Using the Diversity, Taxonomic and Functional Attributes of a Zooplankton Community to Determine Lake Environmental Typology in the Natural Southern Boreal Lakes (Québec, Canada)
Abstract
1. Introduction
2. Material and Methods
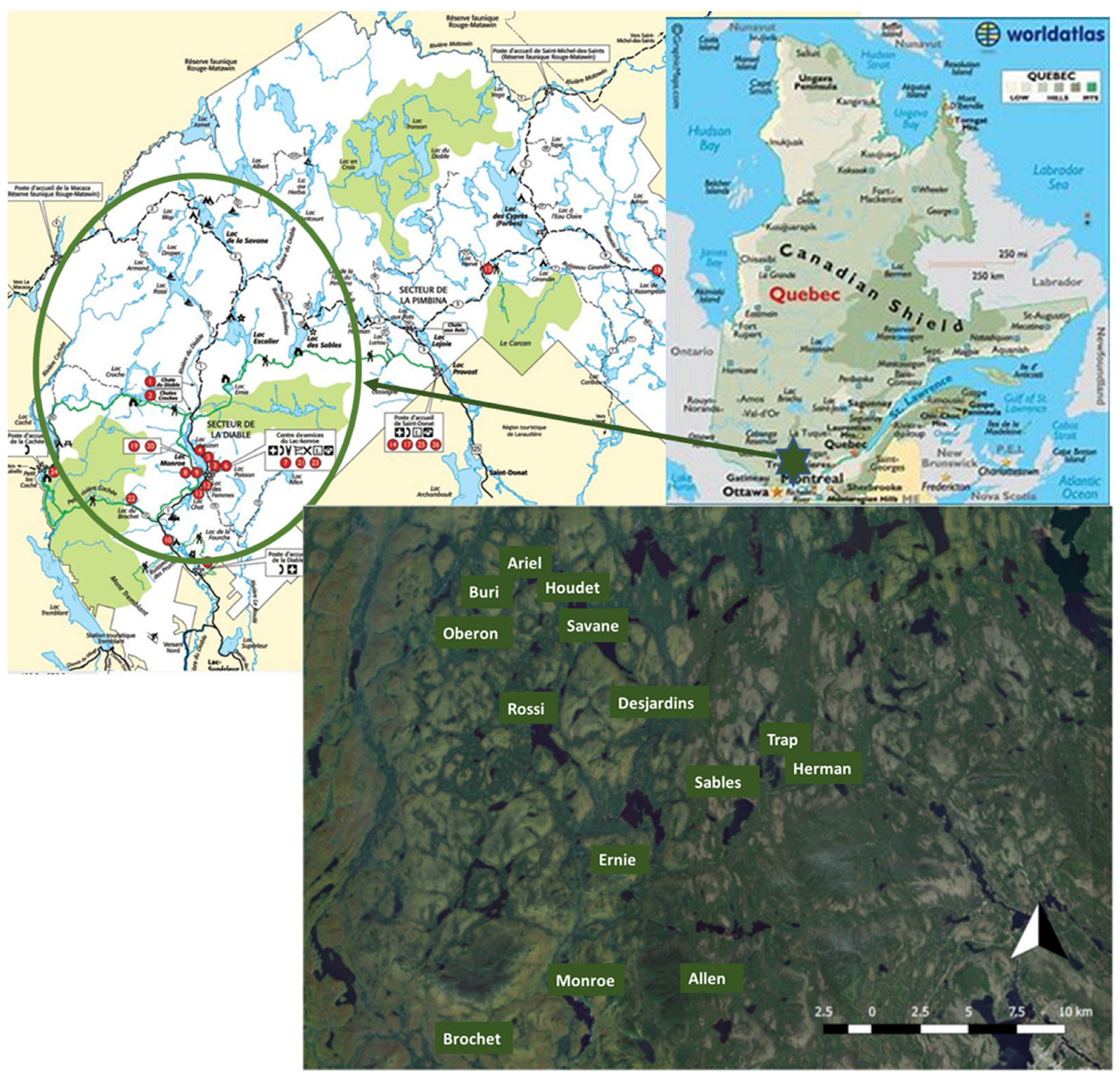
2.1. Study Sites and Limnological Characteristics
2.2. Sampling and Analysis of Zooplankton
2.3. Statistical Analyses
3. Results
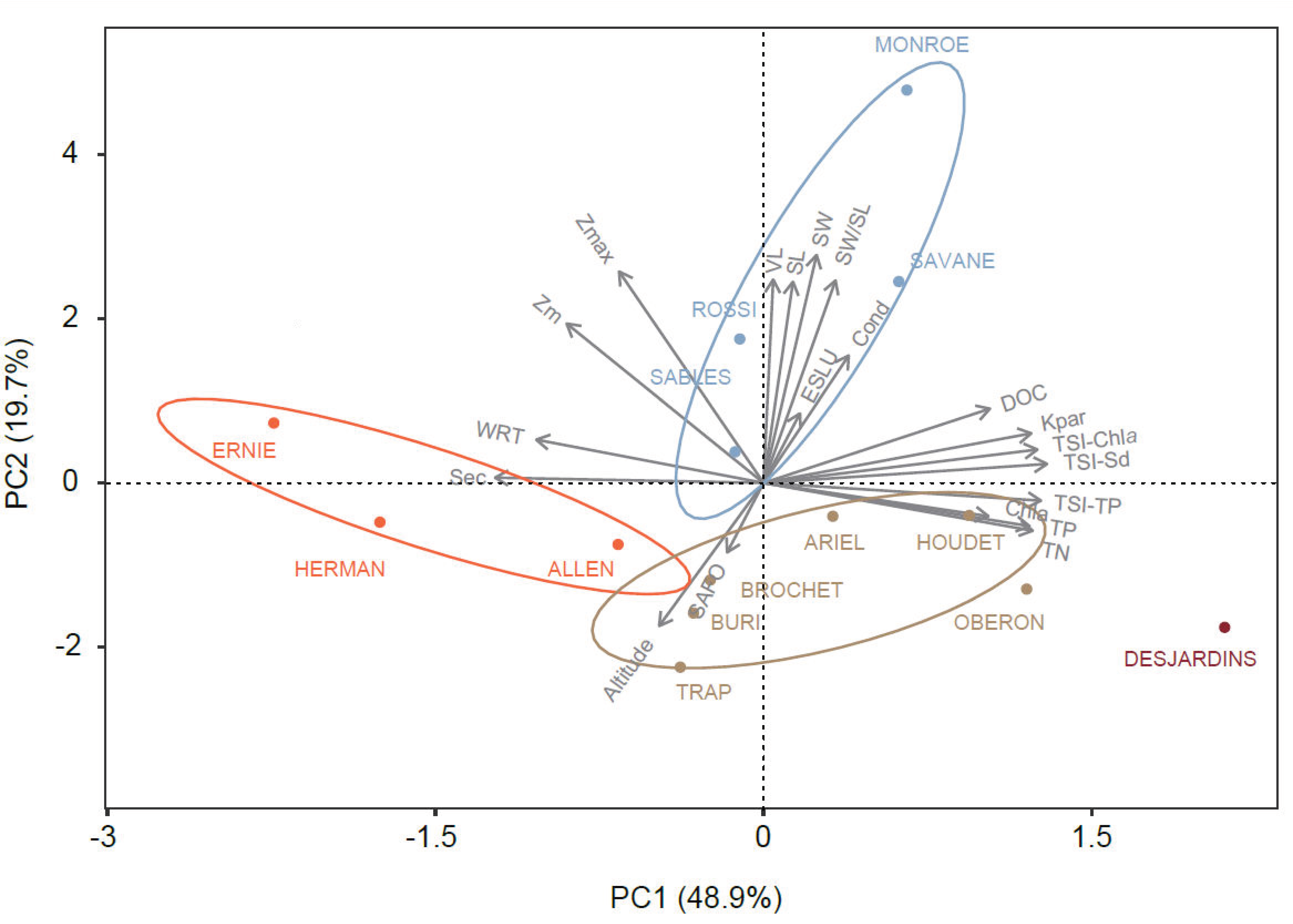
3.1. Environmental Typology of the Lakes
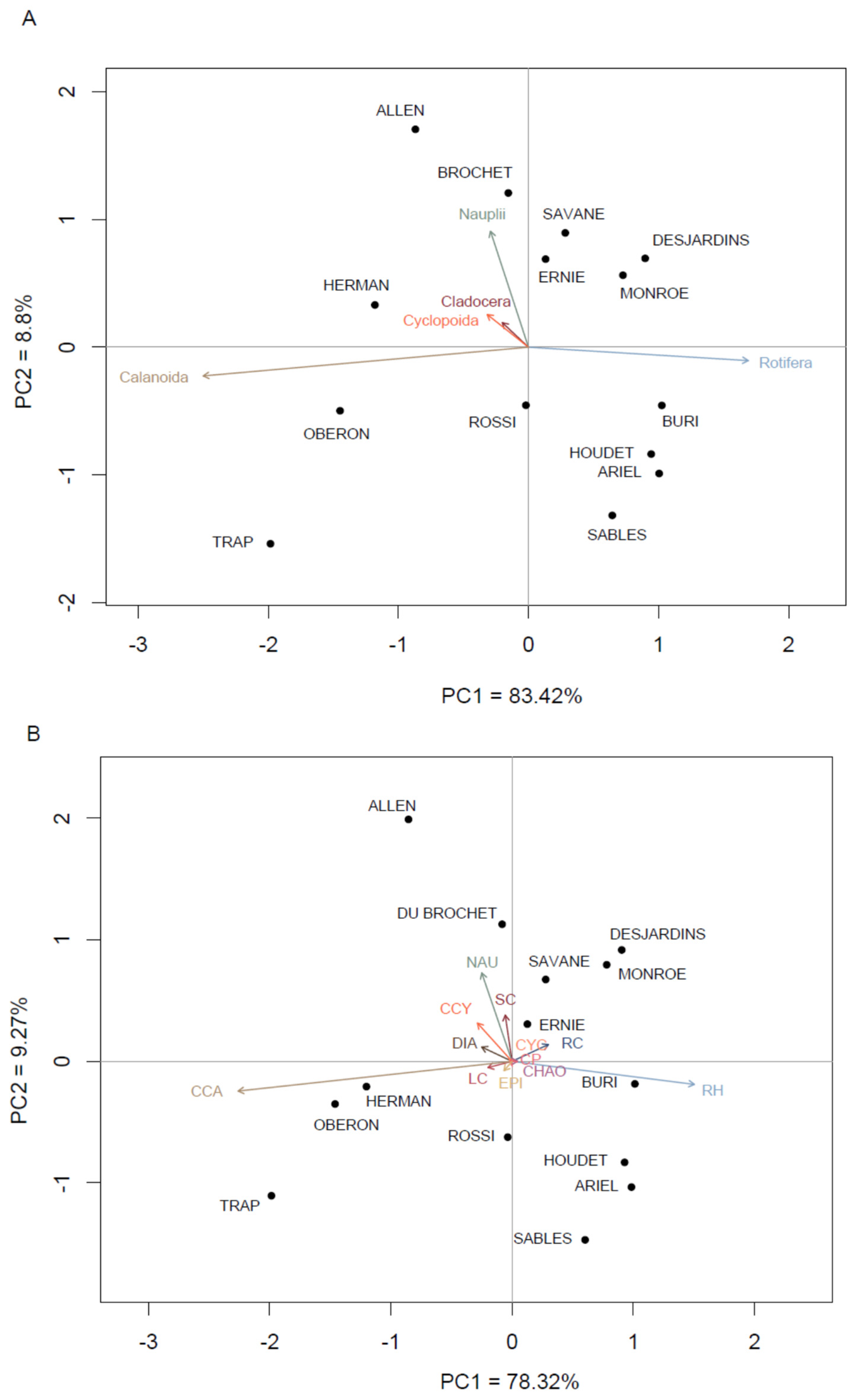
3.2. Spatio-Temporal Variation in the Zooplankton Community
3.3. Species Contribution to Zooplankton Diversity and Abundance
