Tuning the Fe(II)/hydroxide Ratio during Synthesis of Magnetite Nanoparticles to Maximize Cr(VI) Uptake Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis
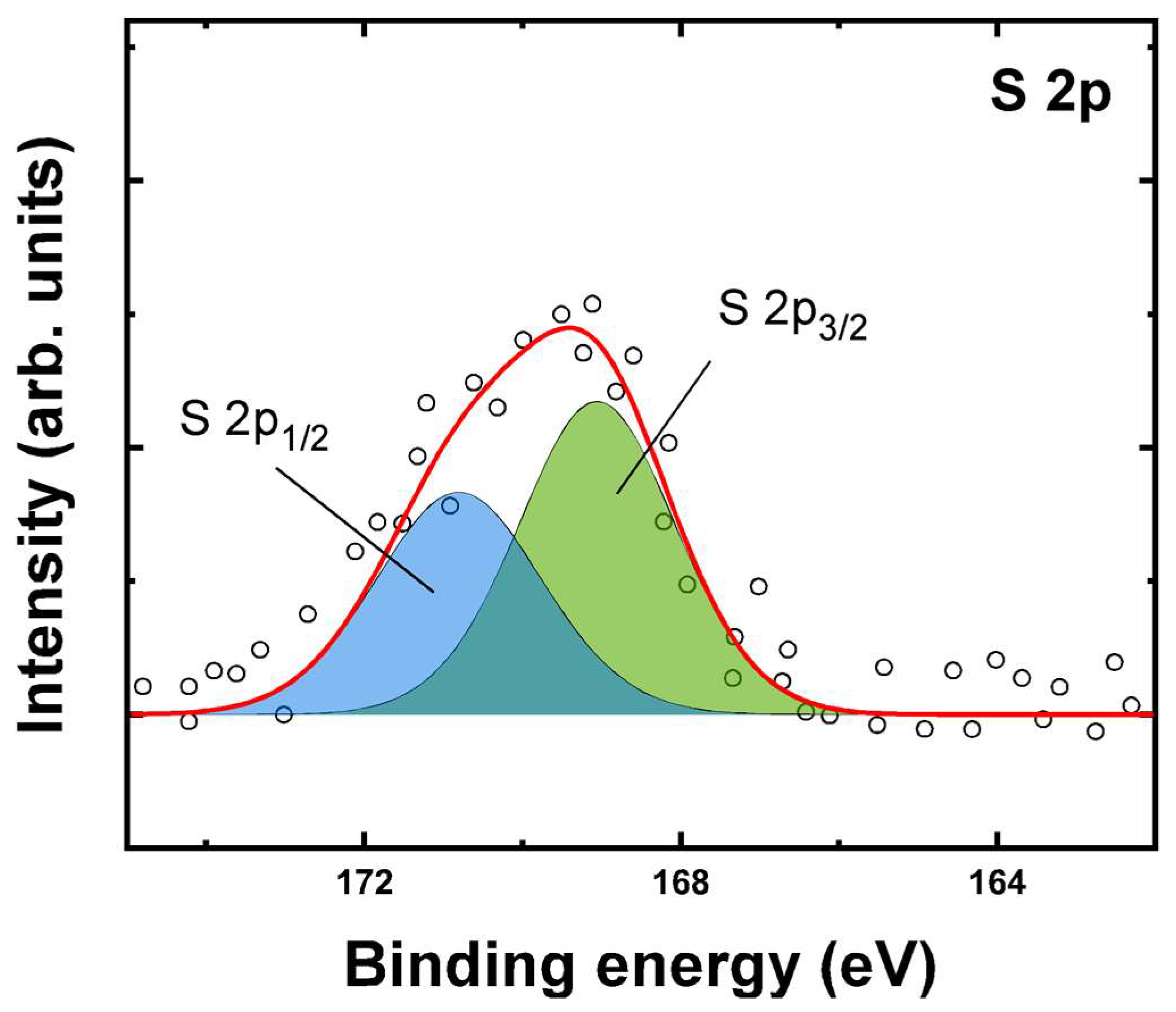
2.3. Characterization
2.4. Cr(VI) Uptake Evaluation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simeonidis, K.; Martinez-Boubeta, C.; Zamora-Pérez, P.; Rivera-Gil, P.; Kaprara, E.; Kokkinos, E.; Mitrakas, M. Implementing nanoparticles for competitive drinking water purification. Environ. Chem. Lett. 2019, 17, 705–719. [Google Scholar] [CrossRef]
- Yadav, N.; Garg, V.K.; Chhillar, A.K.; Rana, J.S. Detection and remediation of pollutants to maintain ecosustainability employing nanotechnology: A review. Chemosphere 2021, 280, 130792. [Google Scholar] [CrossRef] [PubMed]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2016, 2, 43–70. [Google Scholar] [CrossRef] [Green Version]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ. Health Perspect. 2009, 117, 1823–1831. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Pastora, J.; Bringas, E.; Ortiz, I. Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications. Chem. Eng. J. 2014, 256, 187–204. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; Zhu, X.; Huang, W.E.; Zhang, D. Application of magnetic nanoparticles in drinking water purification. Environ. Eng. Manag. J. 2014, 13, 2023–2029. [Google Scholar]
- Ahmed, T.; Imdad, S.; Yaldram, K.; Butt, N.M.; Pervez, A. Emerging nanotechnology-based methods for water purification: A review. Desalin. Water Treat. 2014, 52, 4089–4101. [Google Scholar] [CrossRef]
