Abstract
Plants, fungi, bacteria and protozoa are highly interconnected in constructed wetlands. These heterogeneous groups of organisms constitute a single system with complex internal trophic interactions. Thus, the joint activity of micro- and macroorganisms in constructed wetlands provides highly efficient wastewater treatment: both nutrients and complex organic substances can be effectively removed in branched trophic chains. The bacterial community of constructed wetlands has recently received much attention, while the fungal component remains less studied, particularly saprotrophic fungi. This paper reveals a taxonomic analysis of the cultivated saprotrophic fungi combined with the bacterial community in vertical flow constructed wetlands (VSCWs) operated by the Azoé-NP® process. These systems have unique features to affect the microbial community, which results in a high treatment efficiency and nitrogen removal. There are very few studies of saprotrophic fungi in VFCWs, while this work shows their abundance and diversity in VFCWs. We found 62 species of cultivated microscopic fungi and described the taxonomic composition of bacterial and fungal community at all wastewater treatment stages. In the studied VFCWs, we identified the species of micromycetes, which proved effective in the removal of contaminants. The data obtained can provide a deeper insight into the characteristics of Azoé-NP® systems and the treatment processes occurring in constructed wetlands.
1. Introduction
The problem of wastewater treatment in the modern world is extremely acute. The development of industry and agriculture has led to different wastewater types with a wide range of pollutants. To date, there are many approaches to wastewater treatment: (i) various types of membrane biofilters and rotating biological contactors that surpass conventional activated sludge systems in treatment quality [1]; (ii) advanced oxidation processes (AOPs) that degrade refractory organic pollutants from petrochemical industrial wastewater using highly reactive radicals [2]; (iii) adsorption methods have also proven to be effective in removing toxic heavy metals from wastewater [3].
One of the modern and cost-effective nature-based solutions for wastewater treatment is a Vertical Flow Constructed Wetland (VFCW) [4,5,6,7]. This system can have various modifications. The classical French system is widespread and designed to treat wastewater for small communities (less than 4000 p.e.). It is very effective in removing organic matter, but less effective in removing nitrogen and phosphorus [8,9,10,11]. To improve denitrification and phosphorus removal, the company SCIRPE developed the Azoé-NP® process (EP1857419A1): the incoming wastewater passes through a biological aerobic trickling filter before the first stage of the treatment [10,12].
The wastewater treatment in a VFCW is based on the biological processes of degradation and adsorption of pollutants by microorganisms and plants [13]. The efficiency of pollutant removal is determined by the activity of specific functional groups of microorganisms [14,15,16,17]. Nitrogen (N) is removed from wastewater mainly microbially by nitrification/denitrification and anammox processes [18]. Bacteria and fungi play an important role in the removal of phosphorus (P) [19].
The role of bacteria in constructed wetlands is being actively studied, while the contribution of microscopic fungi remains mostly neglected. However, one of the key functions of fungi is the decomposition of complex organic compounds. Fungi have a complex, well-developed enzymatic apparatus and are able to exist under a wide range of conditions, which makes them extremely important in the bioremediation and biodegradation of organic substances [20,21,22]. A large number of studies show that fungi can efficiently remove pollutants from the environment (soil or water), although many of their mechanisms are still unclear [20,21,22,23]. The current pathways of fungal detoxification include: biodegradation, biosorption (immobilization), chemical modification (change in bioavailability) and volatilization [24,25].
Fungi, plants and bacteria form a complex biotic relationship within a constructed wetland. This relationship affects the efficiency of treatment processes. For instance, fungi and plants form mycorrhizae. Due to the mycelial network, they notably increase the absorptive surface area of plant roots, thereby increasing the removal efficiency of pollutants from wastewater [22]. Moreover, fungi can facilitate bioremediation, not only by removing pollutants directly, but also by removing toxic substances and plant growth inhibitors [21]. Plants, in turn, help with the survival of several fungi species involved in water detoxification, for example, such as in the wastewater treatment of the textile industry [21].
It was revealed that the mycorrhization of plants in CWs by arbuscular mycorrhizal fungi (AM-fungi) stimulate plant growth, the activity of antioxidant enzymes (peroxidase and superoxide dismutase) and the content of soluble protein in plants, which increased the efficiency of pollutants removal [26]. A meta-analysis [27] showed that wetland plants significantly benefit from association with AM-fungi. The degree of the arbuscular mycorrhization of plants may be an important factor in the functioning of CWs. Plants and fungi increase each other’s viability, which has a positive impact on biological purification [22]. Nevertheless, there are many phytopathogenic species among fungi that can negatively affect plants. Moreover, many fungal species involved in bioremediation can release toxins into the environment, which can decrease the quality of the treated water [28].
