Abstract
Tropical rivers are strongly influenced by stormwaters, pollutants and agro-pastoral activities. These systems are no longer able to maintain their native biota. Therefore, it seems important to understand how biological assemblages are driven by environmental gradients at different spatial scales. However, information on the relationships between the distribution of aquatic macroinvertebrates and environmental variables remains scarce in Cameroon. To improve our knowledge on the drivers of such distributions, a study was carried out at 11 contrasted sites from three catchments located in West Cameroon. This study aimed at understanding the spatio-temporal variations of 19 taxonomic metrics calculated for the benthic macroinvertebrate assemblages of these sites sampled during 13 months, concurrently with physico-chemical analyses of water quality. Two hundred and twelve taxa were caught. Diptera(rf-S), Shannon-Wiener diversity, 1–GOLD, total richness, Pielou evenness and Heteroptera(rf-S) revealed their high ability to respond to anthropogenic pressures or disturbances. Conductivity, water temperature, dissolved oxygen, nitrates, total hardness and alkalinity were identified as the main physico-chemical drivers of the taxonomic structure of benthic assemblages. These results will allow further exploration of the implementation of a tool for monitoring the ecological quality of West Cameroon rivers.
1. Introduction
Nowadays, running waters are impaired by multiple stressors acting simultaneously [1,2,3,4] or sequentially [5]. Climate change, increasing urbanization and intensive agro-pastoral activities, which have adverse effects on stream biota [6,7], are the most important stressors of small tropical streams. The physico-chemical and hydromorphological stream impairments that specifically occur in urbanized watersheds have been labeled the “urban stream syndrome” [8,9]. These human-induced environmental changes cause local habitat degradation and fragmentation as well as longitudinal connectivity loss, which can profoundly alter the structure and composition of aquatic assemblages and lead to the decline of biodiversity in many rivers of developing countries [10,11]. Water pollution assessment and control are also major and widespread challenges in developing countries because water can indirectly favor the transmission of many diseases [12,13]. Thus, various methods have been developed to analyze water quality impairment. Water temperature, dissolved oxygen, pH, conductivity, suspended matter, nutrients (including ammonium, nitrites, nitrates and orthophosphates (e.g., [14,15])) and sometimes micropollutants (e.g., [16,17]) have been routinely measured in several tropical streams. Unfortunately, such analyses are expensive and routine surveys of pollutant concentrations (which may greatly vary both temporally and spatially) in tropical areas are scarce and cannot allow efficient detection of diffuse and/or episodic contaminations when based only on one-off measures [18,19].
Adding biological parameters related to the structure and the composition of aquatic assemblages to the physico-chemical assessment of rivers has proven its efficiency in fully assessing the impact of anthropogenic stressors [20,21]. Benthic macroinvertebrates are the most frequently used biological indicators of river conditions because they are diverse, long-lived, rather sedentary, and they react rapidly and often predictably to anthropogenic pressures [22,23]. Benthic macroinvertebrates also play an important role in freshwater ecosystem functioning. They contribute to carbon and nitrogen cycles by (i) feeding on algae, coarse detritus or fine particulate organic matter [24,25] and (ii) providing food to higher trophic levels. They are also taxonomically diverse and relatively easy to identify (at least at the genus or family level). Invertebrate assemblages integrate environmental changes over several months [26], and examining the structure and composition of such assemblages has the potential to relevantly inform on the ecological status of water bodies [19,27,28,29,30,31] and to demonstrate the effects of past and present environmental degradation [31,32].
Several studies in sub-Saharan Africa have demonstrated that stream biomonitoring based on benthic macroinvertebrates is clearly in progress (e.g., [33,34,35,36,37,38]). In southeastern Ivory Coast, Edia et al. (2013, [39]) randomly sampled the aquatic invertebrates of eight sites on eight occasions (including the rainy and dry seasons) and related the presence of several genera of Diptera and Ephemeroptera to the most and weakly mineralized sites, respectively. Kaboré et al. (2016, [40]) related the taxonomic and functional structure of macroinvertebrate assemblages to watershed habitat use in 29 semi-arid stream reaches of Burkina Faso. They found (i) a gradual decline of collector-filterers and habitat-sensitive Ephemeroptera and Trichoptera (mainly the Baetidae and Hydropsychidae families) and (ii) a gradual increase in the relative abundance of tolerant Diptera (Chironomidae, Culicidae, Psychodidae and Syrphidae) from the least impaired river reaches to reaches subjected to intensive agriculture or urban pressure. However, taxonomic richness, the Shannon-Wiener index, or the proportion of the dominant tolerant Diptera did not distinguish invertebrate assemblages of “protected” or “extensive agriculture” areas from those of “intensive agriculture” because high organic matter and fertilizer inputs in the second group of reaches had increased both macrophytic and periphytic production, enhancing habitat heterogeneity and the development of molluscs and coleopterans [40]. Those species have compensated for the loss of water quality sensitive taxa [40]. In Cameroon, Menbohan et al. (2013, [41]) and Tchakonté et al. (2015, [42]) related the high abundance of EPT taxa to very low anthropogenic pressures in the peri-urban streams of Yaoundé and in the forest streams of Douala, respectively. Tchakonté et al. (2015, [42]) considered (i) the absence of EPT species and some coleopteran and hemipteran families and (ii) the decrease of both shredders (indicators of riparian vegetation health) and predators as good predictors of severe anthropogenic pressures.
In Cameroon, aquatic environment management and protection have taken place slowly due to a lack of cooperation and synergy between the various stakeholders from governmental and non-governmental institutions. Anthropogenic pressures on rivers remain high. There is still direct dumping of solid wastes and liquid wastewaters from households, agriculture and industries into streams. In the Bafoussam region, agro-pastoral activities have accelerated this degradation of natural environments, favoring the leaching and drainage of fertilizers in streams during intense rain events [43,44]. In addition, the lack of wastewater treatment plants often converts streams into dumping grounds of various kinds of wastes, described by Parent-Raoult and Boisson (2007, [45]) as “urban discharges in rainy weather” and representing a certain health risk for local populations (e.g., proliferation of mosquitoes, epidemics and parasitic diseases) and ecosystems (e.g., biodiversity loss).
In this context, understanding how watershed anthropogenic level can influence water quality and macroinvertebrate diversity in a tropical stream and identifying the major environmental drivers of invertebrate community structure could therefore be a major challenge for freshwater managers in Cameroon and other tropical regions.
The aim of our study was to (i) diagnose the water quality in the different river sub-catchments of the Bafoussam region, (ii) highlight the main relationships between water quality descriptors and macroinvertebrate community-based metrics, (iii) identify which metrics could be the best candidates for a regional bioassessment tool of streams in this area, and (iv) rank such metrics according to their ability to discriminate “least impacted” conditions from conditions “significantly impacted” by anthropogenic activities, despite the low knowledge level on life history traits and ecological preferences of local macroinvertebrate species.
2. Material and Methods
2.1. Study Area and Human Pressure-Based Typology of Sites
The western region, whose chief town is Bafoussam (Mifi County), covers about 13,892 km2. Roughly, this area is between the latitudes 5°24′–5°33′ N and the longitudes 10°21′–10°34′ E, with an average altitude of 1450 m (Figure 1). This region benefits mainly from (i) agriculture involving the utilization of natural (compost) and chemical (industrial) fertilizers, (ii) livestock farming and (iii) primary trade of local products. Urban and industrial areas are also developing rapidly at the expense of natural vegetation cover. This region is located in a tropical climate zone [46], characterized by a long and abundant wet season from March to October (rainfall ranging from 84.8 to 271.4 mm month−1), and a dry season from November to February (6.7–50.1 mm month−1). The average annual rainfall and temperature (over the 1995–2005 period) were 1741.8 mm and 21.0 °C, respectively (meteorological station in Bafoussam).
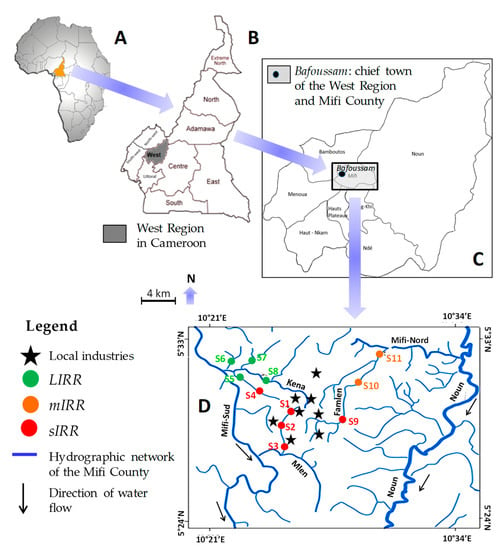
Figure 1.
Locations of the sampling area (A–C) and the sampling sites ((D); S01–S11) in Mifi County of the West Region, Cameroon in Africa (A), the West Region in Cameroon (B), and Mifi County in the West Region (C). LIRR = Least Impacted River Reach, mIRR = moderately Impacted River Reach, sIRR = severely Impacted River Reach. Black stars are the main local industries close to the streams.
Eleven sites were selected in three sub-catchments of Mifi County to optimally illustrate gradients of various anthropogenic pressures occurring in this region (e.g., domestic, industrial, physical and agro-pastoral contaminations), from Least Impacted River Reaches (LIRRs) often located in rural zones with low population density to severely Impacted River Reaches (sIRRs) often located in more urbanized areas (Table 1; Figure 1). Three sampling sites (S01, S02 and S03), expected as severely impaired by anthropogenic pressures, were chosen in the sub-catchment Nlem. They were situated in an urban zone gathering breweries, a soap factory and an agricultural cooperative. These industries, located upstream from riparian residential areas and city drainage services, were directly discharging their wastewaters in the river. Five sampling sites (S04 to S08; Table 1) were located on tributaries of the Kena River, in a rural zone where farming and traditional breeding are the main activities. Except for site S04 draining an intensively cropped area (coffee and vegetables that require fertilizers) with cattle breeding, domestic discharge and boulder extraction, all these sites were subjected to limited anthropogenic pressures (Table 1). Three sampling sites were located on the Famlem stream, which takes its source in an urban zone (S09) before flowing in a least impaired rural area (S10 and S11). Food crop cultivation and local sand extraction (leading to substrate clogging) are the main activities along this stream (Table 1). All the study sites were situated between an altitude of 1117 m (S11) and 1466 m (S01).

Table 1.
Description of the 11 sampling sites. The expected ecological status was evaluated based on a multivariate analysis taking into account anthropogenic pressures at the reach scale (see Section 2.1 for further details). LIRR = Least Impacted River Reach; mIRR = moderately Impacted River Reach; sIRR = severely Impacted River Reach. «High» population density corresponds to “>200 inhabitants km−2” and «Low» to “≤200 inhabitants km−2” (adapted from [50]). Types of waste deposits: “a” = agricultural, “d” = domestic, “i” = industrial and “m” = municipal.
