Fungi in Freshwaters: Prioritising Aquatic Hyphomycetes in Conservation Goals
Abstract
:1. Introduction
2. Threats to Freshwater Ecosystems and Biodiversity of Aquatic Hyphomycetes
2.1. Hydrological Alterations
2.2. Agricultural Practices
2.3. Invasive Species
2.4. Climate Changes
2.5. Other Major Pollutants
3. Biodiversity Decline in Freshwaters Is Alarming but Adequate Knowledge on the Biodiversity of Aquatic Hyphomycetes Is Missing
4. A Responsible Approach towards Understanding the Biodiversity of All Fungi, including Aquatic Hyphomycetes, Is Needed
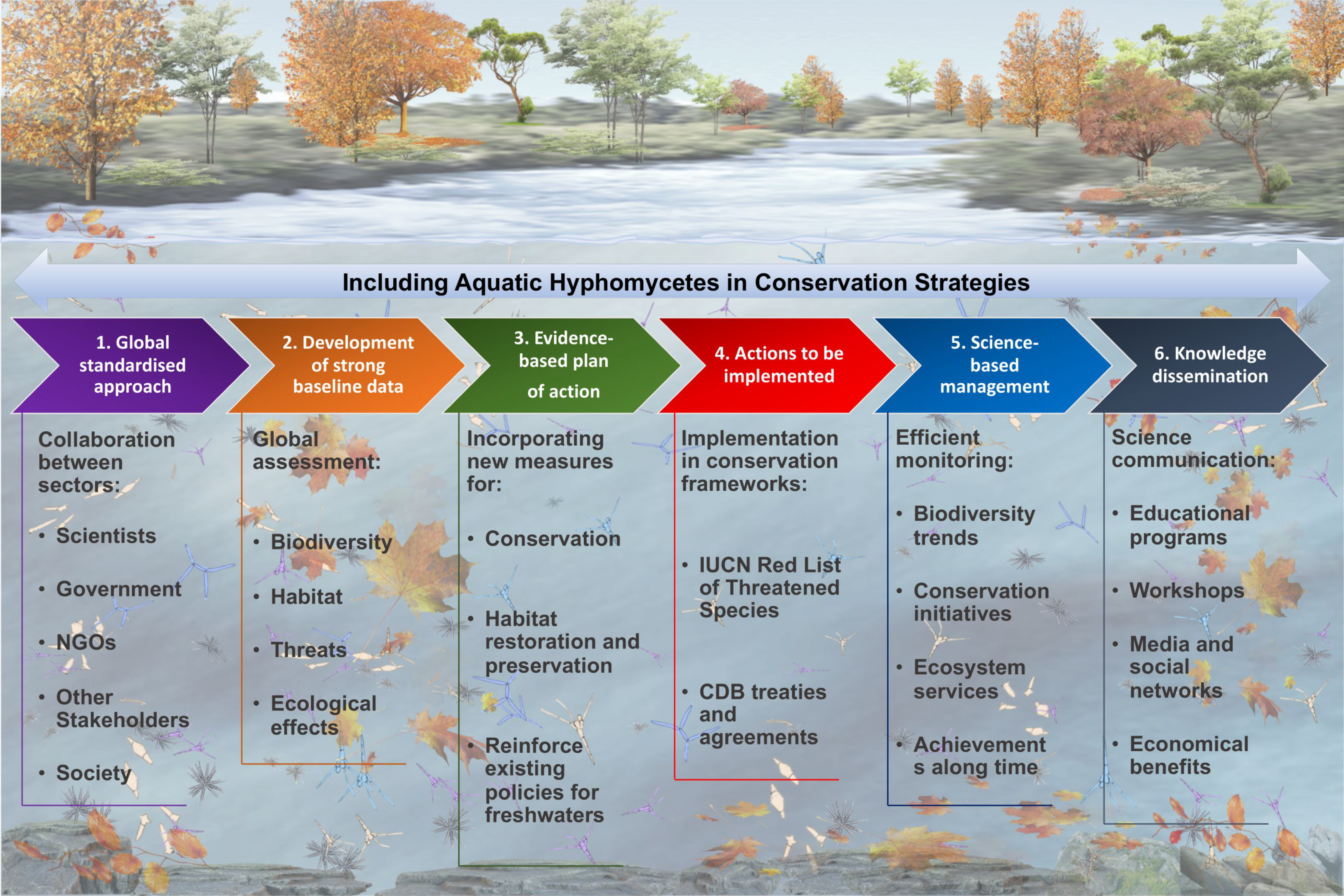
5. Embracing Aquatic Fungi in the Freshwater Biodiversity Conservation Strategies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shiklomanov, I.A. World Fresh Water Resource. In Water Crisis: A Guide to World Fresh Water Resources; Gleick, P.H., Ed.; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Gleick, P.H. Basic Water Requirements for Human Activities: Meeting Basic Needs. Water Int. 1996, 21, 83–92. [Google Scholar] [CrossRef]
- Garcia-Moreno, J.; Harrison, I.J.; Dudgeon, D.; Clausnitzer, V.; Darwall, W.; Farrell, T.; Savy, C.; Tockner, K.; Tubbs, N. Sustaining Freshwater Biodiversity in the Anthropocene BT. In The Global Water System in the Anthropocene: Challenges for Science and Governance; Bhaduri, A., Bogardi, J., Leentvaar, J., Marx, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 247–270. ISBN 978-3-319-07548-8. [Google Scholar]
- Dudgeon, D. Multiple Threats Imperil Freshwater Biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.; Kroll, C.; Lafortune, G.; Fuller, G.; Woelm, F. The Sustainable Development Goals Report 2021; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Naeem, S.; Chazdon, R.; Duffy, J.E.; Prager, C.; Worm, B. Biodiversity and Human Well-Being: An Essential Link for Sustainable Development. Proc. R. Soc. B Biol. Sci. 2016, 283, 20162091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almond, R.E.A.; Grooten, M.; Peterson, T. Living Planet Report 2020-Bending the Curve of Biodiversity Loss; World Wildlife Fund: Gland, Switzerland, 2020. [Google Scholar]
- Flitcroft, R.; Cooperman, M.S.; Harrison, I.J.; Juffe-Bignoli, D.; Boon, P.J. Theory and Practice to Conserve Freshwater Biodiversity in the Anthropocene. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the World’s Free-Flowing Rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Arthington, A.H. Grand Challenges to Support the Freshwater Biodiversity Emergency Recovery Plan. Front. Environ. Sci. 2021, 9, 118. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [Green Version]
- Milardi, M.; Gavioli, A.; Soininen, J.; Castaldelli, G. Exotic Species Invasions Undermine Regional Functional Diversity of Freshwater Fish. Sci. Rep. 2019, 9, 17921. [Google Scholar] [CrossRef]
- Senn-Irlet, B.; Heilmann-Clausen, J.; Genney, D.; Dahlberg, A. Guidance for Conservation of Macrofungi in Europe; ECCF: Strasbourg, France, 2007. [Google Scholar]
- Li, D.-W. Biology of Microfungi; Springer: Berlin/Heidelberg, Germany, 2016; Volume 146. [Google Scholar]