The relationships between fungi and bacteria can be extremely diverse—from complete competitive exclusion to mutual cooperation and endosymbiosis. As a rule, in the natural environment, fungi and bacteria serve as destructors of organic matter. This makes it possible to efficiently decompose complex substrates requiring several stages of transformation [29]. Microorganisms (bacteria and fungi) are the main agents of biological wastewater treatment in VFCWs. Therefore, studying their taxonomic and functional structure is crucial for the improvement and development of VFCWs [30].
The novelty of this study is that fungi from VFCWs with the Azoé-NP® process were studied by cultural methods, which revealed mainly saprotrophic and facultative phytopathogenic fungi. In contrast to metagenomic methods, this approach does not give a complete picture of the fungal composition, but it allows us to isolate pure cultures and form a collection of fungal strains available for the further study of their physiological properties and their potential as pollutant destructors. An analysis of the species composition and possible functions of saprotrophic fungi combined with data on the taxonomic bacterial structure will provide a better understanding of the removal of complex organic pollutants in wastewater treatment plants.
2. Materials and Methods
2.1. Treatment Plants and Sampling
The study included two VFCWs operating by the Azoé-NP® process. (Saint Desert or D and Vercia or V). Saint Desert is located in the Saône et Loire department and Vercia in the Jura department, France. These CWs are used for the treatment of domestic wastewater and seasonal agricultural wastewater from winery production. The treatment capacities Saint Desert and Vercia are 1000- and 1200-person equivalent, respectively. They have the same hydraulic retention time and are planted with common reed (Phragmites australis). The investigated facilities have different operating times; at the time of sampling, Saint Desert and Vercia had been operating for 6 and 12 years, respectively.
The data on the quality of incoming and outgoing waters (total suspended solids or TSS, biological oxygen demand or BOD5, chemical oxygen demand or COD, NH4+, NO3− and total nitrogen or TN) were provided by the company SCIRPE. The data was obtained from several sampling campaigns where three samples per day were taken for several days. The average values were then calculated from the raw data.
The studied CWs have the same technological scheme. The untreated wastewater goes to the aerobic trickling filter, where it is atomized into small drops and flows down the metal mesh. Then, it passes successively the first and second stages of purification. The scheme of the first and second stages of purification is the same and is shown in Figure 1. In order to facilitate nitrogen removal, each stage of treatment is always partially filled with water to create an anoxic zone near the bottom of the filter bed.
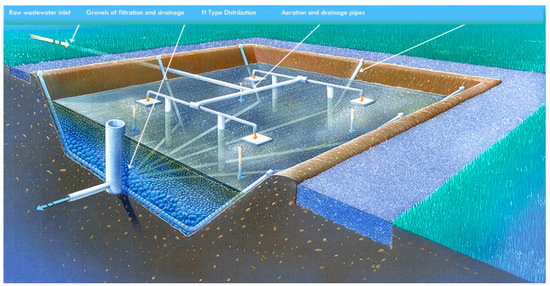
Figure 1.
Scheme of a filter bed in the studied CWs. Rooted soil samples for analysis were taken from the central area of each filter bed. Designed by “Jean Claude Michel”, Copyright 2003.
The samples of microscopic fungi were taken at both stages of wastewater treatment. The samples of surface sludge deposits with rooted soil (about 10 cm deep) were collected in Eppendorf microtubes and sent for storage at a temperature of −28 °C for further analysis.
The composition of microscopic fungi was studied using the cultivation method. For each CW, 10 samples were selected from trickling filters, Stages 1 and 2 (Tri, St 1, St 2). The isolation of micromycetes from the samples was performed by seeding on solid nutrient media from serial dilutions according to the standard method [31]. From the original sample, a series of consecutive dilutions was prepared in sterile water in 10-fold increments. A volume of 0.1 mL of suspension from each dilution was applied to the surface of Petri dishes with agar medium and evenly distributed with a sterile spatula. Two media were used—malt extract agar MEA and Czapek agar CZA with the addition of the ceftriaxone. The Petri dishes with sowing were incubated for seven days at 25 °C.
The total number of colony-forming units (CFU) and the number of different morphotypes in each sample were calculated. The Pure cultures were isolated. The primary identification of cultures was carried out by morphological characters using keys and literature [32]. Additionally, the cultures were verified with Sanger sequencing described below.
For a better understanding of the processes occurring in these treatment facilities, it is necessary to supplement the data obtained with an analysis of the bacterial component of the microbial community. The data for this analysis were taken from Sequence Read Archive (SRA), Bioproject #PRJNA785375: Structure of the microbial communities in vertical flow constructed wetlands (Kharitonov et al., unpublished data).