Because no precise information was locally available for quantifying the intensity of the different sources of anthropogenic pressures on sites, we decided to describe semi-quantitatively the potential impact of anthropogenic pressures using three (low/moderate/high) impact levels for eight pressure categories (Table S1 in Supplementary Material). These pressure categories were related to the importance of “urban” areas, “food crops”, “cash crops”, “cattle and poultry” breeding, untreated “domestic, municipal and agricultural” or “industrial” wastewater effluents, riverine “factories” and “civil engineering and mining” at reach scale. The corresponding array (11 sites × 24 modalities from eight pressure categories) was analyzed by Multiple Correspondence Analysis (MCA, [47]). Then, sampling sites were hierarchically clustered based on Euclidean distances calculated between site locations on the MCA first factorial plane and applying the complete linkage method [48].
2.2. Benthic Macroinvertebrate Sampling and Community Structure Description
Invertebrate sampling was performed by kick sampling using a hand net (30 × 30 cm opening, conical thread, mesh size: 400 μm), applying the multi-habitat approach described by Stark et al. (2001, [49]). Site sampling was performed in a 100 m stretch, for an approximate total sampled surface of 3 m2.
All the available habitats within each study site were sampled, including boulders, gravel, sand, mud, macrophyte beds, leaf packs, branches and rafts. Substrate samples were sieved (mesh size: 400 μm). Large invertebrates were sorted by the naked eye, whereas the smaller ones were sorted under a dissecting microscope. The sorted organisms were fixed with 10% formalin. They were identified to the genus or species level (when possible), based on Durand and Lévêque (1981, [51]), Day et al. (2002, [52]), De Moor and Day (2002, [53]), De Moor et al. (2003a&b, [54,55]) and Tachet et al. (2010, [56]). Invertebrate assemblages were sampled monthly from December 2015 to December 2016 (i.e., 13 sampling campaigns).
Because of the lack of precise information on the biological traits and ecological preferences of the Cameroonian macrobenthic fauna, only 19 metrics describing the taxonomic structure and composition of benthic macroinvertebrate assemblages were selected (Table 2). These taxonomy-based metrics included total richness, the relative richness of thirteen taxonomic groups (e.g., Coleoptera, Ephemeroptera, Odonata, Heteroptera), the richness (EPT) or the abundance (log10(EPTD + 1), 1-GOLD) of combinations of these groups, and the Shannon-Wiener diversity and Pielou evenness indices.

Table 2.
Taxonomy-based metrics calculated on benthic macroinvertebrate assemblages of three river catchments of the West Cameroon Region. rf-S = relative frequency of the taxon in the assemblage in terms of taxonomic richness. The variation of individual metrics was predicted along an increasing anthropogenic pressure gradient (right column). D = Diptera, E = Ephemeroptera, P = Plecoptera and T = Trichoptera.
2.3. Physico-Chemical Parameters
Seventeen physico-chemical variables were measured in the field (i.e., during the 13 invertebrate sampling campaigns) or at the laboratory with standard methods for water (cf. [62,63] for protocols). Dissolved oxygen and conductivity (HACH multiparameter probe, HACH, Dubai, United Arab Emirates), pH (pH-meter CG 818 model SCHOTT Geräte Gmbh, SCHOTT France SAS, Colombes, France) and water temperature (hand-held thermometer; ±0.1 °C) were measured in situ. If daytime variation in dissolved oxygen concentration (DO) was not controlled during the sampling design, the study sites nearest to Bafoussam (e.g., S01 to S03), which belong to sIRRs, were the last sites sampled in the afternoon (i.e., in the most favorable DO measurement conditions) to avoid a confounding effect of daytime variation if a DO deficit was observed. Water samples were collected in polyethylene bottles (1000 mL) and were stored in refrigerated enclosures. Back to the laboratory, concentrations of nitrates, nitrites, ammonium and orthophosphates were measured using a HACH DR/2800 spectrophotometer, as well as suspended matter, water color and turbidity. The Biological Oxygen Demand (BOD5) was measured with a Liebherr BOD analyzer, whereas alkalinity, total hardness, oxidizability (per permanganate method), chlorophyll a and dissolved CO2 were measured by volumetric methods. The concentrations of micropollutants were not measured during the study.
2.4. Environmental and Faunal Data Analyses
Non-parametric Friedman tests with blocks were applied to evaluate the statistical significance of differences among sites for physico-chemical parameters and invertebrate-based metrics. Season was the repeated measure defining block and sites were regarded as different “treatments” within each block. Significant Friedman tests were followed by pairwise comparisons using Nemenyi post-hoc tests for unreplicated blocked data [64].
A normalized Principal Component Analysis (nPCA) was applied to the array [143 samples (i.e., 11 sites × 13 campaigns) × 10 environmental variables with significant between-sites differences based on the Friedman test results] to compare the abiotic characteristics of sampling sites. Five samples were excluded from the final analysis due to extreme values (S02 and S03 in February and May, and S02 in December 2016). Using the same matrix, sampling sites were hierarchically clustered based on Euclidean distances calculated between site locations on the nPCA first factorial plane and applying the complete linkage method [48].
An nPCA was applied also to the array [143 samples × 17 taxonomic metrics with significant between-sites differences based on the Friedman test results]. Preliminary “within-site” and “within-season” nPCA analyses [65] were performed on both datasets. They demonstrated a low effect of “season” on (i) the typology of sites based on taxonomic metrics and (ii) physico-chemical parameter variations (Figures S1 and S2 in Supplementary Material). Sampling sites were also clustered based on the taxonomic structure of their assemblages using the same procedure as above (i.e., applying the complete linkage method on a matrix of Euclidean distances between site locations on the first factorial plane of the taxonomy-based nPCA).
Finally, the relationships between abiotic parameters and taxonomy-based metrics were analyzed with redundancy analysis (RDA; [66]), and sampling sites were clustered based on their locations on the RDA first factorial plane (similarly as above). The Discrimination Efficiency (DE) of each metric was also calculated. The DE of a given metric is the proportion of IRRs that exhibited values (i) lower than the first quartile of the LIRR value distribution (for metrics decreasing in impaired conditions) or (ii) higher than the third quartile of the LIRR value distribution (for metrics increasing in impaired conditions).
Analyses were conducted with the packages ade4 [67] and vegan [68] using the software R (version 3.4.1 [69]).
3. Results
3.1. Human Pressure-Based Typology of Sites
The MCA provided a typology of sampling sites based on the description of anthropogenic pressures potentially impairing water quality and habitat integrity (Figure 2). The first factorial plane (F1-F2; Figure 2A) explained 59.1% of the total inertia. Axis F1 discriminated S01-S03, S09 (F1 < 0; F2 > 0) and S04 (F1 and F2 < 0) by high population density and moderate to high level of pressure for most of the anthropogenic pressure categories (see Figure S3), S05 to S08 (F1 and F2 > 0) by low population density and low level of pressure for most of the pressure categories, and S10 and S11 (F1 > 0; F2 < 0) by only highly impaired by sediment mining and civil engineering. According to the correlations ratios (cr; [70]), factories (cr = 0.948), industrial and domestic/municipal/agricultural (cr = 0.909) or industrial (cr = 0.908) wastewater effluents and cattle/poultry breeding (cr = 0.763) best discriminated the sites along the F1 axis. Riverine factories (cr = 0.870), cash crop (cr = 0.842), civil engineering and sediment mining (cr = 0.692) and urban areas (cr = 0.621) best discriminated the sites along the F2 axis.
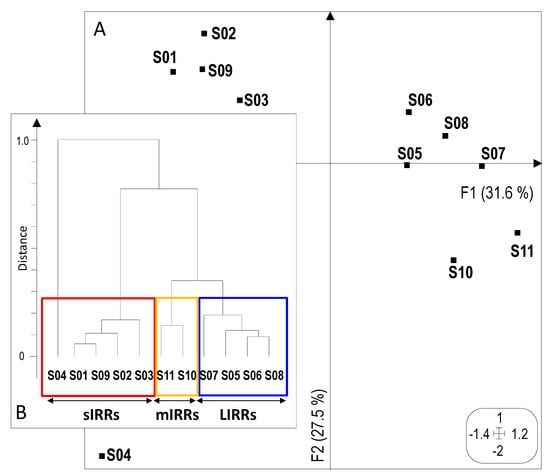
Figure 2.
Ordination of sites by Multiple Correspondence Analysis (MCA) based on eight anthropogenic pressure categories. (A) Projection of 11 sites (S01 to S11) on the first factorial plane (F1-F2). (B) Dendrogram, based on the Euclidean distance (calculated between sites according to their coordinates on the first factorial plane of the MCA) and the complete linkage method, highlighting the similarity of the 11 study sites considering the intensity of anthropogenic pressure categories. LIRRs = Least Impacted River Reaches, mIRRs = moderately Impacted River Reaches, sIRRs = severely Impacted River Reaches. See Table S1, Figure S3 and the text for further details.
The cluster analysis (Figure 2B) highlighted three main groups of sites. The first group gathered sites weakly urbanized and located in rural zones (S05 to S08), which could be considered as “Least Impacted River Reaches” (LIRRs). The second group consisted of two sites (S10 and S11) only moderately impacted by anthropogenic pressures (mIRRs). The third group included five sites (S01 to S04 and S09) more severely impaired by different combinations of anthropogenic pressures (sIRRs). S04, the most impacted site, was included in this third group.
3.2. Physico-Chemical Characteristics of Streams
The highest electrical conductivity values were obtained in the tributary of the Nlem stream, the site S02 exhibiting values up to 1470 µS cm−1 in December 2016. Similarly, the pH values in this sub-catchment reached 11.3 at site S02 and 10.8 at site S03, reflecting the basic character of the effluents received by this stream. Dissolved oxygen concentration was low in this sub-catchment (<50%; Table 3). In contrast, the Kena tributaries (S05 to S08) exhibited the highest dissolved oxygen concentrations (>50%). Significantly higher dissolved oxygen concentration, and lower temperature, conductivity and total hardness were observed in sites with the LIRR status (Figure 3, Table 3). It was more difficult to relate significant variation of other parameters to the LIRR vs. IRR status based on Nemenyi post-hoc test results (Figure S4 in Supplementary Material). No significant between-sites differences in turbidity, suspended matter, dissolved CO2, orthophosphates, chlorophyll a and BOD5 were identified.

Table 3.
Mean values (±standard deviation) of physico-chemical parameters for the 11 sampling sites, over the study period. Asterisks indicate significant differences among sites, based on Friedman’s tests (*** p < 0.001; ** p < 0.01; * p < 0.05). Parameters exhibiting significant differences are those included in the nPCA (see Section 3.2) and RDA (see Section 3.5). See text for further details.
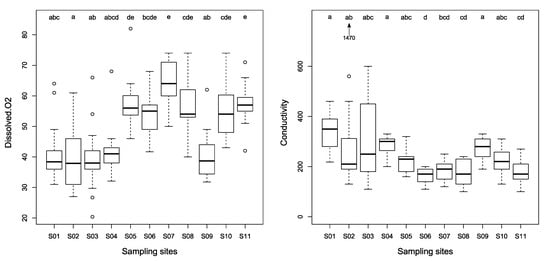
Figure 3.
