Abstract
To improve business performance and achieve sustainable development through the concept of hot spring resource reuse, this study investigated the antibacterial effect of alginate-coated tea tree essential oil microcapsules and the effect of alginate microcapsules on the release of tea tree essential oil. The results revealed that 450 μm alginate/tea tree essential oil microcapsules (containing 720 ppm of tea tree essential oil) prepared using microfluidic assemblies effectively inhibited total bacteria, Escherichia coli, and Staphylococcus aureus in hot spring water. For alginate/tea tree essential oil microcapsules prepared under different conditions, at a fixed concentration of cross-linking reagents, the release time increased with the cross-linking time (10 min > 5 min > 1 min). At a fixed cross-linking time, the release time increased with the concentrations of cross-linking reagents (1 M > 0.5 M > 0.1 M). When the concentrations of cross-linking reagents and the cross-linking time were the same, the release time of cross-linking reagents increased with the strength of metal activity (Ca > Zn).
1. Introduction
Water is essential to sustain life on earth and is used for drinking and irrigation purposes. Moreover, water regulates body temperature in humans [1]. Water is available in diverse forms. Hot spring resources can be divided into hot, cold, mud, and submarine according to their temperature, type, and landform [2]. Hot springs are a destination for tourism and leisure and recreational activities. In addition, hot spring resources have medical treatment, agriculture, geothermal system, and biotechnology applications [3]. Therefore, hot springs have become a crucial resource for the development of the tourism industry worldwide [4].
Surrounded by seas, Taiwan has abundant geothermal resources, including 18 sulfur, sulfuric acid, sodium bicarbonate, salt mud, and mineral-free hot spring areas [5]. Before the COVID-19 pandemic, these hot spring areas attracted a steady stream of visitors. According to official statistics, 6,063,000 tourists visited hot spring areas in Taiwan in 2021, thus providing substantial business opportunities and effectively promoting the development of local tourism-related industries and the urban economy [6]. Thus, hot spring water resources are crucial for decision making related to tourism in Taiwan and local economic development.
Total hot spring water resources in all areas are not unlimited. The government and firms collect, use, store, and transport water from hot spring sources or dig wells to exploit hot spring sources [4,6]. However, hot spring sources should be strictly protected because factors such as artificial development and overuse can adversely affect landforms and water quality, cause pollution, and reduce water temperature, thus decreasing the quality of hot springs [7,8]. Moreover, because of the COVID-19 pandemic, the demand for safe public areas and high-quality hot spring water has gradually increased [9]. In addition, although hot spring water resources are limited, numerous individuals bathe in hot springs; thus, changing the water in hot spring pools at any time is impossible. Because of repeated use by abundant tourists, unchanged hot spring water resources are likely to promote the growth of various bacteria, such as Escherichia coli, which can affect the health and safety of individuals using these hot springs [4,10]. Therefore, maintaining the quality of hot spring water, solving the problem of bacterial growth, and developing sustainable methods to use and manage hot spring water resources are necessary.
The goal of the sustainable management of hot spring water resources is to maintain their safety by using bactericidal methods that do not increase the redox potential of spring water, do not change the water quality of hot springs, are inexpensive, and exert a strong antibacterial effect [11]. However, effective bactericidal methodologies are not yet available, and the quantitative relationship between various bactericidal methods and pollution in hot spring water remains uncertain. In addition, in hot spring pools, a positive result should not be obtained if five tubes of 10 mL of E. coli samples are cultured in every 100 mL of water, and the total plate count should not exceed 500 per mL after culture for 24 h at 37 °C [12]. The bactericidal effect differs between raw hot spring pool water and circulated filtered water [13]. In this study, we attempted to develop a bactericidal method that can meet health management standards, is suitable for sustainable water resource management, and can maintain spring water quality without changing water temperature.
1.1. Microcapsule Technology
Research on microcapsule technology began in the United States in 1940; however, it was used from the 1950s after commercial product availability and patent applications [14,15]. Capsules refer to protective capsule-like containers formed by filling fine, coated materials, such as nuclei and core materials, into one or more skin or shell walls to isolate them from the external environment [16]. Capsules are prepared using physicochemical, chemical, and mechanical methods. Natural or synthetic polymer materials are typically used to prepare shells, and highly dispersed nuclear substances are used as coating materials. The particle size ranges between 1 μm and 100 mm [17]. Low-cost polymer materials that easily form films are typically used for shells. Such shell materials should be more stable than nuclear substances, should not cause decomposition or side reactions, and should be able to surround nuclear substances, such as dispersed fine solid or liquid, and polymerize to form strong capsules [18]. Moreover, the oil-soluble liquid protoplasm is often used as the nuclear substance. If the solid protoplasm is used as the fine coating material, an appropriate solvent can be added according to dissolution characteristics to liquidize it and fully mix it with oily shell material monomers. This process can improve the reaction rate and is favorable for the interface contact for the polymerization reaction and the formation of high-quality capsules [17,18]. Therefore, encapsulated materials exhibit improved physical properties, stability, and compatibility; demonstrate reduced photosensitivity; and are isolated from air [15]. Microcapsules are widely used in many industrial products.
1.2. Capsule Preparation Technology and Dru-Release System
Capsules can be prepared using physicochemical, chemical, and mechanical methods; among them, interfacial condensation polymerization and in situ condensation polymerization are the most commonly used [19]. Capsules with different coated states and particle size distributions can be prepared through interfacial condensation polymerization and in situ condensation polymerization by using synthetic polymer monomers as raw materials and by controlling formulation and operating conditions [20].
Various polymer compounds can be used as shell materials. The selected polymers should not react with nuclear substances chemically and should be stable, permeable, tough, and low-cost. The polymers polyamide, polyester, and cellulose are commonly used as shell materials, and different polymer materials can form shell layers of different materials [19,20].
Controlled-release drug-carrier materials are beneficial for disease treatment [21]. Controlled-release drugs are transmitted to the target point regularly and quantitatively. Thus, reduced drug decomposition during the controlled transmission process avoids the loss of drugs, increasing drug efficacy and preventing drug decomposition due to high temperature [22]. Therefore, capsule preparation should be conducted according to the drug-release system (Figure 1).
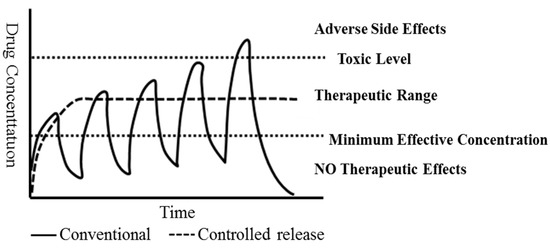
Figure 1.
Drug of controlled release and dosage of tradition.
1.3. Sodium Alginate, Chitosan, and Tea Tree Essential Oil
Sodium alginate, a natural polysaccharide substance extracted from seaweeds, can cross-link with divalent cations and can be concentrated in solutions and form gels and films [23]. Therefore, sodium alginate is widely used for immobilization in foods, medical dressings, drug carriers, heavy metal adsorption, and biological enzymes and is employed as a liquid homogenizer and thickener in the food industry [24,25]. Furthermore, calcium alginate exhibits high biocompatibility and moisture absorption capacity; it can easily remove substances and adsorb heavy metals. Thus, calcium alginate is suitable for coating living organisms to prevent their harm under special environmental conditions or when transferring matter into living organisms.
Chitosan, a positive cation, electrostatically interacts with anions on the surface of bacteria, changes the permeability of bacterial cell membranes, causes abnormal access of materials in and out of cells, and exerts an antibacterial effect. Moreover, chitosan promotes the synthesis of inducible nitric oxide synthase (iNOS) by macrophages to produce nitric oxide (NO) [26], enabling macrophages to engulf bacteria and attack cancer cells [27] and exerting antibacterial effects. In particular, chitosan is the most effective in controlling the growth of Staphylococcus aureus and Salmonella. Chitosan with an MIC50 value of > 5mg/mL exerted the strongest inhibitory effect on E. coli, Pseudomonas aeruginosa, S. aureus, Candida albicans, Streptococci, and S. enteritidis [28].
