MIMS as a Low-Impact Tool to Identify Pathogens in Water
Abstract
1. Introduction
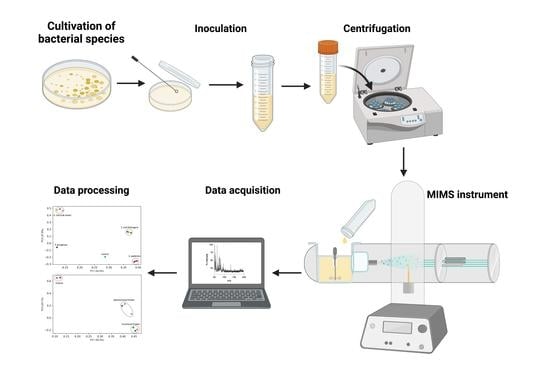
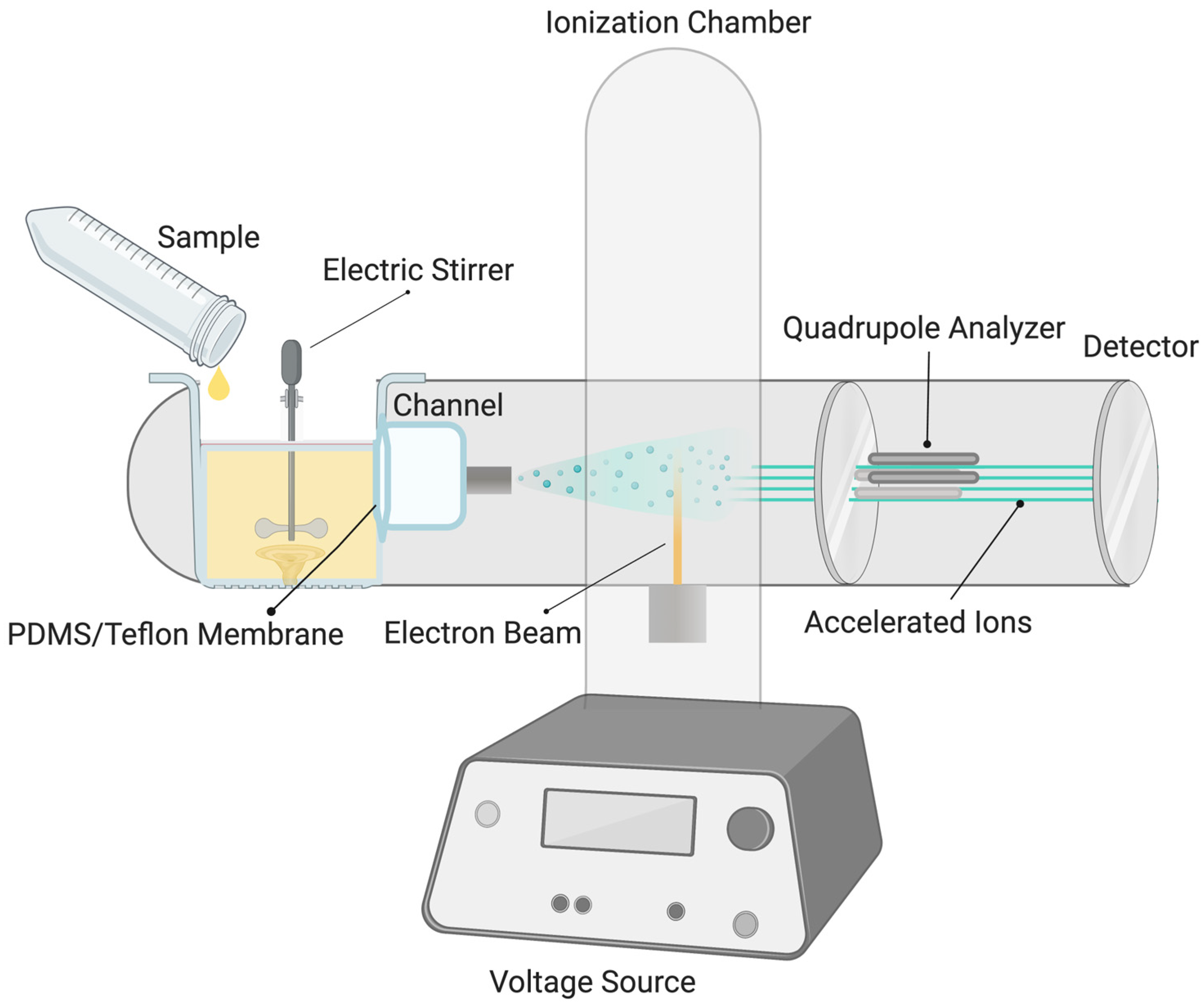
2. Materials and Methods
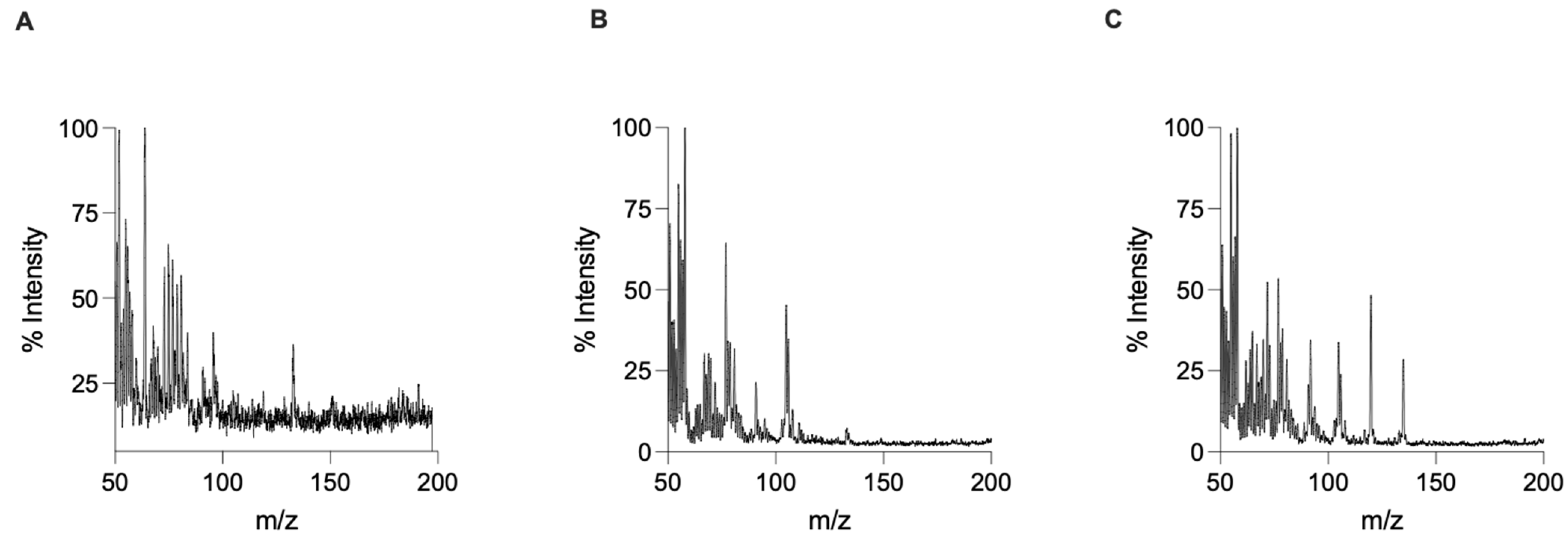
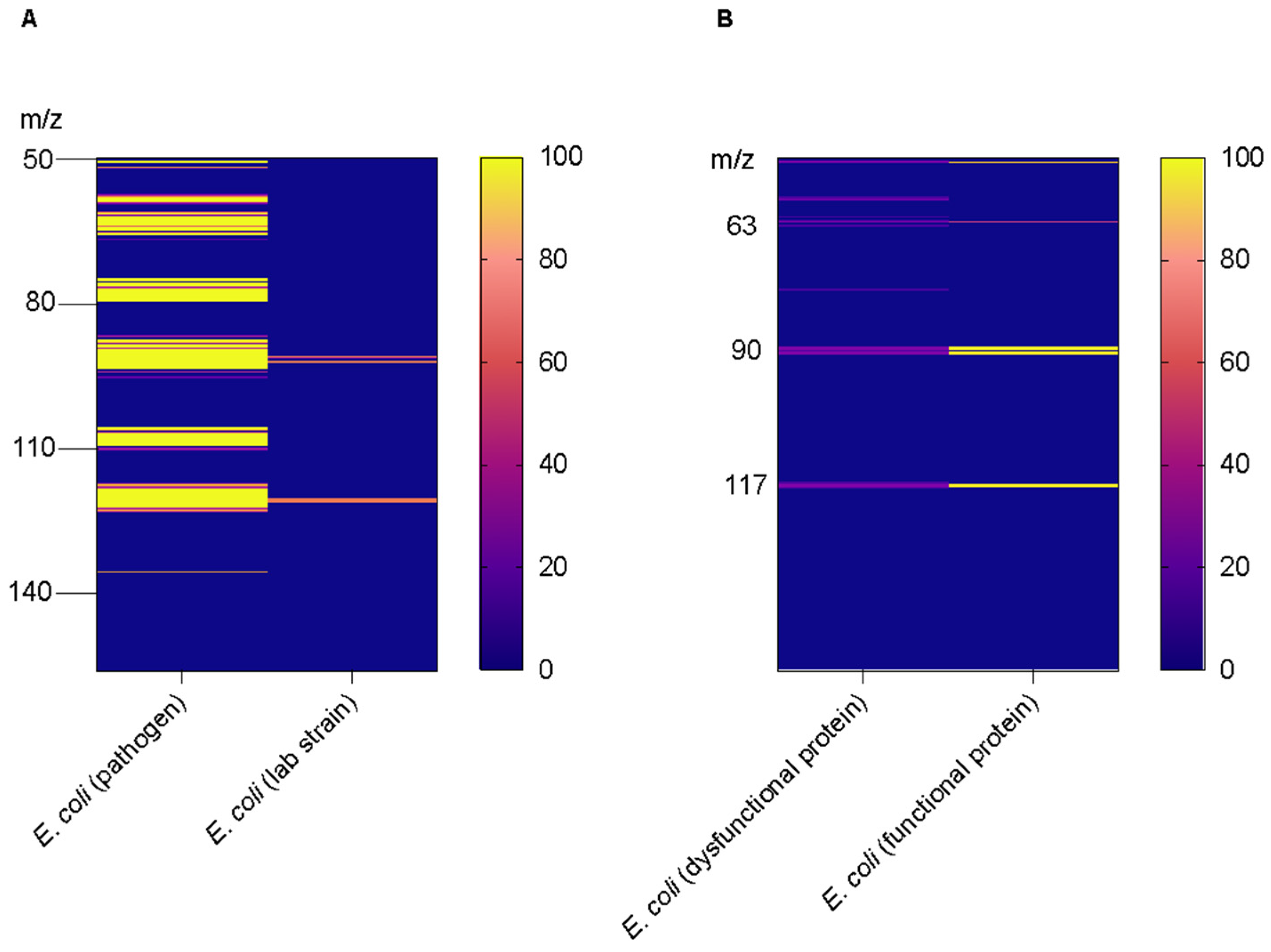
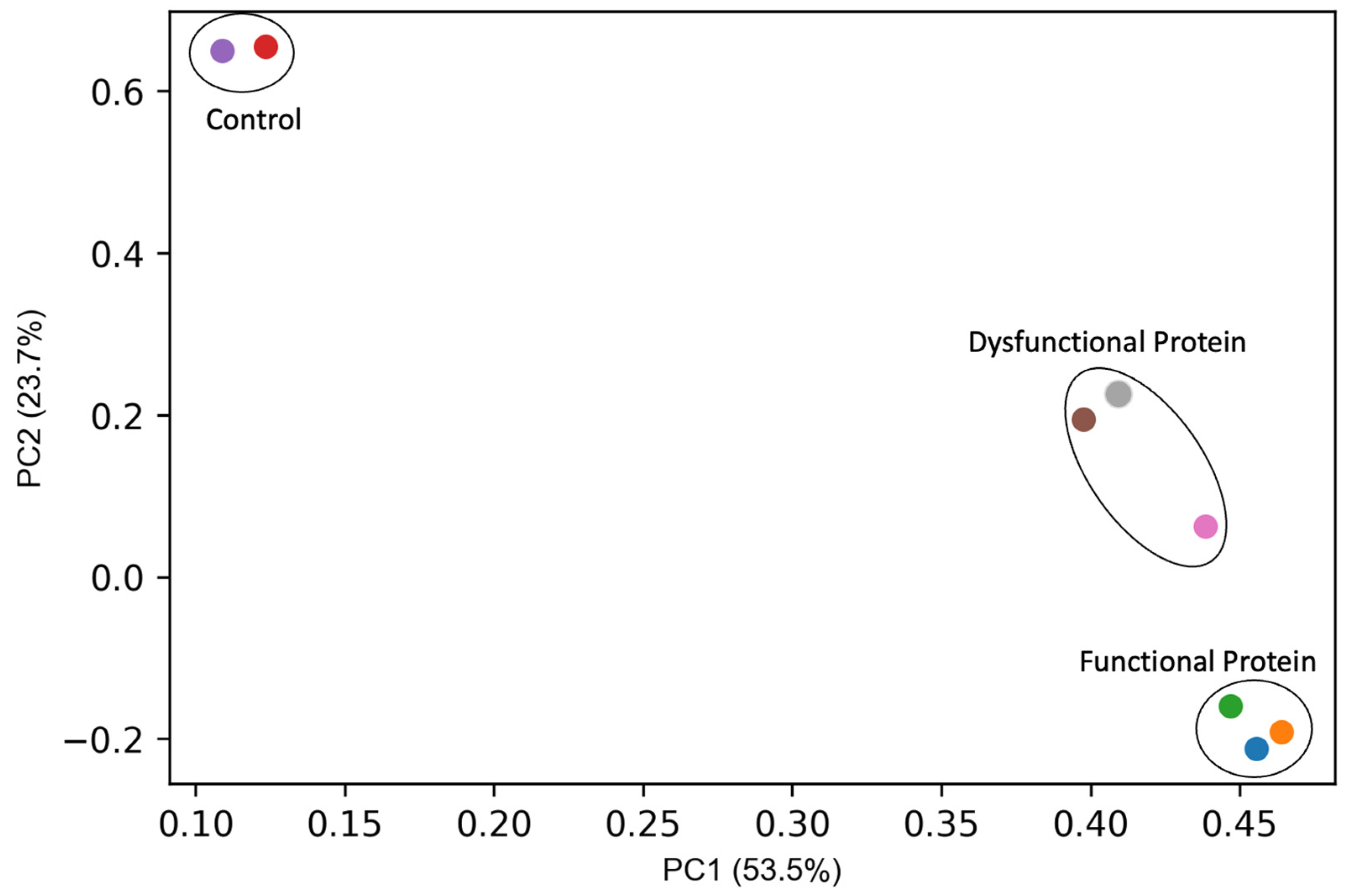
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harbor Persp. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Umber, B.J.; Shin, H.-W.; Meinardi, S.; Leu, S.-Y.; Zaldivar, F.; Cooper, D.M.; Blake, D.R. Gas Signatures from Escherichia coli and Escherichia coli-Inoculated Human Whole Blood. Clin. Transl. Med. 2013, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.L.; Bean, H.D. Dependence of the Staphylococcal Volatilome Composition on Microbial Nutrition. Metabolites 2020, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, L.; Marco, M.-P. Phenazines as Potential Biomarkers of Pseudomonas Aeruginosa Infections: Synthesis Regulation, Pathogenesis and Analytical Methods for Their Detection. Anal. Bioanal. Chem. 2020, 412, 5897–5912. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Holland, L.; Morrin, A. An Investigation of Stability and Species and Strain-Level Specificity in Bacterial Volatilomes. Front. Microbiol. 2021, 12, 693075. [Google Scholar] [CrossRef]
- Nizio, K.D.; Perrault, K.A.; Troobnikoff, A.N.; Ueland, M.; Shoma, S.; Iredell, J.R.; Middleton, P.G.; Forbes, S.L. In Vitro Volatile Organic Compound Profiling Using GC× GC-TOFMS to Differentiate Bacteria Associated with Lung Infections: A Proof-of-Concept Study. J. Breath Res. 2016, 10, 026008. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Chaturvedi, A.; Kaplan, P.D.; Libardoni, M.; Mundada, M.; Patel, U.; Zhang, X. Detection of an Extended Human Volatome with Comprehensive Two-Dimensional Gas Chromatography Time-of-Flight Mass Spectrometry. PLoS ONE 2013, 8, e75274. [Google Scholar] [CrossRef]
- Heddergott, C.; Calvo, A.M.; Latgé, J.P. The Volatome of Aspergillus Fumigatus. Eukaryot. Cell 2014, 13, 1014–1025. [Google Scholar] [CrossRef]
- Orban, A.; Weber, A.; Herzog, R.; Hennicke, F.; Rühl, M. Transcriptome of Different Fruiting Stages in the Cultivated Mushroom Cyclocybe Aegerita Suggests a Complex Regulation of Fruiting and Reveals Enzymes Putatively Involved in Fungal Oxylipin Biosynthesis. BMC Genom. 2021, 22, 324. [Google Scholar] [CrossRef]
- Freihorst, D.; Brunsch, M.; Wirth, S.; Krause, K.; Kniemeyer, O.; Linde, J.; Kunert, M.; Boland, W.; Kothe, E. Smelling the Difference: Transcriptome, Proteome and Volatilome Changes after Mating. Fungal Genet. Biol. 2018, 112, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Smolinska, A.; van Berkel, J.J.B.N.; Fijten, R.R.R.; Stobberingh, E.E.; Boumans, M.L.L.; Moonen, E.J.; Wouters, E.F.M.; Dallinga, J.W.; Van Schooten, F.J. Identification of Microorganisms Based on Headspace Analysis of Volatile Organic Compounds by Gas Chromatography–mass Spectrometry. J. Breath Res. 2014, 8, 027106. [Google Scholar] [CrossRef] [PubMed]
- Beale, D.J.; Pinu, F.R.; Kouremenos, K.A.; Poojary, M.M.; Narayana, V.K.; Boughton, B.A.; Kanojia, K.; Dayalan, S.; Jones, O.A.H.; Dias, D.A. Review of Recent Developments in GC-MS Approaches to Metabolomics-Based Research. Metabolomics 2018, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Nalbantoglu, S. Metabolomics: Basic Principles and Strategies. In Molecular Medicine; Nalbantoglu, S., Amri, H., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Kotiaho, T.; Lauritsen, F.R.; Choudhury, T.K.; Cooks, R.G.; Tsao, G.T. Membrane Introduction Mass Spectrometry. Anal. Chem. 1991, 63, 875A–883A. [Google Scholar] [CrossRef]
- Kotiaho, T.; Lauritsen, F.R. Chapter 16 Membrane Inlet Mass Spectrometry. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2002; Volume 37, pp. 531–557. [Google Scholar]
- Giannoukos, S.; Brkić, B.; Taylor, S.; France, N. Membrane Inlet Mass Spectrometry for Homeland Security and Forensic Applications. J. Am. Soc. Mass Spectrom. 2015, 26, 231–239. [Google Scholar] [CrossRef]
- Johnson, R.C.; Cooks, R.G.; Allen, T.M.; Cisper, M.E.; Hemberger, P.H. Membrane Introduction Mass Spectrometry: Trends and Applications. Mass Spectrom. Rev. 2000, 19, 1–37. [Google Scholar] [CrossRef]
- Kinani, S.; Richard, B.; Souissi, Y.; Bouchonnet, S. Analysis of Inorganic Chloramines in Water. Trends Anal. Chem. 2012, 33, 55–67. [Google Scholar] [CrossRef]
- Degn, H. Membrane Inlet Mass Spectrometry in Pure and Applied Microbiology. J. Microbiol. Methods 1992, 15, 185–197. [Google Scholar] [CrossRef]
- Burlacot, A.; Burlacot, F.; Li-Beisson, Y.; Peltier, G. Membrane Inlet Mass Spectrometry: A Powerful Tool for Algal Research. Front. Plant Sci. 2020, 11, 1302. [Google Scholar] [CrossRef]
- Douchi, D.; Liang, F.; Cano, M.; Xiong, W.; Wang, B.; Maness, P.-C.; Lindblad, P.; Yu, J. Membrane-Inlet Mass Spectrometry Enables a Quantitative Understanding of Inorganic Carbon Uptake Flux and Carbon Concentrating Mechanisms in Metabolically Engineered Cyanobacteria. Front. Microbiol. 2019, 10, 1356. [Google Scholar] [CrossRef]