- Veintemillas-Verdaguer, S.; Marciello, M.; Morales, M.D.P.; Serna, C.J.; Andrés-Vergés, M. Magnetic nanocrystals for biomedical applications. Prog. Cryst. Growth Charact. Mater. 2014, 60, 80–86. [Google Scholar] [CrossRef]
- Asimakidou, T.; Makridis, A.; Veintemillas-Verdaguer, S.; Morales, M.P.; Kellartzis, I.; Mitrakas, M.; Vourlias, G.; Angelakeris, M.; Simeonidis, K. Continuous production of magnetic iron oxide nanocrystals by oxidative precipitation. Chem. Eng. J. 2020, 393, 124593. [Google Scholar] [CrossRef]
- Barceloux, D.G. Chromium. J. Toxicol. Clin. Toxicol. 1999, 37, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Linos, A.; Petralias, A.; Christophi, C.A.; Christoforidou, E.; Kouroutou, P.; Stoltidis, M.; Veloudaki, A.; Tzala, E.; Makris, K.C.; Karagas, M.R. Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece—An ecological study. Environ. Health A Glob. Access Sci. Source 2011, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Costa, M. Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol. 2003, 188, 1–5. [Google Scholar] [CrossRef]
- Beaumont, J.J.; Sedman, R.M.; Reynolds, S.D.; Sherman, C.D.; Li, L.H.; Howd, R.A.; Sandy, M.S.; Zeise, L.; Alexeeff, G.V. Cancer mortality in a Chinese population exposed to hexavalent chromium in drinking water. Epidemiology 2008, 19, 12–23. [Google Scholar] [CrossRef]
- California Office of Administrative Law. Chromium-6 Drinking Water MCL.; California State Water Quality Control Board: Sacramento, CA, USA, 2020.
- European Union. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Off. J. Eur. Union 2020, 1–62. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 14 March 2022).