Tea tree, also known as Melaleuca alternifolia, originates from Australia and belongs to the family Myrtaceae. Tea tree oil is composed of terpenes, mainly monoterpenes, sesquiterpenes, and related alcohols, which are volatile aromatic hydrocarbons. The antibacterial activity of tea tree oil is mainly attributed to terpinene-4-ol, the main component in the essential oil. Tea tree essential oil has poor hydrophilicity. At a high concentration, tea tree oil exerts a bactericidal effect and can inhibit tumor necrosis factor [29]; thus, tea tree oil can be used as a natural bactericide. Moreover, tea tree oil exerts antiviral effects and can inhibit mold growth, stimulate leukocyte proliferation, increase immune cell activity, enhance immunity, prevent infection, inhibit infection spread, promote wound healing, relieve pain and discomfort, and reduce inflammation [29,30,31]. Tea tree oil is often used for anti-inflammatory purposes in surgery and dental operations [32,33] and is widely used in aromatherapy [34].
Because of the antibacterial and anti-inflammatory properties of chitosan, polycaprolactone nonwoven mats (PCLNM) prepared using tea tree essential oil (TTO) exhibit anti-inflammatory and bactericidal effects [35,36,37]. After being injected into capsules and placed in water at 50 °C, the time required for the release of tea tree essential oil is similar to that at 70 °C–90 °C [38]. Therefore, sodium alginate, chitosan, and tea tree essential oil can eliminate pollutants from used hot spring water and thus achieve the goal of the sustainable management of hot spring water.
In summary, microcapsules prepared using sodium alginate, chitosan, and tea tree essential oil exert a bactericidal effect without causing any side effects to humans. Thus, they can be used for water resource management to achieve the sustainable management of hot spring water resources.
2. Materials and Methods
2.1. Drugs, Experiment Equipment, and Instruments
This research used Ultraviolet-Visible Spectrophotometer (DU800); Syringe Pump (kd Scientific 200 Series, Scientific Hightek Co., Taipei, Taiwan); Inverted microscope (Leica DM IL LED, Major Instruments Co., Taipei, Taiwan); Syringe (50 mL, TERUMO, Taipei, Taiwan); Needle (18Gx1 1/2″(1.20 × 38 mm), TERUMO, Taipei, Taiwan); Polyethylene Tubing (PE190, I.D. 1.19 mm (0.047″), O.D. 1.70 mm (0.067″)), INTRAMEDIC CLAY ADAMS Brand, Hondwen Co., Taipei, Taiwan); pipette tip (0.1–10 μL) (I.D. 0.47 mm, O.D. 0.91 mm), 1–10 μL (I.D. 0.42 mm, O.D. 0.667 mm); pipette tip (10–200 μL); and other instruments for experimental tests and microcapsule production experiments.
Moreover, it used eight kinds of chemicals (including sodium alginate) produced in the United States, Japan, Belgium, Australia, Taiwan, and other countries as the main raw materials for experiments. Details on the raw materials, sources, and places of origin are shown in Table 1.

Table 1.
Introduction of sources and places of origin of experimental materials and instruments.
2.2. Experimental Procedure—Preparation of Composite Alginate/Chitosan Microcapsules
Two-tube or three-tube microfluidic devices can use different arrangements and combinations to fabricate single-droplet and double-droplet microfluidic elements. Alternatively, the diameter of the pipette tip material can be changed to achieve any desired microfluidic element [39]. Therefore, we use the characteristics of this device to separate and configure the elements.
The experimenter used the two-tube microfluidic device to mix the alginate and the aqueous solution of chitosan. In the injection tube, the injection of the isooctane oil transfer tube was used to cut the mixed aqueous solution of alginate and chitosan to form water-in-oil forms. Finally, they entered the aqueous solution of curing agents (CaCl2, ZnCl2) to form composite microcapsules. In order to reduce experimental variables, the composite microcapsules were controlled at 450 μm with the use of the microfluidic technology (the flow rate of the injection tube was fixed at 100 μL/min, and the flow rate of the transfer tube was fixed at 1000 μL/min).
The microfluidic device consisted of two polypropylene pipette tips. The microfluidic body was arranged in a coaxial alignment, and the tips of the two pipette tips were 1.5 cm apart. The plastic tube (I.D. 1.19 mm) was connected to the outer tube and fixed on the glass plate. Furthermore, the pipette tips (I.D. 0.47 mm, O.D. 0.91 mm) were used for sealing and fixation, as shown in Figure 2.
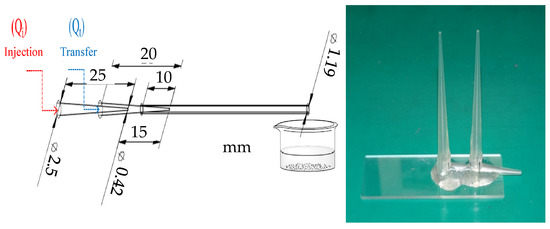
Figure 2.
Two-tube microfluidic device (left) chart, (right) entity picture.
The 1.5% alginate (containing 2% chitosan) was injected into the two-tube microfluidic device through the injection tube by the microsyringe, and isooctane was injected into the two-tube microfluidic device using the transfer tube. After that, the flow rate of the injection tube was fixed at 100 μL/min, and the flow rate of the transfer tube was fixed at 1000 μL/min. The alginate mixture was cut into droplets by isooctane and flowed into the aqueous solution of curing agents (CaCl2, ZnCl2) to form alginate/chitosan microcapsules through curing. In the process of suction and filtration, deionized water was used for cleaning to obtain composite wet pellets of alginate/chitosan. Finally, they were vacuumized and dried to obtain composite microcapsules of alginate/chitosan. The diameter of the composite microcapsules of alginate/chitosan was about 450 μm, as shown in Figure 3.
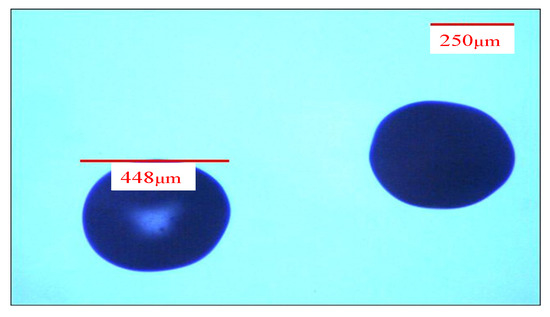
Figure 3.
Microcapsules of alginate/chitosan.
2.3. Preparation of Composite Microcapsules of Alginate/Tea Tree Essential Oil
Sodium dodecyl sulfate (SDS) was added to make the tea tree essential oil evenly mixed with the alginate. The three-tube microfluidic device was used to prepare composite microcapsules, and an additional layer of alginate was added to block the release of tea tree essential oil during curing. Then, the injection of the isooctane oil transfer tube was used to cut the composite aqueous solution of alginate and tea tree essential oil to form water-in-oil forms. Finally, they entered the aqueous solution of curing agents (CaCl2, ZnCl2) to form composite microcapsules. In order to reduce experimental variables, the composite microcapsules were controlled at 450 μm with the use of the microfluidic technology (with the flow rate of the injection tube 1 fixed at 100 μL/min, the flow rate of the injection tube 2 fixed at 100 μL/min, and the flow rate of the transfer tube fixed at 1000 μL/min).
The microfluidic device consisted of 3 polypropylene pipette tips. The microfluidic body was arranged in a coaxial alignment, and the tips of the 3 pipette tips were 1.5 cm apart. The plastic tube (I.D. 1.19 mm) was connected to the outer tube and fixed on the glass plate. Furthermore, the pipette tips (I.D. 0.47 mm, O.D. 0.91 mm) were used for sealing and fixation, as shown in Figure 4.
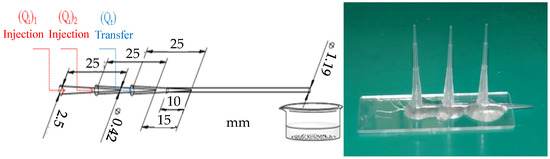
Figure 4.
Three-tube microfluidic device (left) chart, (right) entity picture.