- Lauritsen, F.R.; Lloyd, D. Direct Detection of Volatile Metabolites Produced by Microorganisms. In Mass Spectrometry for the Characterization of Microorganisms; ACS Symposium Series; American Chemical Society: New York, NY, USA, 1993; Volume 541, pp. 91–106. ISBN 9780841227378. [Google Scholar]
- Cabral, J.P.S. Water Microbiology. Bacterial Pathogens and Water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef] [PubMed]
- Payment, P.; Locas, A. Pathogens in Water: Value and Limits of Correlation with Microbial Indicators. Ground Water 2011, 49, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Ismat, F.; Szakonyi, G.; Rahman, M.; Mirza, O. Probing the Putative Active Site of YjdL: An Unusual Proton-Coupled Oligopeptide Transporter from E. Coli. PLoS ONE 2012, 7, e47780. [Google Scholar] [CrossRef] [PubMed]
- Saporito, P.; Vang Mouritzen, M.; Løbner-Olesen, A.; Jenssen, H. LL-37 Fragments Have Antimicrobial Activity against Staphylococcus epidermidis Biofilms and Wound Healing Potential in HaCaT Cell Line. J. Pept. Sci. 2018, 24, e3080. [Google Scholar] [CrossRef]
- Saporito, P.; Biljana, M.; Løbner Olesen, A.; Jenssen, H. Antibacterial Mechanisms of GN-2 Derived Peptides and Peptoids against Escherichia coli. Biopolymers 2019, 110, e23275. [Google Scholar] [CrossRef]
- Hughes, S.S.; Nielsen, M.M.K.; Jonsbo, R.V.; Nielsen, C.U.; Lauritsen, F.R.; Prabhala, B.K. BeerMIMS: Exploring the Use of Membrane-Inlet Mass Spectrometry (MIMS) Coupled to KNIME for the Characterization of Danish Beers. Eur. J. Mass Spectrom. 2021, 27, 266–271. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Vickerman, J.C. Secondary Ion Mass Spectrometry: Characterizing Complex Samples in Two and Three Dimensions. Anal. Chem. 2013, 85, 610–639. [Google Scholar] [CrossRef]