- Simeonidis, K.; Kaprara, E.; Samaras, T.; Angelakeris, M.; Pliatsikas, N.; Vourlias, G.; Mitrakas, M.; Andritsos, N. Optimizing magnetic nanoparticles for drinking water technology: The case of Cr (VI). Sci. Total Environ. 2015, 535, 61–68. [Google Scholar] [CrossRef]
- Lv, X.; Xu, J.; Jiang, G.; Tang, J.; Xu, X. Highly active nanoscale zero-valent iron (nZVI)-Fe 3O 4 nanocomposites for the removal of chromium (VI) from aqueous solutions. J. Colloid Interface Sci. 2012, 369, 460–469. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Lo, I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Tong, Y.; Xu, X. Chromium (VI) reduction in aqueous solutions by Fe3O4-stabilized Fe0 nanoparticles. J. Hazard. Mater. 2009, 172, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Salas, G.; Casado, C.; Teran, F.J.; Miranda, R.; Serna, C.J.; Morales, M.P. Controlled synthesis of uniform magnetite nanocrystals with high-quality properties for biomedical applications. J. Mater. Chem. 2012, 22, 21065. [Google Scholar] [CrossRef]
- Sugimoto, T.; Matijević, E. Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. J. Colloid Interface Sci. 1980, 74, 227. [Google Scholar] [CrossRef]