2.2. DNA Extraction and Sequencing
The total DNA was isolated from the samples of axenic cultures of fungi. Sample lysis was performed using a MagNA Lyser 230B (Roche, Basel, Switzerland). The total DNA concentration was estimated using NanoDrop ND-1000 isolated DNA stored at −28 °C until following procedures. Regions ITS1-ITS4 were subjected to Sanger sequencing with primers TCC GTA GGT GAA CCT GCG G (ITS1) and TCC TCC GCT TAT TGA TAT GC (ITS4) [33,34]. The sequence of the amplified regions was read using an automatic sequencer at Evrogen JSC (https://evrogen.com/index.shtml, 1 July 2021). The read length was 650 base pairs. Species names and taxonomy are concordant with the Index Fungorum database (http://www.indexfungorum.org, 1 August 2021).
2.3. Bioinformatic Analysis
The primary analysis of the results of 16S rRNA genes sequencing was carried out using DADA2 package for R (Bioconductor project) [35]. The analysis included the following steps: (1) primer sequences were removed using cutadapt [36]; (2) reads were trimmed and filtered by quality; (3) error distribution models were derived from read quality profiles; (4) the sequencing errors were corrected; (5) ribosomal sequence variants (RSV) were inferred; (6) forward and reverse RSVs were merged; and (7) chimeric RSVs were eliminated (they accounted for 2.2% RSVs and 0.5% reads). Next, the taxonomic annotation of the RSVs was carried out (7551 RSVs were obtained after the removal of chimeras) using DADA and the Silva v.132 reference sequence database [37].
3. Results
3.1. Chemical Analysis
The data obtained showed the efficiency of wastewater pretreatment with a trickling filter. In Azoé-NP® facilities, the efficiency of nitrogen removal was relatively high for constructed wetlands. Both facilities effectively removed organic matter from wastewater. The results are shown in Table 1. Detailed information on the behavior of carbon and nitrogen in the studied VFCWs has been published by Maciejewski et al. (2022) [38].

Table 1.
The quality of incoming and outgoing waters in the two studied CWs. Provided by the company SCIRPE.
3.2. Taxonomic Analysis of Bacterial Community
The results of the taxonomic analysis of the bacterial community (including Archaea) are presented in Table 2.

Table 2.
The taxonomic structure of the two studied VFCWs Saint Desert (D) and Vercia (V) by stages (Inflow—incoming wastewater, T—trickling filter, St 1—first stage of treatment, St 2—second stage of treatment, Outflow—outgoing treated water). The values are expressed in sequencing reads count. Table is color-scaled according to the values in cells.
Five phyla dominated the incoming wastewater: Proteobacteria, Firmicutes, Actinobacteria, Verrucomicrobia and Bacteroidetes, which is common for domestic wastewaters. It is worth noting that the microbial community composition of the incoming wastewater was the same in both studied CWs. Since these facilities are located in the same area and planted with the same macrophytes, the treatment efficiency and microbial community composition is likely to be affected only by the operating time of the VFCWs.
The composition of bacterial community in the samples from stages 1 and 2 was very similar in the studied CWs; however, these samples showed a significantly higher biodiversity compared to the inflow wastewater. The most represented phyla were Proteobacteria, Bacteroidetes, Acidobacteria, Chloroflexi, Verrucomicrobia, Planctomycetes, Actinobacteria and Nitrospirae. In the outgoing treated water, biodiversity dramatically decreased: more than 80% of the remaining bacteria were Proteobacteria and Bacteroidetes with a small proportion of Patescibacteria, Firmicutes and Actinobacteria.
The trickling filters demonstrated some unique features. They contained a significant proportion of Proteobacteria and Bacteroidetes, similar to the incoming wastewater, stage 1 and 2, but there were groups not found in the other samples. In the St Desert trickling filter, we found Chloroflexi and Epsilonbacteraeota. In turn, the Vercia trickling filter contained a surprisingly high number of Cyanobacteria.
3.3. Fungi Biodiversity
Microscopic fungi were detected in all the studied samples. In total, 61 species of cultivated micromycetes belonging to 39 genera were found in the studied VFCWs. The most abundant were representatives of the Ascomycota. Basidiomycota included four yeasts, Apiotrichum sp., Filobasidium magnum, Rhodotorula mucilaginosa and Trichosporon sp. The mucoromycota was represented by three species of the genus Mucor and two species of Mortierella. One mycophilic species, Piptocephalis xenophila, was identified from the Zoopagomycota.
The species diversity of microscopic fungi was different in all stages. It was the lowest in the trickling filter—16 types for the St Desert and 11 for Vercia. This may be due to uniform conditions on the filter surface, and the absence of plants and soil. The first stage of treatment revealed that the micromycete species significantly increased in number: 32 and 41, respectively. This stage was found to be the richest in organic matter and heterogeneous in terms of microhabitats. At the second stage, the species diversity decreased again—11 and 14 species, which could be associated with decreased organic matter availability.
