Combination of Microscopic Tests of the Activated Sludge and Effluent Quality for More Efficient On-Site Treatment
Abstract
:1. Introduction
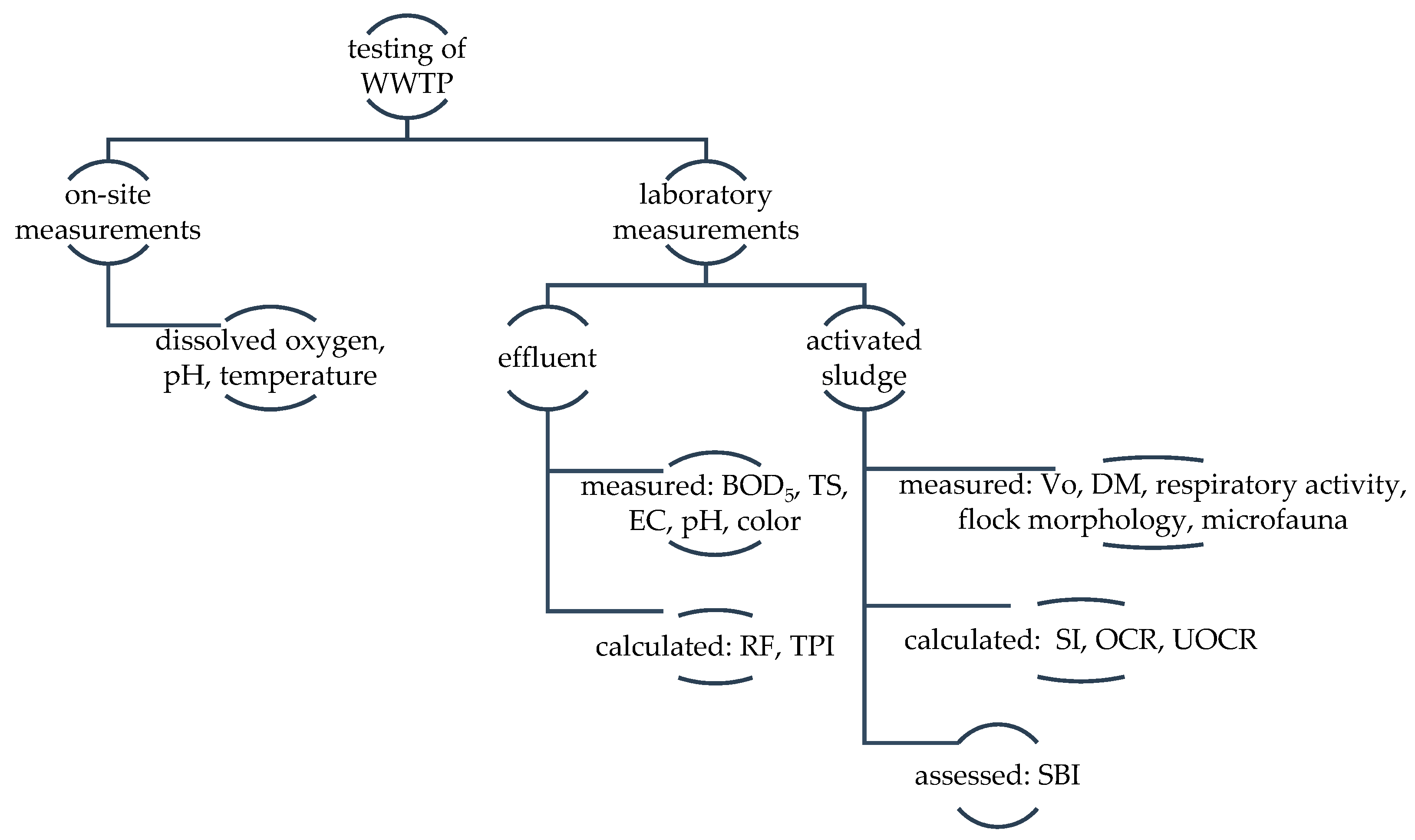
2. Materials and Methods
2.1. Effluent Characteristics
2.2. Activated Sludge Characteristic
2.2.1. At-Site Dissolved Oxygen Measurement in Aeration Chamber