- Smith, F.T.; DeRuiter, J.; Abdel-Hay, K.; Randall Clark, C. GC–MS and FTIR Evaluation of the Six Benzoyl-Substituted-1-Pentylindoles: Isomeric Synthetic Cannabinoids. Talanta 2014, 129, 171–182. [Google Scholar] [CrossRef]
- Morris, H.R.; Taylor, G.W.; Masento, M.S.; Jermyn, K.A.; Kay, R.R. Chemical Structure of the Morphogen Differentiation Inducing Factor from Dictyostelium Discoideum. Nature 1987, 328, 811–814. [Google Scholar] [CrossRef]
- Dickschat, J.S. Capturing Volatile Natural Products by Mass Spectrometry. Nat. Prod. Rep. 2014, 31, 838–861. [Google Scholar] [CrossRef]
- Bunge, M.; Araghipour, N.; Mikoviny, T.; Dunkl, J.; Schnitzhofer, R.; Hansel, A.; Schinner, F.; Wisthaler, A.; Margesin, R.; Märk, T.D. On-Line Monitoring of Microbial Volatile Metabolites by Proton Transfer Reaction-Mass Spectrometry. Appl. Environ. Microbiol. 2008, 74, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, K.; Hunsicker, E.; Ratcliffe, E.; Lindley, M.R.; Leonard, J.; Hitchens, J.R.; Turner, M.A. Volatile Atmospheric Pressure Chemical Ionisation Mass Spectrometry Headspace Analysis of E. coli and S. aureus. Anal. Methods 2021, 13, 5441–5449. [Google Scholar] [CrossRef] [PubMed]
- Key, M. A Tutorial in Displaying Mass Spectrometry-Based Proteomic Data Using Heat Maps. BMC Bioinform. 2012, 13 (Suppl. S16), S10. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.K.; Bae, H. Identification of New Metabolites of Bacterial Transformation of Indole by Gas Chromatography-Mass Spectrometry and High Performance Liquid Chromatography. Int. J. Anal. Chem. 2014, 2014, 239641. [Google Scholar] [CrossRef]