The taxonomic composition in all the studied samples appeared to be specific. Only Trichoderma harzianum was identified in all locations. In the five habitats, Aspergilus tubingensis, Fusarium culmorum, Humicola homopilata and Penicillium polonicum were found. The results are summarized in Table S1.
The trickling filters of the studied facilities were very different from each other (Figure 2). Bionectria solani, Penicillium roqueforti, Pseudallescheria boydii and Trichoderma harzianum prevailed in St Desert. For Vercia, the most common species were Rhodotorula mucilaginosa, Filobasidium magnum and Penicillium polonicum. Only seven species were common to the trickling filters of both facilities. In St Desert, the fungal composition on the trickling filter was closer to the first stage, but in Vercia it was remarkably different from all the studied habitats due to the abundance of Basidiomycetes.
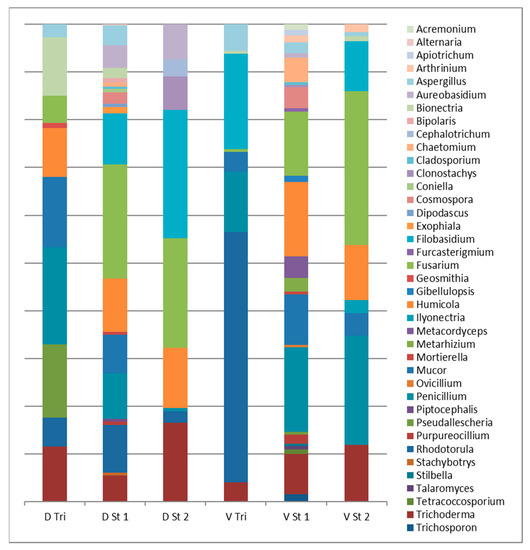
Figure 2.
Taxonimic composition of the cultivated fungi genera in CWs operated by Azoé-NP® process (St Desert and Vercia).
The first stages at the two facilities were similar in the species composition and taxonomic structure of the micromycetes. They had 15 species in common. The most common of both St Desert and Vercia were the phytopathogenic and cellulolytic Fusarium culmorum and Humicola homopilata. The second stages of St Desert and Vercia were also quite similar as both were dominated by Filobasidium magnum, Fusarium culmorum and Humicola homopilata.
In the investigated VFCWs localities, the fungi of various functional groups were identified (Figure 3). The most represented were the groups of saprotrophic fungi capable of assimilating complex polymers, cellulolytics and hydrolithics. These species belong to the genera Trichoderma, Humicola, Aspergillus and Penicillium. A significant proportion of phytopathogenic species were also found: Alternaria alternata, Bionectria solani, Bipolaris sorokiniana, Cladosporium cladosporioides, Clonostachys rosea, Coniella diplodiopsis, Fusarium culmorum, Fusarium oxysporum, Fusarium solani, Ilyonectria destructans, etc. The presence of potentially pathogenic species for humans and animals should also be noted: Apiotrichum sp., Dipodascus geotrichum, Exophiala alcalophila, Filobasidium magnum, Pseudallescheria boydii and Trichosporon sp.
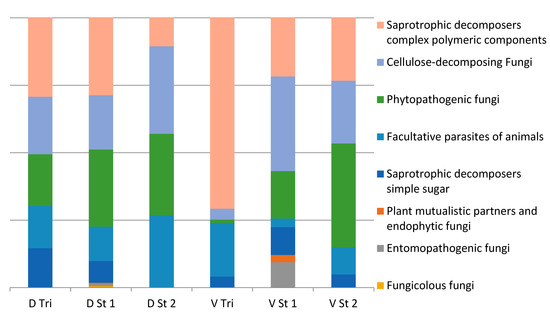
Figure 3.
Functional composition of the cultivated fungi genera in St Desert and Vercia.
The group of entomopathogenic species was not numerous: Metacordyceps chlamydosporia and Metarhizium anisopliae, both found in Versia stage 1, and one mycophilic species—Piptocephalis xenophila from the Zoopagomycota, was found only in St Desert stage 1. Interestingly, both CWs were revealed to contain Penicillium roqueforti—the species used for cheese production.
In terms of the ratio of functional groups, there were no significant differences between stages and structures. The only exception was the trickling filter in Vercia characterized by a sharp predominance of hydrolytics with an insignificant proportion of phytopathogens.