2.2.2. Activated Sludge—Physical Parameters
2.2.3. Activated Sludge—Respiratory Activity
2.2.4. Microscopic Analysis of Activated Sludge
3. Results and Discussion
3.1. Quality of Effluent
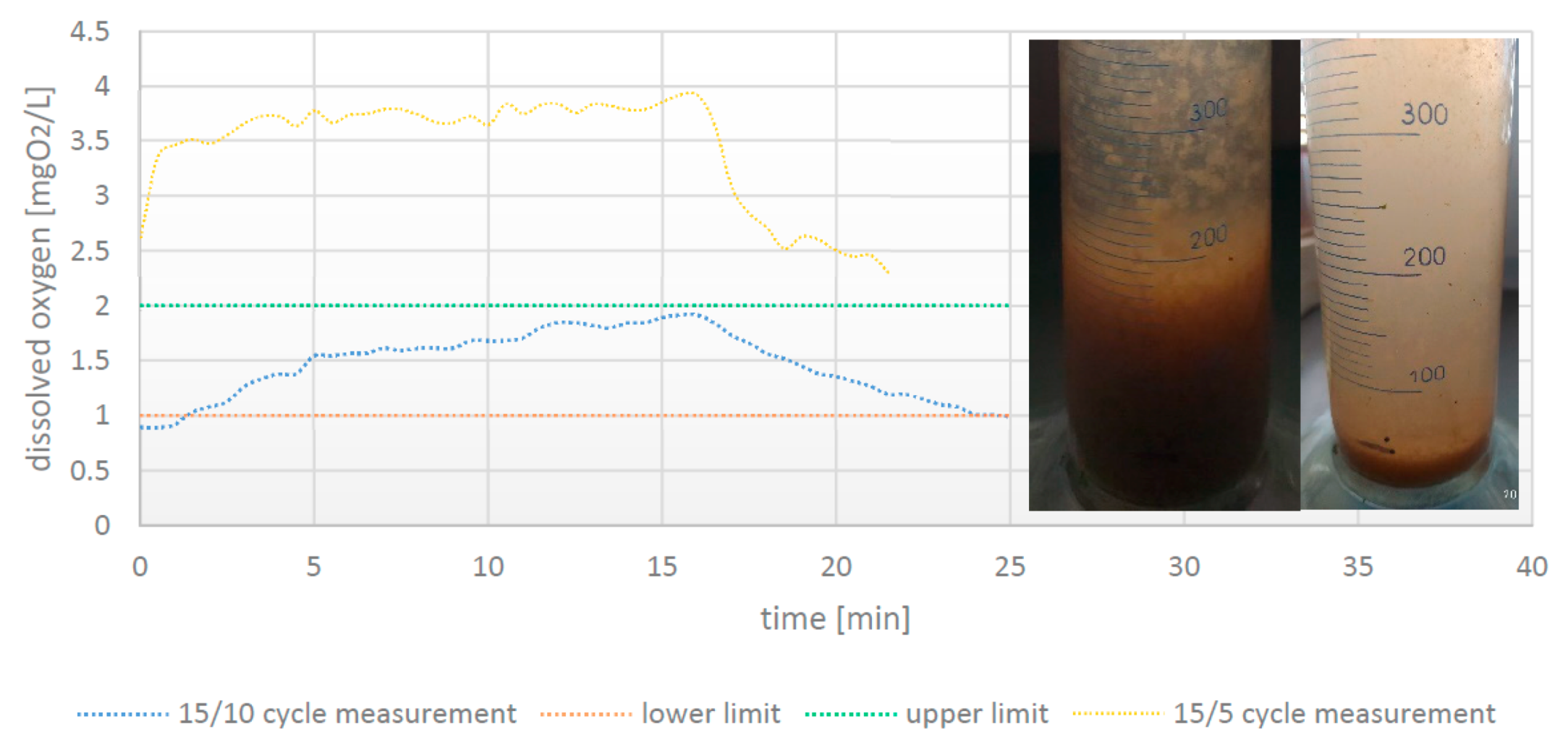
3.2. Dissolved Oxygen Content in Aeration Chamber
3.3. Respiratory Activity
3.4. Physical Parameters of Sludge
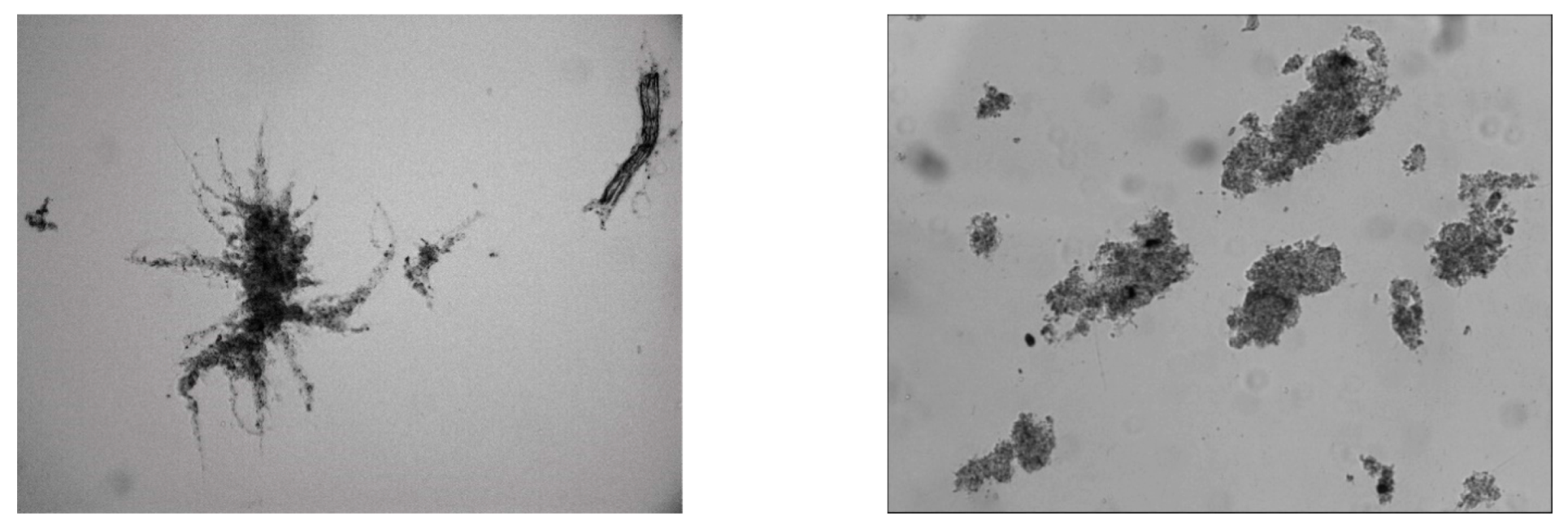