- Verges, M.A.; Costo, R.; Roca, A.G.; Marco, J.F.; Goya, G.F.; Serna, C.J.; Morales, M.P.; Andrés Vergés, M.; Costo, R.; Roca, A.G.; et al. Uniform and water stable magnetite nanoparticles with diameters around the monodomain-multidomain limit. J. Phys. D-Appl. Phys. 2008, 41, 134003. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Morales, M.; Del, P.; Mazarío, E. Effect of the surface charge on the adsorption capacity of chromium (VI) of iron oxide magnetic nanoparticles prepared by microwave-assisted synthesis. Water 2019, 11, 2372. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, T.; Iqbal, M.; Zulfiqar, S.; Sarwar, M.I. Trimellitic acid functionalized magnetite nanoparticles for the efficient removal of Pb (II) and Cr (VI) from wastewater streams. Korean J. Chem. Eng. 2019, 36, 860–868. [Google Scholar] [CrossRef]
- Peterson, M.L.; White, A.F.; Brown, G.E.; Parks, G.A. Surface passivation of magnetite by reaction with aqueous Cr (VI): XAFS and TEM results. Environ. Sci. Technol. 1997, 31, 1573–1576. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data. Powder Diffraction File; Joint Center for Powder Diffraction Studies, Ed.; International Centre for Diffraction Data: Newtown Square, PA, USA, 2004. [Google Scholar]
