]
- Wu, S.; Anumol, T.; Gandhi, J.; Snyder, S.A. Analysis of haloacetic acids, bromate, and dalapon in natural watersby ion chroma-tography–tandem mass spectrometry. J. Chromatogr. A 2017, 1487, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Rohrer, J. Targeted Quantitation Mode Comparison of Haloacetic Acids, Bromate, and Dalapon in Drinking Water Using Ion Chromatography Coupled to High-Resolution (Orbitrap) Mass Spectrometry. J. Chromatogr. A 2020, 1630, 461538. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Farhang, M.; Zarei, A. Data on the level of haloacetic acids in indoor swimming pools of Iran: A case study of Tehran. Data Brief 2018, 19, 326–330. [Google Scholar] [CrossRef]
- Liu, Y.; Mou, S. Simultaneous determination of trace level bromate and chlorinated haloacetic acids in bottled drinking water by ion chromatography. Microchem. J. 2003, 75, 79–86. [Google Scholar] [CrossRef]
- Cidu, R.; Frau, F.; Tore, P. Drinking water quality: Comparing inorganic components in bottled water and Italian tap water. J. Food Compos. Anal. 2011, 24, 184–193. [Google Scholar] [CrossRef]
- Shamsa, M.; Qasemi, M.; Afsharnia, M.; Mohammadzadeh, A.; Zarei, A. Chemical and microbial quality of bottled drinking water in Gonabad city, Iran: Effect of time and storage conditions on microbial quality of bottled waters. MethodsX 2019, 6, 273–277. [Google Scholar] [CrossRef]
- Ikem, A.; Odueyungbo, S.; Egiebor, N.O.; Nyavor, K. Chemical quality of bottled waters from three cities in eastern Alabama. Sci. Total Environ. 2002, 285, 165–175. [Google Scholar] [CrossRef]
- Güler, C. Evaluation of maximum contaminant levels in Turkish bottled drinking waters utilizing parameters reported on manufacturer’s labeling and government-issued production licenses. J. Food Compos. Anal. 2007, 20, 262–272. [Google Scholar] [CrossRef]
- Palomo, M.; Penalver, A.; Borrull, F.; Aguilar, C. Measurement of radioactivity in bottled drinking water in Spain. Appl. Radiat. Isot. 2007, 65, 1165–1172. [Google Scholar] [CrossRef]
- Radwan, E.K.; Barakat, M.H.; Ibrahim, M.B.M. Hazardous inorganic disinfection by-products in Egypt’s tap drinking water: Occurrence and human health risks assessment studies. Sci. Total Environ. 2021, 797, 149069. [Google Scholar] [CrossRef]
- Djam, S.; Najafi, M.; Ahmadi, S.H.; Shoeibi, S. Bottled water safety evaluations in IRAN: Determination of bromide and oxyhalides (chlorite, chlorate, bromate) by ion chromatography. J. Environ. Health Sci. Eng. 2020, 18, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Guo, W.; Mo, J.; He, Y.; Liu, Y.; Liu, W.; Liang, Y.; Yang, X. The occurrence of disinfection by-products in municipal drinking water in China’s Pearl River Delta and a multipathway cancer risk assessment. Sci. Total Environ. 2013, 447, 108–115. [Google Scholar] [CrossRef] [PubMed]
- IRIS. Integrated Risk Information System (2005). Available online: http://www.epa.gov/iris (accessed on 10 December 2022).
- Liu, S.; Zhu, Z.; Fan, C.; Qiu, Y.; Zhao, J. Seasonal variation effects on the formation of trihalomethane during chlorination of water from Yangtze River and associated cancer risk assessment. J. Environ. Sci. 2011, 23, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Guo, H.; Lam, S.; Lau, S. Multipathway risk assessment on disinfection byproducts byproducts of drinking water in Hong Kong. Environ. Res. 2004, 94, 47–56. [Google Scholar] [CrossRef] [PubMed]
- TURKSTAT, Turkish Statistical Institute. Available online: https://www.tuik.gov.tr/ (accessed on 10 December 2022).