4. Discussion
The constructed wetlands operating by the Azoé-NP® process demonstrate a high level of nitrogen removal from wastewater. However, the structures of the bacterial community at stages 1 and 2 (where filtration and pollutant removal processes occur) are similar in the studied facilities and common for vertical flow constructed wetlands, which typically demonstrate relatively low nitrogen removal. This can be partly explained by the similarity of the incoming wastewater, since the incoming wastewater significantly determines the microbiome of the facility. According to the chemical analysis data, the incoming wastewaters in St Desert and Vercia are very close in terms of organic matter and nitrogen content. The composition of the microbial community of incoming wastewater in the studied facilities is also very similar. However, there are slight differences: wastewater entering Vercia has a higher content of organic matter than St Desert, and this is reflected in the microbiome. In Vercia, we observe higher levels of Bacteroidetes and Proteobacteria than in St Desert. It should be noted that our samples were taken from the surface area of CWs (about 10–15 cm in depth). Moreover, the Azoé-NP® process involves partially saturated filter beds to facilitate denitrification (Stage 1 and 2) so that water level is regulated and maintained at a certain height from the bottom. Therefore, denitrification processes should be most active under anaerobic conditions, near the bottom of the filter bed, which is not covered in our study.
In addition, the Azoé-NP® process involves the pretreatment of incoming wastewater on a trickling filter with ferric chloride. As it was previously established, a trickling filter makes a significant contribution to the denitrification processes [10]. Nevertheless, it is unknown how exactly the trickling filter stimulates nitrogen removal and which groups of microorganisms are involved in this process. Moreover, the filters contain a few phyla poorly presented in other locations: Chloroflexi and Epsilonbacteraeota in St Desert and Cyanobacteria in Vercia. A more detailed analysis of the species composition of Bacteria and Archaea on filters could shed light on the mechanism of nitrogen removal in similar VFCWs. The trickling filter is likely to create conditions favorable for aerobic nitrogen reduction coupled to the Fe3+/Fe2+ reduction–oxidation cycle [39].
The study of fungi in CWs has recently received more scientific attention. However, research has mainly focused on obligate biotrophic AM-fungi inhabiting plant roots and forming arbuscular mycorrhiza. Most of the information about fungi in CWs has been accumulated about that specific small group [26,27,40,41,42] and there are very few works on saprotrophic fungi. In some works, saprotrophs are discussed as an additional result of metagenomic studies aimed at AM-fungi [41] due to their importance for plant life. AM-fungi are a very specific group that cannot be cultivated on nutrient media; they can be studied directly in plant roots or with metagenomics methods.
Zhouying, X. U et al. [41] provided a detailed review of studies devoted to mycorrhization of plants in natural wetland habitats by AM-fungi. The authors concluded that AM-fungi can have a positive, negative or neutral effect on the productivity of different wetlands under various conditions. The effects of the artificial mycorrhization of plants in CWs by AM-fungi grown on roots under laboratory conditions have also been studied [42]. The physiological functions of wetland plants with high AM-fungi colonization can be improved under certain water conditions [42].
There are very few studies on saprotrophic fungi, but this work reveals their abundance and diversity in CWs. The data obtained show a relatively wide variety of microscopic fungi in CWs, both taxonomic and functional, including saprotrophs. Fungi produce a wide range of extracellular enzymes that decompose almost all types of organic matter, mainly of plant origin; this ability helps them to degrade various pollutants of anthropogenic origin. Many types of fungi can function as a biosorbent of toxic compounds and metals, accumulating them inside and on the surface of the mycelium [43], which makes them crucial for bioremediation. In addition, they form various relationships with plants [44], which is a very important consideration for constructed wetlands.
Fungi play an important role in the biodegradation of pollutants. In the studied VFCWs, we identified the species of micromycetes that proved effective in the removal of contaminants. Thus, the representatives of the genus Trichoderma can remove phenolic compounds [45], in particular, Trichoderma harzianum, and destroy pentachlorophenol [46]. The members of the genera Mucor, Aspergillus and Penicillium can remove crude oil hydrocarbons [47,48]. For many micromycetes, the ability to eliminate detergents and surfactants was noted, including the species of the genera Aspergillus, Curvularia, Drechslera, Fusarium, Mucor, Penicillium and Trichoderma found in the studied VFCWs [49,50,51,52]. Another purification mechanism is the biosorption of metals by mycelium. Mucor, Alternaria, and Aspergillus were found to be capable of removing toxic metals such as Cd Pb, Cu and Zn [53,54].
The study of fungal role at each treatment stage requires further research. However, it can be noted that endophytic fungi may increase the effectiveness of CWs. Pietro-Souza W. et al. showed that endophytic fungi Aspergillus sp., Curvularia geniculata, Lindgomycetaceae and Westerdykella sp. were effective in removing mercury in conjunction with the host plant. The fungi promoted the growth of macrophytes, their resistance to mercury and accumulation of pollutants in their tissues. Endophytes are known to have a positive effect on plant growth through the production of phytohormones (e.g., indoleacetic acid), which increases the nutrient availability and protects against biotic and abiotic stress [25].
Moreover, it should be emphasized that phytopathogenic fungi were present, mostly at stages 1 and 2, where plants were the main component of the system. The most hazardous species included the members of the genus Fusarium (F. culmorum, F. oxysporum and F. solani) as they can cause root rot and the vascular wilting of plants [55].