- Amy, G.; Chen, H.W.; Drizo, A.; Von Gunten, U.; Brandhuber, P.; Hund, R.; Chowdhury, Z.; Kommineni, S.; Sinha, S.; Jekel, M.; et al. Adsorbent Treatment Technologies for Arsenic Removal, 1st ed.; AWWA Research Foundation: Washington, WA, USA, 2005. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses; John and Wiley and Sons: Hoboken, NJ, USA, 2003; ISBN 9783527302741. [Google Scholar]
- Luengo, Y.; Morales, M.P.; Gutiérrez, L.; Veintemillas-Verdaguer, S. Counterion and solvent effects on the size of magnetite nanocrystals obtained by oxidative precipitation. J. Mater. Chem. C 2016, 4, 9482–9488. [Google Scholar] [CrossRef]
- Finck, N.; Radulescu, L.; Schild, D.; Rothmeier, M.; Huber, F.; Lützenkirchen, J.; Rabung, T.; Heberling, F.; Schlegel, M.L.; Dideriksen, K.; et al. XAS signatures of Am (III) adsorbed onto magnetite and maghemite. In Journal of Physics: Conference Series, Proceedings of the 16th International Conference on X-ray Absorption Fine Structure (XAFS16), Karlsruhe, Germany, 23–28 August 2015; IOP Publishing: Bristol, UK, 2016; Volume 712, p. 712. [Google Scholar]