Box-plots (Min/Q25/Median/Q75/Max) describing the distribution of values of “dissolved oxygen” (in % saturation) and conductivity (in μS cm−1) over a period of thirteen months, for 11 study sites (S01 to S11). «a», «b», «c», «d» and «e» are distinct groups identified by post-hoc Nemenyi tests applied after identifying, for a given parameter, a significant difference among sites by a non-parametric Friedman test. Outliers (open circles) are out of the 1.5 interquartile range. See text for further details.
For alkalinity and nitrites, even if the Friedman test identified a significant heterogeneity among sites, Nemenyi post-hoc tests were unable to identify precisely the corresponding significant differences (probably due to beta errors in post-hoc test statistical decisions).
3.3. Abiotic Typology of Sampling Sites
The nPCA provided a typology of sampling sites based on their physico-chemical characteristics (Figure 4). The first factorial plane (F1-F2) explained 38.5% of the total inertia. Axis F1 discriminated sites exhibiting high percentage of dissolved oxygen (S05, S07 and S08; F1 > 0) from sites with high conductivity and total hardness (S01 and S04; F1 < 0). Axis F2 mainly opposed S09 (F2 > 0) sites with rather low pH and oxidizability values to the other sites.
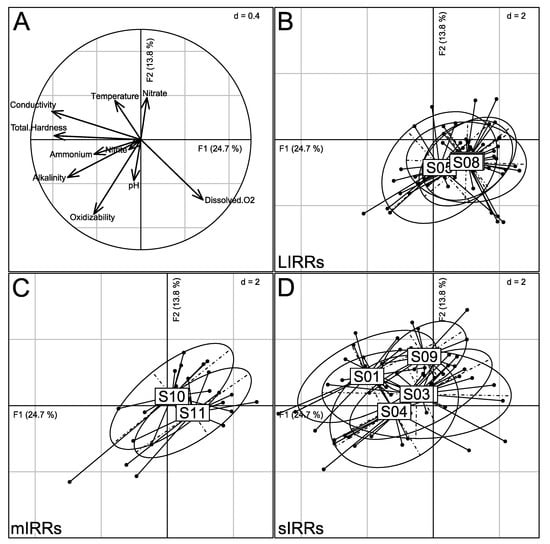
Figure 4.
Ordination of sites by nPCA based on 10 physico-chemical parameters. (A) Projection of physico-chemical parameters on the first correlation circle. (B–D) Locations of sites (S01 to S11) on the first factorial plane at the weighted average of the locations of corresponding monthly sampling events (solid points). Each sampling event is linked by a line to the mean location of the corresponding site. Ellipses of inertia (95%) are provided by site. Sites are gathered according to anthropogenic pressure level: LIRRs = Least Impacted River Reaches; mIRRs = moderately Impacted River Reaches; sIRRs = severely Impacted River Reaches. See Table 1 and Figure 1 for the description and location of sites.
The cluster analysis (Figure S5) highlighted two main groups of sites. The first group gathered sites weakly urbanized and located in rural zones, i.e., S05 to S08 (LIRRs) and S11 (mIRR). The second group consisted of sites located in more urbanized areas, with two sub-groups: S01 and S04 (sIRRs) on the one hand, and S02 and S03 (sIRRs) with S09 and S10 (mIRRs) on the other hand.
3.4. Taxonomic Structure of Stream Benthic Macroinvertebrate Assemblages
A total of 31,099 organisms were sorted and identified, distributed in 5 phyla, 8 classes, 25 orders and 98 families. Insects, the most diversified group (11 orders, 76 families and 177 taxa), represented 86.5% of the total abundance.
Among the most representative taxa, the genus Chironomus (Diptera) was found in almost all the sampling sites (except S05, S07 and S08), with a global occurrence frequency (OF) of 76%. The genus Coenagrion (Odonata) was also highly common (57%), and mainly found in S05 to S08, and S11. The genera Physa (Gastropoda), Caenis, Baetis (Ephemeroptera), Polymorphanisus (Trichoptera), Eurymetra, Rhagovelia, Limnogonus (Heteroptera), Orthetrum, Pseudagrion (Odonata), Dineutus (Coleoptera), and the families Lumbriculidae (Oligochaeta) and Simuliidae (Diptera) were rather commonly found (25% < OF < 50%). Significantly higher values of taxonomic richness and diversity (Shannon-Wiener index), evenness (Pielou index), EPT richness (mainly due to Ephemeroptera), 1-GOLD index and significantly lower values of Diptera richness seemed related to the LIRR status of sites (Table 4; Figure 5 and Figure S6 in Supplementary Material). Even if taxonomic richness of Ephemeroptera, Odonata, Heteroptera, Hirudinea and Gastropoda significantly varied among sites, it was much more difficult to relate these variations to clear differences in site status (Figure S6 in Supplementary Material).

Table 4.
Mean values (±standard deviation) of invertebrate-based metrics for the 11 sampling sites over the study period. Asterisks indicate significant differences among sites based on Friedman tests (*** p < 0.001; ** p < 0.01; * p < 0.05). Metrics exhibiting significant differences are those included in the taxonomy-based nPCA (see Section 3.4) and RDA (see Section 3.5). Richnesses were expressed in terms of relative frequencies (rf-S). See text for further details.
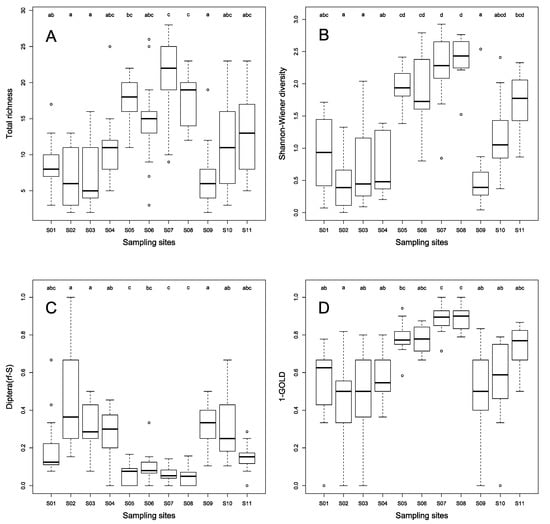
Figure 5.
Box-plots (Min/Q25/Median/Q75/Max) describing the distribution of values of taxonomic richness (A), Shannon-Wiener index (B), Diptera richness (Diptera(rf-S); in proportion of the total richness) (C) and 1-GOLD index (D) over a period of thirteen months, for 11 study sites (S01 to S11). «a», «b», «c» and «d» are distinct groups identified by post-hoc Nemenyi tests applied after identifying, for a given metric, a significant difference among sites by a non-parametric Friedman test. Outliers (open circles) are out of the 1.5 interquartile range. See text for further details.
The nPCA first factorial plane providing the ordination of site assemblages according to 17 taxonomic metrics (Table 2) explained 45.3% of the total inertia (Figure 6). Three groups of sites were identified. Along the first axis, S05-S08 and S11 (F1 < 0), exhibiting the highest values of Shannon-Wiener diversity, Pielou evenness, total and EPT Family richness, 1-GOLD, Heteroptera(rf-S) and Trichoptera(rf-S), were opposed to S01–S04 and S09 (F1 > 0), having the highest values of Diptera(rf-S) and Oligochaeta(rf-S). Axis F2 mainly opposed S10-S11 (F2 < 0), with the highest values of Ephemeroptera(rf-S), to S06 (F2 > 0), which exhibited a high proportion of Odonata.
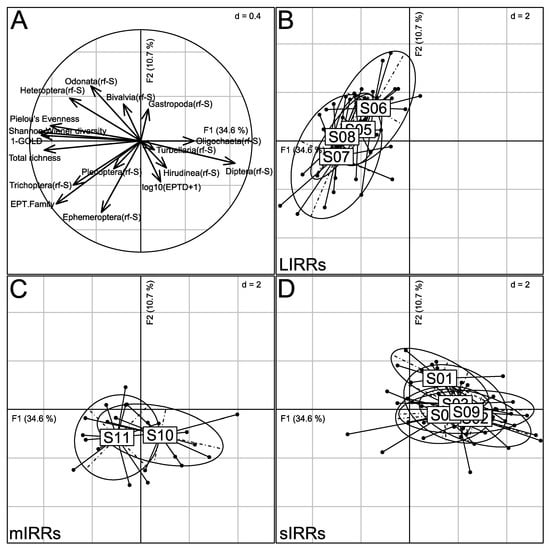
Figure 6.
Ordination of sites by nPCA, based on 17 taxonomy-based metrics. (A) Projection of taxonomic metrics on the first correlation circle; (B–D) Locations of sites (large labels, S01 to S11) on the first factorial plane at the weighted average of the locations of corresponding monthly sampling events (solid points). Each sampling event is linked by a line to the mean location of the corresponding site. Ellipses of inertia (95%) are provided by site. Sites are gathered according to anthropogenic pressure level: LIRRs = Least Impacted River Reaches; mIRRs = moderately Impacted River Reaches; sIRRs = severely Impacted River Reaches. See Table 1 and Figure 1 for the description and location of sites.
The cluster analysis based on taxonomic criteria (Figure S7) clearly separated sites with an expected LIRR status (i.e., S05 to S08) from severely (sIRRs; S01–S04 and S09) or moderately (mIRRs; S10–S11) impaired sites.
3.5. Relationships between Physico-Chemical Parameters and the Taxonomic Metrics of Benthic Macroinvertebrate Assemblages
The influence of physico-chemical parameters on the taxonomic metrics describing stream invertebrate assemblages was examined using redundancy analysis (RDA; Figure 7). The first two factorial axes accounted for 87.9% of total inertia, and ANOVA-like permutation tests showed that both the model and the first axis were significant (p-value(model) < 0.001; p-value(F1) < 0.001). The model explained 20.8% of the variance in taxonomic metric values (adjusted R2).
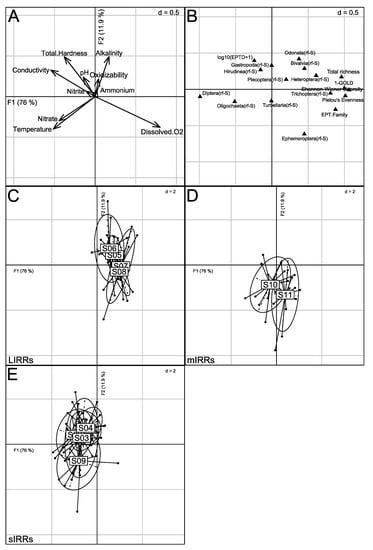
Figure 7.
Ordination of sites by Redundancy Analysis (RDA) based on 17 taxonomic metrics. Factorial axes are linear combinations of 10 physico-chemical parameters. (A) Canonical weights of physico-chemical parameters. Only the main parameters are labeled; (B) location of metrics on the first factorial plane; (C–E) locations of sites (large labels) on the first factorial plane at the weighted average of the locations of corresponding monthly sampling events (solid points). Each event is linked by a line to the mean location of the corresponding site. Labels corresponding to the mean locations of S01 and S02 are partially masked by S03 and S04 labels, respectively. Ellipses of inertia (95%) are provided by site. Sites are gathered according to anthropogenic pressure level: LIRRs = Least Impacted River Reaches; mIRRs = moderately Impacted River Reaches; sIRRs = severely Impacted River Reaches. See Table 1 and Figure 1 for the description and location of sites.