This leads us to the conclusion that the revealed species of fungi play a considerable role in wastewater treatment processes. Consequently, their contribution to the functioning of CWs cannot be underestimated and deserves no less attention than bacteria. A greater awareness of the role of fungi in removing pollutants may help to develop more advanced and cost-effective nature-based technologies for biological wastewater treatment.
Our findings confirm that a comprehensive study of CW microbiota is important at all treatment stages. Molecular methods of high-throughput and Sanger sequencing combined with classical microbial cultural methods allow us to obtain more data on all components of the microbiocenosis. At the same time, this provides a collection of pure cultures to study their physiology and biodegrading ability in more detail.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w14050698/s1, Table S1: The list of the found fungi species in the two VFCWs operated by Azoé-NP® process.
Author Contributions
S.K.: Writing—Original Draft, Review & Editing, Investigation, Conceptualization. N.S.: Project administration, Conceptualization, Methodology. K.M.: Investigation, Validation. P.M.: Methodology, Validation, Resources. M.G.: Supervision, Conceptualization. R.G.: Supervision, Conceptualization. M.S.: Methodology, Conceptualization. G.K.: Software, Formal analysis, Data Curation. A.A.: Review & Editing, Investigation, Formal analysis. A.S.: Investigation, Formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Russian Foundation for Basic Research, project no. 18-29-25027.
Informed Consent Statement
Not applicable.
Data Availability Statement
Hight-throughput sequencing data is submitted to Sequence Read Archive (SRA), Bioproject #PRJNA785375: Structure of the microbial communities in vertical flow constructed wetlands (Kharitonov et al., unpublished data); Fungi Sanger sequencing data is submitted to GenBank database, SUB10747933.
Acknowledgments
The authors would like to thank the company SCIRPE for their active cooperation in research, sampling and the data provided.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Waqas, S.; Bilad, M.R.; Man, Z.B.; Suleman, H.; Nordin, N.A.H.; Jaafar, J.; Othman, M.H.D.; Elma, M. An Energy-Efficient Membrane Rotating Biological Contactor for Wastewater Treatment. J. Clean. Prod. 2021, 282, 124544. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Sillanpää, M. A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Dang, J.; Wang, H.; Wang, C. Adsorption of Toxic Zinc by Functionalized Lignocellulose Derived from Waste Biomass: Kinetics, Isotherms and Thermodynamics. Sustainability 2021, 13, 10673. [Google Scholar] [CrossRef]
- Gourdon, R.; Kania, M.; Gautier, M.; Kim, B.; Michel, P. Treatment of domestic wastewater from small cities on vertical flow constructed wetlands (VFCWs). In Proceedings of the 4th Congrès International de Géotechnique—Ouvrages -Structures, Ho Chi Minh City, Vietnam, 26–27 October 2017; pp. 1066–1073. [Google Scholar] [CrossRef]
- Abou-Elela, S.I.; Hellal, M. Municipal wastewater treatment using vertical flow constructed wetlands planted with Canna, Phragmites and Cyprus. Ecol. Eng. 2012, 47, 209–213. [Google Scholar] [CrossRef]
- Torrens, A.; Molle, P.; Boutin, C.; Salgot, M. Impact of design and operation variables on the performance of vertical-flow constructed wetlands and intermittent sand filters treating pond effluent. Water Res. 2009, 43, 1851–1858. [Google Scholar] [CrossRef]
- Molle, P.; Liénard, A.; Grasmick, A.; Iwema, A. Effect of reeds and feeding operations on hydraulic behaviour of vertical flow constructed wetlands under hydraulic overloads. Water Res. 2006, 40, 606–612. [Google Scholar] [CrossRef]
- Brix, H.; Arias, C.A. The use of vertical flow constructed wetlands for on-site treatment of domestic wastewater: New Danish guidelines. Ecol. Eng. 2005, 25, 491–500. [Google Scholar] [CrossRef]
- Kadlec, R.; Knight, R.; Vymazal, J.; Brix, H.; Cooper, P.; Haberl, R. Constructed Wetlands for Pollution Control; IWA Publishing: London, UK, 2000. [Google Scholar]
- Kim, B.; Gautier, M.; Prost-Boucle, S.; Molle, P.; Michel, P.; Gourdon, R. Performance evaluation of partially saturated vertical-flow constructed wetland with trickling filter and chemical precipitation for domestic and winery wastewaters treatment. Ecol. Eng. 2014, 71, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Molle, P. French vertical flow constructed wetlands: A need of a better understanding of the role of the deposit layer. Water Sci. Technol. 2013, 69, 106–112. [Google Scholar] [CrossRef]