- Cîrcu, M.; Nan, A.; Borodi, G.; Liebscher, J.; Turcu, R. Refinement of magnetite nanoparticles by coating with organic stabilizers. Nanomaterials 2016, 6, 228. [Google Scholar] [CrossRef] [Green Version]
- Al Mamun, A.; Onoguchi, A.; Granata, G.; Tokoro, C. Role of pH in green rust preparation and chromate removal from water. Appl. Clay Sci. 2018, 165, 205–213. [Google Scholar] [CrossRef]
- Qiu, L.; Burton, G.R.; Rousseau, S.; Qian, J. Kinetics and thermodynamics of sulfate adsorption on magnetite at elevated temperatures. J. Solut. Chem. 2019, 48, 1488–1502. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Pliatsikas, N.; Vourlias, G.; Patsalas, P.; Mitrakas, M. The role of so 4 2—Surface distribution in arsenic removal by iron oxy-hydroxides. J. Solid State Chem. 2014, 213, 145–151. [Google Scholar] [CrossRef]
- Bierla, A.; Fromentin, G.; Minfray, C.; Martin, J.M.; Le Mogne, T.; Genet, N. Mechanical and physico-chemical study of sulfur additives effect in milling of high strength steel. Wear 2012, 286, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Baltrusaitis, J.; Cwiertny, D.M.; Grassian, V.H. Adsorption of sulfur dioxide on hematite and goethite particle surfaces. Phys. Chem. Chem. Phys. 2007, 9, 5542–5554. [Google Scholar] [CrossRef]