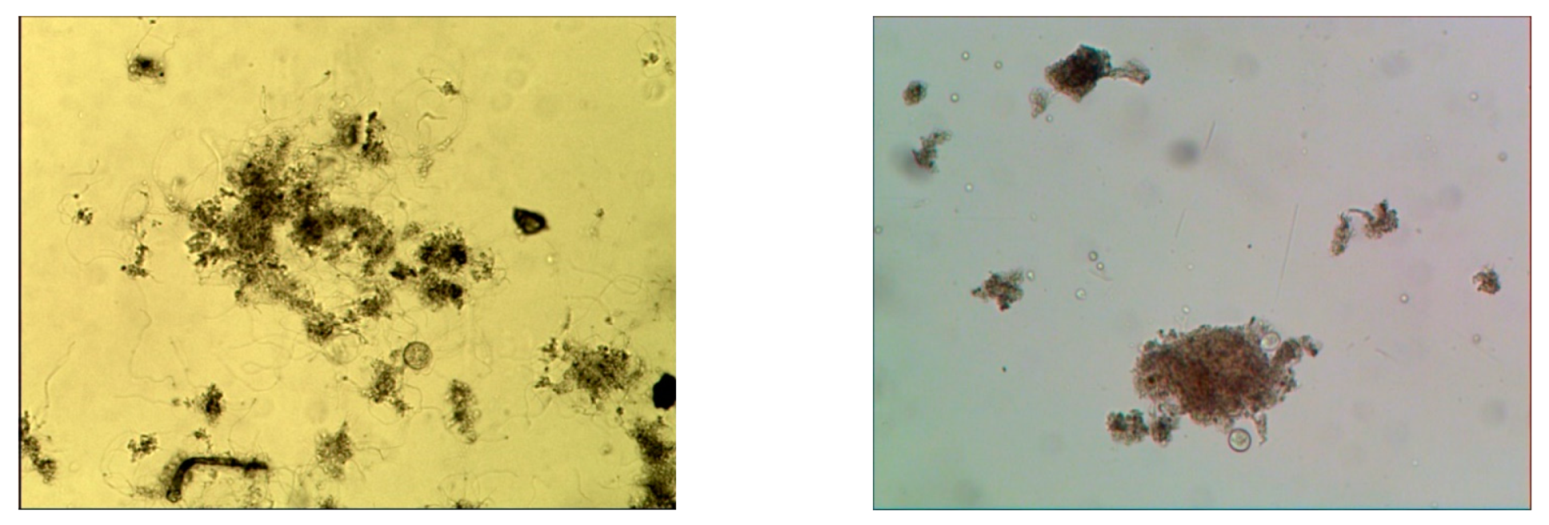
3.5. Microscopic Analysis
3.5.1. Flocks Morphology
3.5.2. Indication of Microfauna
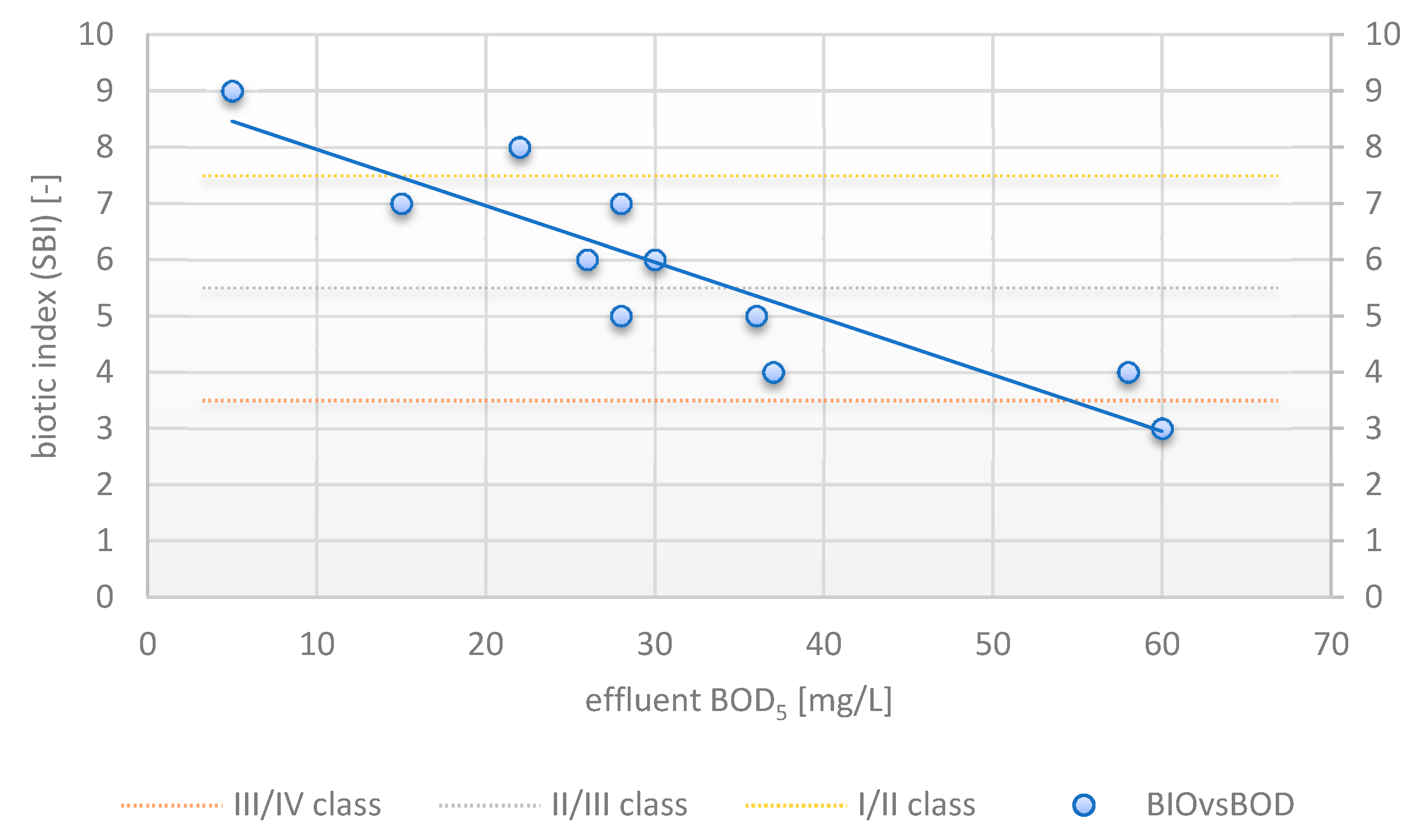
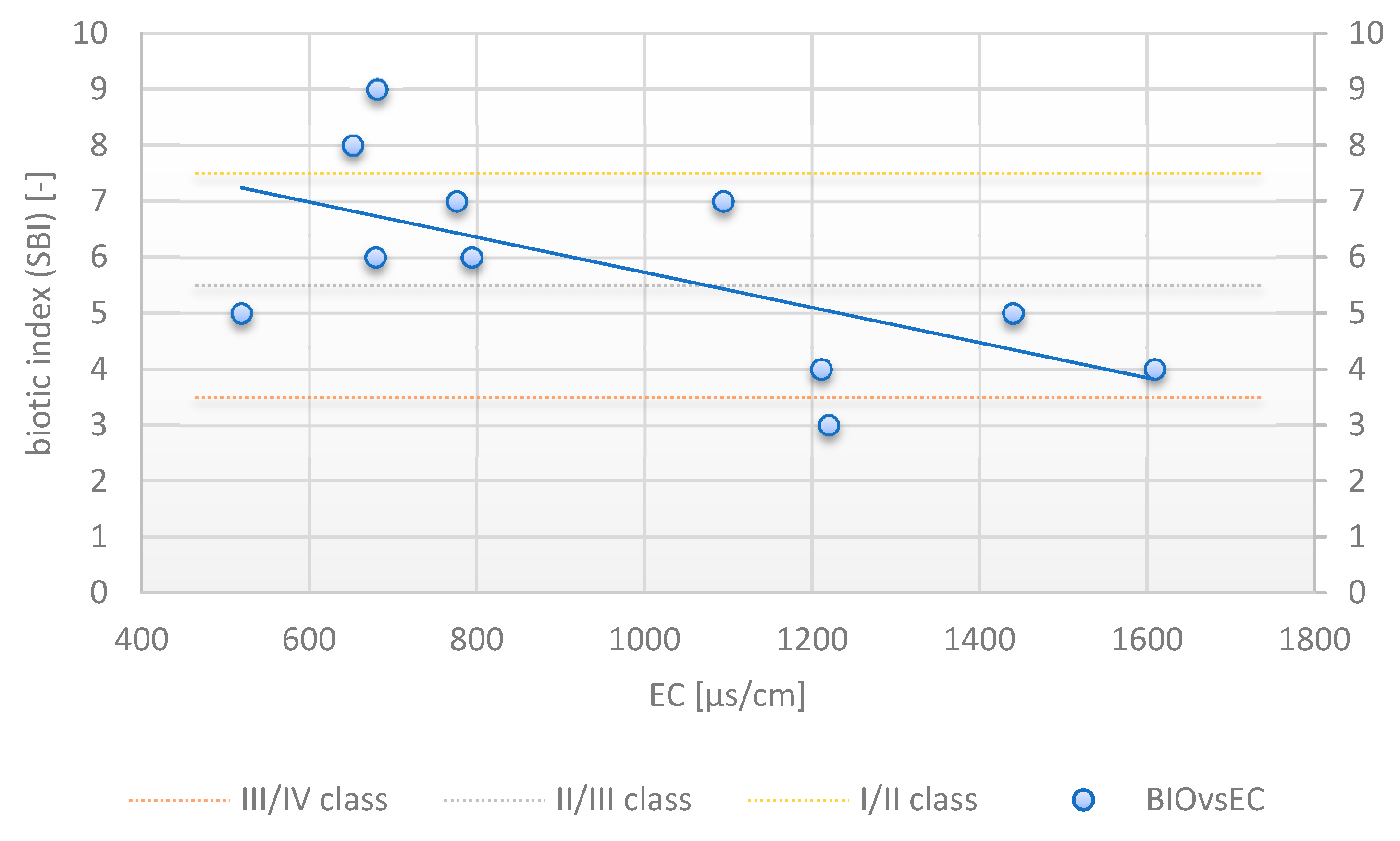
3.6. Correlation between SBI and Effluent Quality
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surerus, V.; Giordano, G.; Teixeira, L.A.C. Activated Sludge Inhibition Capacity Index. Braz. J. Chem. Eng. 2014, 31, 385–392. [Google Scholar] [CrossRef]
- Holenda, B.; Domokos, E.; Rédey, Á.; Fazakas, J. Dissolved Oxygen Control of the Activated Sludge Wastewater Treatment Process Using Model Predictive Control. Comput. Chem. Eng. 2008, 32, 1270–1278. [Google Scholar] [CrossRef]
- European Standards. EN 12566-3 Small Wastewater Treatment Systems for up to 50 PT Packaged and/or Site Assembled Domestic Wastewater Treatment Plants. Available online: https://www.en-standard.eu/bs-en-12566-3-2016-small-wastewater-treatment-systems-for-up-to-50-pt-packaged-and-or-site-assembled-domestic-wastewater-treatment-plants/ (accessed on 28 December 2021).