- Molle, P.; Liénard, A.; Boutin, C.; Merlin, G.; Iwema, A. How to treat raw sewagewith constructed wetlands: An overview of the French systems. Water Sci. Technol. 2005, 51, 11–21. [Google Scholar] [CrossRef]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Müller, R.A.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef]
- Faulwetter, J.L.; Gagnon, V.; Sundberg, C.; Chazarenc, F.; Burr, M.D.; Brisson, J.; Camper, A.K.; Stein, O.R. Microbial processes influencing performance of treatment wetlands: A review. Ecol. Eng. 2009, 35, 987–1004. [Google Scholar] [CrossRef]
- Scholz, M.; Lee, B.H. Constructed wetlands: A review. Int. J. Environ. Res. 2005, 62, 1256–1261. [Google Scholar] [CrossRef]
- Huang, D.L.; Zeng, G.M.; Feng, C.L.; Hu, S.; Jiang, X.Y.; Tang, L.; Su, F.F.; Zhang, Y.; Zeng, W.; Liu, H.L. Degradation of lead-contaminated lignocellulosic waste by Phanerochaete chrysosporium and the reduction of lead toxicity. Environ. Sci. Technol. 2008, 42, 4946–4951. [Google Scholar] [CrossRef]
- Cheng, M.; Huang, C.; Wu, H.P.; Qin, L. Synthesis of surface molecular imprinted TiO2/graphene photocatalyst and its highly efficient photocatalytic degradation of target pollutant under visible light irradiation. Appl. Surf. Sci. 2016, 390, 368–376. [Google Scholar]
- Wallace, S.; Austin, D. Emerging models for nitrogen removal in treatment wetlands. J. Environ. Health 2008, 71, 10–16. [Google Scholar] [PubMed]
- Oehl, F.; Sieverding, E.; Dubois, D.; Ineichen, K.; Boller, T.; Wiemken, A. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 2004, 138, 574–583. [Google Scholar] [CrossRef]
- Samer, M. Biological and chemical wastewater treatment processes. Wastewater Treat. Eng. 2015, 150, 1–50. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, R.; Khardenavis, A.A.; Purohit, H.J. Diverse Metabolic Capacities of Fungi for Bioremediation. Indian J. Microbiol. 2016, 56, 247–264. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Tripathi, R.; Ranjan, A.; Srivastava, A.K. Fungi as potential candidates for bioremediation. In Abatement of Environmental Pollutants: Trends and Strategies; Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–191. [Google Scholar] [CrossRef]
- Gazem, M.A.H.; Nazareth, S. Sorption of lead and copper from an aqueous phase system by marine-derived Aspergillus species. Ann. Microbiol. 2013, 63, 503–511. [Google Scholar] [CrossRef]
- Singh, M.P.; Vishwakarma, S.K.; Srivastava, A.K. Bioremediation of Direct Blue 14 and Extracellular Ligninolytic Enzyme Production by White Rot Fungi: Pleurotus Spp. BioMed Res. Int. 2013, 2013, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Pietro-Souza, W.; de Campos Pereira, F.; Mello, I.S.; Stachack, F.F.F.; Terezo, A.J.; da Cunha, C.N.; Whitee, J.F.; Lif, H.; Soares, M.A. Mercury resistance and bioremediation mediated by endophytic fungi. Chemosphere 2020, 240, 124–874. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Hu, S.; Chen, Z.; Vymazal, J. Employ of arbuscular mycorrhizal fungi for pharmaceuticals ibuprofen and diclofenac removal in mesocosm-scale constructed wetlands. J. Hazard. Mater. 2021, 409, 124524. [Google Scholar] [CrossRef] [PubMed]
- Viga, T.K.R.; Aguilar, R.; Castillo-Argüero, S.; Chiappa-Carrara, X.; Guadarrama, P.; Ramos-Zapata, J. Wetland plant species improve performance when inoculated with arbuscular mycorrhizal fungi: A meta-analysis of experimental pot studies. Mycorrhiza 2018, 28, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, B. Fungi: Ecological importance and impact on humans. eLS 2011. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiol. Ecol. 2011, 78, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Shchegolkova, N.; Krasnov, G.S.; Belova, A.A.; Dmitriev, A.; Kharitonov, S.; Klimina, K.; Melnikova, N.V.; Kudryavtseva, A. Microbial Community Structure of Activated Sludge in Treatment Plants with Different Wastewater Compositions. Front. Microbiol. 2016, 7, 90. [Google Scholar] [CrossRef] [Green Version]