3.4. Site Contribution to Beta Diversity and the Species That Defined Lake Typologies
3.5. Changes in the Taxonomic and Functional Groups of Zooplankton through Space
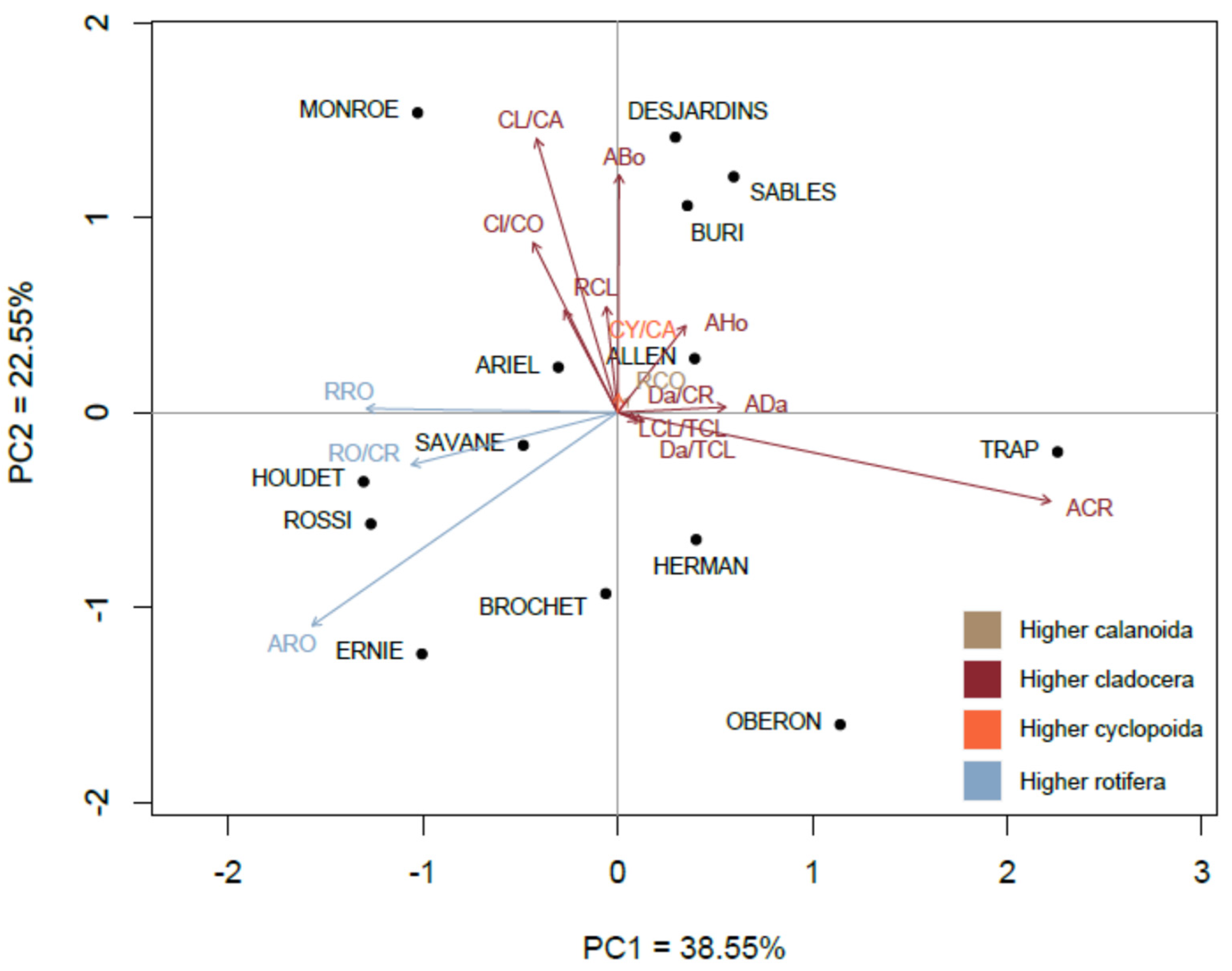
3.6. Zooplankton Biotic Indices
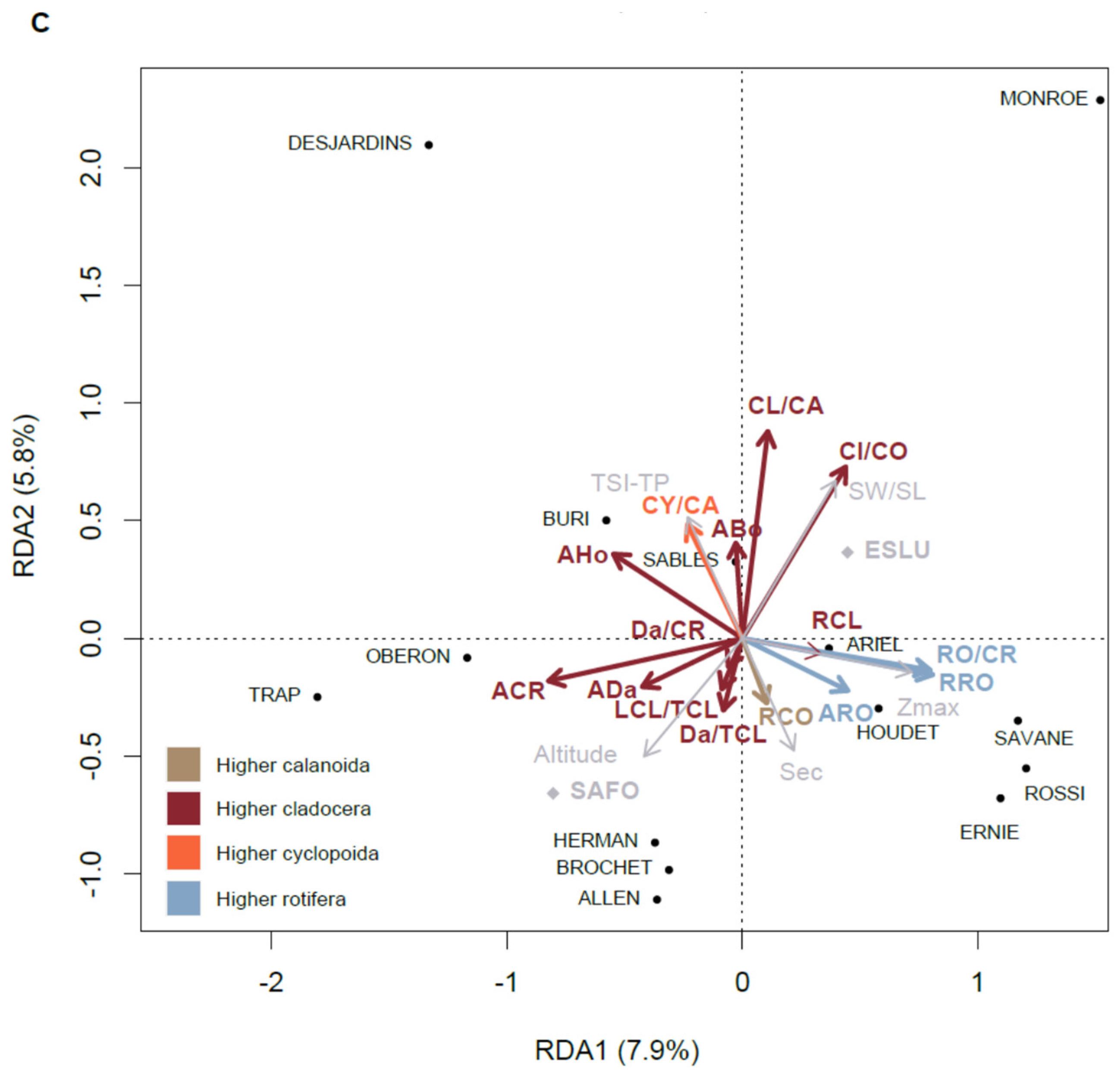
3.7. Relationships of the Zooplankton Community with Environmental Variables
4. Discussion
4.1. Environmental Typology
4.2. Zooplankton Species Assemblages and Typology
4.3. Identification of Lakes and Species with the Greatest Spatial Turnover
4.4. Relationship of Zooplankton Communities with Environmental Typology
4.5. Conclusions and Implications for Lake Monitoring in National Parks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Héritier, S.; Moumaneix, C. Protection et gestion dans les parcs canadiens et états-uniens. Quelques enjeux actuels. Cahiers De Géographie Du Québec 2007, 51, 155–176. [Google Scholar] [CrossRef][Green Version]
- Agence Parcs Canada—Ministère des Travaux Publics et Services Gouvernementaux. 2013. Chapitre 7—L’intégrité Ecologique Dans les Parcs Nationaux. Rapport du Commissaire à L’environnement et au Développement Durable. N° de Catalogue FA1-2/2013-1-7F-PDF, ISBN 978-0-660-21225-8, ISSN 1495-0790. Available online: www.oag-bvg.gc.ca (accessed on 1 October 2021).