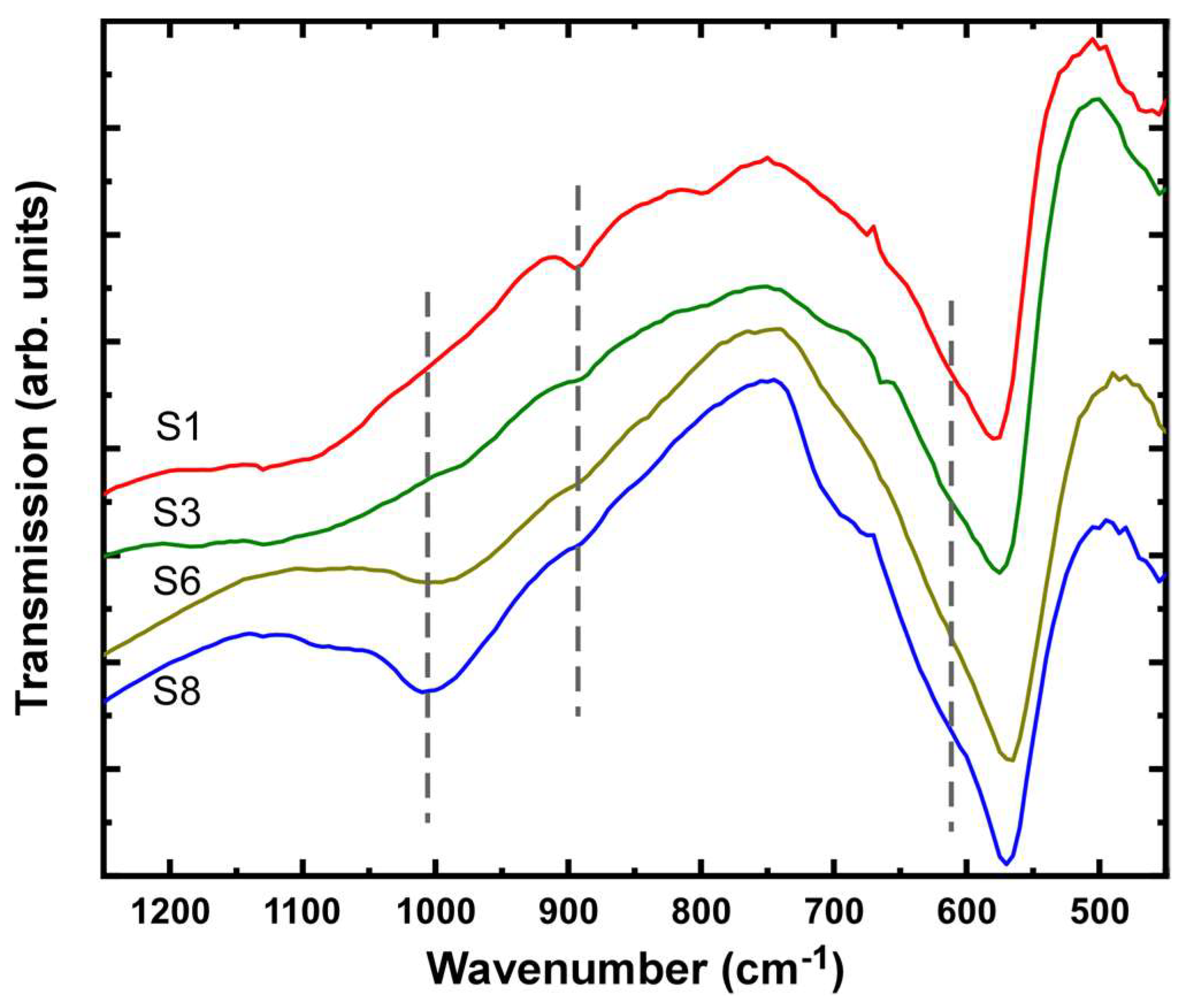
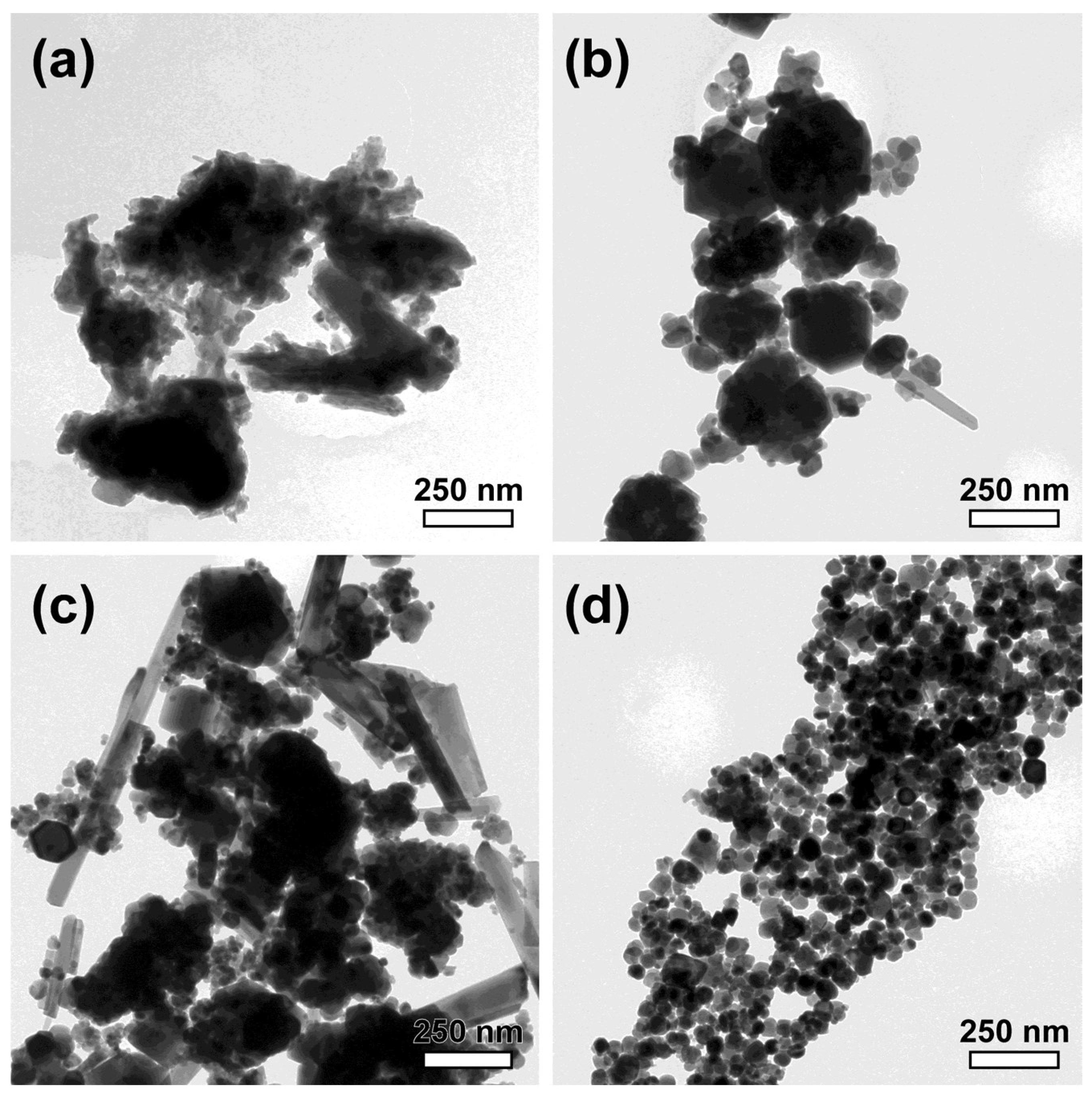
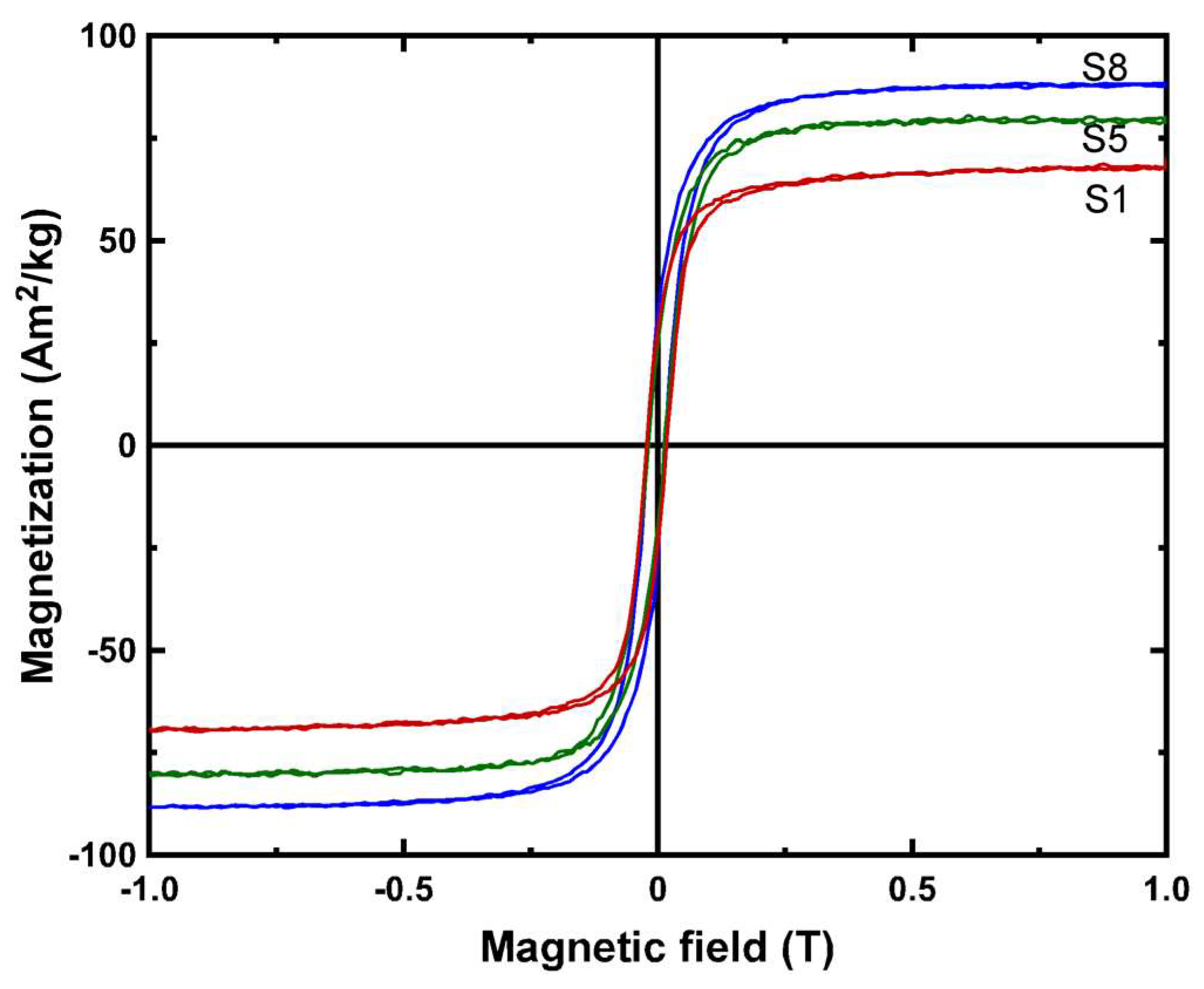



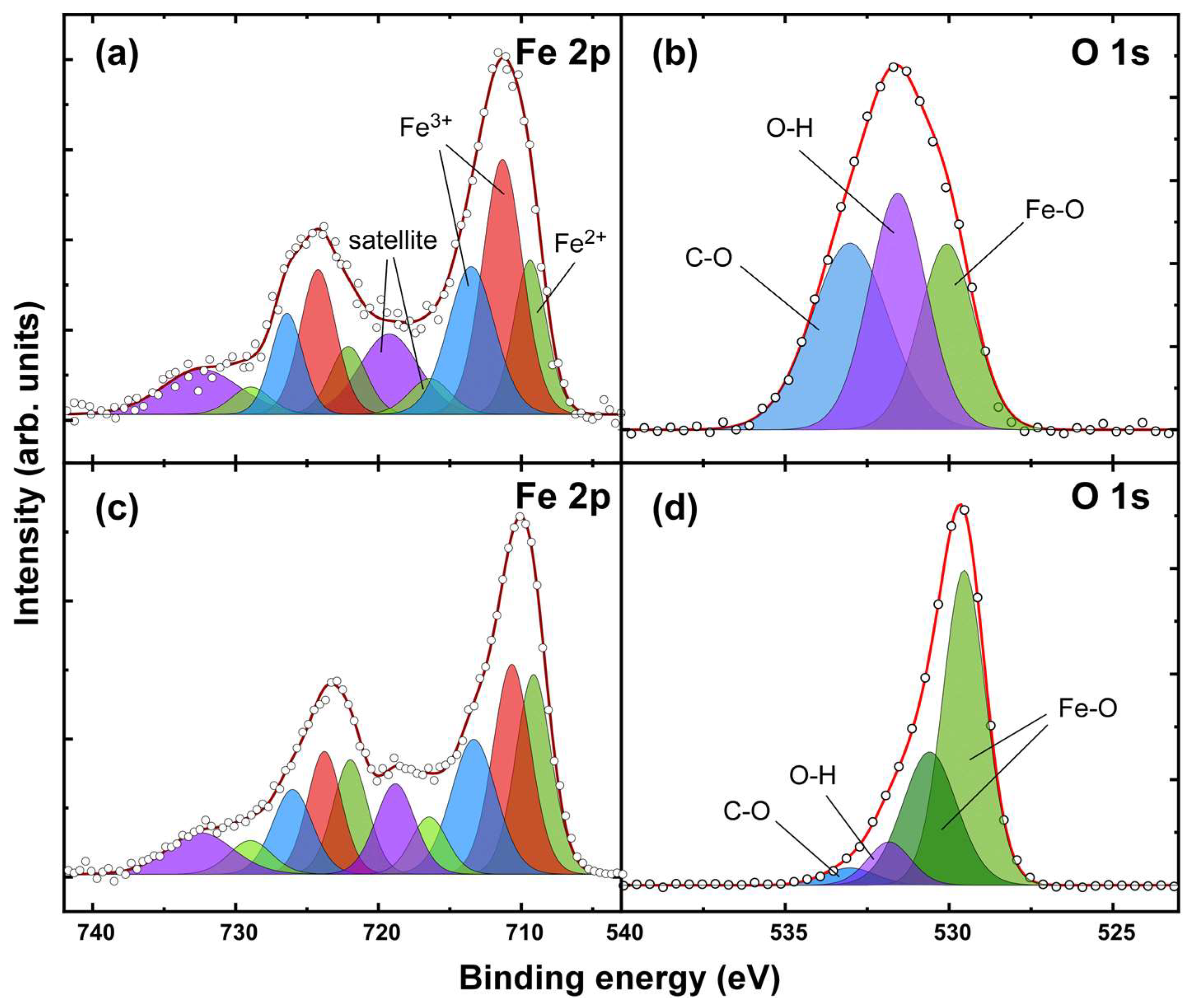
| Sample | OH−excess (M) | Fe2+/Fe3+ | Adsorbed SO42– (%wt.) | Na/Fe | Magnetization (Am2/kg) | Mean Crystal Size (nm) |
|---|---|---|---|---|---|---|
| S1 | −0.10 | 0.25 | 0.7 | 0.006 | 69 | 42 |
| S2 | −0.07 | 0.28 | 0.4 | 0.012 | 72 | 47 |
| S3 | −0.04 | 0.26 | 0.1 | 0.007 | 73 | 39 |
| S4 | −0.02 | 0.29 | 0.1 | 0.011 | 75 | 41 |
| S5 | −0.01 | 0.32 | - | 0.019 | 79 | 48 |
| S6 | +0.01 | 0.33 | - | 0.022 | 80 | 36 |
| S7 | +0.02 | 0.36 | - | 0.041 | 84 | 22 |
| S8 | +0.03 | 0.42 | - | 0.054 | 88 | 29 |
| Sample | KF (μg/mg)/(μg/L)1/n | 1/n | R2 |
|---|---|---|---|
| S1 | 0.627 | 0.329 | 0.994 |
| S2 | 0.408 | 0.371 | 0.996 |
| S3 | 0.087 | 0.341 | 0.995 |
| S4 | 0.073 | 0.407 | 0.978 |
| S5 | 0.084 | 0.420 | 0.996 |
| S6 | 0.073 | 0.333 | 0.994 |
| S7 | 0.981 | 0.172 | 0.989 |