Next, 70 g (1%) alginate aqueous solution, 0.2 g sodium dodecyl sulfate, and 30 g tea tree essential oil were mixed evenly. This aqueous solution was injected into the three-tube microfluidic device through injection tube 1 using the microsyringe. Then, the (1%) alginate aqueous solution was injected into the three-tube microfluidic device through injection tube 2 using the microsyringe. Isooctane was injected into the three-tube microfluidic device through the transfer tube using the microsyringe. The flow rate of injection tube 1 was fixed at 100 μL/min, the flow rate of injection tube 2 was fixed at 100 μL/min, and the flow rate of the transfer tube was fixed at 1000 μL/min. At this time, the fluid in injection tube 1 and the fluid in injection tube 2 flowed in a laminar flow mode, and they were cut into droplets by isooctane. Then, they flowed into the aqueous solution of curing agents (CaCl2, ZnCl2) for curing to form composite microcapsules of alginate/tea tree essential oil. Finally, in the process of suction and filtration, deionized water was used for cleaning to obtain composite microcapsule wet pellets of alginate/tea tree essential oil. The diameter of the composite microcapsules of alginate/tea tree essential oil was about 450 μm, as shown in Figure 5.
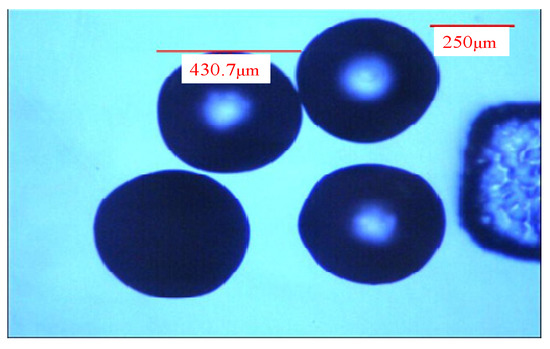
Figure 5.
Microcapsules of alginate/tea tree essential oil.
2.3.1. Establishment of the Calibration Curve of Tea Tree Essential Oil
In the UV-visible spectrum, it can be found that tea tree essential oil has an obvious characteristic peak of absorption at λ = 268 nm, as shown in Figure 6. This characteristic peak of absorption was used to make a decline curve for 0–2500 ppm, as shown in Figure 7.
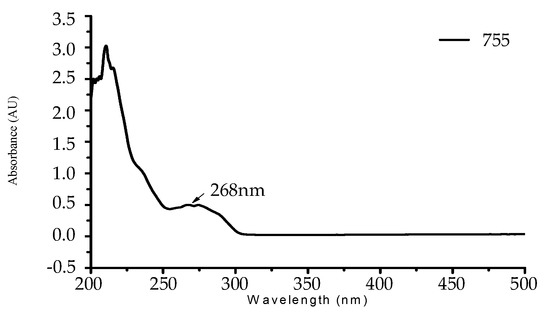
Figure 6.
The UV-visible spectrum of tea tree essential oil.
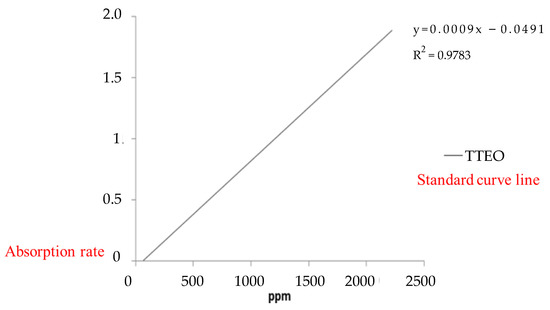
Figure 7.
The calibration curve of tea tree essential oil by UV-visible spectroscopy.
2.3.2. Release of Composite Microcapsules of Alginate/Tea Tree Essential Oil
Tea tree essential oil is a volatile substance. However, in order to meet the properties of “not increasing its redox potential”, “not changing the water quality of hot springs”, and “having a high efficiency in antibacterial effect”, tea tree essential oil is safer and more effective than the commonly used chlorine compounds. In order to conform to the form of hot spring water in the open space, the open-system release experiment was performed [40,41]. It can be seen that in the open-system environment, the essential oil in the water began to volatilize into the air, and the release amount was gradually stable. At this time, the release rate was basically the same as the volatilization rate. However, after the fourth hour, the release curve in the open system gradually decreased, indicating that the release rate at this time was smaller than the volatilization rate. In the closed-system environment, since the essential oil did not evaporate out of the system, although the release amount continued to increase after 1.5 h, the release balance was basically reached after the third hour. From this test, it could be seen that the volatilization and release effect of the essential oil may be affected by the environmental system. Therefore, the environment may be a limiting or influencing factor of the experiment, as shown in Figure 8.
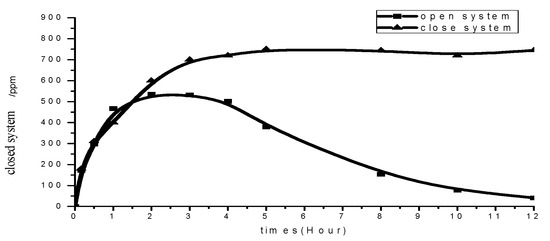
Figure 8.
The essential oil released from microcapsules of alginate/tea tree essential oil in opened and closed system.
Then, an appropriate amount of the mixed solution of alginate/tea tree essential oil was taken to replicate the microcapsules. They were put into 1 L of sodium bicarbonate hot spring water at 45 °C with a stirring speed of 100 rpm. Samples were periodically measured at 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5 h, respectively. The absorbance was analyzed with the Ultraviolet-Visible Spectrophotometer, and the released amount was converted and interpreted according to the standard curve. In addition, two factors were designed to test a total of 18 (2 × 3 × 3) experiments to explore the effect of cross-linking agent type, cross-linking agent concentration, and cross-linking time on the release time of the essential oil. As shown in Table 2.

Table 2.
Factors and variables in this study.
2.3.3. FTIR
Figure 9 presents the FT-IR results of calcium alginate composite microspheres. Figure 3 shows that the characteristic peaks of Ca-alginate at 1400 cm−1 and 1600 cm−1 are weaker than those of alginate, mainly because the cross-linking of calcium forms a –C–O–Ca–O–C– group structure. This indicates that the sodium ion (Na+) of sodium alginate is exchanged with the calcium ion (Ca2+), and the carboxyl group and Ca2+ are cross-linked to form a network molecule. The characteristic peak of alginate–chitosan at 1600 cm−1 appears wider and stronger, indicating that the carboxyl and amine groups are cross-linked to form a network molecule.
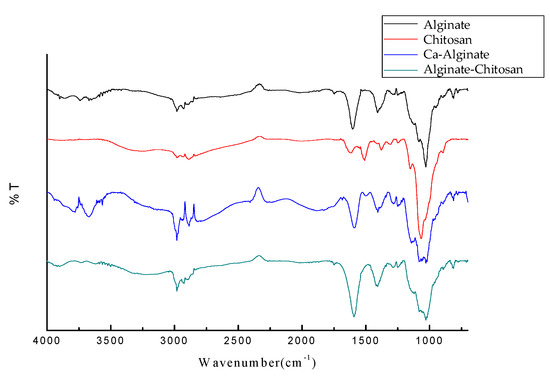
Figure 9.
FT-IR spectrum of sodium alginate, chitosan, and calcium alginate composite microspheres.
In the figure, the characteristic peak of alginate–chitosan at 1600 cm−1 appears wider and stronger, indicating that the carboxyl and amine groups are cross-linked to form a network molecule. As shown in Table 3.

Table 3.
Changes in functional groups at different wavenumbers in FT-IR spectra.
2.3.4. TGA
Figure 10 shows the TGA results of calcium alginate composite microspheres. It is known from Table 4 that the thermal cracking temperature of alginate–chitosan is 314 °C when the weight loss is 30%, which is 14 °C higher than that of Ca-alginate. It indicated that adding chitosan could increase the thermal properties of calcium alginate composite microspheres. Figure 4 shows that the TGA loss of alginate–chitosan is 38%, and that of Ca-alginate is 31% at 800 °C. It means that alginate–chitosan has a less thermogravimetric loss, proving that the connection between amine groups and calcium ions leads to more inorganic substances.
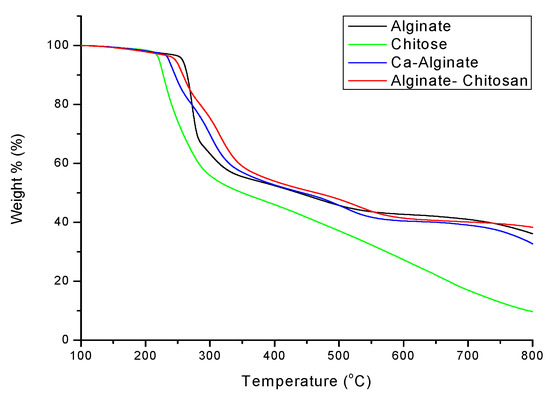
Figure 10.