- PSIE. Programme de Suivi de L’intégrité Ecologique. Rapport 2003–2012. Réseau Parcs Québec—SÉPAQ. 2014. Available online: www.parcsquebec.com/intégriteecologique (accessed on 1 October 2021).
- McQueen, D.J.; Post, J.R.; Mills, E.L. Trophic relationships in freshwater pelagic ecosystems. Can. J. Fish. Aquat. Sci. 1986, 43, 1571–1581. [Google Scholar] [CrossRef]
- Stemberger, R.S.; Larsen, D.P.; Kincaid, T.M. Sensitivity of zooplankton for regional lake monitoring. Can. J. Fish. Aquat. Sci. 2001, 58, 2222–2232. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Lauridsen, T.; Søndergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as indicators in lakes: A scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Hessen, D.O.; Faafeng, B.A.; Andersen, T. Replacement of herbivore zooplankton species along gradients of ecosystem productivity and fish predation pressure. Can. J. Fish. Aquat. Sci. 1995, 52, 433–742. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness biodiversity in Danish lakes: Changes along phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Pinto-Coelho, R.; Pinel-Alloul, B.; Méthot, G.; Havens, K. Relationships of crustacean zooplankton with latitude and trophic gradients in lakes and reservoirs of temperate and tropical regions. Can. J. Fish. Aquat. Sci. 2005, 62, 348–361. [Google Scholar] [CrossRef]
- Haberman, J.; Laugaste, R.; Noges, T. The role of cladocerans reflecting the trophic status of two large and shallow Estonian lakes. Hydrobiologia 2007, 584, 157–166. [Google Scholar] [CrossRef]
- Ejsmont-Karabin, J. The usefulness of zooplankton as lake ecosystem indicators: Rotifer trophic state index Pol. J. Ecol. 2012, 60, 339–350. [Google Scholar]
- Ejsmont-Karabin, J.; Karabin, A. The suitability of zooplankton as lake ecosystem indicators: Crustacean trophic state index. Pol. J. Ecol. 2013, 61, 561–573. [Google Scholar]
- Pinel-Alloul, B.; Méthot, G.; Verreault, G.; Vigneault, Y. Zooplankton species associations in Quebec lakes: Variation with abiotic factors, including natural and anthropogenic acidification. Can. J. Fish. Aquat. Sci. 1990, 47, 110–121. [Google Scholar] [CrossRef]
- Locke, A. Factors influencing community structure along stress gradients: Zooplankton responses to acidification. Ecology 1992, 73, 903–909. [Google Scholar] [CrossRef]
- Havens, K.E.; Yan, N.D.; Keller, W. Lake acidification: Effects on crustacean zooplankton populations. Environ. Sci. Technol. 1993, 27, 1621–1624. [Google Scholar] [CrossRef]
- Yan, N.D.; Strus, R. Crustacean zooplankton communities of acidic, metal contaminated lakes near Sudbury, Ontario. Can. J. Fish. Aquat. Sci. 1980, 37, 2282–2293. [Google Scholar] [CrossRef]
- Yan, N.D.; KSomers, M.; Girard, R.E.; Paterson, A.M.; Keller, W.; Ramcharan, C.W.; Rusak, J.A.; Ingram, R.; Morgan, G.E.; Gunn, J.M. Long-term trends on zooplankton of Dorset, Ontario lakes: The probable interactive effects of change in pH, total phosphorus, dissolved organic carbon, and predators. Can. J. Fish. Aquat. Sci. 2008, 65, 862–877. [Google Scholar] [CrossRef]
- Wissel, B.; Boeing, W.J.; Ramcharan, C.W. Effects of water color on predation regimes and zooplankton assemblages in freshwater lakes. Limnol. Oceanogr. 2003, 48, 1965–1976. [Google Scholar] [CrossRef]
- Stemberger, R.S.; Miller, E.K. Cladoceran body length and Secchi disk transparency in northeastern U.S. lakes. Can. J. Fish. Aquat. Sci. 2003, 60, 1477–1486. [Google Scholar] [CrossRef]
- Patoine, A.; Pinel-Alloul, B.; Prepas, E.E.; Carignan, R. Do logging and forest fires influence zooplankton biomass in Canadian Shield lakes? Can. J. Fish. Aquat. Sci. 2000, 57 (Suppl. S2), 155–164. [Google Scholar] [CrossRef]
- Patoine, A.; Pinel-Alloul, B.; Prepas, E.E. Effects of catchment perturbations by logging and wildfires on zooplankton species richness and composition in Boreal Shield lakes. Freshw. Biol. 2002, 47, 1996–2014. [Google Scholar] [CrossRef]
- Winkler, G.; Leclerc, V.; Sirois, P.; Archambault, P.; Bérubé, P. Short-term impact of forest harvesting on water quality and zooplankton communities in oligotrophic lakes of the eastern Canadian Boreal Shield. Boreal Environ. Res. 2009, 14, 323–337. [Google Scholar]
- Gélinas, M.; Pinel-Alloul, B. Relating crustacean zooplankton community structure to residential development and land-cover disturbance near Canadian Shield lakes. Can. J. Fish. Aquat. Sci. 2008, 65, 2689–2702. [Google Scholar] [CrossRef]
- Pinel-Alloul, B.; Mimouni, E.A. Are cladoceran diversity and community structure linked to spatial heterogeneity in urban landscapes and pond environments? Hydrobiologia 2013, 715, 195–212. [Google Scholar] [CrossRef]
- Mimouni, E.A.; Pinel-Alloul, B.; Beisner, B. Assessing aquatic biodiversity of zooplankton communities in an urban landscape. Urban Ecosyst. 2015, 18, 1353–1372. [Google Scholar] [CrossRef]