| S8 | 1.151 | 0.232 | 0.986 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalaitzidou, K.; Chioti, E.; Asimakidou, T.; Karfaridis, D.; Vourlias, G.; Mitrakas, M.; Simeonidis, K. Tuning the Fe(II)/hydroxide Ratio during Synthesis of Magnetite Nanoparticles to Maximize Cr(VI) Uptake Capacity. Water 2022, 14, 1335. https://doi.org/10.3390/w14091335
Kalaitzidou K, Chioti E, Asimakidou T, Karfaridis D, Vourlias G, Mitrakas M, Simeonidis K. Tuning the Fe(II)/hydroxide Ratio during Synthesis of Magnetite Nanoparticles to Maximize Cr(VI) Uptake Capacity. Water. 2022; 14(9):1335. https://doi.org/10.3390/w14091335
Chicago/Turabian StyleKalaitzidou, Kyriaki, Evangelia Chioti, Theopoula Asimakidou, Dimitrios Karfaridis, George Vourlias, Manassis Mitrakas, and Konstantinos Simeonidis. 2022. "Tuning the Fe(II)/hydroxide Ratio during Synthesis of Magnetite Nanoparticles to Maximize Cr(VI) Uptake Capacity" Water 14, no. 9: 1335. https://doi.org/10.3390/w14091335
APA StyleKalaitzidou, K., Chioti, E., Asimakidou, T., Karfaridis, D., Vourlias, G., Mitrakas, M., & Simeonidis, K. (2022). Tuning the Fe(II)/hydroxide Ratio during Synthesis of Magnetite Nanoparticles to Maximize Cr(VI) Uptake Capacity. Water, 14(9), 1335. https://doi.org/10.3390/w14091335