- Ingold, C.T. Aquatic Hyphomycetes of Decaying Alder Leaves. Trans. Br. Mycol. Soc. 1942, 25, 339–417. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The Ecology of Running Waters; Liverpool University Press: Liverpool, UK, 1970; Volume 555. [Google Scholar]
- Graça, M.A.S. The Role of Invertebrates on Leaf Litter Decomposition in Streams—A Review. Int. Rev. Hydrobiol. 2001, 86, 383–393. [Google Scholar] [CrossRef]
- Graça, M.A.S.; Canhoto, C. Leaf Litter Processing in Low Order Streams. Limnetica 2006, 25, 1–10. [Google Scholar] [CrossRef]
- Duarte, S.; Bärlocher, F.; Pascoal, C.; Cássio, F. Biogeography of Aquatic Hyphomycetes: Current Knowledge and Future Perspectives. Fungal Ecol. 2016, 19, 169–181. [Google Scholar] [CrossRef]
- Shearer, C.A.; Descals, E.; Kohlmeyer, B.; Kohlmeyer, J.; Marvanová, L.; Padgett, D.; Porter, D.; Raja, H.A.; Schmit, J.P.; Thorton, H.A.; et al. Fungal Biodiversity in Aquatic Habitats. Biodivers. Conserv. 2007, 16, 49–67. [Google Scholar] [CrossRef]
- Duarte, S.; Pascoal, C.; Cássio, F.; Bärlocher, F. Aquatic Hyphomycete Diversity and Identity Affect Leaf Litter Decomposition in Microcosms. Oecologia 2006, 147, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Seena, S.; Casotti, C.; Cornut, J. Inter- and Intraspecific Functional Variability of Aquatic Fungal Decomposers and Freshwater Ecosystem Processes. Sci. Total Environ. 2020, 707, 135570. [Google Scholar] [CrossRef]
- Handa, I.T.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O.; Chauvet, E.; Gessner, M.O.; Jabiol, J.; Makkonen, M.; et al. Consequences of Biodiversity Loss for Litter Decomposition across Biomes. Nature 2014, 509, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Boyero, L.; Pearson, R.G.; Gessner, M.O.; Barmuta, L.A.; Ferreira, V.; Graça, M.A.S.; Dudgeon, D.; Boulton, A.J.; Callisto, M.; Chauvet, E.; et al. A Global Experiment Suggests Climate Warming Will Not Accelerate Litter Decomposition in Streams but Might Reduce Carbon Sequestration. Ecol. Lett. 2011, 14, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity Meets Decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Gessner, M.O.; Chauvet, E. A Case for Using Litter Breakdown to Assess Functional Stream Integrity. Ecol. Appl. 2002, 12, 498–510. [Google Scholar] [CrossRef]
- Young, R.G.; Matthaei, C.D.; Townsend, C.R. Organic Matter Breakdown and Ecosystem Metabolism: Functional Indicators for Assessing River Ecosystem Health. J. N. Am. Benthol. Soc. 2008, 27, 605–625. [Google Scholar] [CrossRef]
- Water, U.N. The United Nations World Water Development Report 2021: Valuing Water; UNESCO: Paris, France, 2021. [Google Scholar]
- 2030 Water Resources Group. Charting Our Water Future Economic Frameworks to Inform Decision-Making; McKinsey & Company: Hong Kong, China, 2009. [Google Scholar]
- Alcamo, J. Environmental Futures: The Practice of Environmental Scenario Analysis; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Covich, A.P.; Austen, M.C.; BÄRlocher, F.; Chauvet, E.; Cardinale, B.J.; Biles, C.L.; Inchausti, P.; Dangles, O.; Solan, M.; Gessner, M.O.; et al. The Role of Biodiversity in the Functioning of Freshwater and Marine Benthic Ecosystems. BioScience 2004, 54, 767–775. [Google Scholar] [CrossRef]