- Haberman, J.; Haldna, M. Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: Long-term study of Lake Võrtsjärv. J. Limnol. 2014, 73, 263–273. [Google Scholar] [CrossRef]
- Anas, M.U.M.; Scott, K.A.; Wissel, B. Suitability of presence vs absence indicator species to characterize stress gradients: Lessons from zooplankton species of boreal lakes. Ecol. Ind. 2013, 30, 90–99. [Google Scholar] [CrossRef]
- Anas, M.U.M.; Scott, K.A.; Cooper, R.N.; Wissel, B. Zooplankton communities are good indicators of potential impacts of Athabasca oil sands operations on downwind boreal lakes. Can. J. Fish. Aquat. Sci. 2014, 71, 719–732. [Google Scholar] [CrossRef]
- Couture, S.; Hudon, C.; Gagnon, P.; Taranu, Z.; Pinel-Alloul, B.; Houle, D.; Aldamman, L.; Beauvais, C.; Lachapelle, M. Zooplankton communities in Precambrian Shield lakes (Québec, Canada): Responses to spatial and temporal gradients in water chemistry and climate. Can. J. Fish. Aquat. Sci. 2021, 78, 567–579. [Google Scholar] [CrossRef]
- Pinel-Alloul, B.; Patoine, A.; Marty, J. Multi-scale and multi-system perspectives of zooplankton structure and function in Canadian freshwaters. Can. J. Fish. Aquat. Sci. 2021, 78, 1543–1562. [Google Scholar] [CrossRef]
- Nogrady, T. Canadian rotifers II. Parc du Mont-Tremblant, Québec. Hydrobiologia 1980, 71, 35–46. [Google Scholar] [CrossRef]
- Carignan, R. Rapport d’activités au Parc National du Mont-Tremblant Durant la Saison 2009; Université de Montréal: Montréal, QC, Canada, 2010; 20p. [Google Scholar]
- Tremblay, R.; Pienitz, R. Caractéristiques limnologiques de 56 lacs du Québec méridional en lien avec l’état trophique. Rev. Des Sci. De L’eau 2015, 28, 139–162. [Google Scholar]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 432–471. [Google Scholar]
- Sommer, U.; Adrian, R.; Domis, L.D.; Elser, J.L.; Gaedke, U.; Ibelings, B.; Lürling, E.J.M.; Molinero, J.C.; Mooij, W.M.; van Donk, E.; et al. Beyond the Plankton Ecology Group (PEG) model: Mechanisms driving plankton succession. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 429–448. [Google Scholar] [CrossRef]
- Filion, J.M.; Chain, P.; Futter, M. Cantilevering vertical tow nets to reduce tow-line induced zooplankton avoidance. J. Plankton Res. 1993, 15, 581–587. [Google Scholar] [CrossRef][Green Version]
- Ward, J. A description of new zooplankton counter. Quaterly J. Microsc. Sci. 1955, 96, 371–373. [Google Scholar] [CrossRef]
- Edmondson, W.T. Rotifera. In Freshwater Biology; Ward, H.B., Whipple, G.C., Eds.; John Wiley and Sons: New York, NY, USA, 1959; pp. 420–494. [Google Scholar]
- Stemberger, R.S. A Guide to Rotifers of the Laurentian Great Lakes; EPA-600/4-79-021; United States Environmental Protection Agency, Environmental Monitoring and Support Laboratory: Cincinnati, OH, USA, 1979. [Google Scholar]
- Chengalath, R.; Koste, W. Rotifera from northeastern Quebec, Newfoundland and Labrador, Canada. Hydrobiologia 1983, 104, 49–56. [Google Scholar] [CrossRef]
- Chengalath, R.; Koste, W. Rotifera from Northwestern Canada. Hydrobiologia 1987, 147, 49–56. [Google Scholar] [CrossRef]
- Nogrady, T.; Pourriot, R.; Segers, H. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World. In 8. Rotifera Volume 3: Notommatidae and Scaridiidae; Nogrady, T., Dumont, H.J., Eds.; SPB Academic Publishing, BV: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Brooks, J.L. The systematics of North American Daphnia. Mem. Conn. Acad. Arts Sci. 1957, 13, 1–180. [Google Scholar]
- Brooks, J.L. Cladocera. Freshwater Biology; Ward, H.B., Whipple, G.C., Eds.; John Wiley and Sons: New York, NY, USA, 1959; pp. 587–656. [Google Scholar]
- Brandlova, J.; Brandl, Z.; Fernando, C.H. The Cladocera of Ontario with remarks on some species and distribution. Can. J. Zool. 1972, 50, 1373–1403. [Google Scholar] [CrossRef]
- Chengalath, R. A faunistic and ecological survey of the littoral Cladocera of Canada. Can. J. Zool. 1982, 60, 2668–2682. [Google Scholar] [CrossRef]
- De Melo, R.; Hebert, P.D.N. A taxonomic reevaluation of North American Bosminidae. Can. J. Zool. 1994, 72, 1808–1825. [Google Scholar] [CrossRef]
- Hebert, P.D.N. The Daphnia of North America: An Illustrated Fauna; CD-ROM; University of Guelph: Guelph, ON, Canada, 1995. [Google Scholar]
- Smith, K.; Fernando, C.H. A Guide to Freshwater Calanoid and Cyclopoid Copepod Crustacea of Ontario; Department of Biology, University of Waterloo: Waterloo, ON, Canada, 1978. [Google Scholar]
- Dussart, B. Crustacés Copépodes de l’Ontario; Document Inédit Photocopié, Université de Waterloo: Waterloo, ON, Canada, 2000; p. 107. [Google Scholar]
- Hudson, P.L.; Lesko, L.T. Free-Living and Parasitic Copepods of the Laurentian Great Lakes: Keys and Details on Individual Species; Great Lakes Science Center Home Page: Ann Arbor, MI, USA, 2003. Available online: http://www.glsc.usgs.gov/greatlakescopepods/ (accessed on 5 December 2021).