Three groups of sites were distinguished on the first factorial plane. Axis 1 opposed S05–S08 (F1 > 0) to S01–S04 (F1 < 0). S05–S08 exhibited mainly high water oxygenation. They were associated with the highest values of the Shannon-Wiener and Pielou indices, total richness, 1-GOLD index, Trichoptera(rf-S), Heteroptera(rf-S) and EPT Family richness (see also Table S2 in Supplementary Material). In contrast, S01-S04 were characterized by high mineralization and high nitrate load, and exhibited the highest values of Diptera(rf-S). Along the F2 axis, S09–S11 (F2 < 0) were characterized by the highest levels of temperature, nitrate concentration and dissolved oxygen concentration. S10 and S11 were associated with the highest proportion of Ephemeroptera(rf-S) and opposed mainly to S01, S04 and S06.
The cluster analysis based on the RDA results (Figure S8) clearly separated S01–S04 expected as severely impacted sites from other sites subdivided in two sub-groups. The first sub-group gathered sites expected as least impacted sites (i.e., S05–S08) and the second sub-group gathering sites expected as only moderately (S10–S11) or more severely impacted (S09) by anthropogenic activities.
3.6. The Discrimination Efficiency (DE) of the Metrics Describing Benthic Invertebrate Assemblages
The DE of the 19 calculated metrics ranged from 0.000 for Bivalvia(rf-S) and Plecoptera(rf-S) to 0.934 for Diptera(rf-S). Only 10 out of 19 metrics exhibited a DE value greater than 0.5, which are (in decreasing order): Diptera(rf-S) (0.934), Shannon-Wiener diversity (0.879), 1-GOLD (0.835), total richness (0.813), Heteroptera(rf-S) and Pielou evenness (0.802), Odonata(rf-S) (0.593), EPT Family and Ephemeroptera(rf-S) (0.527) and Oligochaeta(rf-S) (0.505). Among these 10 metrics, only Diptera(rf-S) and Oligochaeta(rf-S) increased with site degradation (Figure 8), as expected in Table 2.
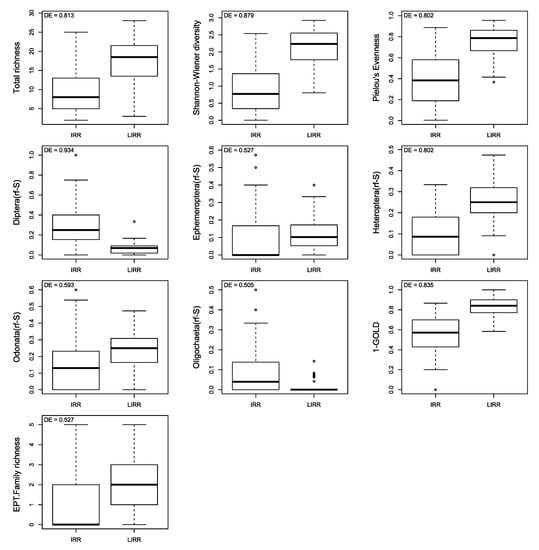
Figure 8.
Box-plots (Min/Q25/Median/Q75/Max) of the most discriminant (DE > 0.5) macroinvertebrate-based metrics in the LIRRs (Least Impacted River Reaches) and IRRs (moderately and severely Impacted River Reaches). Discrimination Efficiency (DE) values are given in the top-left part of each subplot. Outliers are out of the 1.5 interquartile range.
4. Discussion
4.1. Physico-Chemical Characteristics of Streams
Some river reaches of the streams in Mifi County (western region of Cameroon) are clearly impacted by anthropogenic activities occurring in or near the city of Bafoussam. The studied sites were mainly discriminated according to their degree of contamination by factory effluents and waste waters (both domestic/municipal/agricultural and industrial), cattle/poultry breeding and their location in either densely or sparsely populated areas.
Based on the pressure description (MCA in Section 3.1) and physico-chemical criteria (nPCA in Section 3.2), sites located on the Kena stream and its tributaries (S04 to S08) were globally distinguished from the other sites by better water quality, illustrated by significantly higher oxygen concentration and lower mineralization. In this catchment, only S04 exhibited poor water quality. Thus, S05 to S08 represented the less polluted and impacted reaches within the sampling design, and seemed to be good candidates for defining reference conditions for wadeable rivers in this area.
The upper reaches of the Famlem stream (S09), located in an urban area, were subjected to fertilized agriculture and various forms of domestic and commercial activities. In S10 and mainly S11, located in a rural area, the water quality increased due to decantation or sedimentation of matter on the stream bed and dilution of watershed inputs along the stream. The Nlem tributary (S01 to S03) was subjected to high anthropogenic pressure, mainly related to industrial and domestic effluents, which resulted in high values of conductivity and nutrient concentrations. These sites were located in the densely populated surroundings of Bafoussam City, where several industries were known to release wastewaters in the stream. Moreover, many domestic effluents (e.g., latrines in the riparian area [50]) were directly discharged into the stream, enriching it with nitrogen and organic matter. The IRR vs. LIRR status allocated to sites based on MCA results was confirmed by physico-chemical characterization for most of the sites. Only S11, considered as moderately impaired (mIRR) based on potential anthropogenic pressures, was gathered with LIRRs based on physico-chemical criteria (Figure S5).
The accuracy of this physico-chemical characterization indeed relies on the availability of data used to characterize anthropogenic pressures. One of the caveats of our characterization is that micropollutant concentrations were not measured due to the expensive cost of analyses. Data on water concentrations of pesticides are extremely rare in Cameroon. However, Galani et al. (2018, [71]) measured the residues of 99 pesticides in samples of 12 local agricultural products collected in the western highlands of Cameroon, including the Bafoussam region. They found in the Bafoussam region 18.2% of measured pesticide residue concentrations above the Maximum Residue Levels fixed by the European Union, including three fungicides (captan, hexachlorobenzene and matalaxyl), three herbicides (alachlor, chlorotoluron and terbuthylazine) and seven insecticides (aldrin, cypermethrin, dieldrin, malathion, methoxychlor, p,p’-DDT and γ-HCH), indicating that such micropollutants were used in the agricultural areas of the studied watersheds. They could therefore be present in some sections of the rivers of the Bafoussam region and should be taken into account in further studies.
4.2. Invertebrate-Based Metrics and Their Relationships with Physico-Chemical Parameters
Overall, the benthic fauna present in the different sites during this study exhibited a composition rather similar to that of invertebrate communities found in other African rivers [72,73]. Insects had the highest diversity in the three catchments, due to their high capacity to colonize various ecological niches with specific mesohabitat conditions [74,75,76]. Diptera, Coleoptera, Heteroptera, Ephemeroptera, Odonata (Insecta) and gastropods were the most abundant groups.
Site typologies provided by multivariate analyses based on physico-chemical parameters (nPCA, Figure S5), taxonomy-based metrics (nPCA, Figure S7) and taxonomy-based metrics related to physico-chemical parameters (RDA, Figure S8), were not similar. Sites moderately impacted by anthropogenic activities (mIRRs) were indeed gathered with least impacted (S11 based on physico-chemical criteria, Figure S5) or severely impacted (S11 based on taxonomic criteria, Figure S7) river reaches. However, relating taxonomy-based metrics to the physico-chemical parameters by RDA highlighted three groups of sites highly coherent with their expected ecological status (Figure S8).
The first group gathered weakly mineralized and well oxygenated sites (S05 to S08) from the Kena stream, located in rural areas, where anthropogenic pressures were low (e.g., low nutrient concentrations and conductivity). These sites hosted pollution-sensitive organisms (high EPT family richness, Shannon-Wiener diversity, Heteroptera(rf-S) and 1-GOLD values), highlighting their good water quality. Similarly, Qu et al. (2010, [77]), Rawson et al. (2010, [78]), Arimoro et al. (2010, [79]), Shelly et al. (2011, [80]), Myers et al. (2011, [81]) and Kaboré et al. (2016, [82]) identified higher abundance of pollution-sensitive insects in reaches with predominantly preserved habitats. Moreover, Duka et al. (2017, [83]) already observed a drastic decrease in the taxonomic richness of sensitive organisms with increasing watershed urbanization. In Cameroon, Menbohan et al. (2013, [41]) and Tchatcho et al. (2014, [84]) reported similar results in the peri-urban forested rivers of Yaoundé.
The second group, aggregated to the first one, was represented by the sites on the Famlem stream (S09–S11), with increasing water quality along the upstream/downstream longitudinal gradient explained by both the self-purifying capacity of the river and the dilution of anthropogenic inputs along the longitudinal gradient. Diptera was the most abundant group of organisms upstream (in S09, situated in the urban zone), while a higher taxonomic diversity was observed downstream due to partial recolonization of the lower reaches by more pollution-sensitive taxa. This pattern was confirmed by the high richness of Ephemeroptera at S11.
The third group included the sites located on the tributaries of the Nlem (S01 to S03) and Kena (S04) streams. These sites hosted mainly pollution-tolerant taxa (e.g., high Diptera(rf-S)), and can be considered as disturbed reaches with poor water quality, due to domestic and industrial waste contamination. Solid and liquid wastes were dumped into the streams, impairing water quality and favoring resistant organism proliferation (Diptera). Such pollution has already been associated with macroinvertebrate local extinctions and/or biodiversity decline [85]. Rawson et al. (2010, [78]) and Dar and Reshi (2014, [86]) also considered that urbanization-related variations in water physico-chemical characteristics reduce the abundance of sensitive taxa and promote the development of more tolerant organisms, including Oligochaeta (Lumbriculidae), Diptera and Gastropoda. Moreover, habitat degradation contributes in many cases to benthic mosaic homogenization. Mud was the dominant substrate in most of these stream sites, offering a very low diversity of potential ecological niches for benthic invertebrates. Similarly, Doretto et al. (2018, [87]) observed a reduction in taxonomic richness and abundance of macroinvertebrates (especially EPT) when a high level of siltation occurred in a stream reach.