TGA profile of sodium alginate, chitosan, and calcium alginate composite microspheres.

Table 4.
Thermal cracking temperature (Td) of TGA spectrum.
2.3.5. DSC
Figure 11 shows the DSC results of calcium alginate composite microspheres. Table 5 shows that the melting point temperature of Ca-alginate and alginate–chitosan is similar. Figure 11 shows that the alginate–chitosan peak is wider than the Ca-alginate peak, resulting from the cross-linking of chitosan and calcified alginate.
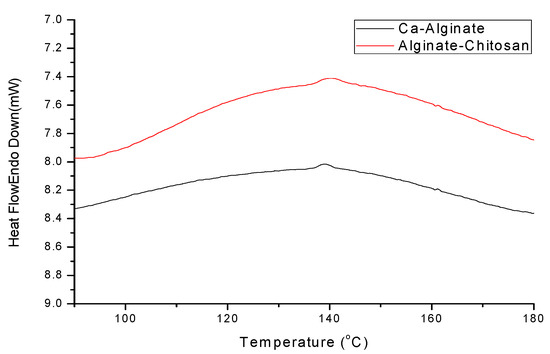
Figure 11.
DSC pattern of calcium alginate composite microspheres.

Table 5.
Melting temperature (Tm) of DSC spectrum.
3. Analysis Methods
3.1. Instrumental Analysis
With the use of the Inverted Stereomicroscope (Leica DM IL LED, Wetzlar, Germany), the object to be observed directly was placed directly under the microscope objective. The Ultraviolet-Visible Spectrophotometer (HITACHI U-3900 Spectrophotometer type, Sano, Japan) was used to detect the situation where the electrons in the orbitals were excited to generate transitions after the solvent was placed into the square quartz tube and the sample slot of the instrument, and then, the desired spectrum was obtained.
3.2. Bacterial Culture, Detection, and Analysis
The pre-work was conducted by disinfecting the sterile bench with ethanol and irradiating it with UV light for 1 h before the operation. After each use, the bench was cleaned and covered with a dust cloth to avoid contamination. Microbiological testing instruments were sterilized in a sterilizer before each usage. After use, the reusable instruments were collected, washed, and dried with the cleaning agent with a disinfecting effect after the inspection to prevent the spread of contaminants.
In the operation steps, the sterilization process of equipment and medicines was completed. After labeling the Petri dishes, we began microbiological testing. TTC (2,3,5-Triphenyl tetrazolium chloride) was added to PCA (Plate count agar) and mixed well. The PCA with TTC was poured into the Petri dish. After solidification, 9 mL of MLB (Modified Letheen broth) was pipetted into the test tube for use. Before the test tube was used, a slight cauterization was performed at the mouth of the Bunsen burner. The bottle was cauterized with a slight rotation to remove the internal moisture. After adding MLB, we sprayed the sample with 75% ethanol and placed it on the operating table.
After the sample was well-mixed with MLB, 0.1 mL was pipetted into the Petri dish and shaken slightly to mix well. After the culture substrate had absorbed the dilution, the surface of the Petri dish substrate was smeared with a smear stick to cause the sample dilution to evenly adhere to the surface. Finally, the Petri dish was put into the bag and then put into an oven at 33–35 °C for 48 h. Finally, the calculation and analysis were carried out according to the theoretical standard and the actual test data (addition 30%, release rate 47%). The calculation formula is as follows:
Q actual: the amount actually released in the hot spring water.
Q theory: the theoretical amount released in hot spring water.
η: release rate.
4. Results
4.1. Antibacterial Test of Composite Microcapsules
In the experiment, the unused hot spring water (uncontaminated) and the used hot spring water (contaminated) were tested, including the total bacteria count, as well as tests of mold, Escherichia coli, and Staphylococcus aureus. The composite microcapsules were added to the used (contaminated) hot spring water for antibacterial testing. Alginate composite microcapsules disintegrate in the presence of sodium ions at pH > 7 [42]. Therefore, our study will not consider discussing the state where the SDS content is less than 0.1%.
4.1.1. Strain Test and Antibacterial Test of Composite Microcapsules in Unused (Uncontaminated) Hot Spring Water
The alginate/tea tree essential oil microcapsules and alginate/chitosan microcapsules were put into the unused hot spring water, respectively, to test the antibacterial ability. Through the test, it can be seen that the hot spring water experienced an antibacterial effect after adding alginate/tea tree essential oil microcapsules, and the release amount of tea tree essential oil was 0.7 mg/mL, which was greater than the minimum antibacterial dose of 0.08 mg/mL. In contrast, the addition of alginate/chitosan microcapsules to hot spring water had no antibacterial effect. As shown in Table 6.

Table 6.
The antibacterial effect of unused hot spring water by the microcapsules.
4.1.2. Strain Test and Antibacterial Test of Composite Microcapsules in Used (Contaminated) Hot Spring Water
The test of used hot spring water showed that the total number of bacterial colonies was greater than 250 CFU/g, the number of Escherichia coli colonies was 50 CFU/g, and the number of Staphylococcus aureus colonies was 8 CFU/g, indicating that the used hot spring water had been contaminated with Escherichia coli and Staphylococcus aureus. Then, the used hot spring water was applied as the matrix of the polluted water to add composite microcapsules for the antibacterial test.
It was added to 50 g sterile water of alginate/tea tree essential oil microcapsules, mixed well, and placed for 4 h before testing. The test results showed that the total bacterial colonies of the alginate/tea tree essential oil microcapsules were all 0 CFU/g, indicating that the alginate/tea tree essential oil microcapsules were not contaminated. It was put into the used hot spring water, mixed well, and placed for 4 h before testing. The test results showed that the total bacteria, Escherichia coli, and Staphylococcus aureus colonies of the alginate/tea tree essential oil microcapsules were all 0 CFU/g, indicating that the alginate/tea tree essential oil microcapsules had an obvious antibacterial effect, as shown in Table 7.

Table 7.
The antibacterial effect of used hot spring water by the microcapsules of alginate/tea tree essential oil.
4.2. Analysis of the Release of Microcapsules Obtained with the Same Concentration of Cross-Linking Agent for Different Cross-Linking Time
4.2.1. Effects of Cross-Linking Time on the Release of Calcium Alginate/tea Tree Essential Oil Microcapsules with Different Cross-Linking Agent Concentrations of CaCl2
The release of microcapsules in hot spring water obtained by using CaCl2 as the cross-linking agent for the cross-linking time of one, five, and ten minutes are shown as follows. According to the analysis of the release curve, in the presence of the cross-linking agent of 0.1 M CaCl2, the microcapsules showed the slowest release rate after ten minutes of cross-linking, and the release equilibrium time was about 2 h after the start of the release. The microcapsules showed the second slowest release rate after five minutes of cross-linking, and the release equilibrium time was about 1.6 h after the start of the release. The microcapsules showed the fastest release rate after one minute of cross-linking, and the release equilibrium time was about 1.3 h after the start of the release. The experimental data showed that the longer the cross-linking time, the thicker the calcification thickness and the smaller the porosity, and the longer the tea tree essential oil encapsulated in the microcapsules may be released in the hot spring water, as shown in Figure 12.
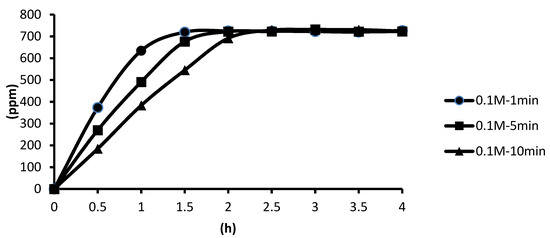
Figure 12.
The effect of cross-linking time by 0.1 M CaCl2 on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
The 0.5 M cross-linking agent was used instead, and the cross-linking time was unchanged. The microcapsules showed the slowest release rate after ten minutes of cross-linking, and the release equilibrium time was about 3.4 h after the start of the release. The microcapsules showed the second slowest release rate after five minutes of cross-linking, and the release equilibrium time was about 2.8 h after the start of the release. The microcapsules showed the fastest release rate after one minute of cross-linking, and the release equilibrium time was about 2.4 h after the start of the release. These results were not significantly different from the 0.1 M group, as shown in Figure 13.