- Ratiu, I.-A.; Ligor, T.; Bocos-Bintintan, V.; Al-Suod, H.; Kowalkowski, T.; Rafińska, K.; Buszewski, B. The Effect of Growth Medium on an Escherichia Coli Pathway Mirrored into GC/MS Profiles. J. Breath Res. 2017, 11, 036012. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.; Lauritsen, F.R.; Degn, H. The Parasitic Flagellates Trichomonas Vaginalis and Tritrichomonas Foetus Produce Indole and Dimethyl Disulphide: Direct Characterization by Membrane Inlet Tandem Mass Spectrometry. J. Gen. Microbiol. 1991, 137, 1743–1747. [Google Scholar] [CrossRef]
- Etzkorn, J.M.; Davey, N.G.; Thompson, A.J.; Creba, A.S.; Leblanc, C.W.; Simpson, C.D.; Krogh, E.T.; Gill, C.G. The Use of MIMS-MS-MS in Field Locations as an on-Line Quantitative Environmental Monitoring Technique for Trace Contaminants in Air and Water. J. Chromatogr. Sci. 2009, 47, 57–66. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, L. A Review on Dimension Reduction. Int. Stat. Rev. 2013, 81, 134–150. [Google Scholar] [CrossRef]
- Simmons, S.; Peng, J.; Bienkowska, J.; Berger, B. Discovering What Dimensionality Reduction Really Tells Us About RNA-Seq Data. J. Comput. Biol. 2015, 22, 715–728. [Google Scholar] [CrossRef]
- Harder, D.; Stolz, J.; Casagrande, F.; Obrdlik, P.; Weitz, D.; Fotiadis, D.; Daniel, H. DtpB (YhiP) and DtpA (TppB, YdgR) Are Prototypical Proton-Dependent Peptide Transporters of Escherichia coli. FEBS J. 2008, 275, 3290–3298. [Google Scholar] [CrossRef]