- Karczmarczyk, A.; Bus, A.; Baryła, A. Assessment of the Efficiency, Environmental and Economic Effects of Compact Type On-Site Wastewater Treatment Plants—Results from Random Testing. Sustainability 2021, 13, 982. [Google Scholar] [CrossRef]
- Scott, W.C.; Breed, C.S.; Haddad, S.P.; Burket, S.R.; Saari, G.N.; Pearce, P.J.; Chambliss, C.K.; Brooks, B.W. Spatial and Temporal Influence of Onsite Wastewater Treatment Systems, Centralized Effluent Discharge, and Tides on Aquatic Hazards of Nutrients, Indicator Bacteria, and Pharmaceuticals in a Coastal Bayou. Sci. Total Environ. 2019, 650, 354–364. [Google Scholar] [CrossRef]
- Schneider, M.Y.; Furrer, V.; Sprenger, E.; Carbajal, J.P.; Villez, K.; Maurer, M. Benchmarking Soft Sensors for Remote Monitoring of On-Site Wastewater Treatment Plants. Environ. Sci. Technol. 2020, 54, 10840–10849. [Google Scholar] [CrossRef]
- Du, B.; Price, A.E.; Scott, W.C.; Kristofco, L.A.; Ramirez, A.J.; Chambliss, C.K.; Yelderman, J.C.; Brooks, B.W. Comparison of Contaminants of Emerging Concern Removal, Discharge, and Water Quality Hazards among Centralized and on-Site Wastewater Treatment System Effluents Receiving Common Wastewater Influent. Sci. Total Environ. 2014, 466–467, 976–984. [Google Scholar] [CrossRef]
- Gunady, M.; Shishkina, N.; Tan, H.; Rodriguez, C. A Review of On-Site Wastewater Treatment Systems in Western Australia from 1997 to 2011. J. Environ. Public Health 2015, 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, P.; Ghangrekar, M.M.; Rao, S. Low efficiency of sewage treatment plants due to unskilled operations in India. Environ. Chem. Lett. 2016, 14, 407–416. [Google Scholar] [CrossRef]
- Naughton, O.; Hynds, P.D. Public awareness, behaviours and attitudes towards domestic wastewater treatment systems in the Republic of Ireland. J. Hydrol. 2014, 518, 108–119. [Google Scholar] [CrossRef]
- Madoni, P. A Sludge Biotic Index (SBI) for the Evaluation of the Biological Performance of Activated Sludge Plants Based on the Microfauna Analysis. Water Res. 1994, 28, 67–75. [Google Scholar] [CrossRef]
- de Celis, M.; Belda, I.; Ortiz-Álvarez, R.; Arregui, L.; Marquina, D.; Serrano, S.; Santos, A. Tuning up Microbiome Analysis to Monitor WWTPs’ Biological Reactors Functioning. Sci. Rep. 2020, 10, 4079. [Google Scholar] [CrossRef]
- Ostoich, M.; Serena, F.; Zacchello, C.; Falletti, L.; Zambon, M.; Tomiato, L. Discharge Quality from Municipal Wastewater Treatment Plants and the Sludge Biotic Index for Activated Sludge: Integrative Assessment. Water Pract. Technol. 2017, 12, 857–870. [Google Scholar] [CrossRef]
- Sowinska, A.; Pawlak, M.; Mazurkiewicz, J.; Pacholska, M. Comparison of the Results from Microscopic Tests Concerning the Quality of Activated Sludge and Effluent. Water 2017, 9, 918. [Google Scholar] [CrossRef] [Green Version]
- Pedrazzani, R.; Menoni, L.; Nembrini, S.; Manili, L.; Bertanza, G. Suitability of Sludge Biotic Index (SBI), Sludge Index (SI) and Filamentous Bacteria Analysis for Assessing Activated Sludge Process Performance: The Case of Piggery Slaughterhouse Wastewater. J. Ind. Microbiol. Biotechnol. 2016, 43, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, J.; Moreno, B.; Pérez, J.; Gómez, M.A. Applicability of the Sludge Biotic Index (SBI) for MBR Activated Sludge Control. J. Hazard. Mater. 2009, 167, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Drzewicki, A.; Kulikowska, D. Limitation of Sludge Biotic Index Application for Control of a Wastewater Treatment Plant Working with Shock Organic and Ammonium Loadings. Eur. J. Protistol. 2011, 47, 287–294. [Google Scholar] [CrossRef]
- Dymaczewski, Z. (Ed.) A Sewage Treatment Plant Operator’s Guide; PZITS Poznań: Poznań, Poland, 2011; ISBN 978-83-89696-38-X. (In Polish) [Google Scholar]
- VH TECH 2014. Work Regulations (for the Functioning of the Process) Biological Sewage Treatment Plant Type: VH4L-VH50L. Available online: http://www.ekohouse-oczyszczalnie.pl/przydomowe-oczyszczalnie-sciekow/typoszereg-vh4l-v12l.html (accessed on 30 December 2021).