- Crous, P.W.; Verkley, G.J.M.; Groenewald, J.Z.; Samson, R.A. Fungal Biodiversity; CBS Laboratory Manual Series; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2009; p. 269. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag: Ehing, Germany, 2007; p. 672. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Manter, D.K.; Vivanco, J.M. Use of the ITS primers, ITS1F and ITS4, to characterize fungal abundance and diversity in mixed-template samples by qPCR and length heterogeneity analysis. J. Microbiol. Methods 2007, 71, 7–14. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Mulder, M.; Radjabzadeh, D.; Jong, J.C.K.-D.; Uitterlinden, A.G.; Kraaij, R.; Stricker, B.H.; Verbon, A. Long-term effects of antimicrobial drugs on the composition of the human gut microbiota. Gut Microbes 2020, 12, 1795492. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, K.; Gautier, M.; Kim, B.; Michel, P.; Gourdon, R. Effect of trickling filter on carbon and nitrogen removal in vertical flow treatment wetlands: A full-scale investigation. J. Environ. Manag. 2022, 303, 114159. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Hou, L.; Li, H. Effects of pollution load and salinity shock on nitrogen removal and bacterial community in two-stage vertical flow constructed wetlands. Bioresour. Technol. 2021, 342, 126031. [Google Scholar] [CrossRef]
- Calheiros, C.S.C.; Pereira, S.I.A.; Franco, A.R.; Castro, P.M.L. Diverse Arbuscular Mycorrhizal Fungi (AMF) Communities Colonize Plants Inhabiting a Constructed Wetland for Wastewater Treatment. Water 2019, 11, 1535. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Ban, Y.; Jiang, Y.; Zhang, X.; Liu, X. Arbuscular Mycorrhizal Fungi in Wetland Habitats and Their Application in Constructed Wetland: A Review. Pedosphere 2016, 26, 592–617. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Z.; Vosátka, M.; Vymazal, J. Arbuscular mycorrhizal fungi colonization and physiological functions toward wetland plants under different water regimes. Sci. Total Environ. 2020, 716, 137040. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef] [Green Version]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. 2019, 94, 1443–1476. [Google Scholar] [CrossRef] [Green Version]
- Divya, L.M.; Prasanth, G.K.; Sadasivan, C. Potential of the salt-tolerant laccase-producing strain Trichoderma viride Pers. NFCCI-2745 from an estuary in the bioremediation of phenol-polluted environments. J. Basic Microbiol. 2013, 54, 542–547. [Google Scholar] [CrossRef]
- Vacondio, B.; Birolli, W.; Ferreira, I.M.; Seleghim, M.H.; Gonçalves, S.; Vasconcellos, S.P.; Porto, A.L.M. Biodegradation of pentachlorophenol by marine-derived fungus Trichoderma harzianum CBMAI 1677 isolated from ascidian Didemnun ligulum. Biocatal. Agric. Biotechnol. 2015, 4, 266–275. [Google Scholar] [CrossRef]
- Hickey, P. Toxicity of water-soluble fractions of crude oil on some bacteria and fungi Isolated from marine water. Am. J. Anim. Res. 2013, 3, 24–29. [Google Scholar]
- Maruthi, Y.; Hossain, K.; Thakre, S. Aspergillus flavus: A potential Bioremediator for oil contaminated soils. Eur. J. Sustain. Dev. 2013, 2, 57. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-T.; Lee, J.-F.; Liu, K.-H.; Liao, Y.-F.; Yang, V. Immobilization of fungal laccase onto a nonionic surfactant-modified clay material: Application to PAH degradation. Environ. Sci. Pollut. Res. 2016, 23, 4024–4035. [Google Scholar] [CrossRef]
- Balaji, V.; Arulazhagan, P.; Ebenezer, P. Enzymatic bioremediation of polyaromatic hydrocarbons by fungal consortia enriched from petroleum contaminated soil and oil seeds. J. Environ. Biol. 2014, 35, 521–529. [Google Scholar]
- Lladó, S.; Covino, S.; Solanas, A.; Viñas, M.; Petruccioli, M.; D’Annibale, A. Comparative assessment of bioremediation approaches to highly recalcitrant PAH degradation in a real industrial polluted soil. J. Hazard. Mater. 2013, 248, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, R.; Cordero, P.R.; Bautista, G.S.; Dedeles, G.R. Reduction of hexavalent chromium using fungi and bacteria isolated from contaminated soil and water samples. Chem. Ecol. 2013, 29, 320–328. [Google Scholar] [CrossRef]
- Sousa, N.R.; Ramos, M.A.; Marques, A.P.; Castro, P.M. A genotype dependent-response to cadmium contamination in soil is displayed by Pinus pinaster in symbiosis with different mycorrhizal fungi. Appl. Soil Ecol. 2014, 76, 7–13. [Google Scholar] [CrossRef]
- Krishnamurthy, Y.L.; Naik, B.S. Endophytic fungi bioremediation. In Endophytes: Crop Productivity and Protection; Maheshwari, D.K., Annapurna, K., Eds.; Springer: Cham, Switzerland, 2017; pp. 47–60. [Google Scholar] [CrossRef]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).