4.3. Metrics of Interest for Future Biomonitoring Tool
The total taxonomic richness, EPT family richness, Shannon-Wiener diversity, Pielou evenness, 1-GOLD, Ephemeroptera(rf-S), Heteroptera(rf-S), Odonata(rf-S), Diptera(rf-S) and Oligochaeta(rf-S) were the ten metrics best responding to anthropogenic pressures commonly acting on streams of the West Cameroon region, based on their efficiency in the discrimination of LIRRs from IRRs (DE; Figure 8). Six of these metrics exhibited a DE value greater than 0.8, indicating their especially high capacity to discriminate IRRs from LIRRs. They can be ranked along a decreasing order of discrimination efficiency: Diptera(rf-S) > Shannon-Wiener diversity > 1-GOLD > total richness > Pielou evenness = Heteroptera(rf-S). These metrics should provide the best overview of the water quality of sampled sites, due also to their high relationship with some of the physico-chemical variables measured (Table S2). They could correspond to the best set of candidate taxonomic metrics for inclusion in an invertebrate-based multimetric bioassessment tool for the wadeable streams of the West Cameroon region. Following Mondy et al. (2012; [88]), reference values (i.e., values expected in reference conditions) and four ecological quality class boundaries (delimiting ‘high’, ‘good’, ‘moderate’, ‘poor’ and ‘bad’ classes) were defined for each of these metrics (the detailed procedure and corresponding values are provided in Table S3 in the Supplementary Material). Such values will help in the ecological diagnostics of river reaches in the Bafoussam region based on the taxonomic structure of macroinvertebrate assemblages, taking into account the original ecological information provided by each of these metrics.
The total taxonomic richness, Shannon–Wiener diversity and Pielou evenness describe the taxonomic characteristics of an assemblage driven by both habitat complexity and stability [88,89,90,91]. As expected (Table 2), the sites less exposed to anthropogenic pressures (S05 to S08) had far higher values of these three metrics than sites moderately and severely exposed (S10–S11 and S01–S04 + S09, respectively).
Based on EPT family richness, Tchatcho et al. (2014, [84]) and Kaboré et al. (2016, [82]) already efficiently monitored forest streams in the peri-urban area of Yaoundé (Cameroon) and streams in protected zones of Burkina Faso, respectively. This metric has been used worldwide for assessing the biological condition of streams [57] in North America [92], South America [93], Europe [94,95,96], Asia [97] and Australia [98], often in combination with Ephemeroptera(rf-S) [99,100]. However, this metric exhibited a rather low discrimination efficiency (DE = 0.527) in the Bafoussam region, probably due to the low family richness of the EPT assemblage (zero to five families), even in reference conditions.
Dias-Silva et al. (2010, [101]) and Vieira et al. (2014, [102]) already considered Heteroptera as indicators of good water quality in Brazilian streams because they are sensitive to the anthropogenic alteration of the riparian aquatic vegetation that would provide them food and offer shelter against predators or during heavy rains. Tchakonté et al. (2015, [42]) concluded similarly based on the invertebrate assemblages of forest streams of Douala (Cameroon). We confirmed the high efficiency of this metric in the discrimination of LIRRs from IRRs in the Bafoussam region (DE > 0.8).
1-GOLD and Diptera(rf-S) were two metrics best reacting to stream degradation (DE = 0.835 and 0.934, respectively). The 1-GOLD metric gathers information from Gastropoda, Oligochaeta and Diptera: three groups of rather pollution-tolerant organisms. Gastropoda and Oligochaeta were involved in the multimetric index evaluating the ecological quality of drainage ditch systems in the Netherlands [103] or siliceous river systems in Portugal [104], and in the identification of metal contamination of soft sediments in French and Swiss rivers [105,106,107]. Diptera, dominated by Chironomidae (especially Chironomus), were found in almost all the sites. This wide distribution can be explained by their ubiquitous traits and their capacity to rapidly colonize new environments. Moreover, Chironomus’s ability to capture in water and carry dissolved oxygen with hemolymphic hemoglobin gives it a high physiological tolerance even in environments with very low oxygen concentration [28,108,109]. This collector-filterer is already considered as highly tolerant to pollution in many African tropical streams [110,111], as are the Oligochaeta [41].
Odonata, generally exhibiting a long period of larval development, are good witnesses of river habitat quality and stability, and are extremely diverse in tropical areas [112]. Odonata(rf-S) can provide a reliable measure of anthropogenic impact. In particular, disturbance of the riparian vegetation has a direct effect on the structure of the adult dragonfly community, potentially impairing larval populations [113]. Miguel et al. (2017, [114]) and De Oliveira-Junior et al. (2017, [115]) noticed their high sensitivity to the degradation of riparian vegetation, potentially altered by the local use of streams by riverine human populations.
4.4. Seasonal Effect on Macroinvertebrate-Based Metrics
Our results demonstrate only a moderate influence of “season” on invertebrate-based metric variations in the studied streams of West Cameroon. Even though Tonkin et al. (2017, [116]) and Dalu et al. (2017, [117]) pointed out the driving force of seasonality on stream community dynamics, its effects on invertebrate assemblages of West Cameroon rivers seem to be modulated by the high predictability of the successive hydrological events (rainy season vs. dry season). However, such events can lead to some structural and compositional changes within benthic communities (e.g., a decrease in taxonomic richness and total abundance) via the physical disturbance of bottom substrates due to faster current velocity and higher water level in the rainy season [118]. This rather moderate effect of season on aquatic invertebrate community structure may be explained also by the absence of strong between-season variation in the physico-chemical characteristics of water. If Masese et al. (2014, [111]) found a high influence of season on the structural and functional organization of macroinvertebrate assemblages in Kenyan highland streams (due to the exacerbation of differences in water quality and habitat characteristics), a moderate effect of season was already pointed out by Tonkin et al. (2016, [119]) and Dalu et al. (2017, [120]) in Afrotropical Nigerian streams and in an austral South African stream, respectively. This rather low influence of seasonality on the taxonomic structure of invertebrate assemblages should facilitate the development of a future biotic index for West Cameroon rivers.
5. Conclusions
In conclusion, human activities in and around streams (farming, vegetation clearance, sand extraction, bathing, laundry, riparian culture, and effluent discharge from households and industries) drastically reduce the within-stream invertebrate biodiversity in the West Region of Cameroon. An urgent need for the conservation and wise use of the watercourses in this area was highlighted. We identified the main relationships between pressure-sensitive invertebrate-based metrics and physico-chemical characteristics of streams. This identification will facilitate the selection of the scale at which efforts for protection and/or rehabilitation should be directed to best improve and/or maintain sustainable ecological conditions in West Cameroon streams. Invertebrate-based biomonitoring of these streams seems very promising, several taxonomic metrics clearly varying according to the intensity of human impacts. Diptera(rf-S), Shannon-Wiener diversity, 1-GOLD, total richness, Pielou evenness and Heteroptera(rf-S) seem the most promising metrics for identifying a significant anthropogenic impairment. The selection of these best candidate metrics is the first significant step towards the construction of an invertebrate-based multimetric index efficiently assessing the ecological status of streams in this region.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w14091490/s1, Figure S1: Ordination of sites by a “within-season” nPCA based on 17 taxonomy-based metrics. (A) Projection of taxonomic metrics on the first correlation circle (F1-F2); (B, C and D) Locations of sites (large labels) on the first factorial plane F1-F2 at the weighted average of the locations of corresponding monthly sampling events (solid points). Each event is linked by a line to the mean position of the corresponding site. Ellipses of inertia (95%) are provided by site. Sites are gathered according to anthropogenic pressure level; Figure S2: Ordination of sites by a «within-season» nPCA, based on 10 physico-chemical parameters. (A) Projection of physico-chemical parameters on the first correlation circle (F1-F2); (B, C and D) Locations of sites (large labels) on the first factorial plane at the weighted average of the locations of corresponding monthly sampling events (solid points); Figure S3: Ordination of impact levels (three categories: low/moderate/high) of eight pressure categories by Multiple Correspondence Analysis (MCA) based on the description of 11 study sites; Figure S4: Box-plots (Min/Q25/Median/Q75/Max) describing the distribution of values of the remaining physico-chemical parameters exhibiting significant differences among sites, over a period of thirteen months, for 11 study sites (S01 to S11). « a », « b », « c » and « d » are distinct groups identified by post-hoc Nemenyi tests applied after identifying, for a given parameter, a significant difference among sites by a non-parametric Friedman test; Figure S5: Dendrogram, based on the Euclidean distance (calculated between sites according to their coordinates on the first factorial plane of the nPCA) and the complete linkage method, highlighting the similarity of the 11 study sites (S01 to S11) considering their physico-chemical characteristics during the study period; Figure S6: Box-plots (Min/Q25/Median/Q75/Max) describing the distribution of values of the remaining taxonomy-based metrics exhibiting significant differences among sites, over a period of thirteen months, for 11 study sites (S01 to S11). “a”, “b”, “c” and “d” are distinct groups identified by post-hoc Nemenyi tests applied after identifying, for a given parameter, a significant difference among sites by a non-parametric Friedman test; Figure S7: Dendrogram, based on the Euclidean distance (calculated between sites according to their coordinates on the first factorial plane of the nPCA) and the complete linkage method, highlighting the similarity of the 11 study sites (S01 to S11) considering the structure of their macroinvertebrate assemblage described by 17 taxonomy-based metrics; Figure S8: Dendrogram, based on the Euclidean distance and the complete linkage method applied to sampling events coordinates on the first factorial plane of the RDA, highlighting the similarity of the 11 study sites (S01 to S11) based on the structure of their macroinvertebrate assemblage described by 17 taxonomy-based metrics explained by 10 physico-chemical variables; Table S1: Semi-quantitative description of eight categories of anthropogenic pressures for 11 sampling sites (S01-S11) in three catchments of the Bafoussam region (West Cameroon). The potential intensity of each pressure category has been described using three modalities: 1 = low, 2 = moderate and 3 = high. The eight pressure categories are related to the importance of “urban” areas, “food crop”, “cash crop”, “cattle and poultry” breeding, untreated “domestic, municipal and agricultural” or “industrial” wastewater effluents, riverine “factories” and “civil engineering and mining” at reach scale; Table S2: Correlations (Spearman’s rho coefficient) between the 19 tested macroinvertebrate-based metrics and the environmental parameters; Table S3: Proposed reference values and ecological quality class boundaries for the ten metrics exhibiting significantly different ranges of values between LIRRs and IRRs in wadeable rivers of the Bafoussam Region. Ecological class boundaries have been defined following Mondy et al. (2012; [88]). As recommended by the WFD, ecological quality class boundaries (i.e. delimiting ‘high’, ‘good’, ‘moderate’, ‘poor’ and ‘bad’ classes) have been defined based on the distribution of each metric values from the LIRR data set. For each metric decreasing with increasing anthropogenic pressures (i.e., all the metrics except Diptera(rf-S) and Oligochaeta(rf-S); cf. Figure 8), the 95th, 75th and 25th percentiles of the metric value distribution in the LIRRs were calculated. These values have been considered as the reference value, the ‘high–good’ boundary and the ‘good–moderate’ boundary, respectively. For the two metrics increasing with increasing anthropogenic pressures (i.e. Diptera(rf-S) and Oligochaeta(rf-S)), the 5th, 25th and 75th percentiles of the metric value distribution in the LIRRs were considered as the reference value, the ‘high–good’ boundary and the ‘good–moderate’ boundary, respectively. For defining the ‘moderate–poor’ and the ‘poor–bad’ boundaries, we divided the metric scoring range between the minimal possible value (decreasing metric) or the maximal possible value (increasing metric) and the ‘good–moderate’ boundary in three equal classes.