- Regulation of The Minister of Maritime Economy and Inland Navigation of July 12, 2019 on Substances Particularly Harmful to the Aquatic Environment and Conditions to Be Met when Discharging Wastewater into Waters or Land, and also When Draining Rainwater or Meltwater to Waters or to Water Devices; Journal of Laws: Warsaw, Poland, 2019; Volume item 1311. (In Polish)
- Cupak, A.; Chmielowski, K.; Bugajski, P.; Dacewicz, E. Assessment of Efficiency of Rural Sewage Treatment Plant with Bioreactor. Acta Sci. Pol. Form. Circumiectus 2019, 18, 137–143. [Google Scholar] [CrossRef]
- Stier, E.; Fischer, M. A Handy Guide to the Operation of a Sewage Treatment Plant; Seidel-Przywecki: Poznań, Poland, 1998; ISBN 83-909960-0-6. [Google Scholar]
- Eikelboom, D.H. Process Control of Activated Sludge Plants by Microscopic Invastigation; IWA Publishing: London, UK, 2000. [Google Scholar]
- Wilén, B.-M.; Balmér, P. The Effect of Dissolved Oxygen Concentration on the Structure, Size and Size Distribution of Activated Sludge Flocs. Water Res. 1999, 33, 391–400. [Google Scholar] [CrossRef]
- Lehtoranta, S.; Vilpas, R.; Mattila, T.J. Comparison of carbon footprints and eutrophication impacts of rural on-site wastewater treatment plants in Finland. J. Clean. Prod. 2014, 65, 439–446. [Google Scholar] [CrossRef]
- Dubber, D.; Gill, L. Application of On-Site Wastewater Treatment in Ireland and Perspectives on Its Sustainability. Sustainability 2014, 6, 1623–1642. [Google Scholar] [CrossRef] [Green Version]
- Głodniok, M.; Zdebik, D. Biological control of industrial wastewater treatment using in-situ respirometric measuring of activated sludge. Inżynieria Sr. 2011, 142, 63–74. Available online: https://zbc.uz.zgora.pl/dlibra/publication/51738/edition/45497 (accessed on 30 December 2021). (In Polish).
- Madoni, P. Protozoa in Wastewater Treatment Processes: A Minireview. Ital. J. Zool. 2011, 78, 3–11. [Google Scholar] [CrossRef]
- Miksch, K. (Ed.) Wastewater Biotechnology; Wydawnictwo Politechniki Sląskiej: Gliwice, Poland, 2000; ISSN 0434-0825. (In Polish) [Google Scholar]
- Kołwzan, B.; Adamiak, W.; Grabas, K.; Pawełczyk, A. Fundamentals of Microbiology in Environmental Protection; Oficyna Wydawnicza Politechniki Wrocławskiej: Wrocław, Poland, 2006; ISBN 83-7085-992-5. (In Polish) [Google Scholar]

| Microoganisms | Flocks | Sludge Characteristic | Turbidity | Effluent BOD5 (mg/L) | ||
|---|---|---|---|---|---|---|
| Group of Protozoa | Genus/Species | Size/Structure/Color | Sedimentation Capacity | |||
| amoebae | naked | - | - | overloaded | high | >150 |
| flagellates | Bodo | small to medium, open | low | overloaded | high | >100 |
| Mastigophora | small to medium, open | low | overloaded | high | >75 | |
| floating ciliates | Paramecium | small to medium, light brown | sufficient to good | slightly overloaded | moderately high | 20 ÷ 50 |
| Uronema nigricans | small to medium, light brown | sufficient | overloaded | moderate | 40 ÷ 50 | |
| Tetrahymena pyriformis | small to medium, light brown to brown | sufficient to poor | overloaded | moderate to low | 30 ÷ 60 | |
| Aspidisca costata | medium to large, brown to dark brown | sufficient to good | slightly overloaded | low | 20 ÷ 40 | |
| attached ciliates | Vorticella | medium to large, dark brown | good to very good | very good | - | 10 ÷ 30 |
| Opercularia | medium to small, dark brown | very good to excellent | very good | - | 5 ÷ 15 | |
| Epistylis | medium to small, dark brown | very good to excellent | very good | - | 5 ÷ 15 | |
| Suctoria | small to medium, light brown | excellent | underloaded | insignificant | 2 ÷ 10 | |
| Parameter | Value | Unit |
|---|---|---|
| Population Equivalent (PE) | Up to 4 | PE |
| Average daily flow (Q) | 0.54 | m3/d |
| Load of BOD5 (LBOD5) | 0.24 | kg/d |
| Diameter (D) | 1.00 | m |
| Height of the tank (H) | 1.50 | m |
| Height of the inlet (Vp) | 1.30 | m |
| Height of the outlet (Vo) | 1.15 | m |
| Quality Index | Assumed by the Manufacturer [1] | PL Regulation * [20] | |