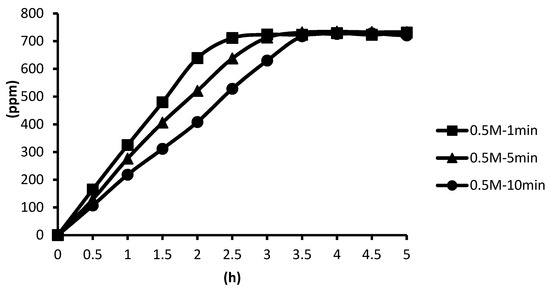
Figure 13.
The effect of cross-linking time by 0.5 M CaCl2 on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
The 1 M cross-linking agent was used instead, and the cross-linking time was unchanged. In the presence of the cross-linking agent of 1 M CaCl2, the release curves of the microcapsules obtained after the cross-linking time of one minute, five minutes, and ten minutes were slightly different, but the difference was not obvious. The release equilibration time was approximately 3.5 h after the start of the release. In addition, from the release curves of microcapsules obtained by the cross-linking agent of 0.1 M and 0.5 M CaCl2 in hot spring water, it can be seen that in the presence of the cross-linking agent of 0.5 M CaCl2, the release time of microcapsules obtained after the cross-linking time of ten minutes was 3.4 h, which fully indicated that the alginate was almost completely cross-linked. According to the above experimental results, all the total releases were around 720 ppm regardless of the cross-linking time, as shown in Figure 14.
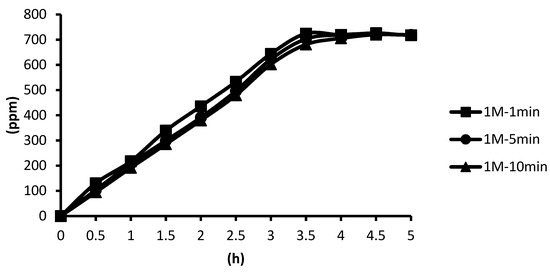
Figure 14.
The effect of cross-linking time by 1 M CaCl2 on releasing of tea tree essential oil from.
4.2.2. Effects of Cross-Linking Time on the Release of Zinc Alginate/Tea Tree Essential Oil Microcapsules with Different Cross-Linking Agent Concentrations of ZnCl2
The release of microcapsules in hot spring water obtained by using ZnCl2 as the cross-linking agent for the cross-linking time of one, five, and, ten minutes are shown as follows. According to the release curve, in the presence of the cross-linking agent of 0.1 M ZnCl2, the microcapsules showed the slowest release rate after ten minutes of cross-linking, and the release equilibrium time was about 1.7 h after the start of the release. The microcapsules showed the second slowest release rate after five minutes of cross-linking, and the release equilibrium time was about 1.3 h after the start of the release. The microcapsules showed the fastest release rate after one minute of cross-linking, and the release equilibrium time was about 1 h after the start of the release. The experimental data showed that the longer the cross-linking time, the thicker the zincization thickness, and the smaller the porosity, and the longer the tea tree essential oil encapsulated in the microcapsules may be released in the hot spring water, as shown in Figure 15.
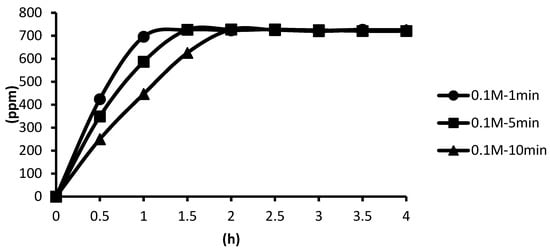
Figure 15.
The effect of cross-linking time by 0.1 M ZnCl2 on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
The 0.5 M cross-linking agent was used instead, and the cross-linking time was unchanged. In the presence of the cross-linking agent of 0.5 M ZnCl2, the microcapsules showed the slowest release rate after ten minutes of cross-linking, and the release equilibrium time was about 3 h after the start of the release. The microcapsules showed the second slowest release rate after five minutes of cross-linking, and the release equilibrium time was about 2.4 h after the start of the release. The microcapsules showed the fastest release rate after one minute of cross-linking, and the release equilibrium time was about 1.9 h after the start of the release. These results were the same as 0.1 M ZnCl2, as shown in Figure 16.
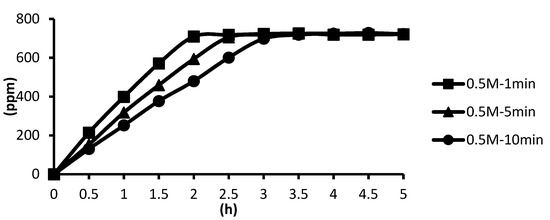
Figure 16.
The effect of cross-linking time by 0.5 M ZnCl2 on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
The cross-linking agent of 1 M ZnCl2 was used instead, and the cross-linking time was unchanged. In the presence of the cross-linking agent of 1 M ZnCl2, the release curves of the microcapsules obtained after the cross-linking time of one minute, five minutes, and ten minutes were slightly different, but the difference was not obvious. The release equilibration time was approximately 2.9 h to 3.5 h after the start of the release. In addition, from the release curves of microcapsules obtained by the cross-linking agent of 0.5 M ZnCl2 in hot spring water, it can be seen that in the presence of the cross-linking agent of 0.1 M and 0.5 M ZnCl2, the release time of microcapsules obtained after the cross-linking time of ten minutes was 3 h, which indicated that the alginate was almost completely cross-linked. According to the above experimental results, all the total releases were around 720 ppm regardless of the cross-linking concentration, as shown in Figure 17.
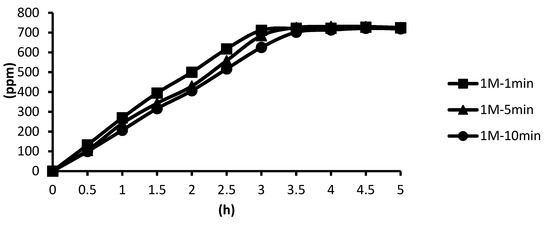
Figure 17.
The effect of cross-linking time by 1 M ZnCl2 on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
4.3. Analysis of the Release with Different Concentrations of Cross-Linking Agent for the Same Cross-Linking Time
4.3.1. Release of Cross-Linking Agents with Different CaCl2 Concentrations for the Same Cross-Linking Time
The release situations of microcapsules in hot spring water obtained by using CaCl2 with different concentrations as cross-linking agents for the cross-linking time of one minute are shown as follows. In the presence of the cross-linking agent of 1 M CaCl2, the microcapsules showed the slowest release rate after one minute of cross-linking, and the release equilibrium time was about 3.3 h after the start of the release. In the presence of the cross-linking agent of 0.5 M CaCl2, the microcapsules showed the second slowest release rate after one minute of cross-linking, and the release equilibrium time was about 2.4 h after the start of the release. In the presence of the cross-linking agent of 0.1 M CaCl2, the microcapsules showed the fastest release rate after one minute of cross-linking, and the release equilibrium time was about 1.3 h after the start of the release. The experimental data showed that the higher the concentration, the thicker the calcification thickness and the smaller the porosity, and the longer the tea tree essential oil encapsulated in the microcapsules may be released in the hot spring water, as shown in Figure 18.
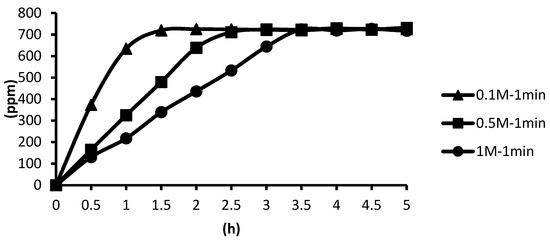
Figure 18.