Author Contributions
J.K.F.: conceptualization, data acquisition, data analysis, writing—original draft and validation; S.F.M.: data acquisition, writing—original draft and validation; A.M.: conceptualization, methodology, data analysis, writing—original draft and validation; A.L.: writing—original draft and validation; P.U.-P.: conceptualization, methodology, data analysis, writing—original draft and validation. All authors have read and agreed to the published version of the manuscript.
Funding
This study was made possible by the funding accorded to Josephine Kengne Fotsing by the mobility program Knowledge, Integration and Transparency in Education (KITE) Scholarship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Contact the correspondence author for data.
Acknowledgments
We would like to thank all the staff of the “Hydrobiology and Environment” Laboratory of the Université of Yaoundé 1 in Cameroon, especially Eric Biram and Donald Nyame, for their help during the sampling campaigns. We would like also to thank Serge H. Zébaze Togouet, Gideon A. Ajeagah and Roger Feumba for giving us the possibility of carrying out the physico-chemical survey of sites by lending their measuring devices.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Townsend, C.R.; Uhlmann, S.S.; Matthaei, C.D. Individual and combined responses of stream ecosystems to multiple stressors. J. Appl. Ecol. 2008, 45, 1810–1819. [Google Scholar] [CrossRef]
- Ormerod, S.J.; Dobson, M.; Hildrew, A.G.; Townsend, C.R. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010, 55, 1–4. [Google Scholar] [CrossRef]
- Nõges, P.; Argillier, C.; Borja, A.; Garmendia, J.M.; Hanganu, J.; Kodes, V.; Pletterbauer, F.; Sagouis, A.; Birk, S. Quantified biotic and abiotic responses to multiple stress in freshwater, marine and ground waters. Sci. Total Environ. 2015, 540, 43–52. [Google Scholar] [CrossRef] [PubMed]
- De Castro, D.M.P.; de Carvalho, D.R.; dos Santos Pompeu, P.; Moreira, M.Z.; Nardoto, G.B.; Callisto, M. Land use influences niche size and the assimilation of resources by benthic macroinvertebrates in tropical headwater streams. PLoS ONE 2016, 11, e0150527. [Google Scholar]
- Christensen, M.R.; Graham, M.D.; Vinebrooke, R.D.; Findlay, D.L.; Paterson, M.J.; Turner, M.A. Multiple anthropogenic stressors cause ecological surprises in boreal lakes. Glob. Chang. Biol. 2006, 12, 2316–2322. [Google Scholar] [CrossRef]
- Sirombra, M.G.; Mesa, L.M. A method for assessing the ecological quality of riparian forests in subtropical Andean streams: QBRy index. Ecol. Indic. 2012, 20, 324–331. [Google Scholar] [CrossRef]
- Taniwaki, R.H.; Piggott, J.J.; Ferraz, S.F.; Matthaei, C.D. Climate change and multiple stressors in small tropical streams. Hydrobiologia 2017, 793, 41–53. [Google Scholar] [CrossRef]
- Meyer, J.L.; Paul, M.J.; Taulbee, W.K. Stream ecosystem function in urbanizing landscapes. J. N. Am. Benthol. Soc. 2005, 24, 602–612. [Google Scholar] [CrossRef]
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M.; Morgan II, R.P. The urban stream syndrome: Current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Cuffney, T.F.; Brightbill, R.A.; May, J.T.; Waite, I.R. Responses of benthic macroinvertebrates to environmental changes associated with urbanization in nine metropolitan areas. Ecol. Appl. 2010, 20, 1384–1401. [Google Scholar] [CrossRef]
- Holland-Clift, S.; O’Dowd, D.J.; Mac Nally, R. Impacts of an invasive willow (Salix × rubens) on riparian bird assemblages in south-eastern Australia. Austral Ecol. 2011, 36, 511–520. [Google Scholar] [CrossRef]
- Kouam Kenmogne, G.R.; Rosillon, F.; Nono, A.; Nzeukou Nzeugang, A.; Mpakam, H.G. Les maladies hydriques à l’épreuve de la gestion des ressources en eau dans une zone urbaine d’un pays en développement: Cas de la ville de Yaoundé (Centre-Cameroun). Eur. J. Water Qual. 2011, 42, 35–49. [Google Scholar] [CrossRef]
- Aazami, J.; Esmaili-Sari, A.; Abdoli, A.; Sohrabi, H.; Van den Brink, P.J. Monitoring and assessment of water health quality in the Tajan River, Iran using physicochemical, fish and macroinvertebrates indices. J. Environ. Health Sci. Eng. 2015, 13, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Aweng, E.R.; Ismid, M.S.; Maketab, M. The effect of land uses on physicochemical water quality at three rivers in Sungai Endau watershed, Kluang, Johor, Malaysia. Aust. J. Basic Appl. Sci. 2011, 5, 923–932. [Google Scholar]
- Zarei, H.; Pourreza Bilondi, M. Factor analysis of chemical composition in the Karoon River basin, southwest of Iran. Appl. Water Sci. 2013, 3, 753–761. [Google Scholar] [CrossRef]
- Bere, T.; Dalu, T.; Mwedzi, T. Detecting the impact of heavy metal contaminated sediment on benthic macroinvertebrate communities in tropical streams. Sci. Total Environ. 2016, 572, 147–156. [Google Scholar] [CrossRef]
- Unyimadu, J.P.; Osibanjo, O.; Babayemi, J.O. Selected persistent organic pollutants (POPs) in water of River Niger: Occurrence and distribution. Environ. Monit. Assess. 2018, 190, 6. [Google Scholar] [CrossRef]
- Heatherly, T.; Whiles, M.R.; Royer, T.V.; David, M.B. Relationships between water quality, habitat quality, and macroinvertebrate assemblages in Illinois streams. J. Environ. Qual. 2007, 36, 1653–1660. [Google Scholar] [CrossRef]
- Deborde, D.D.D.; Hernandez, M.B.M.; Magbanua, F.S. Benthic macroinvertebrate community as an indicator of stream health: The effects of land use on stream benthic macroinvertebrates. Sci. Diliman 2016, 28, 5–26. [Google Scholar]
- Borisko, J.P.; Kilgour, B.; Stanfield, L.W.; Jones, F.C. An evaluation of rapid bioassessment protocols for stream benthic invertebrates in Southern Ontario, Canada. Water Qual. Res. J. Can. 2007, 42, 184–193. [Google Scholar] [CrossRef]
- Buss, D.F.; Vitorino, A.S. Rapid bioassessment protocols using benthic macroinvertebrates in Brazil: Evaluation of taxonomic sufficiency. J. N. Am. Benthol. Soc. 2010, 29, 562–571. [Google Scholar] [CrossRef]
- Gupta, M.; Paliwal, A. Role of aquatic insects of water quality in related to physico-chemical parameters in Yamuna River at district Firozabad (U.P.). Adv. Biores. 2010, 1, 70–73. [Google Scholar]
- Mackintosh, T.J.; Davis, J.A.; Thompson, R.M. The influence of urbanisation on macroinvertebrate biodiversity in constructed stormwater wetlands. Sci. Total Environ. 2015, 536, 527–537. [Google Scholar] [CrossRef]
- Mermillod-Blondin, F. The functional significance of bioturbation and biodeposition on biogeochemical processes at the water–sediment interface in freshwater and marine ecosystems. J. N. Am. Benthol. Soc. 2011, 30, 770–778. [Google Scholar] [CrossRef]
- Mereta, S.T.; Boets, P.; Bayih, A.A.; Malu, A.; Ephrem, Z.; Sisay, A.; Endale, H.; Yitbarek, M.; Jemal, A.; De Meester, L.; et al. Analysis of environmental factors determining the abundance and diversity of macroinvertebrate taxa in natural wetlands of Southwest Ethiopia. Ecol. Inform. 2012, 7, 52–61. [Google Scholar] [CrossRef]
- Floury, M.; Usseglio-Polatera, P.; Férréol, M.; Delattre, C.; Souchon, Y. Global climate change in large European rivers: Long-term effects on macroinvertebrate communities and potential local confounding factors. Glob. Chang. Biol. 2013, 19, 1085–1099. [Google Scholar] [CrossRef]
- Friberg, N.; Bonada, N.; Bradley, D.C.; Dunbar, M.J.; Edwards, F.K.; Grey, J.; Hayes, R.B.; Hildrew, A.G.; Lamouroux, N.; Trimmer, M.; et al. Biomonitoring of human impacts in freshwater ecosystems: The good, the bad and the ugly. Adv. Ecol. Res. 2011, 44, 1–68. [Google Scholar]
- Colas, F.; Archaimbault, V.; Férard, J.-F.; Bouquerel, J.; Roger, M.-C.; Devin, S. Benthic indicators of sediment quality associated with run-of-river reservoirs. Hydrobiologia 2013, 703, 149–164. [Google Scholar]
- Ferreira, A.; Cyrino, J.E.P.; Duarte-Neto, P.J.; Martinelli, L.A. Permeability of riparian forest strips in agricultural, small subtropical watersheds in south-eastern Brazil. Mar. Freshw. Res. 2013, 63, 1272–1282. [Google Scholar] [CrossRef]
- Dallas, H.F.; Rivers-Moore, N. Ecological consequences of global climate change for freshwater ecosystems in South Africa. S. Afr. J. Sci. 2014, 110, 1–11. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Z.; Duan, X.; Pan, B. Effects of pollution on macroinvertebrates and water quality bio-assessment. Hydrobiologia 2014, 729, 247–259. [Google Scholar] [CrossRef]
- Graeber, D.; Jensen, T.M.; Rasmussen, J.J.; Riis, T.; Wiberg-Larsen, P.; Baattrup-Pedersen, A. Multiple stress response of lowland stream benthic macroinvertebrates depends on habitat type. Sci. Total Environ. 2017, 599–600, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Tafangenyasha, C.; Dzinomwa, T. Land-use impacts on river water quality in lowveld sand river systems in south-east Zimbabwe. Land Use Water Resour. Res. 2005, 5, 3.1–3.10. [Google Scholar]
- Adandedjan, D.; Lalèyè, P.; Ouattara, A.; Gourene, G. Distribution of benthic insect fauna in a West African lagoon: The Porto-Novo Lagoon in Bénin. Asian J. Biol. Sci. 2011, 4, 116–127. [Google Scholar] [CrossRef][Green Version]
- Kilonzo, F.; Masese, F.O.; Van Griensven, A.; Bauwens, W.; Obando, J.; Lens, P.N.L. Spatial–temporal variability in water quality and macro-invertebrate assemblages in the Upper Mara River basin, Kenya. Phys. Chem. Earth Parts A/B/C 2014, 67–69, 93–104. [Google Scholar] [CrossRef]