- Haney, J.F.; Aliberti, M.A.; Allan, E.; Allard, S.; Bauer, D.J.; Beagen, W.; Bradt, S.R.; Carlson, B.; Carlson, S.C.; Doan, U.M.; et al. An Image-Based Key to the Zooplankton of the Northeast, USA Version 4.0 Released 2010; University of New Hampshire Center for Freshwater Biology: Durham, NH, USA, 2010; Available online: http://cfb.unh.edu/cfbkey/html/ (accessed on 5 December 2021).
- Saether, O.A. Chaoboridae. In Das Zooplankton der Binnengewasser; Binnengewasser, I., Ed.; Schweizerbart’sche: Stuttgart, Germany, 1972; Volume 26, pp. 257–280. [Google Scholar]
- Gaston, K.J.; Spicer, J.I. Biodiversity: An Introduction, 2nd ed.; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Soininen, J.; Lennon, J.J.; Hillerand, H. A multivariate analysis of beta diversity across organisms and environments. Ecology 2007, 88, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; de Càceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011. [Google Scholar]
- Legendre, P.; Cáceres, M.D.; Borcard, D. Community surveys through space and time: Testing the space-time interaction in the absence of replication. Ecology 2010, 91, 262–272. [Google Scholar] [CrossRef]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward selection of explanatory variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; ISBN 3-900051-07-0. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bertolo, A.; Magnan, P. Spatial and environmental correlates of fish community structure in Canadian Shield lakes. Can. J. Fish. Aquat. Sci. 2006, 63, 2780–2792. [Google Scholar] [CrossRef]
- Swadling, K.M.; Pienitz, R.; Nogrady, T. Zooplankton community composition of lakes in the Yukon and Northwest Territories (Canada): Relationship to physical and chemical limnology. Hydrobiologia 2000, 431, 211–224. [Google Scholar] [CrossRef]
- Rusak, J.A.; Yan, N.D.; Sommers, K.M.; Cottingham, K.L.; Micheli, F.; Carpenter, S.R.; Frost, T.M.; Paterson, M.J.; McQueen, D.J. Temporal, spatial, and taxonomic patterns of crustacean zooplankton variability in unmanipulated north-temperate lakes. Limnol. Oceanogr. 2002, 47, 613–625. [Google Scholar] [CrossRef]
- Larson, G.L.; Hoffman, R.; McIntire, C.D.; Lienkaemper, G.; Samora, B. Zooplankton assemblages in montane lakes and ponds of Mount Rainier National parks, Washington State, USA. J. Plankton Res. 2009, 31, 273–285. [Google Scholar] [CrossRef]
- Méthot, G.; Pinel-Alloul, B. Composition taxonomique du zooplancton des stations pélagiques et littorales du lac La Pêche dans le Parc de la Gatineau. In Rapport Présenté à la Commission de la Capitale Nationale; Ottawa, ON, Canada, 2008; 13p, Available online: https://papyrus.bib.umontreal.ca/xmlui/bitstream/handle/1866/19129/Chemli_Abir_2017_memoire.pdf?sequence=2&isAllowed=y (accessed on 20 December 2021).
- Méthot, G.; Pinel-Alloul, B. Composition taxonomique du zooplancton des stations pélagiques et littorales du lac Philippe dans le Parc de la Gatineau. In Rapport Présenté à la Commission de la Capitale Nationale; Ottawa, ON, Canada, 2009; 28p, Available online: https://papyrus.bib.umontreal.ca/xmlui/bitstream/handle/1866/19129/Chemli_Abir_2017_memoire.pdf?sequence=2 (accessed on 20 December 2021).
- Méthot, G.; Pinel-Alloul, B. Composition taxonomique du zooplancton des stations pélagiques et littorales du lac Meech situé dans le Parc de la Gatineau. In Rapport Présenté à la Commission de la Capitale Nationale; Ottawa, ON, Canada, 2010; p. 40. Available online: https://ccn-ncc.gc.ca/endroits/conservation-au-parc-de-la-gatineau (accessed on 20 December 2021).
- Méthot, G.; Pinel-Alloul, B. Composition taxonomique du zooplancton des stations pélagiques et littorales du lac Pink situé dans le Parc de la Gatineau. In Rapport Présenté à la Commission de la Capitale Nationale; Ottawa, ON, Canada, 2012; p. 42. Available online: https://oraprdnt.uqtr.uquebec.ca/pls/public/docs/GSC543/F_2146344899_RA_10_14_GRIL_protege_interactif_144ppi.pdf (accessed on 20 December 2021).
- Gannon, J.E.; Stemberger, R.S. Zooplankton (especially crustaceans and rotifers) as indicators of water quality. Trans. Am. Microsc. Soc. 1978, 97, 16–35. [Google Scholar] [CrossRef]
- Pejler, B. Taxonomical and ecological studies on planktonic Rotatoria from Central Sweden. K. Sven. Vetensk. Akad. Handl. 1957, 6, 52. [Google Scholar]
- Jeziorski, A.; Tanentzap, A.J.; Yan, N.D.; Paterson, A.M.; Palmer, M.E.; Korosi, J.B.; Rusak, J.A.; Arts, M.T.; Keller, W.; Ingram, R.; et al. The jellification of north temperate lakes. Proc. Biol. Sci. 2015, 282, 2014–2449. [Google Scholar] [CrossRef] [PubMed]
- Arnott, S.E.; Magnuson, J.J.; Yan, N.D. Crustacean zooplankton species richness: Single and multiple-years estimates. Can. J. Fish. Aquat. Sci. 1998, 55, 1573–1582. [Google Scholar] [CrossRef]