- Prabhala, B.K.; Aduri, N.G.; Iqbal, M.; Rahman, M.; Gajhede, M.; Hansen, P.R.; Mirza, O. Several hPepT1-transported Drugs Are Substrates of the Escherichia coli Proton-Coupled Oligopeptide Transporter YdgR. Res. Microbiol. 2017, 168, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Ernst, H.A.; Wang, X.; Hald, H.; Ditta, A.C.; Ismat, F.; Rahman, M.; Mirza, O. Functional Investigation of Conserved Membrane-Embedded Glutamate Residues in the Proton-Coupled Peptide Transporter YjdL. Protein Pept. Lett. 2012, 19, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Robinson, D.K. Industrial Choices for Protein Production by Large-Scale Cell Culture. Curr. Opin. Biotechnol. 2001, 12, 180–187. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajid, S.; Aryal, I.; Chaudhri, S.F.; Lauritsen, F.R.; Jørgensen, M.G.; Jenssen, H.; Prabhala, B.K. MIMS as a Low-Impact Tool to Identify Pathogens in Water. Water 2023, 15, 184. https://doi.org/10.3390/w15010184
Sajid S, Aryal I, Chaudhri SF, Lauritsen FR, Jørgensen MG, Jenssen H, Prabhala BK. MIMS as a Low-Impact Tool to Identify Pathogens in Water. Water. 2023; 15(1):184. https://doi.org/10.3390/w15010184
Chicago/Turabian StyleSajid, Salvia, Ishika Aryal, Suleman Farooq Chaudhri, Frants Roager Lauritsen, Mikkel Girke Jørgensen, Håvard Jenssen, and Bala Krishna Prabhala. 2023. "MIMS as a Low-Impact Tool to Identify Pathogens in Water" Water 15, no. 1: 184. https://doi.org/10.3390/w15010184
APA StyleSajid, S., Aryal, I., Chaudhri, S. F., Lauritsen, F. R., Jørgensen, M. G., Jenssen, H., & Prabhala, B. K. (2023). MIMS as a Low-Impact Tool to Identify Pathogens in Water. Water, 15(1), 184. https://doi.org/10.3390/w15010184