- Mutonkole Senga, P.; Tshitenge Mbuebue, J.-M.; Masamba Lulendo, N. Benthic macroinvertebrates as indicators of water quality: A case-study of urban Funa stream (in Kinshasa, Democratic Republic of Congo). Open J. Water Pollut. Treat. 2015, 2, 8–24. [Google Scholar]
- Onana, F.M.; Zébazé Togouet, S.H.; Koji, E.; Nyamsi Tchatcho, N.L.; Tchakonté, S. Influence of municipal and industrial pollution on the diversity and the structure of benthic macro-invertebrates community of an urban river in Douala, Cameroon. J. Biodivers. Environ. Sci. 2016, 8, 120–133. [Google Scholar]
- Koji, E.; Noah Ewoti, O.V.; Onana, F.M.; Tchakonté, S.; Lontsi Djimeli, C.; Tamsa Arfao, A.; Bricheux, G.; Sime-Ngando, T.; Nola, M. Influence of anthropogenic pollution on the abundance dynamics of some freshwater invertebrates in the coastal area of Cameroon. J. Environ. Prot. 2017, 8, 810–829. [Google Scholar] [CrossRef]
- Edia, O.E.; Bony, K.Y.; Konan, K.F.; Ouattara, A.; Gourène, G. Distribution of aquatic insects among four costal river habitats (Côte d’Ivoire, West-Africa). Bull. Env. Pharmacol. Life Sci. 2013, 2, 68–77. [Google Scholar]
- Kaboré, I.; Moog, O.; Alp, M.; Guenda, W.; Koblinger, T.; Mano, K.; Ouéda, A.; Ouédraogo, R.; Trauner, D.; Melcher, A.H. Using macroinvertebrates for ecosystem health assessment in semi-arid streams of Burkina Faso. Hydrobiologia 2016, 766, 57–74. [Google Scholar] [CrossRef]
- Foto Menbohan, S.; Tchakonté, S.; Ajeagah, G.A.; Zébazé Togouet, S.H.; Bilong Bilong, C.F.; Njiné, T. Water quality assessment using benthic macroinvertebrates in a peri-urban stream (Cameroon). Int. J. Biotechnol. 2013, 2, 91–104. [Google Scholar]
- Tchakonté, S.; Ajeagah, G.A.; Camara, A.I.; Diomandé, D.; Nyamsi Tchatcho, N.L.; Ngassam, P. Impact of urbanization on aquatic insect assemblages in the coastal zone of Cameroon: The use of biotraits and indicator taxa to assess environmental pollution. Hydrobiologia 2015, 755, 123–144. [Google Scholar] [CrossRef]
- Heino, J.; Virkkala, R.; Toivonen, H. Climate change and freshwater biodiversity: Detected patterns, future trends and adaptations in northern regions. Biol. Rev. 2009, 84, 39–54. [Google Scholar] [CrossRef]
- Poulton, B.C.; Graham, J.L.; Rasmussen, T.J.; Stone, M.L. Responses of macroinvertebrate community metrics to a wastewater discharge in the Upper Blue River of Kansas and Missouri, USA. J. Water Resour. Prot. 2015, 7, 1195–1220. [Google Scholar] [CrossRef]
- Parent-Raoult, C.; Boisson, J.C. Impacts des rejets urbains de temps de pluie (RUTP) sur les milieux aquatiques: État des connaissances. Rev. Sci. Eau 2007, 20, 229–239. [Google Scholar]
- Olivry, J.C. Fleuves et Rivières du Cameroun; Edition Mesres-Orstom: Paris, France, 1986; 733p. [Google Scholar]
- Abdi, H.; Valentin, D. Multiple Correspondence Analysis. In Encyclopedia of Measurement and Statistics; Salkind, N., Ed.; Sage: Thousand Oaks, CA, USA, 2007. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011; 319p. [Google Scholar]
- Stark, J.D.; Boothroyd, K.G.; Harding, J.S.; Maxted, J.R.; Scarsbrook, M.R. Protocols for Sampling Macroinvertebrates in Wadeable Streams. New Zealand Macro-Invertebrates Working Group, Report No.1, Ministry for the Environment and Sustainable Management, Fund Project n° 5103. 2001. Available online: https://riversgroup.org.nz/wp-content/uploads/2018/06/4.1.3-macroinvertebrate-sampling.pdf (accessed on 5 September 2015).
- Mpakam, H.G. Water Resource Vulnerability to Pollution in the Bafoussam Region (West Cameroon) and Socio-Economic and Health Effects: Sanitation Procedures. Ph.D. Thesis, University of Yaoundé I, Yaoundé, Cameroon, 2009. [Google Scholar]
- Durand, J.R.; Lévêque, C. Flore et Faune Aquatiques de l’Afrique Sahélo-Soudanienne. Tome II; Edition de l’ORSTOM: Paris, France, 1981; 517p. [Google Scholar]
- Day, J.A.; Harrison, A.D.; de Moor, I.J. Guides to the freshwater invertebrates of Southern Africa, Volume 9: Diptera. In Water Research Commission Report; No. TT 201/02; Water Research Commission: Pretoria, South Africa, 2002; 210p. [Google Scholar]
- De Moor, I.J.; Day, J.A. Guides to the freshwater invertebrates of Southern Africa, Volume 6: Arachnida & Mollusca. Chapter 3: Mollusca. In Water Research Commission Report; No. TT 182/02; Water Research Commission: Pretoria, South Africa, 2002; pp. 42–125. [Google Scholar]
- De Moor, I.J.; Day, J.A.; De Moor, F.C. Guides to the freshwater invertebrates of Southern Africa, Volume 7: Insecta I. Ephemeroptera, Odonata & Plecoptera. In Water Research Commission Report; No. TT 207/03; Water Research Commission: Pretoria, South Africa, 2003; 301p. [Google Scholar]
- De Moor, I.J.; Day, J.A.; De Moor, F.C. Guides to the freshwater invertebrates of Southern Africa, Volume 8: Insecta II. Hemiptera, Megaloptera, Neuroptera, Trichoptera & Lepidoptera. In Water Research Commission Report; No. TT 214/03; Water Research Commission: Pretoria, South Africa, 2003; 219p. [Google Scholar]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés d’Eau Douce. Systématique, Biologie, Écologie; CNRS Éditions: Paris, France, 2010; 600p. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish (Volume 339); US Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999.
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Feld, C.K.; de Bello, F.; Dolédec, S. Biodiversity of traits and species both show weak responses to hydromorphological alteration in lowland river macroinvertebrates. Freshw. Biol. 2014, 59, 233–248. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Buffagni, A.; Erba, S.; Cazzola, M.; Murray-Bligh, J.; Soszka, H.; Genoni, P. The STAR common metrics approach to the WFD intercalibration process: Full application for small, lowland rivers in three European countries. Hydrobiologia 2006, 566, 379–399. [Google Scholar] [CrossRef]
- APHA Standard Method for Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998; 1150p.
- Rodier, J.; Legube, B.; Marlet, N.; Brunet, R. L’Analyse de l’Eau, 9th ed.; Dunod: Paris, France, 2009; 1579p. [Google Scholar]
- Siegel, S.; Castellan, N.J. Non Parametric Statistics for the Behavioral Sciences; McGraw-Hill: New York, NY, USA, 1988; 399p. [Google Scholar]
- Dolédec, S.; Chessel, D. Rythmes saisonniers et composantes stationnelles en milieu aquatique. II. Prise en compte et élimination d’effets dans un tableau faunistique. Acta Oecol. Oecol. Gen. 1989, 10, 207–232. [Google Scholar]
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Chevenet, F.; Dolédec, S.; Chessel, D. A fuzzy coding approach for the analysis of long-term ecological data. Freshw. Biol. 1994, 31, 295–309. [Google Scholar] [CrossRef]
- Galani, J.H.; Houbraken, M.; Wumbei, A.; Djeugap, J.F.; Fotio, D.; Spanoghe, P. Evaluation of 99 pesticide residues in major agricultural products from the Western Highlands Zone of Cameroon using QuEChERS method extraction and LC-MS/MS and GC-ECD analyses. Foods 2018, 7, 184. [Google Scholar] [CrossRef]
- Oscoz, J.; Galicia, D.; Miranda, R. (Eds.) Identification Guide of Freshwater Macroinvertebrates of Spain; Springer: Dordrecht, The Netherlands, 2011; 45p. [Google Scholar]
- Morrissey, C.A.; Boldt, A.; Mapstone, A.; Newton, J.; Ormerod, S.J. Stable isotopes as indicators of wastewater effects on the macroinvertebrates of urban rivers. Hydrobiologia 2013, 700, 231–244. [Google Scholar] [CrossRef]
- Varandas, S.G.; Cortes, R.M.V. Evaluating macroinvertebrate biological metrics for ecological assessment of streams in northern Portugal. Environ. Monit. Assess. 2010, 166, 201–221. [Google Scholar] [CrossRef]
- Hepp, L.U.; Restello, R.M.; Milesi, S.V.; Biasi, C.; Molozzi, J. Distribution of aquatic insects in urban headwater streams. Acta Limnol. Bras. 2013, 25, 1–9. [Google Scholar] [CrossRef]
- Prommi, T.; Payakka, A. Aquatic insect biodiversity and water quality parameters of streams in Northern Thailand. Sains Malays. 2015, 44, 707–717. [Google Scholar] [CrossRef]
- Qu, X.; Wu, N.; Tang, T.; Cai, Q.; Park, Y.S. Effects of heavy metals on benthic macroinvertebrate communities in high mountain streams. Ann. Limnol. 2010, 46, 291–302. [Google Scholar] [CrossRef]
- Rawson, C.A.; Lim, R.P.; Tremblay, L.A.; Warne, M.S.J.; Ying, G.G.; Laginestra, E.; Chapman, J.C. Benthic macroinvertebrate assemblages in remediated wetlands around Sydney, Australia. Ecotoxicology 2010, 19, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Arimoro, F.O.; Muller, W.J. Mayfly (Insecta: Ephemeroptera) community structure as an indicator of the ecological status of a stream in the Niger Delta area of Nigeria. Environ. Monit. Assess. 2010, 166, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Shelly, S.Y.; Mirza, Z.B.; Bashir, S. Comparative ecological study of aquatic macroinvertebrates of Mangla dam and Chashma barrage wetland areas. J. Anim. Plant Sci. 2011, 21, 340–350. [Google Scholar]
- Myers, L.W.; Kondratieff, B.C.; Mihuc, T.B.; Ruiter, D.E. The mayflies (Ephemeroptera), stoneflies (Plecoptera), and caddisflies (Trichoptera) of the Adirondack Park (New York State). Trans. Am. Entomol. Soc. 2011, 137, 63–140. [Google Scholar] [CrossRef]
- Kaboré, I.; Ouédraogo, I.; Tampo, L.; Ouéda, A.; Moog, O.; Guenda, W.; Melcher, A.H. Composition and dynamic of benthic macroinvertebrates community in semi-arid area rivers of Burkina Faso (West Africa). Int. J. Biol. Chem. Sci. 2016, 10, 1542–1561. [Google Scholar] [CrossRef]
- Duka, S.; Pepa, B.; Keci, E.; Paparisto, A.; Lazo, P. Biomonitoring of water quality of the Osumi, Devolli, and Shkumbini rivers through benthic macroinvertebrates and chemical parameters. J. Environ. Sci. Health Part A 2017, 52, 471–478. [Google Scholar] [CrossRef]