- Anderson, S. Crustacean plankton communities of 340 lakes and ponds in and near the National Parks of the Canadian Rocky Mountains. J. Fish. Res. Board Can. 1974, 31, 855–869. [Google Scholar] [CrossRef]
- Lévesque, D.; Pinel-Alloul, B.; Méthot, G.; Steedman, R. Effects of climate, limnological features and watershed clearcut logging on long-term variation in zooplankton communities of Boreal Shield lakes. Water 2017, 9, 733. [Google Scholar] [CrossRef]
- Pinel-Alloul, B.; André, A.; Legendre, P.; Cardille, J.; Patalas, K.; Salki, A. Large-scale geographic patterns of diversity and community structure of pelagic crustacean zooplankton in Canadian lakes. Glob. Ecol. Biogeogr. 2013, 22, 784–795. [Google Scholar] [CrossRef]
- Walz, N.; Elster, H.-J.; Mezger, M. The development of the rotifer community structure in Lake Constance during its eutrophication. Arch. Hydrobiol. 1987, 74, 452–487. [Google Scholar]
- Berzins, B.; Pejler, B. Rotifer occurrence and trophic degree. Hydrobiologia 1989, 182, 171–180. [Google Scholar] [CrossRef]
- May, L.; O’Hare, M. Changes in rotifer species composition and abundance along a trophic gradient in Loch Lomond, Scotland, UK. Hydrobiologia 2005, 546, 397–404. [Google Scholar] [CrossRef]
- Drouin, A.; Sirois, P.; Archambault, P. Discriminating zooplankton communities in lakes with brook trout (Salvelinus fontinalis) and in fishless lakes. Ecoscience 2008, 16, 271–281. [Google Scholar] [CrossRef]
- Bertolo, A.; Carignan, R.; Magnan, P.; Pinel-Alloul, B.; Planas, D.; Garcia, E. Decoupling of pelagic and littoral food webs in oligotrophic Canadian Shield lakes. Oikos 2005, 111, 534–546. [Google Scholar] [CrossRef]
- Donald, D.B.; Vinebrooke, R.D.; Anderson, R.S.; Syrgiannis, J.; Graham, M.D. Recovery of zooplankton assemblages in mountain lakes from the effects of introduced sport fish. Can. J. Fish. Aquat. Sci. 2001, 58, 1822–1830. [Google Scholar] [CrossRef]
- Muñoz-Colmenares, M.E.; Sendra, M.D.; Sòria-Perpinya, X.; Vicente, J.M.S. The use of zooplankton metrics to determine the trophic status and ecological potential: An approach in a large Mediterranean watershed. Water 2021, 13, 2382. [Google Scholar] [CrossRef]
- Bertolo, A.; Magnan, P.; Plante, M. Linking the occurrence of brook trout with isolation and extinction in small Boreal Shield lakes. Freshw. Biol. 2008, 53, 304–321. [Google Scholar] [CrossRef]




| Limnological Variables | Mean ± s.d. | Minimum | Maximum |
|---|---|---|---|
| Lake morphometry | |||
| ALT (Altitude m) | 457 ± 52 | 344 | 533 |
| SL (Lake Area km2) | 0.77 ± 0.95 | 0.03 | 3.35 |
| SW (Watershed Area km2) | 41.9 ± 113.1 | 0.82 | 432 |
| SW/SL (Watershed area/Lake area) | 42.4 ± 87.9 | 7.4 | 346.2 |
| Zmax (Maximum depth m) | 14.7 ± 8.8 | 4 | 27 |
| Zm (Mean depth m) | 5.0 ± 2.9 | 1.6 | 11.3 |
| VL (Lake volume km3) | 5.13 ± 7.07 | 0.06 | 23.42 |
| Hydrology | |||
| WRT (Water residence time yr) | 0.64 ± 0.70 | 0.03 | 2.68 |
| Water quality * | |||
| TP (Total phosphorus μg/L) | 7.5 ± 3.4 | 2.7 | 16.6 |
| TN (Total nitrogen μg/L) | 224.6 ± 63.3 | 138.3 | 389 |
| DOC (Dissolved organic carbon mg/L) | 3.6 ± 0.8 | 2.4 | 4.8 |
| Chla (Chlorophyll-a μg/L) | 2.6 ± 2.1 | 0.8 | 9.4 |
| Cond (Conductivity μS/cm) | 17.2 ± 2.9 | 13.2 | 21.4 |
| Sec (Secchi transparency m) | 4.5 ± 1.5 | 2.9 | 8.3 |
| Kpar (Light attenuation m−1) | 0.73 ± 0.25 | 0.33 | 1.09 |
| Trophic state indices (TSI) | |||
| TSI-Sd | 39.2 ± 4.8 | 29.0 | 46.6 |
| TSI-Chla | 37.3 ± 5.7 | 27.4 | 47.7 |
| TSI-TP | 32.6 ± 6.9 | 18.6 | 46.8 |
| Taxonomic Groups | Species | Functional Groups | SCBD | SCBD (%) |
|---|---|---|---|---|
| ROTIFERA | ||||
| Asplanchnidae | Asplanchna herricki | RC | 0.015 | 0.228 |
| Asplanchna brightwelli | RC | 0.013 | 0.200 | |
| Asplanchna priodonta | RC | 0.040 | 0.627 | |
| Brachionidae | Kellicottia longispina | RH | 0.786 | 12.103 |
| Kellicottia bostoniensis | RH | 0.483 | 7.434 | |
| Keratella taurocephala | RH | 0.298 | 4.593 | |
| Keratella cochlearis | RH | 0.375 | 5.774 | |
| Keratella hiemalis | RH | 0.019 | 0.289 | |
| Conochilidae | Conochilus unicornis | RH | 1.355 | 20.862 |
| Conochiloides sp. | RH | 0.006 | 0.091 | |
| Gastropidae | Gastropus stylifer | RH | 0.007 | 0.105 |
| Ascomorpha saltans | RH | 0.029 | 0.444 | |
| Ascomorpha ecaudis | RH | 0.011 | 0.167 | |
| Synchaetidae | Polyarthra vulgaris | RH | 0.278 | 4.282 |
| Polyarthra major | RH | 0.368 | 5.662 | |
| Ploesoma hudsoni | RH | 0.005 | 0.072 | |
| Synchaeta sp. | RC | 0.023 | 0.351 | |
| Trichocercidae | Trichocerca mucosa | RH | 0.053 | 0.821 |
| Trichocerca elongata | RH | 0.033 | 0.515 | |
| Trichocerca cylindrica | RH | 0.025 | 0.387 | |
| Trichocerca multicrenis | RH | 0.007 | 0.110 | |