- Chauvet, E.; Cornut, J.; Sridhar, K.R.; Selosse, M.-A.; Bärlocher, F. Beyond the Water Column: Aquatic Hyphomycetes Outside Their Preferred Habitat. Fungal Ecol. 2016, 19, 112–127. [Google Scholar] [CrossRef] [Green Version]
- Pascoal, C.C.; Marvanová, L. Anthropogenic Stress May Affect Aquatic Hyphomycete Diversity More than Leaf Decomposition in a Low-Order Stream. Arch. Für Hydrobiol. 2005, 162, 481–496. [Google Scholar] [CrossRef]
- Solé, M.; Fetzer, I.; Wennrich, R.; Sridhar, K.R.; Harms, H.; Krauss, G. Aquatic Hyphomycete Communities as Potential Bioindicators for Assessing Anthropogenic Stress. Sci. Total Environ. 2008, 389, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Junk, W.J.; An, S.; Finlayson, C.M.; Gopal, B.; Květ, J.; Mitchell, S.A.; Mitsch, W.J.; Robarts, R.D. Current State of Knowledge Regarding the World’s Wetlands and Their Future under Global Climate Change: A Synthesis. Aquat. Sci. 2013, 75, 151–167. [Google Scholar] [CrossRef] [Green Version]
- Poff, N.L.; Allan, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Richter, B.D.; Sparks, R.E.; Stromberg, J.C.; Junk, W.J.; An, S.; et al. The Impact of Climate Change on Freshwater Ecosystems Due to Altered River Flow Regimes. Nature 2010, 47, 1929. Available online: https://www.fs.fed.us/stream/Poffetal_1997.pdf (accessed on 29 December 2021).
- Chao, B.F.; Wu, Y.H.; Li, Y.S. Impact of Artificial Reservoir Water Impoundment on Global Sea Level. Science 2008, 320, 212–214. [Google Scholar] [CrossRef] [Green Version]
- Phung, D.; Nguyen-Huy, T.; Tran, N.N.; Tran, D.N.; Doan, V.Q.; Nghiem, S.; Nguyen, N.H.; Nguyen, T.H.; Bennett, T. Hydropower Dams, River Drought and Health Effects: A Detection and Attribution Study in the Lower Mekong Delta Region. Clim. Risk Manag. 2021, 32, 100280. [Google Scholar] [CrossRef]
- Matthews, N. People and Fresh Water Ecosystems: Pressures, Responses and Resilience. Aquat. Procedia 2016, 6, 99–105. [Google Scholar] [CrossRef]
- Colas, F.; Baudoin, J.-M.; Chauvet, E.; Clivot, H.; Danger, M.; Guérold, F.; Devin, S. Dam-Associated Multiple-Stressor Impacts on Fungal Biomass and Richness Reveal the Initial Signs of Ecosystem Functioning Impairment. Ecol. Indic. 2016, 60, 1077–1090. [Google Scholar] [CrossRef] [Green Version]
- Martínez, A.; Larrañaga, A.; Basaguren, A.; Pérez, J.; Mendoza-Lera, C.; Pozo, J. Stream Regulation by Small Dams Affects Benthic Macroinvertebrate Communities: From Structural Changes to Functional Implications. Hydrobiologia 2013, 711, 31–42. [Google Scholar] [CrossRef]
- Ferreira, V.; Koricheva, J.; Pozo, J.; Graça, M.A.S. A Meta-Analysis on the Effects of Changes in the Composition of Native Forests on Litter Decomposition in Streams. For. Ecol. Manag. 2016, 364, 27–38. [Google Scholar] [CrossRef]
- Boyero, L.; Pearson, R.G.; Hui, C.; Gessner, M.O.; Pérez, J.; Alexandrou, M.A.; Graça, M.A.S.; Cardinale, B.J.; Albariño, R.J.; Arunachalam, M.; et al. Biotic and Abiotic Variables Influencing Plant Litter Breakdown in Streams: A Global Study. Proc. R. Soc. B 2016, 283, 20152664. [Google Scholar] [CrossRef]