The effect of CaCl2 concentration (cross-linking time of one minute) on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
The release situations of microcapsules in hot spring water obtained by using CaCl2 with different concentrations as cross-linking agents after the cross-linking time of five minutes are shown as follows. In the presence of the cross-linking agent of 1 M CaCl2, the microcapsules showed the slowest release rate after five minutes of cross-linking, and the release equilibrium time was about 3.5 h after the start of the release. In the presence of the cross-linking agent of 0.5 M CaCl2, the microcapsules showed the second slowest release rate after five minutes of cross-linking, and the release equilibrium time was about 2.8 h after the start of the release. In the presence of the cross-linking agent of 0.1 M CaCl2, the microcapsules showed the fastest release rate after five minutes of cross-linking, and the release equilibrium time was about 1.6 h after the start of the release. These results were the same as that after one minute of cross-linking. As for the release time, it is that 1 M > 0.5 M > 0.1 M, as shown in Figure 19.
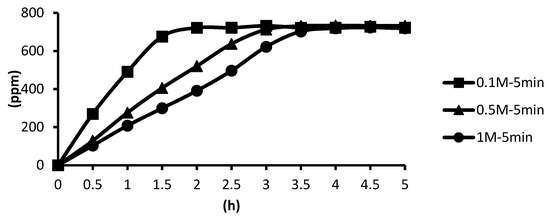
Figure 19.
The effect of CaCl2 concentration (cross-linking time of five minutes) on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
The release situations of microcapsules in hot spring water obtained by using CaCl2 with different concentrations as cross-linking agents after the cross-linking time of ten minutes are shown as follows. In the presence of the cross-linking agent of 0.1 M CaCl2, the microcapsules showed the fastest release rate after ten minutes of cross-linking, and the release equilibrium time was about 2.0 h after the start of the release. In the presence of the cross-linking agent of 0.5 M and 1 M CaCl2, the release curves of microcapsules were similar after ten minutes of cross-linking, and the release equilibrium time was about 3.5 h after the start of the release. This showed that in the presence of the cross-linking agent of 0.5 M CaCl2, the alginate inside the microcapsules was almost completely cross-linked after the cross-linking time of ten minutes. According to the above experimental results, all the total releases were around 720 ppm regardless of the cross-linking time, as shown in Figure 20.
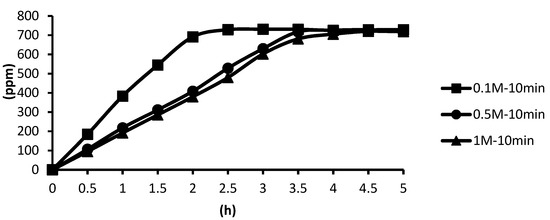
Figure 20.
The effect of CaCl2 concentration (cross-linking time of ten minutes) on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
4.3.2. Release of Cross-Linking Agents with Different ZnCl2 Concentrations for the Same Cross-Linking Time
The release situations of microcapsules in hot spring water obtained by using ZnCl2 with different concentrations as cross-linking agents for the cross-linking time of one minute are shown as follows. In the presence of the cross-linking agent of 1 M ZnCl2, the microcapsules showed the slowest release rate after one minute of cross-linking, and the release equilibrium time was about 2.9 h after the start of the release. In the presence of the cross-linking agent of 0.5 M ZnCl2, the microcapsules showed the second slowest release rate after one minute of cross-linking, and the release equilibrium time was about 1.9 h after the start of the release. In the presence of the cross-linking agent of 0.1 M ZnCl2, the microcapsules showed the fastest release rate after one minute of cross-linking, and the release equilibrium time was about 1 h after the start of the release. This fully showed that the higher the cross-linking agent concentration of the ZnCl2, the longer the tea tree essential oil encapsulated in the microcapsules may be released in the hot spring water. The experimental data showed that the higher the concentration, the thicker the zincization thickness and the smaller the porosity, and the longer the tea tree essential oil encapsulated in the microcapsules may be released in the hot spring water, as shown in Figure 21.
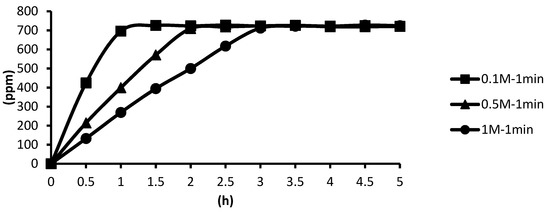
Figure 21.
The effect of ZnCl2 concentration (cross-linking time of one minute) on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
The release situations of microcapsules in hot spring water obtained by using ZnCl2 with different concentrations as cross-linking agents after the cross-linking time of five minutes are shown as follows. In the presence of the cross-linking agent of 1 M ZnCl2, the microcapsules showed the slowest release rate after five minutes of cross-linking, and the release equilibrium time was about 3.1 h after the start of the release. In the presence of the cross-linking agent of 0.5 M ZnCl2, the microcapsules showed the second slowest release rate after five minutes of cross-linking, and the release equilibrium time was about 2.4 h after the start of the release. In the presence of the cross-linking agent of 0.1 M ZnCl2, the microcapsules showed the fastest release rate after five minutes of cross-linking, and the release equilibrium time was about 1.3 h after the start of the release. These results were the same as that after one minute of cross-linking. As for the release time, it is that 1 M > 0.5 M > 0.1 M, as shown in Figure 22.
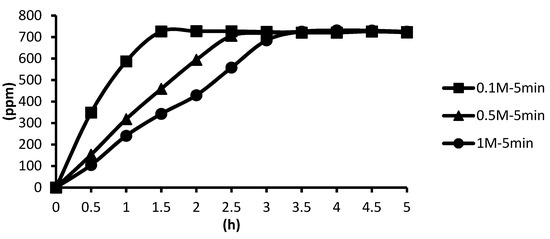
Figure 22.
The effect of ZnCl 3 concentration (cross-linking time of five minutes) on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
The release situations of microcapsules in hot spring water obtained by using ZnCl2 with different concentrations as cross-linking agents after the cross-linking time of ten minutes are shown as follows. In the presence of the cross-linking agent of 0.1 M ZnCl2, the microcapsules showed the fastest release rate after ten minutes of cross-linking, and the release equilibrium time was about 1.7 h after the start of the release. In the presence of the cross-linking agent of 0.5 M and 1 M ZnCl2, the release curves of microcapsules were similar without much difference after ten minutes of cross-linking, and the release equilibrium time was about 3 h to 3.5 h after the start of the release. This showed that in the presence of the cross-linking agent of 0.5 M ZnCl2, the alginate inside the microcapsules was almost completely cross-linked after the cross-linking time of ten minutes. According to the above experimental results, all the total releases were around 720 ppm regardless of the cross-linking time, as shown in Figure 23.
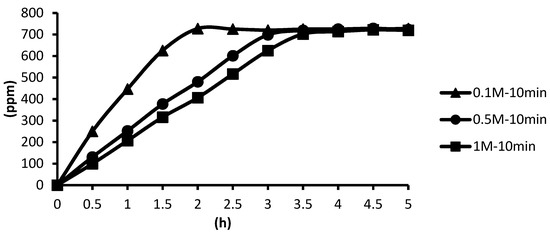
Figure 23.
The effect of ZnCl2 concentration (cross-linking time of ten minutes) on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
4.4. Analysis of the Release of Microcapsules Obtained with Different Types of Cross-Linking Agents
Release of microcapsules with different cross-linking agents of 0.1 M after the cross-linking time of one minute, five minutes, and ten minutes are shown as follows. Although the release curves of microcapsules obtained by the cross-linking agent of 0.1 M CaCl2 and ZnCl2 after the cross-linking time of one minute, five minutes, and ten minutes were very similar, it could still be seen that the obtained release rate of the microcapsules obtained by different cross-linking agents of 0.1 M had been affected after the cross-linking time of one minute, five minutes, and ten minutes. The release curves for the cross-linking time of one minute, five minutes, and ten minutes intersected at approximately 1.5 h, 2 h, and 2.5 h after the start of the release. The release rate of microcapsules is 0.1 M ZnCl2 > that with 0.1 M CaCl2, as shown in Figure 24.
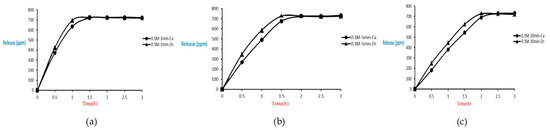
Figure 24.
The effect of different cross-linking reagents (0.1 M, cross-linking time of 1 (a), 5 (b), 10 (c) minute) on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot spring water.