| Testudinellide | Pompholyx sulcata | RH | 0.368 | 5.664 |
| Undertermined Rotifera | RH | 0.005 | 0.079 | |
| CLADOCERA | ||||
| Bosminidae | Bosmina sp. | SC | 0.556 | 8.552 |
| Daphiniidae | Ceriodaphnia quadrangula | SC | 0.014 | 0.212 |
| Ceriodaphnia affinis | SC | 0.005 | 0.071 | |
| Ceriodaphnia sp. | SC | 0.008 | 0.118 | |
| Daphnia catawba | LC | 0.371 | 5.713 | |
| Daphnia ambigua | LC | 0.063 | 0.971 | |
| Daphnia longiremis | LC | 0.037 | 0.575 | |
| Daphnia sp. | LC | 0.003 | 0.049 | |
| Holopediidae | Holopedium gibberum (glacialis) | LC | 0.227 | 3.491 |
| Leptodoridae | Leptodora kindtii | PC | 0.001 | 0.005 |
| Sididae | Diaphanosoma birgei | SC | 0.213 | 3.281 |
| Diaphanosoma brachyurum | SC | 0.193 | 2.975 | |
| COPEPODA CYCLOPOIDA | ||||
| Cyclopidae | Eucyclops speratus | CCY—CYC | 0.025 | 0.383 |
| Orthocyclops modestus | CCY—CYC | 0.003 | 0.042 | |
| Cyclops scutifer | CCY—CYC | 0.021 | 0.319 | |
| Mesocyclops edax | CCY—CYC | 0.034 | 0.522 | |
| COPEPODA CALANOIDA | ||||
| Diaptomidae | Leptodiaptomus minutus | CCA—DIA | 0.035 | 0.543 |
| Leptodiaptomus siciloides | CCA—DIA | 0.003 | 0.053 | |
| Temoridae | Aglaodiaptomus spatulocrenatus | CCA—DIA | 0.049 | 0.757 |
| Epischura lacustris | CCA—EPI | 0.033 | 0.507 |
| Lakes | Species Richness (α) | Contribution to β Diversity (LCBD) | ||||||
|---|---|---|---|---|---|---|---|---|
| Rot. | Clad. | Cop. | Zoo. | Rot. | Clad. | Cop. | Zoo. | |
| Allen | 13 | 5 | 2 | 20 | 3.70 | 1.92 | 0.10 | 5.73 |
| Oberon | 5 | 2 | 1 | 8 | 7.19 | 2.70 | 0.08 | 9.97 |
| Ernie | 13 | 3 | 1 | 17 | 6.96 | 1.17 | 0.05 | 8.18 |
| Herman | 6 | 6 | 0 | 12 | 7.20 | 1.44 | 0.06 | 8.70 |
| Rossi | 7 | 3 | 1 | 11 | 6.17 | 1.40 | 0.08 | 7.65 |
| Savane | 8 | 6 | 2 | 16 | 3.36 | 0.32 | 0.24 | 3.92 |
| Trap | 2 | 5 | 3 | 10 | 3.31 | 5.76 | 0.99 | 10.06 |
| Ariel | 10 | 5 | 4 | 19 | 3.42 | 0.51 | 0.10 | 4.03 |
| Buri | 8 | 5 | 1 | 14 | 3.83 | 1.60 | 0.16 | 5.60 |
| Brochet | 10 | 4 | 2 | 16 | 2.30 | 0.58 | 0.38 | 3.26 |
| Houdet | 10 | 9 | 2 | 21 | 4.34 | 1.26 | 0.11 | 5.71 |
| Desjardins | 5 | 5 | 1 | 11 | 9.63 | 2.20 | 0.09 | 11.93 |
| Monroe | 11 | 4 | 0 | 15 | 6.39 | 1.34 | 0.06 | 7.79 |
| Sables | 4 | 7 | 3 | 14 | 3.06 | 3.79 | 0.62 | 7.47 |
| % | 50 | 37.5 | 12.5 | 100 | 70.86 | 26.01 | 3.13 | 100.00 |
| Mean | 8 | 5 | 2 | 15 | 5.06 | 1.86 | 0.22 | 7.14 |
| Biotic Indices-2009 | Codes | Factors | Mean ± Sd | Min. | Max. |
|---|---|---|---|---|---|
| Large Cladocera/Total Cladocera | LCL/TCL | E-, T+, P- | 0.43 ± 0.28 | 0.00 | 0.85 |
| Daphnia/Total Cladocera | Da/TCL | E-, T+, P- | 0.25 ± 0.21 | 0.00 | 0.59 |
| Cladocera/Copepoda | CL/CO | E+ | 0.97 ± 1.59 | 0.08 | 6.30 |
| Daphnia/Crustacea | Da/CR | E-, T+, A-, P- | 0.06 ± 0.06 | 0.00 | 0.21 |
| Abundance Bosmina | Abo | E+, A+, P+ | 2.81 ± 3.83 | 0.00 | 12.34 |
| Abundance Holopedium | Aho | E-, A+ | 1.44 ± 3.04 | 0.00 | 11.56 |
| Abundance Daphnia | Ada | E-, T+, A-, P- | 1.23 ± 1.14 | 0.00 | 3.57 |
| Cladocera/Calanoida | CL/CA | E+ | 3.07 ± 6.44 | 0.08 | 24.38 |
| Abundance Crustacea | ACR | E+ | 28.33 ± 30.56 | 2.83 | 98.75 |
| Cyclopoida/Calanoida | CY/CA | E+ | 1.12 ± 2.65 | 0.02 | 10.00 |
| Rotifera/Crustacea | RO/CR | E+ | 1.39 ± 1.60 | 0.04 | 6.25 |
| Abundance Rotifera | ARO | E+ | 20.81 ± 17.08 | 0.91 | 49.64 |
| Richness Rotifera | RRO | E+ | 8 ± 3 | 2 | 13 |
| Richness Cladocera | RCL | E-, P-, T+ | 5 ± 2 | 2 | 10 |
| Richness Copepoda | RCO | E+ | 2 ± 1 | 0 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinel-Alloul, B.; Chemli, A.; Taranu, Z.E.; Bertolo, A. Using the Diversity, Taxonomic and Functional Attributes of a Zooplankton Community to Determine Lake Environmental Typology in the Natural Southern Boreal Lakes (Québec, Canada). Water 2022, 14, 578. https://doi.org/10.3390/w14040578
Pinel-Alloul B, Chemli A, Taranu ZE, Bertolo A. Using the Diversity, Taxonomic and Functional Attributes of a Zooplankton Community to Determine Lake Environmental Typology in the Natural Southern Boreal Lakes (Québec, Canada). Water. 2022; 14(4):578. https://doi.org/10.3390/w14040578
Chicago/Turabian StylePinel-Alloul, Bernadette, Abir Chemli, Zofia E. Taranu, and Andrea Bertolo. 2022. "Using the Diversity, Taxonomic and Functional Attributes of a Zooplankton Community to Determine Lake Environmental Typology in the Natural Southern Boreal Lakes (Québec, Canada)" Water 14, no. 4: 578. https://doi.org/10.3390/w14040578
APA StylePinel-Alloul, B., Chemli, A., Taranu, Z. E., & Bertolo, A. (2022). Using the Diversity, Taxonomic and Functional Attributes of a Zooplankton Community to Determine Lake Environmental Typology in the Natural Southern Boreal Lakes (Québec, Canada). Water, 14(4), 578. https://doi.org/10.3390/w14040578