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandagopal, S. Water Resources: Agricultural and Environmental Issues. BioScience 2004, 54, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Pérez, J.; Martínez, A.; Descals, E.; Pozo, J. Responses of Aquatic Hyphomycetes to Temperature and Nutrient Availability: A Cross-Transplantation Experiment. Microb. Ecol. 2018, 76, 328–339. [Google Scholar] [CrossRef]
- Gulis, V.; Ferreira, V.; Graça, M.A.S. Stimulation of Leaf Litter Decomposition and Associated Fungi and Invertebrates by Moderate Eutrophication: Implications for Stream Assessment. Freshw. Biol. 2006, 51, 1655–1669. [Google Scholar] [CrossRef]
- Wilcox, H.S.; Bruce Wallace, J.; Meyer, J.L.; Benstead, J.P. Effects of Labile Carbon Addition on a Headwater Stream Food Web. Limnol. Oceanogr. 2005, 50, 1300–1312. [Google Scholar] [CrossRef]
- Ferreira, V.; Graça, M.A.S. Fungal Activity Associated with Decomposing Wood Is Affected by Nitrogen Concentration in Water. Int. Rev. Hydrobiol. 2007, 92, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekar, K.R.; Kaveriappa, K.M. Effect of Pesticides on the Growth of Aquatic Hyphomycetes. Toxicol. Lett. 1989, 48, 311–315. [Google Scholar] [CrossRef]
- Martínez, A.; Barros, J.; Gonçalves, A.L.; Canhoto, C. Salinisation Effects on Leaf Litter Decomposition in Fresh Waters: Does the Ionic Composition of Salt Matter? Freshw. Biol. 2020, 65, 1475–1483. [Google Scholar] [CrossRef]
- Sridhar, K.R.; Duarte, S.; Cássio, F.; Pascoal, C. The Role of Early Fungal Colonizers in Leaf-Litter Decomposition in Portuguese Streams Impacted by Agricultural Runoff. Int. Rev. Hydrobiol. 2009, 94, 399–409. [Google Scholar] [CrossRef]
- Bull, J.W.; Maron, M. How Humans Drive Speciation as Well as Extinction. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160600. [Google Scholar] [CrossRef] [Green Version]
- Canhoto, C.; Graça, M.A.S. Decomposition of Eucalyptus Globulus Leaves and Three Native Leaf Species (Alnus glutinosa, Castanea sativa and Quercus faginea) in a Portuguese Low Order Stream. Hydrobiologia 1996, 333, 79–85. [Google Scholar] [CrossRef]
- Ferreira, V.; Elosegi, A.; Gulis, V.; Pozo, J.; Graça, M.A.S. Eucalyptus Plantations Affect Fungal Communities Associated with Leaf-Litter Decomposition in Iberian Streams. Arch. Für Hydrobiol. 2006, 166, 467–490. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.; Ferreira, V. Invasion of Native Riparian Forests by Acacia Species Affects In-Stream Litter Decomposition and Associated Microbial Decomposers. Microb. Ecol. 2021, 81, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Leuzinger, S.; Rewald, B. The Who or the How? Species vs. Ecosystem Function Priorities in Conservation Ecology. Front. Plant Sci. 2021, 12, 2442. [Google Scholar] [CrossRef]
- Steiner, F.M.; Schlick-Steiner, B.C.; VanDerWal, J.; Reuther, K.D.; Christian, E.; Stauffer, C.; Suarez, A.V.; Williams, S.E.; Crozier, R.H. Combined Modelling of Distribution and Niche in Invasion Biology: A Case Study of Two Invasive Tetramorium Ant Species. Divers. Distrib. 2008, 14, 538–545. [Google Scholar] [CrossRef]