The release of microcapsules with different cross-linking agents of 0.5 M after the cross-linking time of one minute, five minutes, and ten minutes are shown as follows. Although the release curves of microcapsules obtained by the cross-linking agent of 0.5 M CaCl2 and ZnCl2 after the cross-linking time of one minute, five minutes, and ten minutes were very similar, it could still be seen that the obtained release rate of the microcapsules obtained by different cross-linking agents of 0.5 M had been affected after the cross-linking time of one minute, five minutes, and ten minutes. The release curves for the cross-linking time of one minute, five minutes, and ten minutes intersected at approximately 2.5 h, 3 h, and 3.5 h after the start of the release. The release rate is that microcapsules with 0.5 M ZnCl2 > that with 0.5 M CaCl2, as shown in Figure 25.

Figure 25.
The effect of different cross-linking reagents (0.5 M, cross-linking time of 1 (a), 5 (b), 10 (c) minute) on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the hot.
The release of microcapsules with different cross-linking agents of 1 M after the cross-linking time of one minute, five minutes, and ten minutes are shown as follows. Although the release curves of microcapsules obtained by the cross-linking agent of 1 M CaCl2, aluminum chloride, and ZnCl2 after the cross-linking time of one minute, five minutes, and ten minutes were very similar, it could still be seen that the obtained release rate of the microcapsules obtained by different cross-linking agents of 1 M had been affected after the cross-linking time of one minute, five minutes, and ten minutes. The release curves for the cross-linking time of one minute, five minutes, and ten minutes intersected at approximately 3.5 h, 4 h, and 4 h after the start of the release. The release rate of microcapsules with 1 M ZnCl2 > that with 1 M aluminum chloride > that with 1 M CaCl2 All the total releases were around 720 ppm regardless of the type, time, and concentration of the cross-linking agent (Figure 26).
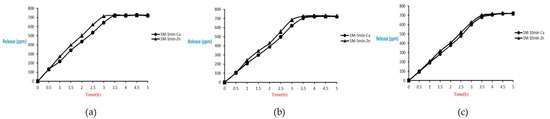
Figure 26.
The effect of different cross-linking reagents (1 M, cross-linking time of 1 (a), 5 (b), 10 (c) minute) on releasing of tea tree essential oil from alginate/tea tree essential oil microcapsules in the.
5. Discussion
5.1. The Antibacterial Effect of Alginate/Tea Tree Essential Oil Microcapsules and Alginate/Chitosan Microcapsules
Sodium alginate is a natural polysaccharide with the ability to concentrate solutions, form gels, and form films [23]. Chitosan also has inhibitory effects on Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans, streptococcus, and salmonella [28]. However, tea tree essential oil can damage cell membranes, prevent cell adhesion, cause irregular aggregation of cytoplasm, and lead to cell disintegration [40]. Therefore, compared with the antibacterial liquid prepared by simply combining alginate substances, the antibacterial effect of microcapsules made of tea tree essential oil is better than that of sodium alginate. This result has been confirmed by [23,28].
5.2. Discussion on the Release of Microcapsules Obtained with the Same Concentration of Cross-Linking Agent for Different Cross-Linking Time
The experiment objective was to meet the properties of “not increasing its redox potential”, “not changing the water quality of hot springs”, and “having a high efficiency in antibacterial effect”. The experiment showed that the longer the cross-linking time, the thicker the zincization thickness and the smaller the porosity, and the longer the tea tree essential oil encapsulated in the microcapsules may be released in the hot spring water. It also revealed that it could be completely cross-linked with the alginate after 3 h. However, since the metal activity of calcium is higher than that of zinc, the time to reach complete cross-linking in the experimental group added with CaCl2 can last for 3.4 h during the experiment. Therefore, in the experiment of adding different concentrations of CaCl2 and ZnCl2 into the microcapsules, the release time of the CaCl2 group was longer, and it could maintain the effective antibacterial effect for a longer time.
5.3. Discussion on the Release with Different Concentrations of Cross-Linking Agents for the Same Cross-Linking Time
The microcapsules extracted from alginate have high water absorption. They can easily remove stains and can absorb heavy metals [41,42]. Moreover, the metal activity of CaCl2 is higher than that of ZnCl2, so it is helpful for the release of microcapsules in hot spring water. However, the temperature of hot spring water is generally at least 40 degrees or above. Although substances such as alginate, chitosan, and tea tree essential oil have the effect of removing heavy metals and sterilizing, their chemical substances are prone to qualitative change under long-term immersion in high temperatures, which reduces their existing functions. As a result, although each of 0.1 M, 0.5 M, and 1 M has antibacterial benefits, the release time of 1 M is longer. In addition, considering factors such as production technology, cost, release time, and antibacterial benefit, when the concentration of the substance loaded in the microcapsules is set above 1 M, the release time and antibacterial effect of 3 h can be achieved.
6. Conclusions
The composite microcapsules of alginate/tea tree essential oil have an obvious antibacterial effect on microorganisms in hot spring water, while the composite microcapsules of alginate/chitosan have no antibacterial effect in hot spring water. When the concentration of the cross-linking agent is fixed, the longer the cross-linking time is (10 min > 5 min > 1 min), the longer the release equilibrium time of the essential oil in the microcapsules in the hot spring water is. When the cross-linking time is fixed, the higher the concentration of the cross-linking agent (1 M > 0.5 M > 0.1 M) and the longer the release equilibrium time of the essential oil in the microcapsules in the hot spring water is. When the concentration of the cross-linking agent and the cross-linking time are fixed, the higher the metal activity of the cross-linking agent (Ca > Zn) is and the longer the release equilibrium time of the essential oil in the microcapsules in the hot spring water is.
Author Contributions
Conceptualization, J.-L.H. and C.-H.H.; methodology, J.-L.H.; software, J.-L.H.; validation, J.-L.H., T.-Y.L. and J.-H.C.; formal analysis, J.-L.H. and A.-C.Y.; investigation, J.-L.H.; resources, J.-H.C.; data curation, J.-L.H.; writing—original draft preparation, C.-H.H.; writing—review and editing, H.-H.L.; visualization, J.-L.H.; supervision, H.-H.L.; project administration, J.-L.H.; funding acquisition, T.-Y.L. and J.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
There was no additional funding for this study.
Institutional Review Board Statement
This research is mainly for hot spring water quality management and testing, and all testing resources are natural components that can decompose or volatilize in water. In addition, there is no need for human trials during the experiment, so there is no need to apply for ethical review.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data are available for this study.
Acknowledgments
All authors and participants agree with the analytical content of this manuscript, as well as the results of the assignment of contributions to the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salamé, L.; Bogardi, J.J.; Sebesvari, Z.; Tockner, K.; Yazici, B.; Turan, F.; Calli, B.; Kerç, A.; Ünver, O.; Walz, Y. Drivers, pressures and stressors: The societal framework of water resources management. In Handbook of Water Resources Management: Discourses, Concepts, and Examples; Springer: Cham, Switzerland, 2021; pp. 329–364. Available online: https://link.springer.com/book/10.1007/978-3-030-60147-8#about (accessed on 16 March 2022).
- Hsu, B.M.; Wu, S.F.; Fan, C.W.; Shih, F.C.; Lin, Y.C.; Ji, D.D. Water quality parameters associated with prevalence of Legionella in hot spring facility water bodies. Water Res. 2010, 44, 4805–4811. [Google Scholar] [CrossRef]
- Lund, J.W.; Toth, A.N. Direct utilization of geothermal energy 2020 worldwide review. Geothermics 2021, 90, 101915. [Google Scholar] [CrossRef]
- Erfurt, P. Hot springs—A final overview. In The Geoheritage of Hot Springs. Geoheritage, Geoparks, and Geotourism (Conservation and Management Series); Springer: Cham, Switzerland, 2021. [Google Scholar]
- Peng, X.R. The Hot Spring Season Is Here! An Introduction to the 10 Hot Springs in Taiwan and the World-Class “Undersea Hot Springs”. Available online: https://www.managertoday.com.tw/eightylife/article/view/721 (accessed on 29 January 2021).
- Organization Structure of the Tourism Bureau. Visitors to the Principal Scenic Spots in Taiwan, January–December 2020. Available online: https://admin.taiwan.net.tw/FileUploadCategoryListC003330.aspx?appname=FileUploadCategoryListC003330 (accessed on 30 January 2022).