- Jan, F.A.; Ishaq, M.; Khan, S.; Ihsanullah, I.; Ahmad, I.; Shakirullah, M. A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir). J. Hazard. Mater. 2010, 179, 1–3, 612–621. [Google Scholar] [CrossRef]
- Simon, V.; Berne, F.; Gallard, H. Disinfection By-products in Drinking Water. In Chloramination and Bromamination of Amino Acids; Thompson, C., Gillespie, S., Goslan, E., Eds.; Disinfection By-Products in Drinking Water: Cambridge, UK, 2015; pp. 70–80. [Google Scholar]
- Shah, A.D.; Liu, Z.-Q.; Salhi, E.; Höfer, T.; Werschkun, B.; von Gunten, U. Formation of disinfection by-products during ballast water treatment with ozone, chlorine, and peracetic acid: Influence of water quality parameters. Environ. Sci. Water Res. Technol. 2015, 1, 465–480. [Google Scholar] [CrossRef]
- Liu, Y.; Mou, S. Determination of bromate and chlorinated haloacetic acids in bottled drinking water with chromatographic methods. Chemosphere 2004, 55, 1253–1258. [Google Scholar] [CrossRef]
- Agori, G. The Behaviour of Haloacetic Acids in Distribution Zones in Scotland. Master Thesis, Cranfield University, Shrivenham, UK, 2014. [Google Scholar]
- Al-shatri, M.A.; Nuhu, A.A.; Basheer, C. Determination of Haloacetic Acids in Bottled and Tap Water Sources by Dispersive Liquid-Liquid Microextraction and GC-MS Analysis. Sci. World J. 2014, 2014, 695049. [Google Scholar] [CrossRef] [Green Version]
- Leivadara, S.V.; Nikolaou, A.D.; Lekkas, T.D. Determination of organic compounds in bottled waters. Food Chem. 2008, 108, 277–286. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, S.; Chen, L.; Xiao, R.; Chu, W. Disinfection byproducts in indoor swimming pool water: Detection and human lifetime health risk assessment. J. Environ. Sci. 2023, 126, 378–386. [Google Scholar] [CrossRef]
- EPA 822-F-18-001; Drinking water standards and health advisories. United States Environmental Protection Agency: Washington, DC, USA, 2018.
- Zhao, H.; Yang, L.; Li, Y.; Xue, W.; Li, K.; Xie, Y.; Meng, S.; Cao, G. Environmental occurrence and risk assessment of haloacetic acids in swimming pool water and drinking water. RSC Adv. 2020, 47, 28267–28276. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Value | Reference |
|---|---|---|
| Rfd (mg/kg/day) | 0.004 (DCAA) | [23] |
| 0.020 (DCAA) | ||
| 0.003 (Dalapon) | ||
| Sf (mg/kg/day) | 0.05 (DCAA) | [23] |
| 0.07 (DCAA) | ||
| C | Measured | In this study |
| IR (L/day) | 2 (Male, female) | [24] |
| 1 (Children) | [21] | |
| EF (days/year) | 365 | [25] |
| ED (year) | 75.9 (Male) | [26] |
| 81.3 (Female) | [27] | |
| 5–17 (Avagere 10) (Children) | ||
| AT (days) | Lifetime×365 (carcinogenic risk) | [20] |
| ED×365 (non-carcinogenic risk) | [25] | |
| BW | 75.8 (Male) | [26] |
| 69.9 (Female) | [27] | |
| 32.7 (Children) |
| Sample No | MCAA | MBAA | DCAA | TCAA | BCAA | BDCAA | DBAA | CDBAA | TBAA | Dalapon |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-SW | 0.85 | <dl | <dl | <dl | <dl | 0.08 | <dl | 1.00 | 1.62 | 11.76 |
| 2-SW | 0.41 | <dl | <dl | <dl | <dl | 0.03 | <dl | 1.00 | 1.45 | 6.16 |
| 3-SW | 0.59 | <dl | <dl | <dl | <dl | 0.03 | <dl | 1.00 | 1.54 | 1.16 |
| 4-SW | 0.57 | <dl | <dl | <dl | <dl | 0.07 | <dl | 1.00 | 1.77 | 12.41 |
| 5-SW | 0.83 | <dl | <dl | <dl | <dl | 0.09 | <dl | 1.00 | 1.32 | 12.48 |