- Döll, P.; Bunn, S.E. The Impact of Climate Change on Freshwater Ecosystems Due to Altered River Flow Regimes. Clim. Chang. 2010, 14, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Singh, A.; Singh, P.; Mishra, V.K. Chapter 4—Impact of Climate Change on Freshwater Ecosystem. In Water Conservation in the Era of Global Climate Change; Thokchom, B., Qiu, P., Singh, P., Iyer, P.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 73–98. ISBN 978-0-12-820200-5. [Google Scholar]
- Kumaraswamy, T.R.; Javeed, S.; Javaid, M.; Naika, K. Impact of Pollution on Quality of Freshwater Ecosystems. In Fresh Water Pollution Dynamics and Remediation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 69–81. [Google Scholar]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global Threats to Human Water Security and River Biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Ferreira, V.; Voronina, E. Impact of Climate Change on Aquatic Hyphomycetes and Terrestrial Macromycetes. Clim. Chang. Microb. Ecol. Curr. Res. Future Trends 2016, 53–72. [Google Scholar] [CrossRef]
- Krauss, G.-J.; Solé, M.; Krauss, G.; Schlosser, D.; Wesenberg, D.; Bärlocher, F. Fungi in Freshwaters: Ecology, Physiology and Biochemical Potential. FEMS Microbiol. Rev. 2011, 35, 620–651. [Google Scholar] [CrossRef] [PubMed]
- Seena, S.; Bärlocher, F.; Sobral, O.; Gessner, M.O.; Dudgeon, D.; McKie, B.G.; Chauvet, E.; Boyero, L.; Ferreira, V.; Frainer, A.; et al. Biodiversity of Leaf Litter Fungi in Streams along a Latitudinal Gradient. Sci. Total Environ. 2019, 661, 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, V.; Gulis, V.; Pascoal, C.; Graça, M.A.S. Chapter 18. Stream Pollution and Fungi. In Freshwater Fungi; De Gruyter: Berlin, Germany, 2014; pp. 389–412. [Google Scholar]
- Woodward, G.; Bonada, N.; Feeley, H.B.; Giller, P.S. Resilience of a Stream Community to Extreme Climatic Events and Long-Term Recovery from a Catastrophic Flood. Freshw. Biol. 2015, 60, 2497–2510. [Google Scholar] [CrossRef]
- Evangeliou, N.; Grythe, H.; Klimont, Z.; Heyes, C.; Eckhardt, S.; Lopez-Aparicio, S.; Stohl, A. Atmospheric Transport Is a Major Pathway of Microplastics to Remote Regions. Nat. Commun. 2020, 11, 3381. [Google Scholar] [CrossRef]
- Du, J.; Qv, W.; Niu, Y.; Yuan, S.; Zhang, L.; Yang, H.; Zhang, Y. Co-Exposures of Acid Rain and ZnO Nanoparticles Accelerate Decomposition of Aquatic Leaf Litter. J. Hazard. Mater. 2022, 426, 128141. [Google Scholar] [CrossRef]
- Niyogi, D.K.; Lewis, W.M., Jr.; McKnight, D.M. Litter Breakdown in Mountain Streams Affected by Mine Drainage: Biotic Mediation of Abiotic Controls. Ecol. Appl. 2001, 11, 506–516. [Google Scholar] [CrossRef]
- Sridhar, K.R.; Raviraja, N.S. Aquatic Hyphomycetes and Leaf Litter Processing in Polluted and Unpolluted. In Trichomycetes and Other Fungal Groups; CRC Press: Boca Raton, FL, USA, 2001; p. 293. [Google Scholar]
- Guimarães-Soares, L.; Felícia, H.; João Bebianno, M.; Cássio, F. Metal-Binding Proteins and Peptides in the Aquatic Fungi Fontanospora fusiramosa and Flagellospora curta Exposed to Severe Metal Stress. Sci. Total Environ. 2006, 372, 148–156. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Cássio, F. Effects of Metals on Growth and Sporulation of Aquatic Fungi. Drug Chem. Toxicol. 2010, 33, 269–278. [Google Scholar] [CrossRef]