- Goldscheider, N.; Mádl-Szőnyi, J.; Erőss, A.; Schill, E. Thermal water resources in carbonate rock aquifers. Hydrogeol. J. 2010, 18, 1303–1318. [Google Scholar] [CrossRef] [Green Version]
- Parise, M.; Ravbar, N.; Živanović, V.; Mikszewski, A.; Kresic, N.; Szőnyi, J.M.; Kukurić, N. Hazards in Karst and managing water resources quality. In Karst Aquifers—Characterization and Engineering. Professional Practice in Earth Sciences; Stevanović, Z., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Hsu, C.H.; Lin, H.H.; Jhang, S.W.; Lin, T.Y. Does environmental engineering help rural industry development? Discussion on the impact of Taiwan’s “special act for forward-looking infrastructure” on rural industry development. Environ. Sci. Pollut. Res. 2020, 28, 40137–40150. [Google Scholar] [CrossRef] [PubMed]
- LaMoreaux, P.E.; Tanner, J.T. Springs and Bottled Waters of the World: Ancient History, Source, Occurrence, Quality, and Use; Springer Science & Business Media: Berlin, Germany, 2001. [Google Scholar]
- Chia Nan University of Pharmacy and Science. Research on The Utilization Enhancement Technology of Hot Spring Resources; Water Resources Department, Ministry of Economic Affairs: Taipei, Taiwan, 2008.
- Su, X.B.; Chen, Y.L.; Zheng, L.R.; Zhou, Z.Y.; Cai, S.F. Stones from other mountains can be used as a mirror—On the management mechanism of Legionella in hot spring water in Japan. Epidem. Rep. 2005, 21, 919–929. [Google Scholar] [CrossRef]
- Leoni, E.; Catalani, F.; Marini, S.; Dallolio, L. Legionellosis associated with recreational waters: A systematic review of cases and outbreaks in swimming pools, spa pools, and similar environments. Int. J. Environ. Res. Public Health 2018, 15, 1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stejskal, V.; Vendl, T.; Aulicky, R.; Athanassiou, C. Synthetic and natural insecticides: Gas, liquid, gel and solid formulations for stored-product and food-industry pest control. Insects 2021, 12, 590. [Google Scholar] [CrossRef]
- Labuschagne, P. Impact of wall material physicochemical characteristics on the stability of encapsulated phytochemicals: A review. Food Res. Int. 2018, 107, 227–247. [Google Scholar] [CrossRef]
- Lin, C.L. Study on the Formulation of Capsule Herbicides Alachlor. Master’s Thesis, Chaoyang University of Technology, Taichung, Taiwan, 2006. [Google Scholar]
- Lin, S.Y.; Kun, B.; Shan, Z.; Shang, W. Microcapsules. Taiwan Sci. 1980, 34, 148–180. [Google Scholar]
- Wu, P.S. The Preparation and Drug-release Study of Nicotine/Sodium Alginate Composite Microcapsules. Master’s Thesis, Chia Nan University of Pharmacy and Science, Tainan, Taiwan, 2007. [Google Scholar]
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49–50, 175–220. [Google Scholar] [CrossRef]
- Simonazzi, A.; Cid, A.G.; Villegas, M.; Romero, A.I.; Palma, S.D.; Bermúdez, J.M. Nanotechnology applications in drug controlled release. Drug Target. Stimuli Sensit. Drug Deliv. Syst. 2018, 81–116. [Google Scholar] [CrossRef]
- Prabakaran, S.; Jeyaraj, M.; Nagaraj, A.; Sadasivuni, K.K.; Rajan, M. Polymethyl methacrylate–ovalbumin@ graphene oxide drug carrier system for high anti-proliferative cancer drug delivery. Appl. Nanosci. 2019, 9, 1487–1500. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin-and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Jeong, H.J.; Koo, H.N.; Oh, E.Y.; Chae, H.J.; Kim, H.R.; Suh, S.B.; Kim, C.H.; Cho, K.H.; Park, B.R.; Park, S.T.; et al. Nitric oxide production by high molecular weight water-soluble chitosan via nuclear factor-kappaB activation. Int. J. Immunopharmacol. 2000, 22, 923–933. [Google Scholar] [CrossRef]
- Seo, W.G.; Pae, H.O.; Kim, N.Y.; Oh, G.S.; Park, I.S.; Kim, Y.H.; Kim, Y.M.; Lee, Y.H.; Jun, C.D.; Chung, H.T. Synergistic cooperation between watersoluble chitosan oligomers and interferongamma for induction of nitric oxide synthesis and tumoricidal activity in murine peritoneal macrophages. Cancer Lett. 2000, 159, 189–195. [Google Scholar] [CrossRef]
- Lee, P.C.; Syu, F.Y.; Yang, W.J.; Chan, C.F. Antioxidant and antimicrobial activity of high molecular weight water-soluble chitosan. Bull. Hungkuang Instit. Technol. 2012, 67, 1–10. [Google Scholar] [CrossRef]
- Aljazy, N.A.S.; Abdulstar, A.R. Potential effects of natural antioxidants in the treatment of some viral diseases. Al-Qadisiyah J. Agric. Sci. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Lupattelli, A.; Koren, G.; Nordeng, H. Safety classification of herbal medicines used in pregnancy in a multinational study. BMC Complement. Altern. Med. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garozzo, A.; Timpanaro, R.; Stivala, A.; Bisignano, G.; Castro, A. Activity of Melaleuca alternifolia (tea tree) oil on Influenza virus A/PR/8: Study on the mechanism of action. Antivir. Res. 2011, 89, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Usachev, E.V.; Pyankov, O.V.; Usacheva, O.V.; Agranovski, I.E. Antiviral activity of tea tree and eucalyptus oil aerosol and vapour. J. Aerosol Sci. 2013, 59, 22–30. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Xu, D.; Wang, R.; Tseng, S.C.G. Treatment of ocular itching associated with ocular demodicosis by 5% tea tree oil ointment. Cornea 2012, 31, 14–17. [Google Scholar] [CrossRef]
- Keragala, R.K.; Kasunsiri, T.D.; Kempitiya, K.S.; Kumarapeli, N.N.; Kumara, K.; Gunathilaka, S.S. A study on the extent, aetiology, and associated factors of dandruff in a group of medical students and the in vitro effects of antidandruff preparations. Sri Lankan J. Infect. Dis. 2020, 10, 134–145. [Google Scholar] [CrossRef]
- Lin, Y.W.; Chang, C.P. Preparation and controlled release properties of alginate microcapsules encapsulated tea-tree oil. J. Hwa Gang Text. 2005, 12, 136–146. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. LWT 2018, 96, 671–678. [Google Scholar] [CrossRef]
- Ge, Y.; Tang, J.; Fu, H.; Fu, Y.; Wu, Y. Characteristics, controlled-release, and antimicrobial properties of tea tree oil liposomes-incorporated chitosan-based electrospun nanofiber mats. Fibers Polym. 2019, 20, 698–708. [Google Scholar] [CrossRef]
- Tsai, J.C. Preparation of Active Ingredient-Containing Chitosan/Polycaprolactone Nonwoven Mats Using Electrospinning Technique: In Vitro and In Vivo Evaluations on Wound Healing. Master’s Thesis, National Taiwan University of Science and Technology, Taipei, Taiwan, 2013. [Google Scholar]
- Chen, Y.-W. Preparation of Calcium Alginate Complex Microspheres with Microfluidics Used in Metal Adsorption and Drug Delivery Systems. Master’s Thesis, Cheng Shiu University, Kaohsiung City, Taiwan, 2012. [Google Scholar]
- Su, P.-J.; Xu, X.-L. Antioxidant activity of methanol extracts from Camellia oleifera leaves with different drying treatments. Taiwanese J. Agric. Chem. Food Sci. 2017, 55, 59–65. [Google Scholar]
- Kikuchi, A.; Kawabuchi, M.; Sugihara, M.; Sakurai, Y. Pulsed dextran release from calcium-alginate gel beads. J. Control. Release 1997, 47, 21–29. [Google Scholar] [CrossRef]
- Jeon, C.; Park, J.Y.; Yoo, Y.J. Novel immobilization of alginic acid for heavy metal removal. Biochem. Eng. J. 2002, 11, 159–166. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).