|---|---|---|---|
| Effluent Discharged to Water | Effluent Discharged to Soil | ||
| BOD5 (mg/L) | 5 | 40 | min. reduction of 20% |
| COD (mg/L) | 50 | 150 | - |
| TS (mg/L) | 15 | 50 | min. reduction of 50% |
| N (mg/L) | - | 30 ** | - |
| P (mg/L) | - | 5 ** | - |
| SBI Value | SBI Class | Sludge Quality and Treatment Effect |
|---|---|---|
| 0–3 | IV | insufficient biological treatment in aeration chambers, poor treatment effect |
| 4–5 | III | poor biological purification in aeration chambers, poor treatment effect |
| 6–7 | II | well inhabited and stable, biological activity slightly reduced, good treatment effect |
| 8–10 | I | very well inhabited and stabilized, excellent biological activity, very high treatment effect |
| Parameter | Effluent Quality Characteristic (mg/L) | Reliability Indicators | |||
|---|---|---|---|---|---|
| N | MEAN | RF | TPI | ||
| BOD5 | 48 | 39.10 | 0.98 | 0.61 | |
| TS | 63 | 18.35 | 0.37 | 0.84 | |
| Parameter | N | MEAN | Optimal Values | |
|---|---|---|---|---|
| Vo (mL/L) | 11 | 108.89 | 300 ÷ 700 | |
| DM (g/L) | 11 | 0.93 | 3 ÷ 7 | |
| SI (mL/g) | 11 | 141.69 | 50 ÷ 150 |
| Sample no. | Dominating Key Groups of Microfauna | SBI Value/Class | Flock Morphology Shape/Structure/Strength/Size |
|---|---|---|---|
| 1 | Swimming bacterivorous ciliates | 4/III | rounded/open/weak/medium |
| 2 | Swimming bacterivorous ciliates | 3/II | rounded/open/weak/medium |
| 3 | Crawling + sessile ciliates and/or testate amoebae | 5/III | irregular/compacted/firm/medium to small |
| 4 | Crawling + sessile ciliates and/or testate amoebae | 6/II | irregular/compacted/firm/large |
| 5 | Crawling + sessile ciliates and/or testate amoebae | 7/II | irregular/open/weak/medium to small |
| 6 | Crawling + sessile ciliates and/or testate amoebae | 6/II | rounded/open/firm/large |
| 7 | Swimming bacterivorous ciliates | 4/III | rounded/compacted/weak/medium |
| 8 | Crawling + sessile ciliates and/or testate amoebae | 9/I | rounded/open/weak/medium to large |
| 9 | Crawling + sessile ciliates and/or testate amoebae | 8/I | rounded/open/weak/medium |
| 10 | Crawling + sessile ciliates and/or testate amoebae | 7/II | rounded/compacted/firm/medium to large |
| 11 | Opercularia spp. | 5/III | rounded/open/weak/medium to large |
| BOD5 (mg/L) | TS (mg/L) | EC (µs/cm) | Vo (mL/L) | DM (g/L) | SI (mL/g) | SBI | |
|---|---|---|---|---|---|---|---|
| BOD5 (mg/L) | 1.000000 | 0.578482 | 0.688517 | 0.271220 | 0.432850 | −0.035482 | −0.927747 |
| TS (mg/L) | 0.578482 | 1.000000 | 0.520251 | −0.359692 | 0.453229 | −0.646216 | −0.564339 |
| EC (µs/cm) | 0.688517 | 0.520251 | 1.000000 | 0.258669 | 0.158856 | 0.129761 | −0.747845 |
| Vo (mL/L) | 0.271220 | −0.359692 | 0.258669 | 1.000000 | 0.176118 | 0.697091 | −0.302407 |
| DM (g/L) | 0.432850 | 0.453229 | 0.158856 | 0.176118 | 1.000000 | −0.546505 | −0.158357 |
| SI (mL/g) | −0.035482 | −0.646216 | 0.129761 | 0.697091 | −0.546505 | 1.000000 | −0.140440 |
| SBI | −0.927747 | −0.564339 | −0.747845 | −0.302407 | −0.158357 | −0.140440 | 1.000000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karczmarczyk, A.; Kowalik, W. Combination of Microscopic Tests of the Activated Sludge and Effluent Quality for More Efficient On-Site Treatment. Water 2022, 14, 489. https://doi.org/10.3390/w14030489
Karczmarczyk A, Kowalik W. Combination of Microscopic Tests of the Activated Sludge and Effluent Quality for More Efficient On-Site Treatment. Water. 2022; 14(3):489. https://doi.org/10.3390/w14030489
Chicago/Turabian StyleKarczmarczyk, Agnieszka, and Weronika Kowalik. 2022. "Combination of Microscopic Tests of the Activated Sludge and Effluent Quality for More Efficient On-Site Treatment" Water 14, no. 3: 489. https://doi.org/10.3390/w14030489
APA StyleKarczmarczyk, A., & Kowalik, W. (2022). Combination of Microscopic Tests of the Activated Sludge and Effluent Quality for More Efficient On-Site Treatment. Water, 14(3), 489. https://doi.org/10.3390/w14030489