- Nyamsi Tchatcho, N.L.; Foto Menbohan, S.; Zébazé Togouet, S.H.; Onana, F.M.; Adandedjan, D.; Tchakonté, S.; Yémélé Tsago, C.; Koji, E.; Njiné, T. Indice multimétrique des macroinvertébrés benthiques yaoundéens (IMMY) pour l’évaluation biologique de la qualité des eaux des cours d’eau de la région du Centre Sud Forestier du Cameroun. Eur. J. Sci. Res. 2014, 123, 412–430. [Google Scholar]
- Cook, S.E.; Fisher, M.J.; Andersson, M.S.; Rubiano, J.; Giordano, M. Water, food and livelihoods in river basins. Water Int. 2009, 34, 13–29. [Google Scholar] [CrossRef]
- Dar, P.A.; Reshi, Z.A. Components, processes and consequences of biotic homogenization: A review. Contemp. Probl. Ecol. 2014, 7, 123–136. [Google Scholar] [CrossRef]
- Doretto, A.; Piano, E.; Bona, F.; Fenoglio, S. How to assess the impact of fine sediments on the macroinvertebrate communities of alpine streams? A selection of the best metrics. Ecol. Indic. 2018, 84, 60–69. [Google Scholar] [CrossRef]
- Mondy, C.P.; Villeneuve, B.; Archaimbault, V.; Usseglio-Polatera, P. A new macroinvertebrate-based multimetric index (I2M2) to evaluate ecological quality of French wadeable streams fulfilling the WFD demands: A taxonomical and trait approach. Ecol. Indic. 2012, 18, 452–467. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. New ‘biodiversity’ measures reveal a decrease in taxonomic distinctness with increasing stress. Mar. Ecol. Prog. Ser. 1995, 129, 301–305. [Google Scholar] [CrossRef]
- Böhmer, J.; Rawer-Jost, C.; Zenker, A. Multimetric assessment of data provided by water managers from Germany: Assessment of several different types of stressors with macrozoobenthos communities. Hydrobiologia 2004, 516, 215–228. [Google Scholar] [CrossRef]
- Dedieu, N.; Clavier, S.; Vigouroux, R.; Cerdan, P.; Céréghino, R. A multimetric macroinvertebrate index for the implementation of the European Water Framework Directive in French Guiana, East Amazonia. River Res. Appl. 2016, 32, 501–515. [Google Scholar] [CrossRef]
- Stoddard, J.L.; Herlihy, A.T.; Peck, D.V.; Hughes, R.M.; Whittier, T.R.; Tarquinio, E. A process for creating multimetric indices for large-scale aquatic surveys. J. N. Am. Benthol. Soc. 2008, 27, 878–891. [Google Scholar] [CrossRef]
- Pereira, P.S.; Souza, N.F.; Baptista, D.F.; Oliveira, J.L.M.; Buss, D.F. Incorporating natural variability in the bioassessment of stream condition in the Atlantic Forest biome, Brazil. Ecol. Indic. 2016, 69, 606–616. [Google Scholar] [CrossRef]
- Brabec, K.; Zahrádková, S.; Nĕmejcová, D.; Pařil, P.; Kokeš, J.; Jarkovský, J. Assessment of organic pollution effect considering differences between lotic and lentic stream habitats. Hydrobiologia 2004, 516, 331–346. [Google Scholar] [CrossRef]
- Ofenböck, T.; Moog, O.; Gerritsen, J.; Barbour, M. A stressor specific multimetric approach for monitoring running waters in Austria using benthic macroinvertebrates. Hydrobiologia 2004, 516, 251–268. [Google Scholar] [CrossRef]
- Hering, D.; Feld, C.K.; Moog, O.; Ofenböck, T. Cook book for the development of a multimetic index for biological condition of aquatic ecosystems: Experiences from the European AQEM and STAR projects and related initiatives. Hydrobiologia 2006, 566, 311–324. [Google Scholar] [CrossRef]
- Chen, K.; Hughes, R.M.; Xu, S.; Zhang, J.; Cai, D.; Wang, B. Evaluating performance of macroinvertebrate-based adjusted and unadjusted multi-metric indices (MMI) using multi-season and multi-year samples. Ecol. Indic. 2014, 36, 142–151. [Google Scholar] [CrossRef]
- Chessman, B.C.; Thurtell, L.A.; Royal, M.J. Bioassessment in a harsh environment: A comparison of macroinvertebrate assemblages at reference and assessment sites in an Australian inland river system. Environ. Monit. Assess. 2006, 119, 303–330. [Google Scholar] [CrossRef]
- Pond, G.J. Biodiversity loss in Appalachian headwater streams (Kentucky, USA): Plecoptera and Trichoptera communities. Hydrobiologia 2012, 679, 97–117. [Google Scholar] [CrossRef]
- Buffagni, A.; Erba, S.; Cazzola, M.; Kemp, J.L. The AQEM multimetric system for the southern Italian Apennines: Assessing the impact of water quality and habitat degradation on pool macroinvertebrates in Mediterranean rivers. Hydrobiologia 2004, 516, 313–329. [Google Scholar] [CrossRef]
- Dias-Silva, K.; Cabette, H.S.R.; Juen, L.; De Marco, P., Jr. The influence of habitat integrity and physical-chemical water variables on the structure of aquatic and semi-aquatic Heteroptera. Zoologia (Curitiba) 2010, 27, 918–930. [Google Scholar] [CrossRef]
- Vieira, T.B.; Dias-Silva, K.; Pacífico, E.S. Effects of riparian vegetation integrity on fish and Heteroptera communities. Appl. Ecol. Environ. Res. 2014, 13, 53–65. [Google Scholar]
- Verdonschot, R.C.M.; Keizer-Vlek, H.E.; Verdonschot, P.F.M. Development of a multimetric index based on macroinvertebrates for drainage ditch networks in agricultural areas. Ecol. Indic. 2012, 13, 232–242. [Google Scholar] [CrossRef]
- Pinto, P.; Rosado, J.; Morais, M.; Antunes, I. Assessment methodology for southern siliceous basins in Portugal. Hydrobiologia 2004, 516, 191–214. [Google Scholar] [CrossRef]
- Lafont, M.; Camus, J.C.; Rosso, A. Superficial and hyporheic oligochaete communities as indicators of pollution and water exchange in the River Moselle, France. Hydrobiologia 1996, 334, 147–155. [Google Scholar] [CrossRef]
- Prygiel, J.; Rosso-Darmet, A.; Laffont, M.; Lesniak, C.; Durbec, A.; Ouddane, B. Use of oligochaete communities for assessment of ecotoxicological risk in fine sediment of rivers and canals of the Artois-Picardie water basin (France). Hydrobiologia 1999, 410, 25–37. [Google Scholar] [CrossRef]
- Vivien, R.; Tixier, G.; Lafont, M. Use of oligochaete communities for assessing the quality of sediments in watercourses of the Geneva area (Switzerland) and Artois-Picardie basin (France): Proposition of heavy metal toxicity thresholds. Ecohydrol. Hydrobiol. 2014, 14, 142–151. [Google Scholar] [CrossRef]
- Failla, A.J.; Vasquez, A.A.; Fujimoto, M.; Ram, J.L. The ecological, economic and public health impacts of nuisance chironomids and their potential as aquatic invaders. Aquat. Invasions 2015, 10, 1–15. [Google Scholar] [CrossRef]
- Machado, N.G.; Nassarden, D.C.S.; dos Santos, F.; Boaventura, I.C.G.; Perrier, G.; de Souza, F.S.C.; de Lima Martins, E.; Biudes, M.S. Chironomus larvae (Chironomidae: Diptera) as water quality indicators along an environmental gradient in a neotropical urban stream. Rev. Ambient. Agua 2015, 10, 298–309. [Google Scholar] [CrossRef]
- Odume, O.N.; Muller, W.J. Diversity and structure of Chironomidae communities in relation to water quality differences in the Swartkops River. Phys. Chem. Earth Parts A/B/C 2011, 36, 929–938. [Google Scholar] [CrossRef]
- Masese, F.O.; Kitaka, N.; Kipkemboi, J.; Gettel, G.M.; Irvine, K.; McClain, M.E. Macroinvertebrate functional feeding groups in Kenyan highland streams: Evidence for a diverse shredder guild. Freshw. Sci. 2014, 33, 435–450. [Google Scholar] [CrossRef]
- Kalkman, V.J.; Clausnitzer, V.; Dijkstra, K.D.B.; Orr, A.G.; Paulson, D.R.; van Tol, J. Global diversity of dragonflies (Odonata) in freshwater. Hydrobiologia 2008, 595, 351–363. [Google Scholar] [CrossRef]
- Samways, M.J.; Steytler, N.S. Dragonfly (Odonata) distribution patterns in urban and forest landscapes, and recommendations for riparian management. Biol. Conserv. 1996, 78, 279–288. [Google Scholar] [CrossRef]
- Miguel, T.B.; Oliveira-Junior, J.M.B.; Ligeiro, R.; Juen, L. Odonata (Insecta) as a tool for the biomonitoring of environmental quality. Ecol. Indic. 2017, 81, 555–566. [Google Scholar] [CrossRef]
- De Oliveira Junior, J.M.B.; Shimano, Y.; Gardner, T.A.; Hughes, R.M.; Marco Júnior, P.; Juen, L. Neotropical dragonflies (Insecta: Odonata) as indicators of ecological condition of small streams in the eastern Amazon. Austral Ecol. 2015, 40, 733–744. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Bogan, M.T.; Bonada, N.; Rios-Touma, B.; Lytle, D.A. Seasonality and predictability shape temporal species diversity. Ecology 2017, 98, 1201–1216. [Google Scholar] [CrossRef]
- Dalu, T.; Wasserman, R.J.; Tonkin, J.D.; Mwedzi, T.; Magoro, M.L.; Weyl, O.L.F. Water or sediment? Partitioning the role of water column and sediment chemistry as drivers of macroinvertebrate communities in an austral South African stream. Sci. Total Environ. 2017, 607–608, 317–325. [Google Scholar] [CrossRef]
- Callisto, M.; Goulart, M.; Medeiros, A.O.; Moreno, P.; Rosa, C.A. Diversity assessment of benthic macroinvertebrates, yeasts, and microbiological indicators along a longitudinal gradient in Serra Do Cipó, Brazil. Braz. J. Biol. 2004, 64, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Tonkin, J.D.; Arimoro, F.O.; Haase, P. Exploring stream communities in a tropical biodiversity hotspot: Biodiversity, regional occupancy, niche characteristics and environmental correlates. Biodivers. Conserv. 2016, 25, 975–993. [Google Scholar] [CrossRef]
- Dalu, T.; Wasserman, R.J.; Tonkin, J.D.; Alexander, M.E.; Dalu, M.T.B.; Motitsoe, S.N.; Manungo, K.I.; Bepe, O.; Dube, T. Assessing drivers of benthic macroinvertebrate community structure in African highlands: An exploration using multivariate analysis. Sci. Total Environ. 2017, 601–602, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).