- Miersch, J.; Tschimedbalshir, M.; Bärlocher, F.; Grams, Y.; Pierau, B.; Schierhorn, A.; Krauss, G.-J. Heavy Metals and Thiol Compounds in Mucor Racemosus and Articulospora Tetracladia. Mycol. Res. 2001, 105, 883–889. [Google Scholar] [CrossRef]
- Seena, S.; Kumar, S. Short-Term Exposure to Low Concentrations of Copper Oxide Nanoparticles Can Negatively Impact the Ecological Performance of a Cosmopolitan Freshwater Fungus. Environ. Sci. Processes Impacts 2019, 21, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Seena, S.; Sobral, O.; Cano, A. Metabolomic, Functional, and Ecologic Responses of the Common Freshwater Fungus Neonectria Lugdunensis to Mine Drainage Stress. Sci. Total Environ. 2020, 718, 137359. [Google Scholar] [CrossRef] [PubMed]
- Abel, T.H.; Bärlocher, F. Effects of Cadmium on Aquatic Hyphomycetes. Appl. Environ. Microbiol. 1984, 48, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Jaeckel, P.; Krauss, G.-J.; Krauss, G. Cadmium and Zinc Response of the Fungi Heliscus lugdunensis and Verticillium Cf. Alboatrum Isolated from Highly Polluted Water. Sci. Total Environ. 2005, 346, 274–279. [Google Scholar] [CrossRef]
- Miersch, J.; Bärlocher, F.; Bruns, I. Effects of Cadmium, Copper, and Zinc on Growth and Thiol Content of Aquatic Hyphomycetes. Hydrobiologia 1997, 346, 77–84. [Google Scholar] [CrossRef]
- Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J.N. Nonylphenol in the Environment: A Critical Review on Occurrence, Fate, Toxicity and Treatment in Wastewaters. Environ. Int. 2008, 34, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Duhalt, R.; Marquez-Rocha, F.; Ponce, E.; Licea, A.F.; Viana, M.T. Nonylphenol, an Integrated Vision of a Pollutant. Appl. Ecol. Environ. Res. 2005, 4, 1–25. [Google Scholar] [CrossRef]
- Bärlocher, F.; Guenzel, K.; Sridhar, K.R.; Duffy, S.J. Effects of 4-n-Nonylphenol on Aquatic Hyphomycetes. Sci. Total Environ. 2011, 409, 1651–1657. [Google Scholar] [CrossRef]
- Adetunde, L.A.; Glover, R.L.K. Bacteriological Quality of Borehole Water Used by Students of University for Development Studies, Navrongo Campus in Upper-East Region of Ghana. Curr. Res. J. Biol. Sci. 2010, 2, 361–364. [Google Scholar]
- Kuburić, M. Tolerance and Co-Tolerance of Microbial Communities on Leaf Litter to Silver Nanoparticles and Antibiotics. Ph.D. Thesis, Universidade de Coimbra, Coimbra, Portugal, 2014. [Google Scholar]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the Curve of Global Freshwater Biodiversity Loss: An Emergency Recovery Plan. BioScience 2020, 70, 330–342. [Google Scholar] [CrossRef]
- Vallero, D.A. Chapter 20—Thermal Pollution. In Waste, 2nd ed.; Letcher, T.M., Vallero, D.A.B.T.-W., Eds.; Academic Press: New York, NY, USA, 2019; pp. 381–404. ISBN 978-0-12-815060-3. [Google Scholar]
- Pradhan, A.; Seena, S.; Pascoal, C.; Cássio, F. Can Metal Nanoparticles Be a Threat to Microbial Decomposers of Plant Litter in Streams? Microb. Ecol. 2011, 62, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, A.; Seena, S.; Dobritzsch, D.; Helm, S.; Gerth, K.; Dobritzsch, M.; Krauss, G.-J.; Schlosser, D.; Pascoal, C.; Cássio, F. Physiological Responses to NanoCuO in Fungi from Non-Polluted and Metal-Polluted Streams. Sci. Total Environ. 2014, 466–467, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Seena, S. Plastisphere in Freshwaters: An Emerging Concern. Environ. Pollut. 2021, 290, 118123. [Google Scholar] [CrossRef] [PubMed]