| 6-SW | 0.85 | <dl | <dl | <dl | <dl | 0.04 | <dl | 1.00 | 1.14 | 12.25 |
| 7-SW | 2.78 | <dl | <dl | 0.93 | 0.10 | 0.78 | 0.77 | 0.98 | 1.23 | <dl |
| 8-SW | 1.05 | <dl | <dl | <dl | 2.58 | 0.06 | 0.65 | 0.98 | 1.18 | 6.21 |
| 9-SW | 0.85 | <dl | <dl | 0.14 | <dl | <dl | 0.70 | 1.00 | 1.11 | 6.73 |
| 10-SW | 0.80 | <dl | <dl | <dl | <dl | 0.03 | <dl | 1.00 | 1.65 | 10.62 |
| 11-SW | 0.30 | <dl | <dl | 0.25 | <dl | 0.06 | 0.68 | 1.00 | 1.22 | 9.32 |
| 12-SW | 0.38 | <dl | <dl | 0.01 | <dl | 0.04 | 0.66 | 1.00 | 1.18 | 11.37 |
| 13-SW | 0.29 | <dl | <dl | 1.32 | 0.57 | 0.06 | 0.65 | 1.02 | 1.25 | 11.37 |
| 14-SW | 0.21 | <dl | <dl | 0.66 | <dl | 0.05 | 0.38 | 0.51 | 1.14 | 11.03 |
| 15-SW | <dl | <dl | <dl | <dl | <dl | 0.03 | 0.33 | 1.00 | 1.18 | 11.35 |
| 16-SW | 0.21 | <dl | <dl | <dl | <dl | 0.07 | 0.67 | 1.00 | 1.24 | 11.76 |
| 17-SW | 0.03 | <dl | <dl | 0.11 | <dl | 0.04 | 0.67 | 1.00 | 0.64 | 11.84 |
| 18-SW | 0.47 | <dl | <dl | <dl | 1.63 | 0.17 | 0.33 | 1.00 | 1.30 | 12.09 |
| 19-SW | 0.47 | <dl | <dl | <dl | <dl | 0.10 | 0.33 | 1.00 | 1.47 | 7.22 |
| 20-SW | 0.44 | <dl | <dl | <dl | <dl | 0.34 | <dl | 1.00 | 1.54 | 8.49 |
| 21-SW | 0.00 | <dl | <dl | <dl | <dl | <dl | <dl | 0.98 | 1.36 | 8.17 |
| 22-SW | 0.47 | <dl | <dl | <dl | <dl | 0.36 | 0.33 | 1.00 | 1.38 | 9.31 |
| 23-SW | 0.16 | <dl | <dl | <dl | <dl | 0.12 | 0.33 | 1.00 | 1.26 | 8.90 |
| 24-SW | 0.18 | <dl | <dl | <dl | <dl | 0.08 | 0.32 | 1.00 | 1.25 | 8.41 |
| 1-MW | 0.35 | <dl | <dl | <dl | <dl | 0.09 | <dl | 1.00 | 1.37 | 9.23 |
| 2-MW | 0.00 | <dl | <dl | <dl | <dl | 0.03 | <dl | 0.98 | 1.13 | 7.68 |
| 3-MW | 0.15 | <dl | <dl | <dl | <dl | 0.09 | 0.33 | 1.00 | 1.62 | 8.66 |
| 4-MW | 0.07 | <dl | <dl | <dl | <dl | 0.08 | <dl | 1.00 | 1.31 | 7.76 |
| Country | MCAA | MBAA | DCAA | TCAA | BCAA | BDCAA | DBAA | CDBAA | TBAA | Dalapon | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| USA | <0.05 | <0.01 | <0.01 | <1.00 | <0.02 | <0.01 | <0.02 | <0.01 | <0.01 | nd | [10] |
| Saudi Arabia | 0.71–0.75 | 0.57–0.76 | 0.53–0.65 | 0.58–0.74 | 0.55–0.82 | nd | 0.59–1.58 | nd | nd | nd | [32] |
| Greece | nd | nd | nd | 0.1–1.5 | 1.6–2.2 | 1 | 0.6–0.9 | nd | nd | nd | [33] |
| China | nd | nd | 0.4–0.6 | nd | nd | nd | nd | nd | nd | nd | [30] |
| USA | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | [7] |
| USA | <0.1 | <0.1 | <0.1 | nd | nd | nd | nd | nd | nd | nd | [12] |
| Turkey | <dl-2.77 | <dl | <dl | <dl-1.32 | <dl-2.58 | <dl-0.78 | <dl-0.76 | 0.51–1.02 | 0.64–1.77 | <dl-12.4 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulvi, A. The Occurrence of Haloacetic Acids and Dalapon in Bottled Waters and an Assessment of Their Health Risk. Water 2023, 15, 1810. https://doi.org/10.3390/w15101810
Ulvi A. The Occurrence of Haloacetic Acids and Dalapon in Bottled Waters and an Assessment of Their Health Risk. Water. 2023; 15(10):1810. https://doi.org/10.3390/w15101810
Chicago/Turabian StyleUlvi, Arzu. 2023. "The Occurrence of Haloacetic Acids and Dalapon in Bottled Waters and an Assessment of Their Health Risk" Water 15, no. 10: 1810. https://doi.org/10.3390/w15101810
APA StyleUlvi, A. (2023). The Occurrence of Haloacetic Acids and Dalapon in Bottled Waters and an Assessment of Their Health Risk. Water, 15(10), 1810. https://doi.org/10.3390/w15101810