- Seena, S.; Gutiérrez, I.B.; Barros, J.; Nunes, C.; Marques, J.C.; Kumar, S.; Gonçalves, A.M.M. Impacts of Low Concentrations of Nanoplastics on Leaf Litter Decomposition and Food Quality for Detritivores in Streams. J. Hazard. Mater. 2022, 429, 128320. [Google Scholar] [CrossRef]
- Balian, E.V.; Segers, H.; Lévèque, C.; Martens, K. The Freshwater Animal Diversity Assessment: An Overview of the Results. Hydrobiologia 2008, 595, 627–637. [Google Scholar] [CrossRef]
- Mougi, A.; Kondoh, M. Diversity of Interaction Types and Ecological Community Stability. Science 2012, 337, 349–351. [Google Scholar] [CrossRef]
- Tedesco, P.A.; Beauchard, O.; Bigorne, R.; Blanchet, S.; Buisson, L.; Conti, L.; Cornu, J.-F.; Dias, M.S.; Grenouillet, G.; Hugueny, B.; et al. A Global Database on Freshwater Fish Species Occurrence in Drainage Basins. Sci. Data 2017, 4, 170141. [Google Scholar] [CrossRef] [Green Version]
- Darwall, W.R.T.; Holland, R.A.; Smith, K.G.; Allen, D.; Brooks, E.G.E.; Katarya, V.; Pollock, C.M.; Shi, Y.; Clausnitzer, V.; Cumberlidge, N.; et al. Implications of Bias in Conservation Research and Investment for Freshwater Species. Conserv. Lett. 2011, 4, 474–482. [Google Scholar] [CrossRef]
- Darwall, W.; Smith, K.; Allen, D.; Seddon, M.; Reid, G.M.; Clausnitzer, V.; Kalkman, V. Freshwater Biodiversity: A Hidden Resource under Threat. In Wildlife in a Changing World: An Analysis of the 2008 IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2009; pp. 43–53. [Google Scholar]
- Anderson, J.L.; Shearer, C.A. Population Genetics of the Aquatic Fungus Tetracladium Marchalianum over Space and Time. PLoS ONE 2011, 6, e15908. [Google Scholar] [CrossRef] [Green Version]
- Jabiol, J.; Bruder, A.; Gessner, M.O.; Makkonen, M.; McKie, B.G.; Peeters, E.T.H.M.; Vos, V.C.A.; Chauvet, E. Diversity Patterns of Leaf-Associated Aquatic Hyphomycetes along a Broad Latitudinal Gradient. Fungal Ecol. 2013, 6, 439–448. [Google Scholar] [CrossRef] [Green Version]
- IUCN. The IUCN Red List of Threatened Species; IUCN: Grand, Swiss, 2021. [Google Scholar]
- Goffeau, A.; Barrell, B.G.; Bussey, H.; Davis, R.W.; Dujon, B.; Feldmann, H.; Galibert, F.; Hoheisel, J.D.; Jacq, C.; Johnston, M.; et al. Life with 6000 Genes. Science 1996, 274, 546–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfante, P.; Anca, I.-A. Plants, Mycorrhizal Fungi, and Bacteria: A Network of Interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tittensor, D.P.; Walpole, M.; Hill, S.L.; Boyce, D.G.; Britten, G.L.; Burgess, N.D.; Butchart, S.H.; Leadley, P.W.; Regan, E.C.; Alkemade, R.; et al. A mid-term analysis of progress toward international biodiversity targets. Science 2014, 346, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Leadley, P.; Proença, V.; Fernández-Manjarrés, J.; Pereira, H.M.; Alkemade, R.; Biggs, R.; Bruley, E.; Cheung, W.; Cooper, D.; Figueiredo, J.; et al. Interacting Regional-Scale Regime Shifts for Biodiversity and Ecosystem Services. BioScience 2014, 64, 665–679. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.P.G.; Asner, G.P.; Butchart, S.H.M.; Karanth, K.U. The ‘Why’, ‘What’and ‘How’of Monitoring for Conservation. Key Top. Conserv. Biol. 2013, 2, 327–343. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater Biodiversity Conservation: Recent Progress and Future Challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, S.C.; Haelewaters, D.; Furci, G.; Mueller, G.M. Include All Fungi in Biodiversity Goals. Science 2021, 373, 403. [Google Scholar] [CrossRef]
- Mayers, J. Water Ecosystem Services and Poverty Under Climate Change: Key Issues and Research Priorities: Report of a Scoping Exercise to Help Develop a Research Programme for the UK Department for International Development; IIED: London, UK, 2009. [Google Scholar]
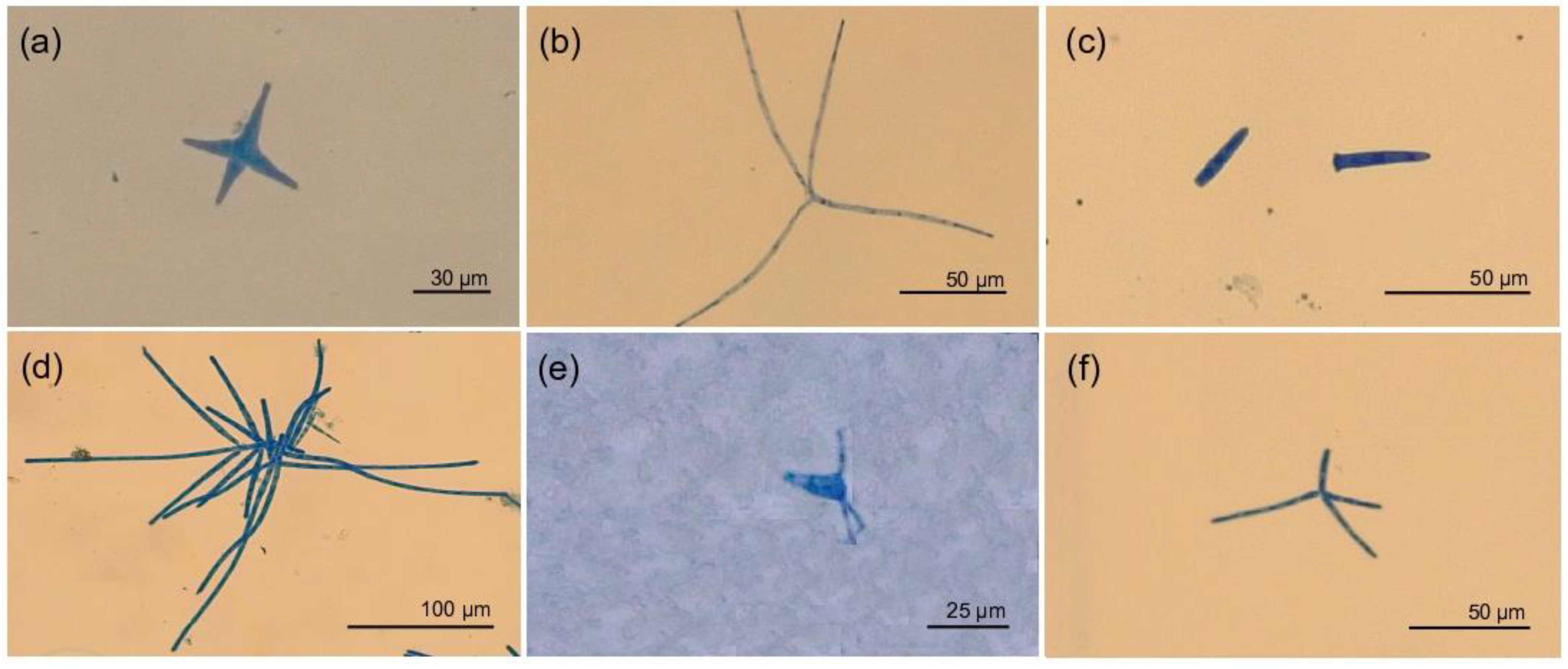
| Year | Milestone | Conservation Strategies |
|---|---|---|
| 1948 | International Union for the Conservation of Nature (IUCN) |
|
| 1961 | World Wildlife Fund for Nature (WWF) |
|
| 1964 | First edition of IUCN Red List |
|
| 1971 | Green Peace |
|
| 1971 * | The Ramsar Convention on wetlands |
|
| 1972 | United Nations Conference on the Human Environment (Stockholm Declaration) |
|
| 1972 | The United Nations Environment Programme (UNEP) |
|
| 1973 | Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) |
|
| 1982 | The World Resources Institute (WRI) |
|
| 1988 | Intergovernmental Panel on Climate Change (IPCC) |
|
| 1991 * | World Water Week |
|
| 1992 | Earth Summit-Convention on Biological Diversity (CBD) |
|
| 2000 | The Cartagena Protocol |
|
| 2000 | The Millennium Development Goals (MDGs) |
|
| 2001–2005 | The Millennium Ecosystem Assessment (MEA) |
|
| 2002 | The World Summit on Sustainable Development |
|
| 2002 | Strategic Plan for the Convention on Biological Diversity |
|
| 2010 | The United Nations World Water Development Report (WWDR) |
|
| 2010 | Strategic Plan for Biodiversity Revision 2011–2020 |
|
| 2010 | The Nagoya Protocol |
|
| 2010 | The Aichi Biodiversity Targets |
|
| 2010 | The Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) |
|
| 2012 | The world’s 100 most threatened species |
|
| 2015 | Sustainable Development Goals (SDGs) |
|
| 2020 | Post-2020 Global Biodiversity Framework |
|
| 2020 | The UN Decade on Ecosystem Restoration |
|
| 2021 | Water and Climate Pavilion at COP 26 |
|
| 2021 * | Water at the IUCN Congress |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, J.; Seena, S. Fungi in Freshwaters: Prioritising Aquatic Hyphomycetes in Conservation Goals. Water 2022, 14, 605. https://doi.org/10.3390/w14040605
Barros J, Seena S. Fungi in Freshwaters: Prioritising Aquatic Hyphomycetes in Conservation Goals. Water. 2022; 14(4):605. https://doi.org/10.3390/w14040605
Chicago/Turabian StyleBarros, Juliana, and Sahadevan Seena. 2022. "Fungi in Freshwaters: Prioritising Aquatic Hyphomycetes in Conservation Goals" Water 14, no. 4: 605. https://doi.org/10.3390/w14040605
APA StyleBarros, J., & Seena, S. (2022). Fungi in Freshwaters: Prioritising Aquatic Hyphomycetes in Conservation Goals. Water, 14(4), 605. https://doi.org/10.3390/w14040605






